Abstract
Objective
To evaluate the prognostic ability of systemic immune-inflammation index (SII) combine with quick Sequential Organ Failure Assessment (qSOFA) criteria in predicting the 28-day mortality of sepsis.
Methods
A retrospective cohort study was conducted, with the population comprised in whom sepsis was confirmed. Clinical and laboratory data recorded were analyzed. The score of Sequential Organ Failure Assessment (SOFA), SII, qSOFA were calculated. Multivariable regression, receiver operating characteristic (ROC) analysis and Kaplan-Meier method were used to identify and compared the predictors of prognosis among SOFA, qSOFA, and the combination of SII with qSOFA.
Results
A total of 349 patients admitted from December 2020 and December 2022 were included in the cohort. 95 (27.2%) of whom had died by day 28. The SII, SOFA, and qSOFA scores were significant higher in the non-survivors than that of survivors (P < 0.05), and identified as independent predictors of sepsis mortality. The addition of SII to qSOFA shown an area under receiver operator characteristic (AUROC) of 0.840 (95% CI: 0.787–0.884), manifested an effective ability in predicting poor outcome than other scoring systems. The optimum cutoff for SII (>1.7668) and qSOFA (>1) represented a high risk level in 28-day mortality of sepsis, were performed and identified in Kaplan-Meier survival curves (log-rank test, HR: 6.942, 95% CI: 3.976–12.121; P < 0.0001).
Conclusion
The SII in addition to qSOFA provided an effective prognostic tool for predicting mortality in sepsis.
Keywords: Sepsis, Mortality, Systemic immune-inflammation index, Quick sequential organ failure assessment, Scoring system
1. Introduction
Sepsis, recognized as an infection-induced disorder of the host's response, results in the life-threatening condition, induces dysfunction of organs, and poses a considerable challenge [1]. As per empirical findings [2], sepsis is projected to have engendered 48.9 million instances, wherein nearly 11 million individuals succumbed to the condition, constituting 19.7% of the total fatalities recorded across the globe in 2017. Due to its intricate pathophysiology and consequential high morbidity, sepsis has emerged as a significant clinical research area within critical care medicine. Despite the existing knowledge gap regarding the pathophysiology of sepsis, the majority of research indicates a direct association with alterations in immunological function and the dysregulation of the inflammatory and anti-inflammatory systems [3]. Concurrently, early assessment and proactive management in sepsis patients are critical for expeditious and efficacious therapeutic measures and improved prognosis [4].
Given the high mortality of this disease, the early identification of sepsis patients who are at a higher risk of mortality is a critical step towards enhancing sepsis management, which serves to guide clinicians in developing individualized treatment strategies, appropriate to the specific risk tiers of patients, thereby enabling timely and suitable interventions [5]. To this end, grading systems were designed and developed to aid in predicting sepsis patients' prognoses in recent years. From 1992, the Systematic Inflammatory Response Syndrome (SIRS) criteria was applied at an initial stage of infection to discern patients with sepsis. The Sequential Organ Failure Assessment (SOFA), which can reflect the variations in organ function dynamically [6], have been recommended and widely employed to evaluate the illness and forecast death in sepsis, with an elevation two-point in the SOFA score being correlated to a 10% rise in mortality [7]. In 2016, the quick Sequential Organ Failure Assessment (qSOFA), a condensed form of SOFA, was recommended as a scoring tool for rapid detection of sepsis in high-risk patient in the third international consensus definition for Sepsis and Septic Shock (Sepsis-3), with a finding of better prognostic ability in SOFA and qSOFA compare to SIRS criteria when predicting intensive care unit (ICU) stay and hospital mortality in sepsis patients [8]. Consequently, studies are being conducted in increasing numbers to evaluate the predictive capacity of qSOFA in an effort to validate the scoring system [1,9,10]. Nonetheless, critiques have arisen regarding the prognostic capability of qSOFA for sepsis. It has demonstrated limited capacity to forecast adverse clinical outcomes in some studies due to its lack of support from laboratory indicators and low sensitivity [[11], [12], [13]]. Currently, some scholars are attempting to combine the qSOFA score with laboratory indicators to enhance the predictive capability for sepsis prognosis [14,15]. Moreover, it remains necessary to conduct additional research and provide substantiation on the predictive capability of these scoring tools for the prognosis of sepsis [16].
