Abstract
This study aimed to investigate the impact of anthropogenic stressors (landfilling, navigation for transport of goods, cooling in fossil fuel, urbanization, industrial expansion, agriculture activities, and recreational activities) on environmental variables, microbiological quality, and sediment properties using benthic macroinvertebrates as a bioindicator within Lagos Lagoon, Nigeria. Four (4) sampling stations were established with respect to their importance/anthropogenic activities within the Lagos Lagoon. Surface water, bottom substrates, and benthic macroinvertebrate fauna samples were collected bimonthly from each sampling station for a year and analyzed using appropriate standard methods and procedures. The highest pH range of 7.96–8.01 (7.98 ± 2.35) was recorded at Site IV, while the lowest range of 6.41–7.01 (6.15 ± 1.14) was observed at Site II, and there was a significant difference (p < 0.05) among the pH mean values across the sites. High values of salinity, chloride, sodium, COD, BOD, manganese, nickel, cadmium, and nitrate were recorded among the surface water physicochemical parameters, which were above WHO (2011) permissible limits, while the high concentrations of toxic metals (Pb, Cr, Zn, and Cd) was recorded in sediment. A total of 26 species of benthic macroinvertebrates were recorded during this study, which belongs to eight (8) classes. Gastropoda recorded the highest percentage contribution of 39.12%, followed by polychaeta accounting for 30.34%, while malacostraca contributed 2.63%. The highest abundance of macroinvertebrates was recorded at Site I (256 Indiv/m2), followed by Site IV (252 Indiv/m2), and the least was observed at Site II (195 Indiv/m2). Based on the results of the physico-chemical, heavy metals, microbial quality, and macroinvertebrates assemblage obtained from this study revealed the adverse effect of anthropogenic activities on water quality degradation. It plays a significant role in the distribution and diversity of benthic macroinvertebrates in an aquatic environment.
Keywords: Benthic macroinvertebrate, Anthropogenic stressors, Toxic metals, Lagoon, Bioindicator, Physicochemical, Sediment, And aquatic environment
1. Introduction
Coastal regions are the center of economic activities in most countries due to their proximity to the seas, which aids overseas trade [1]. They link the sea and the land, facilitating the international trade of goods, resources, and energy [2]. As a result, neighboring residents in search of employment and success move to the coastal areas, making these regions densely populated. An increase in population leads to land shortage, and numerous land reclamation projects have been implemented to meet the exponential demand for space and land [3]. Several coastal nations, such as the USA, Netherlands, Japan, and Nigeria, have been engaged in extensive sea enclosing and reclamation for urban expansion and industrial activities [[4], [5], [6], [7]]. In Nigeria, the world’s most populous African nation with 202 million people, about 25% of the population lives in the seven coastal states, Lagos, Ondo, Delta, Bayelsa, Rivers, Akwa Ibom, and Cross Rivers, bordering the Atlantic Ocean [8].
Land reclamation is one factor affecting the overall quality of the environment [2]. Their actual effects on aquatic ecosystems are poorly understood, making it difficult to understand their impacts [2]. Studies have indicated that the effects of land reclamation include the formation of new habitats for some species and the permanent loss of protected areas, loss of biodiversity, and severe adverse effects on water quality, sediment structure, and benthic organisms [9,10]. Although reclamation has yielded a vast amount of land for the construction of recreation centers, buildings, and other structures, it has also caused a decline in coastal and intertidal ecosystems as a result of the short- and long-term impacts on benthic macroinvertebrates, sediment characteristics, water quality, and fisheries [11]. Sediment disturbance during land reclamation alters the chemical composition of the sediment, such as a rise in the redox potential (Eh). The sediment's pH decreases due to the oxidation of sulphide due to the oxidation of the anoxic material, which increases microbial (thiobacteria) activity [12]. The degree of oxidation and sulphide concentration affect the sediment's pH level. An alteration in the physicochemical characteristics of the sediment can intricate the assembling and moving of metals, mainly from sulphide-bound complexes (FeS/MnS) [13]. However, if there are no significant changes in Eh and pH, especially during the partial oxidation of sediments, the transfer and movement of metals are negligible [12]. The resuspension of anoxic sediment affects the desorption rates of metals adsorbed to sulphide, such as manganese sulfide and iron sulfide, which are quickly oxidized following sediment resuspension due to their relative solubility in oxic conditions [14]. The sulphide-bound metals, which are more stable, such as CuS and pyrite, are unlikely to be oxidized immediately due to their slower oxidation kinetics [15]. Iron and Manganese are spontaneously reprecipitated and built up as insoluble hydroxides/oxides that can release metals into the aquatic environment and adsorb by organisms at different rates [15]. Studies have shown that metals release are multisegmented, with one process controlling early resuspension and another controlling long-term release [12]. Therefore, periodic or continuous cycling processes result in significant fluctuations in the concentrations of contaminants [16].
Over the years, aquatic ecosystems have been subjected to various human stressors, impacting the hydromorphology, organic compounds build-up, sediment characteristics, and physicochemical qualities of water bodies [17]. Accurate estimation of land reclamation effects is essential for managing aquatic life and ecological preservation since the impact of land reclamation on the coastal environment is irreversible and cannot be overemphasized. The most effective ways to assess the degree of habitat alteration include biomonitoring of water quality and determination of physicochemical properties and contamination levels [15,17,18]. Benthic macroinvertebrates are frequently used as pollution bioindicators to assess anthropogenic operations' impact on aquatic environments, such as dredging and reclamation activities [[19], [20], [21]]. Benthic macroinvertebrates reside on the floor of water bodies, where they either spend their entire life or migrate from the water to become terrestrial insects. [19]. Different benthic macroinvertebrates have varying levels of contamination tolerance, which influences their community structure; therefore, these organisms can be found in all water bodies [19]. Benthic macroinvertebrates are among the quick and inexpensive biomonitoring resources since they are often localized throughout their life and are simple to obtain [20]. One of the most practical and realistic approaches to assessing the ecological quality of water bodies is to determine these macroinvertebrates' abundance, diversity, and distribution.
Quantifying the physicochemical properties of the water body and environmental contaminants does not reflect the exact status of the water. To have a holistic representation, monitoring the biological composition of the water body is essential. Since anthropogenic influences can affect the aquatic environment in many ways, such pollutants may alter the hydrological regime, and the water's physical and chemical nature may change. This study seeks to investigate the impact of anthropogenic stressors on Lagos Lagoon water quality, microbiological quality, and sediment properties using benthic macroinvertebrates as bio-monitors, which will provide information on the intensity of adverse effects of human activities on the aquatic environment. Data obtained from this study will help to understand the present status of the water quality, sediment composition, and distribution of benthic macroinvertebrates and also provide data for further research into the succession of aquatic organisms in polluted water bodies.
2. Materials and methods
2.1. Study area
The study area is the Lagos Lagoon, located on the narrow coastal plain of the Bight of Benin in Southwestern Nigeria. It is bordered on the north and east by Ogun State, on the west by Benin Republic, and spans about 180 km along the Atlantic Ocean coast. The longitude (003°23′ E to 003°40′ E) and the latitude (06°22′ N to 06°38′ N) define the central body of the Lagoon. It is the largest of the Gulf of Guinea Coast's four lagoon systems [22]. The shallow water in the area is between 0.5 and 2 m deep, with some dredged areas of the Lagos harbor reaching 18–25 m. The tide is small, ranging from 0.3 to 1.3 m, and the creeks are interconnected and relatively shallow. It has two climatic seasons (dry and wet), and the ambient temperatures range from 30 °C to 38 °C [23]. It is influenced by a two-fold rainfall pattern between 1400 mm and 1800 mm per year, with a brief break in August [24]. Lagos' population was 325,218 in 1950, but it increased by 493,779 in 2015, representing a 3.44% yearly increase. According to a United Nations study and the State Regional Master Plan, the State's population was predicted to be 14,862,111 in 2021, with a projected population of 24,418,768 in 2035, a growth rate of 3.33%. By 2050, Lagos' population is expected to double, making it the world's third most populated city [25,26].
2.2. Sampling sites
Within the Lagos Lagoon, fourteen (14) sampling stations were established based on their importance/anthropogenic activities, which were classified into four (4), sites I to IV.
Site I: Station before land reclamation (stations 3, 5, 6, and 7) where residential, agricultural, and industrial wastes are discharged directly into the water, and other activities such as fishing, washing, and bathing are carried out with the depth of 0–3 m. Site II (stations 4, 12, and 13): Filling water-filled areas with clay, heavy rock, and/or cement until the desired height for building construction is reached with the maximum depth of 5 m. Site III (stations 1, 2, 8, 9, 10, and 11): Station after land reclamation site where fishing and other recreational activities are the main activities with a depth between 0 and 15 m. Site IV, where there are lesser human activities with a depth of more than 15 m above the surface water. Surface and underwater samples were collected for both seasons (dry and rainy seasons) from all 14 sampling stations within the study region, as shown in Fig. 1.
Fig. 1.
A map showing sampling locations on the Lagos Lagoon Mainland section, Southwest Nigeria.
2.3. Water sampling
Water, sediment, and macroinvertebrates samples were collected bimonthly from each sampling station for a period of a year (2021), in the morning between 7 a.m. and 11 a.m. to cover both seasons (April to October represents the rainy season while November to March represents dry season). 168 water samples (84 surface and 84 under water samples) were collected in pre-sterilized sampling bottles with a capacity of 2 L (plastic container) from 14 sampling locations. To preserve the natural composition of the samples for analysis, all sampling containers were rinsed with the water sample at each sampling point before collection, sealed and suitably marked, and stored in a thermos cooler to prevent further microbial activities or reactions that can place in the samples following the procedure of Idris et al., [27]. Samples collected were transported to the laboratory for physicochemical and microbiological investigations. Before further investigation, water samples for dissolved oxygen analysis were fixed on site, and microbiological samples were collected in sanitised McCartney sample containers, placed in a cooler containing ice blocks, transported to the laboratory, and stored at 4 °C. Standard protocols were used to test and determine each water sample's physical and chemical properties [27].
2.3.1. Physico-chemical properties analysis
Temperature, pH, total dissolved solids (TDS), total suspended solids (TSS), turbidity, and electrical conductivity (EC) were determined in-situ using the MYRONL Ultrameter II 6232682 m at each sampling station at different time intervals. The MYRONL Ultrameter meter was previously calibrated with buffer solutions and was used for measuring pH, while conductivity was measured with a conductivity meter calibrated with potassium chloride solution. The dissolved oxygen (DO) was determined by PCE-PHD-1 meter at the field. Samples for biological oxygen demand (BOD5) determination were collected in amber bottles and kept in a dark cupboard at room temperature (25 °C) for five (5) days, after which its oxygen content was determined by the Winkler method at the end of the incubation period [28]. Nitrate was determined using Brucine sulphanlic acid method [29]. Chloride was analyzed by Mohr's titration method; the spectrophotometric method was adopted in analyzing phosphate, while chemical oxygen demand (COD) and sulphate were analyzed following the standard procedures outlined by APHA [28].
2.3.2. Heavy metals analysis
Water samples for heavy metals analysis were collected in glass bottles and acidified to a pH of around 2. According to APHA et al. [28] procedures, water samples were digested in the laboratory. Heavy metals (Lead, chromium, cadmium, manganese, nickel, iron, copper, and zinc) in the water samples were determined using an Atomic Absorption Spectrophotometer (ASS) PG, 990 Model at specific wavelengths.
2.3.3. Microbiological analysis
Total heterotrophic bacterial count, total coliform bacterial count, and total fungal count were the microbiological parameters assessed using the serial dilution method and pour plate procedures. Pure bacterial isolates were obtained using the streaking method (i.e., which involved sub-culturing from incubated culture onto a freshly prepared sterile plate to obtain pure fungi isolates using the cutting technique). Incubation time for bacteria and fungi in the water samples was determined using established procedures with acceptable quality control measures [28,[30], [31], [32]]. Colonial, morphological, and biochemical identification methods were used to characterise the bacterial isolates. They were then identified using Bergey's handbook of Determinative Bacteriology and established procedures for microscopic and macroscopic identification of fungal isolates [31]. Sediment samples were serially diluted with sterilized old seawater to culture and count total aerobic heterotrophic bacteria and total fungi. Appropriate dilutions for aerobic heterotrophic bacteria and fungi were plated onto Nutrient Agar (NA) and Sabourund Dextrose Agar (SDA), respectively, using the surface spreading technique. The preparations were in triplicates. The maximum colony development after 7 days incubation period for fungi and 48 h incubation period for bacteria at room temperature (∼28 °C) was used to count colony-forming units.
2.4. Sediment sampling
The sediment samples were grabbed 4–5 times using Van-Veen grab of 0.04 m2 area (0.2 m × 0.2 m) from the waterbed at each sampling location. Samples were kept in a well-labeled polyethene bag, stored in a cooler at 4 °C, and transported to the laboratory for further analysis. Air-dried samples and chemical analyses were carried out using laboratory manuals and guides by Ademorti [31], APHA [32], Golterman et al., [33], APHA [34]. Sediment samples were gently crushed with mortar and pestle not to destroy grain size, then 0.05 g of samples (triplicates) were digested with 10 m1 of 70% nitric acid (HNO3) and 1 ml of hydrogen peroxide (H2O2) in a microwave vessel. The process was repeated once to ensure total digestion. After completion of the digestion process, the vessel was cooled to room temperature and carefully vented in a fume hood. The content of the vessel was transferred to a beaker and evaporated to incipient wetness. The residues were re-dissolved in 5 ml of 1.1HNO3 (deionized water v/v and brought to a final volume of 50 ml). The digested samples were analyzed for heavy metals using an Atomic Absorption Spectrophotometer (ASS) PG, 990 Model at specific wavelengths.
2.4.1. Identification of benthic macroinvertebrates
A Van-Veen grab was used to obtain silt samples from the waterbed. Bottom sediment samples were collected at each sampling location, and the grab contents were filtered through a 0.5 mm mesh sieve. The filtrates were washed off in the case of attached benthos and were searched for larvae or smaller species. Using a pair of long forceps, to picked the macrobenthos identified in the sediment samples and collected in sample containers. The specimens were preserved in 90% alcohol, labeled, and transferred to the laboratory for identification. The macro-benthos obtained were sorted and counted in the laboratory. The taxonomic categories were created from the sorted macrobenthos. In the laboratory, the macrobenthos were sorted to generate taxonomic groupings. Identification of specimens to the lowest possible taxa was based on their morphological and anatomical characteristics with the aid of a trinocular zoom Stereo Microscope (Model: BS-2041T), using the identification guides of [[35], [36], [37]] and taxonomic lists of indigenous species of Nigeria [[38], [39], [40]].
2.5. Statistical analyses
To describe the data properties obtained from this study were subjected to descriptive statistics (mean and standard deviation) and inferential analysis. Significant variations in physicochemical parameters of surface water and sediment and microbiological quality were tested using one-way analysis of variance (ANOVA) and Duncan Multiple Range Test (DMRT), respectively. The degree of correlation between the variables was described using Canonical Correspondences Analysis (CCA). To evaluate the abundance and evenness of benthic macroinvertebrates within the sites and seasons, species richness was calculated by diversity indices such as Margalef's diversity index (d) [41], Shannon-Weiner index (H) [42], Evenness of distribution (E) [43], and Simpson dominance index (D) [44]. PAST (version 3.0) and SPSS (Version 26) were used for all statistical analyses.
3. Results
3.1. Physico-chemical parameters of surface water, heavy metals, and microorganisms of Lagos Lagoon
The water temperature ranged from 17.32 °C to 29.30 °C recorded during this study, with the highest mean temperature (24.97 °C ± 1.16) observed at site II. The highest pH range of 7.96–8.01 was recorded at site IV, while the lowest range of 6.41–7.01 was observed at site II, and there was a significant difference (p < 0.05) among the pH mean values across the sites, as shown in Table 1. The mean electrical conductivity was higher at site I (38089.67 μS/cm ± 2411.42) than at other sites. The highest chloride mean was observed at site II (10172.83 mg/L ± 114.49L), while the lowest value occurred at site IV (465.82 mg/L ± 97.18), and there was a significant difference (p < 0.05) in mean chloride between the sampling locations. The highest values of turbidity, TSS, BOD, phosphate, nitrate, and sodium were recorded in site II (5.53 NTU ± 3.13, 5260.21 mg/L ± 1055.43, 40.82 mg/L ± 5.01, 51.80 mg/L ± 49.84, 10172.83 mg/L ± 1141.96, 6.91 mg/L ± 0.47) while the mean values of salinity, TDS, COD were higher at site III. Seasonally, the highest values of water temperature, electrical conductivity, nitrate, and (35.10 °C ± 3.66, 61842.83 μS/cm ± 11777.45, and 6.09 mg/L ± 3.70) were observed at site I during the dry season. Salinity, TDS, TSS, DO, and COD (15.43 ppt ±3.59, 9676.75 mg/L ± 1351.24, 5998.75 mg/L ± 2323.66, 8.27 mg/L ± 3.08 respectively) were higher at site III during the dry season. The pH, turbidity, BOD, and chloride (7.99 ± 0.05, 10.33 NTU ± 0.40, 70.80 mg/L ± 96.84, 11163.33 mg/L ± 1667.10) were also higher in site II during the rainy season (Supplementary Table 1).
Table 1.
Spatial variation of physico-chemical parameters of surface water, heavy metals and microbiological quality of Lagos Lagoon.
| parameters | Site I |
Site II |
Site III |
Site IV |
ANOVA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | F | Sig. | |
| pH | 7.35–8.69 | 7.88 ± 0.11 | 6.41–7.01 | 6.15 ± 1.14 | 7.79–8.32 | 7.97 ± 0.06 | 7.96–8.01 | 7.98 ± 2.35 | 0.92 | 0.027 |
| Water temperature (°C) | 19.60–27.00 | 21.61 ± 1.12 | 18.80–29.30 | 24.97 ± 1.16 | 22.50–2.10 | 22.20 ± 16.79 | 17.30–23.50 | 19.40 ± 15.10 | 0.36 | 0.785 |
| EC (μs/cm) | 5490.00–30100.00 | 38089.67 ± 2411.42 | 3380.00–850.00 | 6450.67 ± 840.73 | 2350.00–33700.00 | 10755.25 ± 4097.49 | 25300.00–27410.00 | 26355.00 ± 1055.00 | 0.58 | 0.636 |
| Salinity (ppt) | 0.00–19.50 | 13.21 ± 1.46 | 11.60–17.10 | 15.00 ± 0.84 | 10.30–31.40 | 15.34 ± 1.29 | 10.70–12.20 | 11.45 ± 0.75 | 0.82 | 0.498 |
| Turbidity (NTU) | 0.20–14.03 | 4.39 ± 1.34 | 0.10–18.20 | 5.53 ± 3.13 | 0.10–10.34 | 4.48 ± 1.40 | 2.56–7.08 | 1.32 ± 0.76 | 0.13 | 0.940 |
| TDS (mg/l) | 3372.00–10982.00 | 8399.75 ± 623.14 | 5340.00–9654.00 | 7197.83 ± 595.95 | 6240.00–11200.00 | 9277.50 ± 536.42 | 1610.00–3204.00 | 2407.00 ± 797.00 | 8.21 | 0.001** |
| TSS (mg/l) | 3.50–20491.00 | 4507.41 ± 2042.14 | 4.14–5693.00 | 5260.21 ± 1055.43 | 4.25–8732.00 | 3002.07 ± 1253.84 | 2.90–4690.00 | 2346.45 ± 2343.55 | 0.30 | 0.822 |