In 2014, a new biomarker, known as the systemic immune-inflammation index (SII), based on platelet counts, lymphocyte counts, and neutrophil counts, was introduced [17]. More recently, given the robust relationship between thrombosis and inflammation with incorporating three independent blood-tested biomarkers into a single index, the SII index has been described as a predictor of adverse outcomes in a variety of conditions, such as oncology, cardiovascular diseases, and intracerebral hemorrhage [[18], [19], [20]]. However, as an easily obtainable biomarker and the equally simple and clinically implementable scoring standard, the capacity of SII combines qSOFA in predicting mortality among sepsis patients still requires in-depth exploration. The objective of this study was to investigate whether combining the qSOFA scores and SII could effectively predict mortality in sepsis.
2. Patients and methods
The present retrospective cohort study analyzed sepsis patient data from our large tertiary hospital in China to compare the predictive capabilities of scoring tools. The study followed the Declaration of Helsinki's guidelines. Ethics committee approval were given from the institutional review committee (No.2019LCSY012). Recorded data were anonymized. Written informed consent for participation was not required in accordance with the national legislation and the institutional requirements.
All the cases enrolled in the study have been diagnosed with sepsis in accordance with the Sepsis-3, which was characterized by severe organ dysfunction resulting from an uncontrolled immune response to infection, and the organ dysfunction was represented by the patient with having at least two points in the SOFA scoring system [21].
The diagnostic criteria for infection in this study was established as the identification of microorganisms in sterile body fluids or cavities. Patients diagnosed with lung infections, abdominal infections, or infections at other sites were eligible for inclusion in the study. The criteria used to identify lung infections in this study were as follows: (1) emerging or progressive invasive pulmonary lesions; (2) fever with a temperature ≥38 °C; (3) indications of pulmonary consolidation with or without wet rales detected via auscultation; (4) white blood cell count (WBC) <4×109 L or >10 × 109/L, with or without a left shift of the nucleus; (5) Newly onset of cough, sputum, purulent sputum, or exacerbation of other respiratory symptoms, with or without chest pain. Patients who met the criterion in item (1) in conjunction with any of items (2) through (5), in addition to the exclusion of tuberculosis, non-infectious pulmonary interrogation disease, lung tumors, atelectasis, pulmonary edema, pulmonary embolism, pulmonary vasculitis, pulmonary eosinophilic infiltration, etc. Diagnosis of abdominal infection should be following two criteria: (1) the infection spread from the cavity organs to the peritoneal cavity; (2) Combined with abscess formation or peritonitis. Infections at other sites were defined as those not affecting the lungs or abdominal cavity, and were identified based on clinical and laboratory evidence supporting the presence of infection, including urological, hematogenous, intracranial, and mucocutaneous infections.
During the raw data collection of the study, the inclusion criteria and exclusion criteria were followed.
2.1. Inclusion criteria
-
(1)
Individuals aged 18 years or older of onset,
-
(2)
Comply with the diagnostic criteria all above.
Exclusion criteria.
-
(1)
Definitively diagnose sepsis beyond 48-h period after onset,
-
(2)
Death within 48 h in-hospital, or the patient refused aggressive treatment,
-
(3)
Complicated with serious underlying diseases such as malignant tumors, liver cirrhosis, chronic renal failure (uremia stage), blood system diseases, and HIV,
-
(4)
Treated with hormones, immunosuppressants or have organ transplantation within 6 months before the onset,
-
(5)
Those who have participated in other clinical trials at the same time or within 30 days before the onset,
-
(6)
Within pregnancy or lactation period.