| THC (mg/l) | 0.00–0.20 | 0.04 ± 0.02 | 0.00–0.01 | 0.01 ± 0.01 | 0.00–0.05 | 0.02 ± 0.01 | 0.07–0.10 | 0.09 ± 0.02 | 2.48 | 0.085 |
| DO (mg/L) | 2.87–9.34 | 4.14 ± 0.56 | 1.04–4.01 | 2.81 ± 0.85 | 3.90–10.85 | 5.12 ± 0.73 | 7.30–8.47 | 7.89 ± 0.58 | 1.76 | 0.181 |
| COD (mg/L) | 0.00–135.00 | 38.36 ± 9.64 | 14.50–26.43 | 10.87 ± 4.16 | 25.00–93.00 | 47.48 ± 9.41 | 15.76–57.00 | 36.38 ± 20.62 | 0.31 | 0.820 |
| BOD (mg/l) | 9.40–32.00 | 17.21 ± 2.23 | 4.50–52.01 | 40.82 ± 5.01 | 5.67–215.00 | 40.62 ± 25.17 | 2.87–18.00 | 15.44 ± 2.57 | 0.77 | 0.522 |
| Sulphate (mg/l) | 17.50–131.00 | 58.41 ± 15.51 | 15.70–32.00 | 24.59 ± 3.01 | 18.30–131.00 | 49.49 ± 17.81 | 130.00–152.00 | 130.00 ± 90.01 | 2.75 | 0.065 |
| Phosphate (mg/l) | 1.02–3.47 | 2.12 ± 0.26 | 1.11–301.00 | 51.80 ± 49.84 | 0.97–2.18 | 1.53 ± 0.17 | 1.02–6.89 | 3.96 ± 2.94 | 1.25 | 0.313 |
| Chloride (mg/l) | 243.56–14176.00 | 7466.76 ± 156.79 | 6549.00–13438.00 | 10172.83 ± 114.96 | 355.47–12750.00 | 8193.59 ± 175.32 | 368.64–563.00 | 465.82 ± 97.18 | 2.15 | 0.021* |
| Nitrate (mg/l) | 2.00–13.01 | 5.66 ± 0.97 | 3.70–6.98 | 6.91 ± 0.47 | 0.98–7.89 | 4.19 ± 0.80 | 1.63–2.00 | 1.82 ± 0.19 | 1.55 | 0.228 |
| Calcium (mg/l) | 1.39–601.00 | 314.46 ± 68.85 | 311.50–657.90 | 496.36 ± 50.66 | 1.44–490.67 | 269.57 ± 64.58 | 1.42–4.98 | 3.20 ± 1.78 | 3.49 | 0.031* |
| Sodium (mg/l) | 0.02–200.67 | 75.87 ± 19.31 | 99.20–1300.09 | 509.55 ± 201.08 | 0.08–87.00 | 58.49 ± 13.06 | 0.78–2.70 | 1.74 ± 0.96 | 5.98 | 0.003 |
| Magnesium (mg/l) | 0.08–80.45 | 27.60 ± 7.31 | 13.80–70.32 | 45.45 ± 9.49 | 0.06–70.10 | 32.78 ± 8.79 | 0.55–2.74 | 1.65 ± 1.19 | 1.78 | 0.177 |
| Potassium (mg/l) | 0.95–23.95 | 9.87 ± 2.23 | 9.70–15.47 | 12.99 ± 0.85 | 1.09–20.50 | 11.98 ± 2.55 | 1.10–6.48 | 3.79 ± 2.69 | 1.12 | 0.360 |
| Manganese (mg/L) | 0.00–0.11 | 0.03 ± 0.01 | 0.00–0.01 | 0.05 ± 0.02 | 0.00–0.26 | 0.03 ± 0.03 | 0.00–0.04 | 0.03 ± 0.01 | 6.19 | 0.003** |
| Lead (mg/l) | 0.00–0.41 | 0.07 ± 0.04 | 0.00–0.21 | 0.09 ± 0.01 | 0.00–0.90 | 0.21 ± 0.14 | 0.02–0.07 | 0.05 ± 0.02 | 1.46 | 0.252 |
| Copper (mg/l) | 0.00–0.36 | 0.06 ± 0.03 | 0.01–0.07 | 0.04 ± 0.01 | 0.00–0.47 | 0.12 ± 0.07 | 0.17–0.42 | 0.30 ± 0.13 | 2.29 | 0.104 |
| Chromium (mg/l) | 0.00–0.01 | 0.01 ± 0.02 | 0.00–0.01 | 0.03 ± 0.01 | 0.00–0.01 | 0.02 ± 0.01 | 0.01–0.02 | 0.02 ± 0.01 | 0.95 | 0.433 |
| Nickel (mg/l) | 0.00–0.94 | 0.01 ± 0.01 | 0.00–0.03 | 0.24 ± 0.11 | 0.01–0.62 | 0.15 ± 0.09 | 0.01–0.03 | 0.01 ± 0.02 | 1.29 | 0.299 |
| Zinc (mg/l) | 0.00–9.86 | 1.80 ± 0.87 | 0.02–6.40 | 1.83 ± 1.06 | 0.00–2.95 | 0.78 ± 0.40 | 0.23–0.51 | 0.23 ± 0.01 | 0.51 | 0.680 |
| Iron (mg/l) | 0.01–4.95 | 1.19 ± 0.51 | 0.00–5.28 | 0.99 ± 0.86 | 0.00–1.09 | 0.31 ± 0.16 | 0.00–8.29 | 4.15 ± 2.03 | 2.06 | 0.133 |
| Cadmium (mg/l) | 0.01–0.21 | 0.03 ± 0.02 | 0.00–0.32 | 0.06 ± 0.05 | 0.00–0.01 | 0.04 ± 0.01 | 0.00–0.02 | 0.02 ± 0.01 | 0.89 | 0.457 |
| THF (Cfu/ml) | 10.00–570.00 | 259.17 ± 62.83 | 220.00–510.00 | 418.33 ± 42.69 | 250.00–4000.00 | 1186.25 ± 516.60 | 0.00–10.00 | 5.00 ± 2.38 | 2.55 | 0.079 |
| THB (Cfu/ml) | 610.00–2.0 × 105 | 35162.42 ± 20957.14 | 1100.00–1900.00 | 1501.67 ± 138.09 | 700.00–2700.00 | 1736.38 ± 240.89 | 7000.00–7020.00 | 7010.00 ± 10.00 | 1.03 | 0.398 |
| Total coliform (Cfu/ml) | 150.00–901.00 | 406.00 ± 80.20 | 90.00–210.00 | 153.50 ± 18.73 | 450.00–810.00 | 572.38 ± 52.08 | 0.00–1500.00 | 750.00 ± 562.01 | 3.11 | 0.045 |
| THUB (%) | 0.00–470.00 | 164.38 ± 47.82 | 140.00–210.00 | 173.33 ± 10.54 | 10.00–430.00 | 205.25 ± 46.06 | 0.00–10.00 | 5.00 ± 3.71 | 1.35 | 0.281 |
| THUF (%) | 0.00–150.00 | 35.42 ± 15.56 | 10.00–170.00 | 98.33 ± 28.80 | 0.00–30.00 | 13.75 ± 3.63 | 0.00–10.00 | 5.00 ± 4.01 | 3.97 | 0.020* |
Note: EC (Electrical conductivity), DO (Dissolved oxygen), TDS (Total dissolved solids), TSS (Total suspended solids), THC (Total hydrocarbon), COD (Chemical oxygen demands), BOD (Biochemical oxygen demands), THF (Total heterotrophic fungi), THB (Total heterotrophic bacteria), THUB (Total hydrocarbon utilizing bacteria) and THUF (Total hydrocarbon utilizing fungi), ANOVA – Analysis of Variance p value.
The lowest mean concentration of calcium and manganese were at site IV (1.82 mg/L ± 0.19 and 0.003 mg/L ± 0.001), and maximum values were recorded at site II (496.36 mg/L ± 50.66 and 0.05 mg/L ± 0.002). The mean values across the stations differed significantly (p < 0.01). The mean concentrations of Mg2+, K+, Pb, Cr, Ni, Zn, and Cd (496.36 mg/L ± 50.66, 509.55 mg/L ± 201.08, 45.45 mg/L ± 9.49, 12.99 mg/L ± 0.85, 0.05 mg/L ± 0.02, 0.09 mg/L ± 0.01, 0.03 mg/L ± 0.01, 0.24 mg/L ± 0.11, 1.83 mg/L ± 1.06, 0.06 mg/L ± 0.05 respectively) were recorded in site II. Seasonally, Pb mean concentration (0.23 mg/L ± 0.45) was higher at site III during the dry season, while only copper (0.13 mg/L ± 0.23) was high at site III during the rainy season. The mean of phosphate, calcium, magnesium, potassium, nickel, zinc, and cadmium (102.02 mg/L ± 172.32, 571.75 mg/L ± 101.85, 45.80 mg/L ± 24.82, 14.51 mg/L ± 0.96, 0.27 mg/L ± 0.01, 3.47 mg/L ± 2.89, 1.93 mg/L ± 2.91, and 0.12 mg/L ± 0.17) were higher in site II during the dry season while calcium, and magnesium (457.57 mg/L ± 425.27, 0.14 mg/L ± 0.10) were also higher in site II during the rainy season (Supplementary Table 1).
The highest total hydrocarbon utilizing fungi (THUF) mean was observed at site II (98.33% ± 28.80). In comparison, the lowest THUF percentage observed at site IV (465.82% ± 97.18), and there was a significant difference (p < 0.05) in the mean values of THUF a crossed the sampling sites. In contrast, the highest mean values of THF and THUB were recorded in site III, and THC and total coliform count were higher in site IV, as presented in Table 1. Seasonally, the highest THB mean value (38174.83 cfu/ml ± 79509.21) was observed at site I during the dry season, while the highest mean of THF and THUB (1342.50 cfu/ml ± 1783.09 and 206.25% ± 170.65) were recorded at site III during the dry season. The total coliform count (801.00 cfu/ml ± 988.54) was higher at site IV during the dry season, while the highest value of THUF (100.00% ± 81.55) was recorded in the dry season. (Supplementary Table 1).
3.2. Physico-chemical parameters of sediment, heavy metals, and microorganisms of Lagos Lagoon
The highest sediment pH range of 6.18–9.18 (7.58 ± 0.27) was recorded at site I, while the lowest mean pH was observed at site II (6.87 ± 0.19), as presented in Table 2. The mean values of permeability, chloride, calcium, potassium, and lead were recorded in site I, while electrical conductivity, clay, porosity, sulphate, chromium, nickel, cadmium, THF, total coliform, THUB, and THUF were higher in site II. The highest silt percentage, total hydrocarbon, sulphate, total nitrogen, magnesium, zinc, iron, copper, and THB, were observed at site III. In contrast, total organic carbon, moisture content, and sand percentage were higher in site IV (Table 2). Seasonally, the lowest sediment mean pH concentration was recorded at site II (5.85 ± 3.94) during the dry season than other sites, while electrical conductivity (625.00 μS/cm ± 22.91) was higher in the dry season at site II (Supplementary Table 2). The highest mean values of calcium, sodium, and chromium (14.30 cmol/kg ± 14.92, 52.38 cmol/kg ± 34.77, and 1.69 cmol/kg ± 2.75) were observed at site I dry seasons compared with other sites. The highest mean values of TOC, clay percentage, porosity, THC, sulphate, phosphorus, lead, nickel, cadmium, total coliform, THUB, and THUF (15.23% ± 4.68, 45.60% ± 32.95, 0.04 cmol/kg ± 0.005, 6.72 μgg−1 ± 2.06, 6.04 μgg−1 ± 1.68, 1.39 μgg−1 ± 0.13, 0.60 μgg−1 ± 1.01, 0.07 μgg−1 ± 0.11, 10171.67 cfug−1 ± 17604.85, 5370.00 cfug−1 ± 8343.57, 5334.61 cfug−1 ± 9236.49 and 6001.27 cfug−1 ± 10391.20) were high at site II during the dry season while chloride value was higher during the rainy season. The mean of silt percentage, total nitrogen, magnesium, iron, copper, THB, and pH were higher at site III during the dry season, while the sand percentage, manganese, and zinc were higher in the rainy season. The mean moisture content and permeability values were higher in the dry season at site IV than at other sites (Supplementary Table 2).
Table 2.