The cases in this study were categorized into survivor group and non-survivor group based on clinical outcomes observed within a period of 28 days. The baseline characteristics of sepsis patients, such as age, gender, weight, height, body temperature, heart rate, systolic blood pressure, major comorbidities, APACHE II score, SOFA score, among others, were collected. Additionally, laboratory test results obtained within the first 24 h of admission were also collected from the patients' medical records.
The researchers involved in this study underwent systematic training to effectively utilize the scoring systems. After collecting the necessary data, the qSOFA score, SIRS criteria, and SII were computed and documented. The qSOFA score comprises three measures, namely Glasgow coma score, systolic blood pressure, and respiratory rate, and ranges from 0 to 3 points. The SIRS criteria includes WBC, heart rate, temperature, and respiration rate/PaCO2 four parameters. The SII was calculated with the formula: SII = [(platelet count × absolute neutrophil count/absolute lymphocyte count)/1000] [22].
2.2. Statistical analysis
The categorical data were analyzed as percentages, whereas continuous variables were expressed as a median with interquartile range (IQR) or a mean with standard deviation (SD). A Mann-Whitney U test or an independent samples t-test was used in the comparison of continuous measures based on data distribution. Fisher's exact test or Pearson's Chi-squared test was used to compare nominal variables.
Before constructing the combined predictive model, patients were randomly allocated to either the training set or the validation set, adhering to a 7:3 ratio. Within the training set, any variables demonstrating a P value of less than 0.1 in the univariate logistic regression analysis were identified as potential candidates for the subsequent stepwise multivariate analysis. Logistic regression analysis was used to analyze the connection between the pertinent parameters and mortality, and measured by the Hosmer-Lemeshow goodness-of-fit test. The clinical predictive performances of qSOFA, qSOFA + SII, and SOFA were performed by receiver operating characteristic (ROC) curves and decision curve analysis (DCA). Area Under the receiver operating characteristic (AUROC) were compared using DeLong's method, which was performed by MedCalc software. A nomogram was utilized to visualize the analysis of qSOFA + SII, while calibration curves were used for internal validation with Bootstrap method. The optimal test cut-offs of qSOFA + SII were calculated by Youden's index. Subsequently, using these cut-offs, all cases were categorized into two groups based on their predicted in-hospital mortality risk: the high-risk group (comprising cases with both qSOFA and SII values exceeding the cut-offs) and the low-risk group (comprising cases with either qSOFA or SII values below the cut-offs). The survival curves of the two groups were performed with the Kaplan-Meier method and measured by the log-rank test. Statistical analyses were carried out and performed using SPSS (version 26.0; IBM Corp.), MedClac (version 20.1; MedCalc Ltd.), GraphPad Prism 9 (version 9.0.0; GraphPad) software, and R software (R Foundation for Statistical Computing, version 4.2.2). The P value < 0.05 was considered to be statistically significant.
3. Results
In this study, 349 patients were enrolled, which based on the data of 372 patients recorded between December 2020 and December 2022. Of these, 23 patients were excluded because of uncertain outcomes or missing data. Among the included cases, 254 (72.8%) survived by day 28, while 95 (27.2%) experienced an adverse outcome. The screening flow illustration was present in Fig. 1.
Fig. 1.
The screening flow chart of study.
In the cohort, 131 (37.5%) females and 218 (62.5%) males were composed. The median age was 76 (67, 85) years in survivor group, and 77 (70, 84) years in non-survivor group, respectively. Lung was the major infection site in both group. There was no significant difference in age, gender, BMI, comorbidities, vital signs, or infection site component between two groups. Compared to the survivor group in laboratory parameters, patients who developed adverse outcome had a higher median platelet (PLT) count (196.6 ± 110.6 vs 172.4 ± 91.5 109/L, P < 0.05). The median APACHE II, SOFA, SII, and qSOFA scores were also higher in the non-survivor group, manifested significant differences (P < 0.05). The baseline information is shown in Table 1. The analysis of logistic regression was conducted to investigated independent predictor for the prognosis of sepsis patients, presented SII, SOFA, and qSOFA scores predict mortality (P < 0.05) (Table 2).