Spatial variation of physico-chemical parameters of sediment, heavy metals and microbiological quality of Lagos Lagoon.
| Parameters | Site I |
Site II |
Site III |
Site IV |
ANOVA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | Min–Max. | Mean ± SD | F | Sig. | |
| pH | 6.18–9.18 | 7.58 ± 0.27 | 6.14–7.53 | 6.87 ± 0.19 | 7.02–8.98 | 7.02 ± 1.03 | 6.76–7.48 | 7.34 ± 0.14 | 0.462 | 0.711 |
| EC (μS/cm) | 210.00–560.00 | 399.58 ± 27.57 | 375.00–650.00 | 545.00 ± 47.54 | 250.00–617.00 | 402.13 ± 44.45 | 340.00–440.00 | 390.00 ± 50.00 | 2.815 | 0.061 |
| Total Organic Carbon (%) | 0.02–0.19 | 0.07 ± 0.14 | 0.04–0.26 | 0.11 ± 0.03 | 0.01–0.20 | 0.09 ± 0.02 | 0.13–0.17 | 0.15 ± 0.02 | 1.267 | 0.308 |
| Moisture Content | 25.20–35.10 | 31.82 ± 20.84 | 0.00–33.27 | 27.19 ± 15.44 | 30.20–36.20 | 32.82 ± 20.71 | 33.14–37.14 | 35.14 ± 22.00 | 1.197 | 0.332 |
| Sand (%) | 67.20–91.80 | 83.66 ± 72.46 | 35.10–97.10 | 73.52 ± 63.69 | 20.30–80.90 | 54.40 ± 48.00 | 85.00–90.00 | 87.50 ± 82.50 | 4.280 | 0.015* |
| Silt (%) | 5.00–28.60 | 12.09 ± 2.19 | 1.50–51.80 | 20.27 ± 9.89 | 10.30–58.50 | 31.75 ± 6.37 | 5.00–10.00 | 7.50 ± 2.50 | 2.938 | 0.054 |
| Clay (%) | 0.00–10.00 | 4.34 ± 1.07 | 14.03–63.10 | 61.22 ± 38.88 | 6.80–21.20 | 13.93 ± 1.67 | 2.00–5.00 | 5.00 ± 0.01 | 9.116 | 0.000 |
| Porosity | 42.90–45.10 | 44.10 ± 0.19 | 40.20–75.40 | 50.57 ± 32.82 | 40.10–46.20 | 43.25 ± 0.69 | 43.30–44.20 | 43.75 ± 0.45 | 0.561 | 0.646 |
| permeability | 0.00–0.01 | 0.03 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0.00–0.01 | 0.03 ± 0.01 | 0.01–0.02 | 0.01 ± 0.01 | 0.941 | 0.437 |
| Total hydrocarbon (cmol/kg) | 0.00–0.10 | 0.01 ± 0.01 | 0.00–0.01 | 0.01 ± 0.002 | 0.01–0.02 | 0.04 ± 0.02 | 0.01–0.02 | 0.01 ± 0.01 | 0.504 | 0.683 |
| Sulphate (μgg−1) | 2.75–7.38 | 4.55 ± 0.43 | 3.62–8.92 | 5.94 ± 0.74 | 3.45–8.21 | 5.72 ± 0.60 | 2.56–3.61 | 3.09 ± 0.53 | 2.476 | 0.086 |
| Phosphorus (μgg−1) | 0.36–6.60 | 4.49 ± 0.54 | 1.90–8.71 | 6.79 ± 0.67 | 4.41–7.80 | 5.74 ± 0.36 | 2.10–3.98 | 3.04 ± 0.94 | 2.553 | 0.079 |
| Chloride (μgg−1) | 1.03–4.03 | 2.10 ± 0.26 | 1.14–2.25 | 1.71 ± 0.18 | 0.80–2.20 | 1.33 ± 0.18 | 0.08–0.83 | 0.46 ± 0.38 | 4.076 | 0.018* |
| Total nitrogen (%) | 1.25–4.95 | 2.97 ± 0.31 | 1.80–3.67 | 2.62 ± 0.31 | 1.32–7.01 | 4.27 ± 0.79 | 3.15–3.25 | 3.20 ± 0.05 | 1.826 | 0.169 |
| Calcium (cmol/kg) | 1.90–37.84 | 13.99 ± 4.13 | 4.89–13.22 | 11.51 ± 0.88 | 1.51–17.23 | 9.19 ± 1.99 | 4.70–5.07 | 4.89 ± 0.19 | 0.784 | 0.514 |
| Magnesium (cmol/kg) | 1.03–4.91 | 3.32 ± 0.38 | 0.45–5.50 | 2.76 ± 0.78 | 1.96–6.21 | 3.98 ± 0.60 | 0.93–1.90 | 1.42 ± 0.49 | 1.710 | 0.192 |
| Potassium (cmol/kg) | 1.20–5.89 | 2.85 ± 0.39 | 1.98–321 | 2.43 ± 0.23 | 1.18–4.50 | 2.73 ± 0.42 | 1.73–2.37 | 2.05 ± 0.32 | 0.381 | 0.767 |
| Sodium (cmol/kg) | 18.41–67.80 | 53.28 ± 44.88 | 25.87–50.12 | 36.82 ± 14.26 | 39.40–71.42 | 44.46 ± 41.12 | 38.70–41.98 | 40.34 ± 31.64 | 1.936 | 0.151 |
| Lead (μgg−1) | 0.001–2.67 | 0.57 ± 0.27 | 0.001–0.25 | 0.73 ± 0.05 | 0.01–0.02 | 0.005 ± 0.002 | 0.00–0.01 | 0.01 ± 0.02 | 7.461 | 0.001** |
| Chromium (μgg−1) | 0.00–7.28 | 1.52 ± 0.72 | 0.00–2.21 | 2.13 ± 0.40 | 0.21–1.98 | 1.23 ± 0.23 | 0.21–0.51 | 0.36 ± 0.15 | 0.365 | 0.779 |
| Manganese (μgg−1) | 0.00–1.78 | 0.93 ± 0.14 | 0.27–1.90 | 0.88 ± 0.24 | 0.27–1.83 | 1.10 ± 0.24 | 0.63–0.89 | 0.76 ± 0.13 | 0.318 | 0.812 |
| Nickel (μgg−1) | 0.001–44 | 0.46 ± 0.18 | 0.001–1.76 | 0.84 ± 0.34 | 0.00–0.06 | 0.02 ± 0.01 | 0.01–0.05 | 0.02 ± 0.02 | 1.789 | 0.176 |
| Zinc (μgg−1) | 0.18–8.76 | 4.32 ± 0.86 | 0.30–12.04 | 4.62 ± 2.06 | 0.87–11.42 | 6.50 ± 1.42 | 0.77–1.79 | 1.28 ± 0.51 | 1.201 | 0.331 |
| Iron (μgg−1) | 30.07–80.23 | 57.93 ± 4.67 | 1.79–86.06 | 54.56 ± 11.74 | 38.63–123.58 | 84.23 ± 10.26 | 49.91–51.57 | 50.74 ± 0.83 | 2.852 | 0.058 |
| Copper (μgg−1) | 0.57–4.36 | 1.93 ± 0.32 | 0.02–3.91 | 1.95 ± 0.68 | 1.42–5.89 | 3.40 ± 0.57 | 0.33–2.54 | 1.44 ± 1.11 | 2.283 | 0.105 |
| Cadmium (μgg−1) | 0.001–0.12 | 0.02 ± 0.01 | 0.02–0.19 | 0.06 ± 0.03 | 0.00–0.04 | 0.01 ± 0.04 | 0.00–0.02 | 0.02 ± 0.01 | 1.855 | 0.164 |
| THF (Cfug−1) | 10.0–45.00 | 23.08 ± 3.72 | 1.50–30500.00 | 10086.40 ± 6376.62 | 4.00–4000.00 | 539.25 ± 494.45 | 10.00–26.00 | 18.00 ± 8.00 | 2.991 | 0.051 |
| THB (Cfug−1) | 100.00–5100.00 | 1007.17 ± 546.22 | 245.00–15000.00 | 7374.17 ± 2475.14 | 200.00–3.00 × 108 | 4.0 × 107 ± 372 × 107 | 100.00–125.00 | 112.50 ± 12.50 | 0.942 | 0.436 |
| Total Coliform (Cfug−1) | 0.02–25000.00 | 3821.84 ± 253.72 | 305.00–15000.00 | 4716.67 ± 2671.15 | 1.10–350.00 | 113.27 ± 50.42 | 1.27–1.30 | 1.29 ± 0.15 | 0.809 | 0.501 |
| THUB (%) | 1.20–6.01 | 2.53 ± 0.35 | 1.71–16000.00 | 5168.09 ± 3269.79 | 1.02–2.67 | 1.76 ± 0.18 | 1.80–1.87 | 1.84 ± 0.04 | 3.138 | 0.044 |
| THUF (%) | 0.87–2.90 | 1.80 ± 0.22 | 1.50–18000.00 | 3042.89 ± 2991.69 | 1.01–2.54 | 1.87 ± 0.17 | 1.90–2.10 | 2.00 ± 0.10 | 1.299 | 0.298 |
Note: THF (Total heterotrophic fungi), THB (Total heterotrophic bacteria), THUB (Total hydrocarbon utilizing bacteria) and THUF (Total hydrocarbon utilizing fungi).
3.3. Benthic macroinvertebrates abundance
26 species of benthic macroinvertebrates were identified during this study, which belongs to 8 classes namely: gastropoda (11), polychaeta (7), malacostraca (3), bivalvia (2), clitellata (1), thecostraca (1) and insecta (1) as presented in Table 3. Gastropoda recorded the highest contribution percentage of 39.12%, followed by polychaeta, which accounted for 30.34%, while malacostraca contributed 2.63% (Table 3). The highest benthic macroinvertebrates abundances were recorded at site I (256 Indiv/m2) followed by site I (252 Indiv/m2), and the least abundance was observed at site II (195 Indiv/m2). Nereis spp. recorded the highest species occurrence of 89, followed by Fistubalanus pallidus (72), whereas Clibanarius africanus showed the least species occurrence (12). Seasonally, the highest benthic macroinvertebrates abundances were recorded during the dry season at site IV (144 Indiv/m2), while the lowest was observed at site II (99 Indiv/m2). During the rainy season highest macroinvertebrates abundance was recorded at site IV (149 Indiv/m2), while the lowest was observed at site III (104 Indiv/m2) (Table 4). Macroinvertebrate diversity indices yield was higher at site I (2.94) for the Shannon-Weiner diversity index (H) and 2.88 at site III. The highest Shannon-Weiner (H) value was recorded at site IV (2.93) during the dry season compared to the 2.17 value observed during the rainy season at site II (Table 5). Likewise, the Evenness index was higher at site II (0.82). In contrast, the highest was recorded during rainy season site II (0.85) compared to the value observed in the dry season (0.82), as presented in Table 5.
Table 3.