Table 1.
Baseline information of Survivor group and Non-survivor group.
| Survivor group (n = 254) | Non-survivor group (n = 95) | P value | |
|---|---|---|---|
| Patient characteristics | |||
| Sex, female, N (%) | 93 (26.3%) | 38 (40.0%) | 0.561 |
| Age, median (interquartile ranges) | 76 (67, 85) | 77 (70, 84) | 0.474 |
| BMI (Mean ± SD) | 21.9 ± 3.0 | 21.2 ± 3.2 | 0.077 |
| Comorbidities, N (%) | |||
| Hypertension | 143 (56.3%) | 52 (54.7%) | 0.794 |
| Diabetes | 79 (31.1%) | 34 (35.8%) | 0.405 |
| Heart disease | 81 (31.9%) | 35 (36.8%) | 0.382 |
| Chronic pulmonary disease | 35 (13.8%) | 9 (9.5%) | 0.281 |
| Cerebrovascular disease | 46 (18.1%) | 14 (14.7%) | 0.457 |
| Chronic mild or severe liver disease | 11 (4.3%) | 5 (5.3%) | 0.711 |
| Chronic kidney disease | 19 (7.5%) | 11 (11.6%) | 0.224 |
| Vital signs (Mean ± SD) | |||
| HR | 88.9 ± 22.0 | 91.5 ± 18.9 | 0.310 |
| SBP | 124.1 ± 21.1 | 123.9 ± 25.6 | 0.929 |
| T | 36.9 ± 0.7 | 37.0 ± 0.7 | 0.421 |
| Infection site, N (%) | |||
| Lung | 191 (75.2%) | 76 (80.0%) | 0.346 |
| Abdomen | 41 (16.1%) | 9 (9.5%) | 0.114 |
| Others | 22 (8.7%) | 10 (10.5%) | 0.591 |
| Laboratory parameters (Mean ± SD) | |||
| WBC ( × 109/L) | 12.3 ± 11.1 | 12.6 ± 5.9 | 0.817 |
| PLT ( × 109/L) | 172.4 ± 91.5 | 196.6 ± 110.6 | 0.039 |
| ALT (U/L) | 61.1 ± 170.7 | 72.8 ± 177.5 | 0.572 |
| TBIL (μmol/L) | 23.2 ± 26.4 | 25.5 ± 36.8 | 0.518 |
| Scr (μmol/L) | 122.5 ± 305.2 | 138.4 ± 122.4 | 0.622 |
| SII (Mean ± SD) | 1.7 ± 2.3 | 7.1 ± 13.2 | <0.0001 |
| Score system (interquartile ranges) | |||
| SIRI | 2 (2, 3) | 2 (2, 3) | 0.208 |
| APACHE II | 13 (9, 19) | 18 (13, 22) | <0.0001 |
| SOFA | 5 (3, 6) | 6 (4, 9) | <0.0001 |
| qSOFA | 1 (1, 1) | 2 (1, 2) | <0.0001 |
Abbreviations: BMI, Body Mass Index; SD, Standard deviation; HR, Heart rate; SBP, Systolic blood pressure; T, Temperature; WBC, White blood cell; PLT, Platelet; ALT, Alanine aminotransferase; TBIL, Total bilirubin; Scr, Serum creatinine; SII, Systemic Immune-Inflammation Index.
Table 2.