Spatial variation of benthic macroinvertebrate abundance of Lagos Lagoon.
| Class | Family | Taxon | Site I | Site II | Site III | Site IV |
|---|---|---|---|---|---|---|
| Polychaeta | Nereididae | Nereis sp. | 31 | 5 | 23 | 30 |
| Capitellidae | Capitella sp | 12 | 20 | 18 | 12 | |
| Spionidae | Polydora ciliata | 13 | 0 | 0 | 13 | |
| Ophelininae | Polypthalmus pitcus | 10 | 7 | 4 | 2 | |
| Nephytyidae | Nephtys sp. | 12 | 22 | 0 | 3 | |
| Hirudinidae | Hirudinaria granulosa | 3 | 22 | 2 | 6 | |
| Amphinomidae | Pherecardia striata | 6 | 2 | 11 | 9 | |
| Clitellata | Lumbriculidae | Lumbriculus variegatus | 9 | 12 | 2 | 20 |
| Gastropoda | Potamididae | Tympanotonus spp | 11 | 10 | 12 | 1 |
| Potamididae | Tympanotamus fuscatus | 6 | 15 | 7 | 1 | |
| Thiaridae | Pachymelania aurita | 0 | 25 | 10 | 5 | |
| Neritidae | Neritina glabrata | 24 | 0 | 21 | 10 | |
| Neritina senegalensis | 20 | 0 | 4 | 10 | ||
| Turritellidae | Turritella ungulina | 7 | 10 | 3 | 15 | |
| Donacidae | Iphigenia truncate | 2 | 5 | 3 | 3 | |
| Planorbidae | Bulinus truncatus | 2 | 4 | 8 | 1 | |
| Bulinus nyassanus | 2 | 3 | 8 | 3 | ||
| Physidae | Physa fontinalis | 4 | 8 | 11 | 20 | |
| Physinae | Physella sp. | 10 | 9 | 2 | 5 | |
| Bivalvia | Arcidae | Andara sp. | 12 | 8 | 8 | 12 |
| Mytilidae | Mytilus edulis | 9 | 10 | 4 | 19 | |
| Thecostraca | Balanidae | Fistubalanus pallidus | 31 | 8 | 18 | 15 |
| Insecta | Chronomidae | Chironomus sp. | 5 | 15 | 8 | 0 |
| Malacostraca | Aoridae | Aora gracilis | 3 | 6 | 7 | 11 |
| Penaeidae | Penaeus notialis | 2 | 0 | 1 | 25 | |
| Diogenidae | Clibanarius africanus | 10 | 0 | 0 | 1 |
Table 4.
Seasonal variation of benthic macroinvertebrate abundance of Lagos Lagoon.
| Class |
Family |
Taxon |
Site I |
Site II |
Site II |
Site IV |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry season | Rainy season | Dry season | Rainy season | Dry season | Rainy season | Dry season | Rainy season | |||
| Polychaeta | Nereididae | Nereis sp. | 9 | 22 | 3 | 2 | 13 | 10 | 10 | 20 |
| Capitellidae | Capitella sp | 3 | 9 | 5 | 15 | 8 | 10 | 5 | 7 | |
| Spionidae | Polydora ciliata | 5 | 8 | 0 | 0 | 0 | 0 | 9 | 4 | |
| Ophelininae | Polypthalmus pitcus | 7 | 3 | 4 | 3 | 3 | 1 | 2 | 0 | |
| Nephytyidae | Nephtys sp. | 10 | 2 | 7 | 15 | 0 | 0 | 2 | 1 | |
| Hirudinidae | Hirudinaria granulosa | 2 | 1 | 12 | 10 | 4 | 6 | 1 | 5 | |
| Amphinomidae | Pherecardia striata | 4 | 2 | 0 | 2 | 9 | 2 | 8 | 1 | |
| Clitellata | Lumbriculidae | Lumbriculus variegatus | 3 | 6 | 1 | 11 | 1 | 1 | 5 | 10 |
| Gastropoda | Potamididae | Tympanotonus spp | 6 | 5 | 3 | 7 | 2 | 10 | 9 | 7 |
| Potamididae | Tympanotamus fuscatus | 3 | 3 | 7 | 8 | 5 | 2 | 1 | 9 | |
| Thiaridae | Pachymelania aurita | 0 | 0 | 20 | 5 | 9 | 10 | 0 | 5 | |
| Neritidae | Neritina glabrata | 19 | 5 | 0 | 0 | 10 | 11 | 9 | 1 | |
| Neritina senegalensis | 13 | 7 | 0 | 0 | 3 | 1 | 2 | 8 | ||
| Turritellidae | Turritella ungulina | 4 | 3 | 4 | 6 | 1 | 2 | 3 | 12 | |
| Donacidae | Iphigenia truncate | 1 | 1 | 1 | 4 | 2 | 1 | 2 | 1 | |
| Planorbidae | Bulinus truncatus | 1 | 1 | 2 | 2 | 3 | 5 | 6 | 1 | |
| Bulinus nyassanus | 2 | 0 | 0 | 3 | 7 | 1 | 0 | 0 | ||
| Physidae | Physa fontinalis | 2 | 2 | 4 | 4 | 5 | 6 | 6 | 14 | |
| Physinae | Physella sp. | 5 | 5 | 4 | 5 | 1 | 1 | 3 | 2 | |
| Bivalvia | Arcidae | Andara sp. | 8 | 4 | 4 | 4 | 9 | 5 | 10 | 2 |
| Mytilidae | Mytilus edulis | 5 | 4 | 5 | 5 | 2 | 2 | 4 | 15 | |
| Thecostraca | Balanidae | Fistubalanus pallidus | 19 | 12 | 3 | 5 | 9 | 9 | 7 | 8 |
| Insecta | Chronomidae | Chironomus sp. | 3 | 2 | 5 | 10 | 3 | 5 | 0 | 0 |
| Malacostraca | Aoridae | Aora gracilis | 2 | 1 | 1 | 5 | 5 | 2 | 1 | 11 |
| Penaeidae | Penaeus notialis | 1 | 1 | 0 | 0 | 1 | 1 | 10 | 15 | |
| Diogenidae | Clibanarius africanus | 7 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | |
Table 5.
Spatial and seasonal diversity indices of benthic macroinvertebrates of Lagos Lagoon.
| Diversity indices parameter | Spatial |
Seasonal |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry season |
Rainy Season |
|||||||||||
| Site I | Site II | Site III | Site IV | Site I | Site II | Site III | Site IV | Site I | Site II | Site III | Site IV | |
| Taxa (d) | 25 | 23 | 23 | 25 | 25 | 21 | 23 | 23 | 24 | 22 | 23 | 22 |
| Individuals (S) | 256 | 195 | 235 | 252 | 144 | 99 | 115 | 125 | 112 | 136 | 104 | 149 |
| Dominance (D) | 0.07 | 0.06 | 0.07 | 0.07 | 0.07 | 0.09 | 0.06 | 0.06 | 0.08 | 0.06 | 0.07 | 0.08 |
| Simpson (1-D) | 0.93 | 0.94 | 0.93 | 0.94 | 0.93 | 0.91 | 0.94 | 0.94 | 0.92 | 0.94 | 0.93 | 0.92 |
| Shannon-weiner (H) | 2.94 | 2.03 | 2.88 | 2.90 | 2.91 | 2.74 | 2.90 | 2.93 | 2.82 | 2.17 | 2.81 | 2.75 |
| Evenness (E) | 0.75 | 0.82 | 0.77 | 0.73 | 0.73 | 0.74 | 0.79 | 0.82 | 0.70 | 0.85 | 0.72 | 0.71 |
| Margalef | 4.33 | 4.03 | 4.17 | 4.34 | 4.83 | 4.35 | 4.64 | 4.56 | 4.87 | 4.28 | 4.74 | 4.20 |
3.4. Correlation matrix and canonical correspondences analysis (CCA) showing the interrelationship between the studied variables
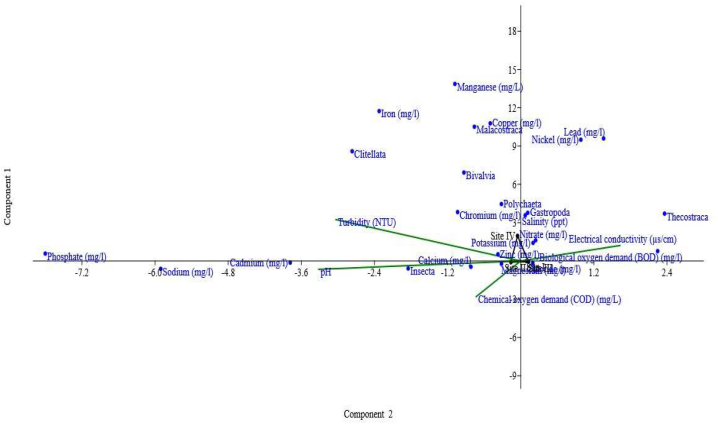
There was a strong positive correlation between Polychaeta and water pH, conductivity, chemical oxygen demands, and salinity, respectively. At the same time, clitellate showed a positive correlation with salinity, manganese, copper, nickel, and iron (Supplementary Table 3). There was a positive relationship between Gastropoda, salinity, BOD, chloride, nitrate, sodium, zinc, and cadmium. At the same time, Bivalvia showed a strong positive correlation with conductivity, sodium, manganese, copper, nickel, and iron. Thecostracan positively correlated with conductivity, BOD, sodium, and nickel, while insecta correlated with pH, salinity, BOD, phosphate, chloride, nitrate, chromium, and zinc. However, Malacostraca positively correlated with conductivity, manganese, lead, copper, nickel, iron, and chloride (Supplementary Table 3). Sediment physicochemical characteristics showed a positive correlation with macroinvertebrates groups. Polychaeta showed a strong positive correlation with sediment pH, sand percentage, and nickel. At the same time, there was a positive correlation between clitellate and sediment pH, TOC, sand percentage, lead, and nickel, as indicated in Supplementary Table 4. Gastropoda positively correlated with sediment pH, silt percentage, phosphorus, Cr, Mn, Zn, Fe, and Cu. In contrast, Bivalvia positively correlated with TOC, sand percentage, lead and nickel respectively. There was a strong positive correlation between thecostracan with pH and chromium. At the same time, Insecta showed a positive correlation with conductivity, silt percentage, sand percentage, zinc, and cadmium. In contrast, Malacostraca showed a strong positive correlation with TOC, nickel, and nickel (Supplementary Table 4). The canonical Correspondences Analysis (CCA) showed a strong positive correlation between salinity, Polychaeta, clitellate, Malacostraca, Gastropoda, Thecostracan, Bivalvia, Fe, Mn, Cu, Pb, K+, nitrate, Zn, BOD, Ca2+, phosphate, while turbidity conductivity, Na+, Cd, COD, Mg2+ and pH correlate with Insecta (Fig. 2). Sediment physicochemical showed interrelationship with benthic macroinvertebrate groups such as Malacostraca, and clitellate, clustered with nickel, THF, and lead. In contrast, Bivalvia clustered with total hydrocarbon, nitrate, TOC and Polychaeta, Gastropoda, Insecta, Thecostracan clustered with Cu, phosphorus, clay percentage, Ca2+, silt percentage, Mg2+, Cd, total coliform, Cr, conductivity, sand percentage and pH as presented in Fig. 3. A high abundance of tolerant species was recorded at site II and I, while moderately tolerant species were observed at site III and a low abundance of intolerant macroinvertebrate species were recorded at site II (Fig. 4).
Fig. 2.
Canonical Correspondences Analysis (CCA) showing interrelationship between the physico-chemical parameters of surface water and benthic macroinvertebrate groups of the Lagos Lagoon.
Fig. 3.
Canonical Correspondences Analysis (CCA) showing interrelationship between the physico-chemical parameters of sediment and benthic macroinvertebrate groups of Lagos Lagoon.
Fig. 4.
Pollution tolerance levels of benthic macroinvertebrate species recorded from Lagos Lagoon.
4. Discussion
The physicochemical parameters of surface water obtained from Lagos Lagoon clearly showed the impact of anthropogenic activities on this waterbody. Spatially, the temperature values ranged from 19.50 °C to 26.30 °C during the study period, with the highest mean temperature observed at site II (35.97 °C ± 1.16), similar to the work of Oyeleke et al. [45], who recorded high-temperature range of 27 °C–30 °C in Lagos Lagoon. The temperature range obtained from this result could be due to the impact of land use within and around this waterbody as well as the climatic change, which could affect water quality through changes in evaporation, temperature, runoff, and rainfall pattern that can affect the mobilization of nutrients, distribution, and mobility of pollutants in aquatic ecosystems [46]. Climate change altered the physical, chemical, and biological functions of any waterbody, which could contribute to increased water temperature and decreased oxygen content [47]. The pH range of 6.41–8.69 observed in the study fell within the range that supports aquatic life. However, any increase above this range can result in rapid unionized ammonia in water, which could be toxic to aquatic organisms [48,49]. The pH range recorded during this study aligns with the report of Emmanuel et al. [50], who recorded a range of 6.00–9.33 in Lagoon Lagos. The low pH mean value record at site II could be due to human activities at this site, which also agrees with the work of Onyena and Okoro [51] reported that water with a low pH is generally more conducive to aquatic life. The highest values of turbidity, TSS, BOD, phosphate, nitrate, Na+, Mg2+, K+, Pb, Cr, Ni, Zn, and Cd observed in site II could be due to high concentrations of these ions entering into waterbody through the runoffs which can alter the hydromorphological and led to an accumulation of organic compounds, and other contaminants [52]. The high mean values of salinity, TDS, COD, copper, THF, and THUB at site III and THC, DO, iron, and total coliform count were higher in Site IV could be attributed to the runoffs water from surrounding communities containing livestock/or human feces flows into this waterbody, as well as stormwater runoff (soil and vegetation). The high THC observed maybe originate from decomposed micro and macro phytoplankton or/from oil and gas seeps. [53]. The high dissolved oxygen recorded maybe from oxygen from the atmosphere dissolving and mixing into the water's surface. Algae and underwater grasses release oxygen during photosynthesis. The high microbial load recorded during this study could lead to an increase in other harmful substances, such as hydrogen sulphide. The salinity range recorded during this study was above WHO (2011) standard (0.5–30 ppt) for brackish water, likewise the conductivity (5000–6000 μs/cm), Phosphorus (<0.5 mg/L) while Biological Oxygen Demand (BOD) and nitrate concentration were below NESREA [49] standard. The continuous discharge of petroleum chemical products from industrial companies around this waterbody or/from ships and other sources could contribute to hydrocarbon concentration in this Lagoon. The form of chemical petroleum compounds occurring in the aquatic environment is complex, diversified, and changeable since natural petroleum crude oil is a multicomponent substance containing several thousands of compounds with varying properties, such as aliphatic hydrocarbon and aromatic compounds. These components are decomposed by various physical, chemical, and microbiological processes, which can increase microbial activities leading to oxygen depletion in this waterbody.
Seasonally, the high mean values of water temperature, electrical conductivity, nitrate, and THB observed at site I during the dry season may be due to water concentration as a result of a reduction influx of fresh water into the Lagoon and/or higher evaporation in the dry season [54]. The low mean DO concentration recorded at site II was below FAO (>4 mg/L) standard for aquaculture and could be due to landfilling materials rich in organic content used at this site, increasing anaerobic microorganisms' activities and causing depletion of oxygen during decomposition [55]. The high mean pH, turbidity, BOD, chloride, calcium, and magnesium values recorded at Site II during the rainy season might be attributed to the high water influx during the rainy season. The mean values of phosphate, ca2+, mg2+, K+, Ni, Zn, Cd, and THUF were high at site II during the dry season compared with the rainy season, while a high concentration of ions recorded in the dry season can be governed by the transport of mineral salts leached from the soil into the waterbody [54]. The mean concentration of Cr, Ni, Zn, and Cd was higher at site II during the dry season might be due to metals released from petrol chemical products from heavy machines or through other anthropogenic activities at this site. Cadmium is one of the most poisonous metals, and the high cadmium content observed during this study may harm the growth and development of aquatic organisms in this waterbody [56]. High BOD recorded during the rainy season could be due to the consumption of oxygen in the water by the bacteria during the breakdown of organic matter. The high turbidity observed during the rainy season at site II could be attributed to the high influx of runoffs with high debris, suspended particles, and disturbance at the bottom sediment causing water cloudiness. The mean total hydrocarbon (THC) was higher at site IV during the rainy season could be a result of petroleum hydrocarbon discharge by ships used for transportation or cooling water discharge into the waterbody by a thermal power station [57].
The sediment texture results showed a low content of silty-clay with average particle composition of sand percentage dominant in all the stations, followed by silt and clay percentages of typical Lagoon sediment. This typical sediment texture provided a large selection of habitats for bottom dwellers or bottom-oriented organisms to burrow into the sediment in unfavorable conditions. Significantly, the higher sand and silt percentages recorded in the rainy season compared to the dry season could be attributed to high siltation or/coarse sediments occurring during the rainy season due to the heavy rainfall from inland water into the Lagoon as well as the low nature of the gradients of the slope of deposition, the higher energy of water movement and the overland runoffs mostly during the rainy season [58]. The sediment pH means recorded at site II fell within the acidic range, maybe due to the breaking down of high organic matter at the site, which makes the sediment acidic as a result of oxidation of sulphide or/anoxic material by microbial (thiobacteria) activity [12,59]. Sediment disturbance during land reclamation alters the chemical composition of the sediment, such as decreases in the dissolved oxygen (DO) content, which results in a rise in the redox potential (Eh) as well as the oxidation of anoxic sediments resulting in the release of associated trace metals, that is often associated with short-term changes in the speciation of metals [60]. The higher organic carbon recorded in the dry season may also be attributed to organic debris arising from the decomposition of mangrove-dropping litter leaves, roots, and dead aquatic plants and animals [61]. The high mean conductivity values recorded in this study might be due to the high concentration of ions. In contrast, low electrical conductivity values indicate a high presence of silicate materials in the sediments [62]. The high Pb mean concentration observed at site II could result from Pb materials used in landfilling at this site and the domestic wastes discharged from surrounding communities into this waterbody. Lead reaches the aquatic environment through industrial and municipal discharges, atmosphere deposition, weathering processes in natural Pb mineralisation areas, and highway runoff. Among aquatic biota, Pb accumulates in algae and benthic organisms and is lower in upper trophic predators. Lead is toxic to all phyla of aquatic biota through effects that are modified significantly by various biological and abiotic variables [63,64]. The exchangeable cations (Ca2+, Mg2+, K+, and Na+) and anions (Cl−) concentrations were higher in the dry season than rainy season except for the sodium, which could be due to runoffs from weathering of rock deposits in sediment [62]. The mean concentration of Pb, Cr, Ni, and Cd was relatively high at site II in the dry season except for Ni, which was higher in the rainy season. Pollutants such as heavy metals, phosphorus, and pesticides are adsorbed by sediment, move with the sediment, and are subject to sedimentation or scour in the waterbody. This could contribute to high concentrations of these metals due to the petrochemical products used in fueling heavy equipment at this site. The elemental concentration of sediments depends on anthropogenic and lithogenic sources and on the textural characteristics of organic matter content, mineralogical composition, and depositional environments of sediments [65]. The highest mean of total heterotrophic fungi, total coliform, total hydrocarbon-utilizing bacteria, and total hydrocarbon-utilizing fungi was recorded at site II during the dry season, while THB was higher at site III. The results obtained from this study revealed that the distribution of heterotrophic bacteria and fungi in the study area maybe influenced by several factors. The highest bacterial density was recorded in the dry season, while the highest heterotrophic fungi were recorded in the rainy season. The bacterial density largely depends upon the amount of utilizable organic matter in the sediments, which is largely controlled by the sedimentation and degradation rates. The minimal hydrodynamic circulation during the dry season allows the deposition of organic matter on the bottom sediment. The result confirms the report of Cavallo et al. [66] that bacterial community in sediment did not vary with seasons. Several studies have revealed that the bacterial community composition in hydrocarbon-polluted sediments tends to comprise mostly bacteria specially adapted to hydrocarbons as carbon sources [67]. Microbial degradation such as bacteria, yeast, and fungi are the major ultimate natural mechanism of clean-up the petroleum hydrocarbon pollutants from the environment. The primary effects of petrochemical products, e.g., diesel fuel toxicity, reduces the species richness, evenness, and phylogenetic diversity, resulting in the community being heavily dominated by few species [68]. During the breaking down of organic content by microorganisms, they consume available oxygen and release gases such as methane and hydrogen sulphide into the aquatic environment, which can cause a decrease in the pH of that environment and oxygen depletion. This led to the loss of certain organisms, destabilization of sediments, and thriving of tolerant benthic macroinvertebrates or/replacement of indigenous benthic macroinvertebrates with pollution-tolerant ones.
Benthic macroinvertebrate assemblage showed instability in abundance across all the stations may be due to the impact of anthropogenic activities, fluctuation of the salinity regime, types of substrata, and abiotic and biotic factors. The disturbance of coastal water has altered the coastal balance, changed the current pattern, waves, and quality of the water, altered sediment characteristics, released contaminants, and increased nutrient mobility [69,70]. Gastropoda recorded the highest percentage contribution of 39.12%, followed by polychaeta account 30.34%, while malacostraca contribution of 2.63% was recorded during this study. The dominance of gastropoda could depend on habitat characteristics, spatial and temporal variability [39]. Nereis Sp had the highest species occurrence of 89 indiv/m2, followed by Fistubalanus pallidus (72 indiv/m2) and Clibanarius africanus with the least occurrence (12 indiv/m2). The low abundance of benthic macroinvertebrates recorded could be attributed to high concentrations of water physicochemical parameters such as salinity, chloride, sodium, pH, COD, BOD, Mn, Ni, Cd, nitrate and Fe, which could affect macroinvertebrate composition, distribution, and diversity. The high sediment physicochemical parameters such as sediment pH, conductivity, total organic carbon (TOC), sand percentage, silt percentage, Pb, Cr, Zn, and Cd, since most of their elements are toxic and absorb/sink into sediment may also contribute to low macroinvertebrate abundance recorded in this study. The high total organic carbon and hydrocarbon recorded in this study could result from petroleum products from the ship used for transportation and industrial waste discharge into this waterbody. The hyponeuston organisms which inhabit the surface microlayer, such as the early development stages of numerous aquatic organisms, commercially valuable fish, are susceptible and highly vulnerable to the toxic effects of petroleum products. A certain proportion of petroleum products is absorbed into the suspension and sink into the sediment, which could be toxic to bottom dweller organisms. The positive correlation between some of these physicochemical parameters and macroinvertebrate groups implies that the high concentrations of these parameters could also affect the diversity of macroinvertebrates in waterbodies, likewise canonical correspondences analysis (CCA) plots also showed a clustered relationship between these parameters and macroinvertebrate groups which implied they are one of the major determinant factors in the distribution of macroinvertebrate in the waterbody. The low abundance of species observed at site II in the dry season compared to the rainy season could be attributed to some ecological imbalance arising from alterations of some important factors governing the distribution of benthic communities such as water quality, substrates type, food availability and the extremely high turbidity at the study could be responsible for the poor macroinvertebrate abundances [71]. The absence of some species, such as Neritina glabrata, Neritina senegalensis, and Polydora ciliata, in site II maybe due to their sensitivity to pollutants associated with this region of the waterbody. The opportunistic pollution-tolerant macroinvertebrate taxa recorded in this study are known to thrive in organic polluted areas of an aquatic environment. High occurrence of Tympanotomus sp, Pachymelania aurita, Physa fontinalis, Fistubalanus pallidus, Andara sp, and Penaeus notialis recorded in both seasons may be attributed to dominant taxa associated with grossly polluted with high organic matter, trace metals, and petroleum hydrocarbons due to their ability to thrive in low oxygen environment [72]. Pachymelania aurita was the most abundant at site II. This might be due to the mode of life indicative of a preference for exposure and short excursion out of the shell for feeding and other activities better undertaken on the mudflats rather than in the shallow water. The finding of this work is similar to reports of Nkwoji et al. [71] that Capitella sp, Nephyts sp, and Hirudinaria granulosa were the most abundant and can be influenced by the type of organic metals and hydrocarbon content in the sediment. The polychaeta are the most dominant group due to their pollution-tolerant, which makes them a good indicator species. Although estuarine macrozoobenthic communities are well known to be dominated by polychaetes and some species of oligochaetes due to their ability to survive in euryhaline water [73]. Studies have shown their high relative dominance over other organisms due to their ability to thrive in a polluted aquatic environment, thereby engendering a shift in macrofauna composition in favour of the more tolerant taxa. The ecological indices recorded in this study revealed low levels of richness, diversity, and evenness of benthic macroinvertebrates in the sites due to high human activities around this waterbody, which greatly impacted the diversity of macroinvertebrates. The Shannon-Weiner index (H) values recorded at site II were within the normal range of 0.0–2.53. The Shannon-Weiner index (H) value decreases with the increased aquatic environment stress [74]. Likewise, the Evenness index, which increases with a decrease in ecosystem stress, was relatively high at site II but still far above the ideal metric value of 1, which indicates macroinvertebrate species population in this study is not evenly distributed. This implies that the species composition was similarly exhibited by the dominance of pollution-tolerant taxa over sensitive taxa.
5. Conclusion
Due to anthropogenic activities around this waterbody, the fluctuation in concentrations of some water physicochemical and sediment quality in this study plays a crucial role in the low abundance and distribution of benthic macroinvertebrates recorded. The effective control of Lagos Lagoon pollution requires further research to evaluate the extent of pollution in this aquatic ecosystem to determine the biological effect of toxicants and the prediction of biological consequences. The principal toxicity criteria of this waterbody should be determined by changes in the nature and the rate of production, anomalous species composition, the disappearance of pollution-sensitive species. The varieties of aquatic organisms and their development stages, blocking of biochemical connecting channels in ecosystems as well as by other ecological disturbances which take place, when the level of chronic pollution is relatively low. The studies of comparative resistance to toxicants by some organisms identified during this study are mostly sensitive species which revealed the abnormalities caused by pollution in the primary and secondary productivity of Lagos Lagoon water. The analysis of present-day pollution in the aquatic environment and the future prediction of biological and ecological consequences arising from it should also determine.
Funding statement
The authors received no specific funding for this work.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19508.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Resources Institute (WRI) 2002. Drylands, People, and Ecosystem Goods and Services: A Web-Based Geo-Spatial Analysis.http://www.wri.org [Google Scholar]
- 2.Lyu H., Song D., Zhang S., Wu W., Bao X. Compound effect of land reclamation and land-based pollutant input on water quality in Qinzhou Bay, China. Sci. Total Environ. 2022;826 doi: 10.1016/j.scitotenv.2022.154183. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S., Xu N., Li Z., Huang C. Satellite derived coastal reclamation expansion in China since the 21st century. Glob. Ecol. Conser. 2021;30 [Google Scholar]
- 4.Kida K., Ibori Y., Kawahigashi M. Impact of farmland reclamation on soil distribution in Japan: the case of Andosols in Nagano Prefecture. J. Soils Sediments. 2021;21(5):1938–1946. [Google Scholar]
- 5.Gyawali B., Shrestha S., Bhatta A., Pokhrel B., Cristan R., Antonious G., Banerjee S., Paudel K.P. Assessing the effect of land-use and land-cover changes on discharge and sediment yield in a rural coal-mine dominated watershed in Kentucky. USA. Water. 2022;14(4):516. [Google Scholar]
- 6.Bakker M. The nature and dynamics of pre-Roman iron age and roman iron age reclamation settlements in the (former) peat and clay-on-peat area of Friesland (The Netherlands) J. Wetl. Archaeol. 2022:1–23. [Google Scholar]
- 7.Fashae O.A., Tijani M.N., Adekoya A.E., Tijani S.A., Adagbasa E.G., Aladejana J.A. Comparative assessment of the changing pattern of land cover along the southwestern Coast of Nigeria using GIS and remote sensing techniques. Scientific African. 2022:1286. [Google Scholar]
- 8.Zabbey N., Giadom F.D., Babatunde B.B. World Seas: an Environmental Evaluation. Academic Press; 2019. Nigerian coastal environments; pp. 835–854. [Google Scholar]
- 9.Ospar Commission . 2008. Assessment of the Environmental Impact of Land Reclamation. Biological Diversity of OSPAR Commission.http://qsr2010.ospar.org/media/assessments/p00368 Land Reclamation.pdf [Google Scholar]
- 10.Cheng Z., Jalon-Rójas I., Wang X.H., Liu Y. Impacts of land reclamation on sediment transport and sedimentary environment in a macro-tidal estuary. Estuar. Coast. Shelf Sci. 2020;242 [Google Scholar]
- 11.Gao C., Zhang S., Liu H., Cong J., Li Y., Wang G. The impacts of land reclamation on the accumulation of key elements in wetland ecosystems in the Sanjiang Plain, northeast China. Environ. Pollut. 2018;237:487–498. doi: 10.1016/j.envpol.2018.02.075. [DOI] [PubMed] [Google Scholar]
- 12.Reible D.D., Fleeger J.W., Pardue J., Tomson M., Kan A., Thibodeaux L. 2002. Contaminant Release during Removal and Resuspension; pp. 1–89. [Google Scholar]
- 13.Calmano W., Hong J., Förstner U. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci. Technol. 1993;28(8–9):223–235. [Google Scholar]
- 14.Caille N., Tiffreau C., Leyval C., Morel J.L. Solubility of metals in an anoxic sediment during prolonged aeration. Sci. Total Environ. 2003;301(1–3):239–250. doi: 10.1016/s0048-9697(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 15.Caetano M., Madureira M.J., Vale C. Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study. Water Air Soil Pollut. 2003;143(1):23–40. [Google Scholar]
- 16.Latimer J.S., Davis W.R., Keith D.J. Mobilization of PAHs and PCBs from in-place contaminated marine sediments during simulated resuspension events. Estuar. Coast Shelf Sci. 1999;49(4):577–595. [Google Scholar]
- 17.Elosegi A., Díez J., Mutz M. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia. 2010;657(1):199–215. [Google Scholar]
- 18.Sumudumali R.G.I., Jayawardana J.M.C.K. A review of biological monitoring of aquatic ecosystems approaches: with special reference to macroinvertebrates and pesticide pollution. Environ. Manag. 2021;67(2):263–276. doi: 10.1007/s00267-020-01423-0. [DOI] [PubMed] [Google Scholar]
- 19.Khatri N., Tyagi S., Rawtani D., Tharmavaram M. Assessment of river water quality through application of indices: a case study River Sabarmati, Gujarat India. Sustain Water Resource Management. 2020;6(6):101. [Google Scholar]
- 20.Parikh G., Rawtani D., Khatri N. Insects as an indicator for environmental pollution. Environ. Claims J. 2021;33(2):161–181. [Google Scholar]
- 21.Patel A., Rastogi N., Gandhi U., Khatri N. Oxidative potential of atmospheric PM10 at five different sites of Ahmedabad, a big city in Western India. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115909. [DOI] [PubMed] [Google Scholar]
- 22.Ajao E.A., Fagade S.O. The benthic macro-fauna of Lagos Lagoon. Zool. 2002;1(2):1–15. [Google Scholar]
- 23.Ojeh V.N., Balogun A.A., Okhimamhe A.A. 2016. Urban-Rural Temperature Differences in Lagos. [Google Scholar]
- 24.Ayeni A.O., Omojola A.S., Fasona M.J. 2008. Urbanization and Water Supply in Lagos State, Nigeria: The Challenges in a Climate Change Scenario; pp. 1–9. [Google Scholar]
- 25.Ayeni A.O. Increasing population, urbanization and climatic factors in Lagos State, Nigeria: the nexus and implications on water demand and supply. J. Glob. Initiat. : Policy Pedag. Persp. 2017;11(2):1–6. [Google Scholar]
- 26.Idris J., Fagbenro A. 2016. Lagos the Mega-City: A Report on How the Metropolis Handled an Outbreak of the Ebola Epidemic; pp. 1–10. [Google Scholar]
- 27.APHA/AWWA/WEF . twenty-third ed. American Public Health Association, American Water Works Association, Water Environment Federation; Denver: 2017. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 28.American Public Health Association . twenty-first ed. American Public Health Association/American Water Works Association/Water Environment Federation; Washington DC: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 29.Marczenko Z. second ed. E. Horwood; Chichester: 1986. Separation and Spectrophotometric Determination of Elements; p. 678. [Google Scholar]
- 30.Ademoroti C.M.A. Foludex Press Ltd.; Ibadan: 1996. Standard Methods for Water and Effluents Analysis; p. 182. [Google Scholar]
- 31.American Public Health Association . In: Standard Methods for the Examination of Water and Wastewater. twentieth ed. Lenore S.C., Arnold E.G., Andrew D.E., editors. America Public Health Association (APHA); Washington D.C: 2001. [Google Scholar]
- 32.Golterman H.L., Clymo R.S., Ohnstand M.A.M. second ed. IBP Handbook No 8., Blackwell Scientific Publication; Oxford: 1978. Methods for Physical and Chemical Analysis of Freshwater; p. 213. [Google Scholar]
- 33.APHA/AWWA/WEF, Standard Methods for the Examination of Water and Wastewater. nineteenth ed., American, .
- 34.Public Health Association (APHA), The American Water Works Association (AWWA) and the Water Environment Federation (WEF). Washington, DC, (1995) page 120.
- 35.I. I. T. A . Vol. 7. 1999. Automated and semi-automated methods for sediment analysis; pp. 33–36. [Google Scholar]
- 36.Brown D.S. second ed. Taylor & Francis; London: 1994. Freshwater Snails of Africa and Their Medical Importance. [Google Scholar]
- 37.Day J.A., Harrison A.D., De Moor I.J. vol. 9. Water Research Commission; Pretoria: 2002. Guides to the Freshwater Invertebrates of Southern Africa. [Google Scholar]
- 38.De Moor I.J., Day J.A., De Moor F.C. vol. 7. Water Research Commission; Pretoria: 2003. Guides to the Freshwater Invertebrates of Southern Africa. [Google Scholar]
- 39.Arimoro F.O., James H.M. Grahamstown: Albany Museum; 2008. Preliminary Pictorial Guide to the Macroinvertebrates of Delta State Rivers, Southern Nigeria. [Google Scholar]
- 40.Arimoro F.O., Obi-Iyeke G., Obukeni P.J.O. Spatiotemporal variation of macroinvertebrates in relation to canopy cover and other environmental factors in Eriora River, Niger Delta, Nigeria. Environ. Monit. Assess. 2012;184:6449–6461. doi: 10.1007/s10661-011-2432-9. [DOI] [PubMed] [Google Scholar]
- 41.Oladejo M.A., Oloyede O.O., Adesakin T.A., Morenikeji M.O. The abundance, distribution and diversity of invasive and indigenous freshwater snails in a section of the Ogunpa River, southwest Nigeria. Molluscan Res. 2021;18(58):1–14. [Google Scholar]
- 42.Margalef R. A new limnology method for investigation of thin-layered Epilithic communities. Hydrobiologia. 1948;29:215–216. [Google Scholar]
- 43.Shannon C.E., Weaver W. The University of Illinois Press; Illinois: 1963. The Mathematical Theory of Communication. [Google Scholar]
- 44.Hill M.O. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54:427–432. http://www.hsrc.org/hsrc/html/sswIssw-contaminant.htn-d [Google Scholar]
- 45.Simpson E.H. Nature. 1949;163:688. doi: 10.1038/163771a0. [DOI] [PubMed] [Google Scholar]
- 46.Oyeleke P., Olatunde B.P., Abiodun O. Assessment of some physico-chemical parameters of Lagos Lagoon, Southwestern, Nigeria. Acad. J. Chem. 2019;4(3):9–11. [Google Scholar]
- 47.Whitehead P.G., Wilby R.L., Battarbee R.W., Kernan M., Wade A.J. A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. Sci. Hydrologiq. 2009;54(1) [Google Scholar]
- 48.Solheim A.L., Austnes K., Eriksen T.E., Seifert I., Holen S. Background Report for EEA European Environment State and Outlook Report. 2010. Climate change impacts on water quality and biodiversity; pp. 1–68. [Google Scholar]
- 49.World Health Organization (WHO) Fourth 565 edition. WHO Library Cataloguing-in-Publication; Switzerland: 2011. Guidelines for Drinking-Water Quality. [Google Scholar]
- 50.National Environmental Standards and Regulations Enforcement Agency (NESREA) 2011. National Environmental (Surface and Ground Water Quality Control) Regulations- Effluent Discharges, Irrigation and Reuse Standards. [Google Scholar]
- 51.Emmanuel B.E., Chukwu L.O., Bakare S.O. Hydro-Chemistry, macro-invertebrate fauna and fish production of Acdja fishing sites in A tropical lagoonal ecosystem. Journal of American Science. 2010;6(1):42–48. [Google Scholar]
- 52.Onyena A.P., Okoro C.A. Spatio-temporal variations in water and sediment parameters of Abule Agege, Abule Eledu, Ogbe, creeks adjoining Lagos Lagoon, Nigeria. J. Ecol. Nat. Environ. 2019;11(4):46–54. [Google Scholar]
- 53.Chen X., Gao W., Liu W., Sun C., Kang L. Characteristics of macrobenthos community structure and its relationship to environmental factors within a typical plain river network. Ecol. Environ. Sci. (in Chinese) 2013;22:1310–1316. [Google Scholar]
- 54.Ayandiran T.A., Fawole O.O., Dahunsi S.O. Water quality assessment of bitumen polluted Oluwa River, Southwestern Nigeria. Water Resour. Ind. 2017;2212–3717(17) [Google Scholar]
- 55.Nkwoji J.A., Edokpayi C.A. Hydrochemistry and community structure of benthic macroinvertebrates of a south western Lagoon, Lagos, Nigeria. Res. J. Pharm. Chem. Sci. 2013;4(1):1119–1131. [Google Scholar]
- 56.Nkwoji J.A., Yakub A.S., Ajani G., Balogun K.J. Seasonal variations in water chemistry and benthic macroinvertebrates of a South Western Lagoon, Lagos, Nigeria. J. Am. Sci. 2014;6(3):85–95. [Google Scholar]
- 57.Davies O.A., Allison M.E., Uyi H.S. Bioaccumulation of heavy metals in water, sediment and periwinkle (Tympanotonus fuscatus var. radula) from the Elechi Creek, Niger Delta. Afr. J. Biotechnol. 2006;5(10):968–973. [Google Scholar]
- 58.Moses S., Wyasu G., Moses T.Y. Physicochemical and bacteriological quality of water collected from Dams and Rivers along gold mining sites in Zamfara state. Sci. World J. 2019;14(1) [Google Scholar]
- 59.Ong M.C., Kamaruzzaman B.Y., Noor M.S. Sediment characteristics studies in the surface sediment from Kemaman Mangrove Forest, Teregganu, Malaysia. Orient. J. Chem. 2012;28(4):1639–1644. pg. [Google Scholar]
- 60.Wondim Y.K., Mosa H.M. Spatial variation of sediment physico-chemical cahracteristics of Lake Tana, Ethiopia. J. Environ. Earth Sci. 2015;5(13):95–109. [Google Scholar]
- 61.Miao S., DeLaune R.D., Jugsujinda A. Influence of sediment redox conditions on release/solubility of metals and nutrients in a Louisiana Mississippi River Deltaic plain freshwater Lake. Sci. Total Environ. 2006;371:334–343. doi: 10.1016/j.scitotenv.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 62.Yong Y., Baipeng P., Chen G., Yan C. Processes of organic carbon in mangrove ecosystem. Acta Ecol. Sin. 2011;31(3):169–173. [Google Scholar]
- 63.Sawant C.P., Marathe R.B., Marathe Y.M. Sediment characteristics of Tapati River, Maharastra, India. Int. J. Chem. Technol. Res. 2011;3(3):1179–1183. [Google Scholar]
- 64.Eilser R. Lead hazards to fish, wildlife and invertebrates: a synoptic review: contaminant hazard reviews report No. 14. Biol. Rep. (Wash. D C) 1988;85(114) [Google Scholar]
- 65.Stefan-Adrian S., Nicoara M., Teodosiru C., Baltag E., Gobanu C., Plavan G. Patterns toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere. 2018;207(1) doi: 10.1016/j.chemosphere.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 66.Parizanganeh A. In: Proceeding of Taal 2007: the 12th World Lake Conference. Sengupta M., Dalwani R., editors. 2008. Grain size effects on trace metals in contaminated sediments along the Iranian Coast of the Caspian Sea; pp. 329–336. [Google Scholar]
- 67.Cavallo R.A., Rizzic C., Vozza T., Stabili L. Viable heterotrophic bacteria in water and sediments in ‘Mar Piccolo’ of Taranto (Ionian Sea, Italy) J. Appl. Microbiol. 1999;86:906–916. doi: 10.1046/j.1365-2672.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 68.a Kasai Y., Kishira H., Harayama S. Bacteria belonging to the genus Cycloclasticus play a primary role in the degradation of aromatic hydrocarbons released in a marine environment. Appl. Environ. Sci. Microbiol. 2002;68:5625–5633. doi: 10.1128/AEM.68.11.5625-5633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sadhana S., JaiVarshini E., Nikita S.R., Shruthi S. Crude oil bioremediation - genetically modified microorganisms for poly-aromatic hydrocarbon degradation. Appl. Ecol. Environ. Sci. 2021;9(8):769–785. [Google Scholar]
- 69.Damasion J., San-Jan M.F., Sanchez-Avila L.S., Prat N., Rieradevall M.S., Amadeu M.V.M., Barata C. Multi-biochemical responses of benthic macroinvertebrate species as a complementary tool to diagnose the cause of community impairment in polluted rivers. Water Res. 2011;45(12):3599–3613. doi: 10.1016/j.watres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Fekadu M.B., Agembe S., Kiptum C.K., Mingist M. Impacts of anthropogenic activities on the benthic macroinvertebrate assemblages during the wet season in Kipsinende River, Kenya. Turk. J. Fish. Aquat. Sci. 2022;22(6) doi: 10.4194/TRJFAS18410. [DOI] [Google Scholar]
- 71.Nkwoji J., Ajani A. Yakub G., Balogun K., Renner K., Igbo J., Ariyo A., Bello B. Seasonal variations in the water chemistry and benthic macro-invertebrates of a south western lagoon, Lagos Nigeria. J. Am. Sci. 2010;6(3):85–92. [Google Scholar]
- 72.Das N., Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol. Res. Int. 2010;2011:1–30. doi: 10.4061/2011/941810. Article ID 941810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chainho P., Costa J.L., Chaves M.L., Lane M.F., Dauer D.M., Costa M.J. Seasonal and Spatial patterns of distribution of subtidal benthic invertebrate communities in the Mondego River, Portugal A Poikilohaline Estuary. Hydrobiologia. 2006;555(1):9–74. [Google Scholar]
- 74.Magurran A.E. third ed. Blackwell; 2004. Measuring Biological Diversity; p. 256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.