Univariate and multivariate analysis of prognostic risk factors in patients with sepsis.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| BMI | 0.930 | 0.859–1.008 | 0.078 | 0.960 | 0.872–1.058 | 0.414 |
| PLT | 1.002 | 1.000–1.005 | 0.041 | 0.998 | 0.994–1.002 | 0.260 |
| SII | 1.428 | 1.276–1.598 | <0.0001 | 1.434 | 1.252–1.642 | <0.0001 |
| APACHE II | 1.102 | 1.059–1.146 | <0.0001 | 1.003 | 0.946–1.064 | 0.908 |
| SOFA | 1.213 | 1.113–1.323 | <0.0001 | 1.169 | 1.031–1.325 | 0.015 |
| qSOFA | 3.495 | 2.392–5.105 | <0.0001 | 2.459 | 1.546–3.912 | <0.0001 |
Next, all cases were randomly allocated to either the training set (242 patients) or validation set (107 patients), with the baseline information for both datasets displayed in Supplementary Table 1, Supplementary Table 2 and Supplementary Table 3. The data from the training set was utilized to plot ROC curves of SOFA, qSOFA, and qSOFA + SII, in order to demonstrate their predictive capacities for sepsis mortality (Fig. 2). AUROC were calculated and compared. The result illustrated SII in addition to qSOFA performed an effective ability in distinguishing poor outcome (AUROC = 0.840, 95% CI: 0.787–0.884) (Table 3), with the coefficients of qSOFA and SII were 1.166 and 0.337 respectively. The nomogram was employed, while calibration curves were utilized for internal validation using the Bootstrap method with 1000 resampling (Fig. 3, Fig. 4). Additionally, the AUROC of qSOFA + SII showed a significant different among the predictors (qSOFA + SII vs qSOFA, P = 0.0001; qSOFA + SII vs SOFA, P = 0.0001) (Table 4).
Fig. 2.
ROC curves for comparing the scoring systems within training set.
Table 3.
ROC of the different scoring systems within training set for predicting the mortality of sepsis patients.
| Scoring system | SE | AUROC | Youden's index | sensitivity | specificity | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|
| qSOFA + SII | 0.028 | 0.840 (0.787–0.884) | 0.583 (0.461–0.676) | 0.710 (0.588–0.813) | 0.873 (0.814–0.919) | 0.690 (0.594–0.772) | 0.883 (0.839–0.916) | 5.584 (3.675–8.486) | 0.332 (0.229–0.483) |
| qSOFA | 0.031 | 0.751 (0.691–0.804) | 0.470 (0.349–0.597) | 0.696 (0.573–0.801) | 0.775 (0.705–0.835) | 0.552 (0.473–0.628) | 0.865 (0.816–0.902) | 3.086 (2.247–4.238) | 0.393 (0.273–0.566) |
| SOFA | 0.040 | 0.654 (0.590–0.714) | 0.287 (0.163–0.400) | 0.420 (0.302–0.545) | 0.867 (0.807–0.914) | 0.558 (0.441–0.669) | 0.789 (0.753–0.822) | 3.161 (1.974–5.062) | 0.669 (0.542–0.824) |
Abbreviations: AUROC, area under the ROC curve; SE, standard error; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
In Brackets: 95% confidence intervals.
Fig. 3.
The nomogram for present the logistic regression analysis results of qSOFA + SII in predicting the risk of adverse outcome.
Fig. 4.
The calibration curves with Bootstrap method for training set.
Table 4.
Pairwise comparison of ROC curves of the different scoring systems within training set.
| Scoring system | Difference between AUROC | SE | 95% CI | Z statistic | P value |
|---|---|---|---|---|---|
| qSOFA + SII ∼ qSOFA | 0.089 | 0.0216 | 0.047–0.131 | 4.112 | 0.0001 |
| qSOFA + SII ∼ SOFA | 0.186 | 0.0478 | 0.092–0.280 | 3.893 | 0.0001 |
| qSOFA ∼ SOFA | 0.097 | 0.0464 | 0.006–0.188 | 2.091 | 0.0365 |
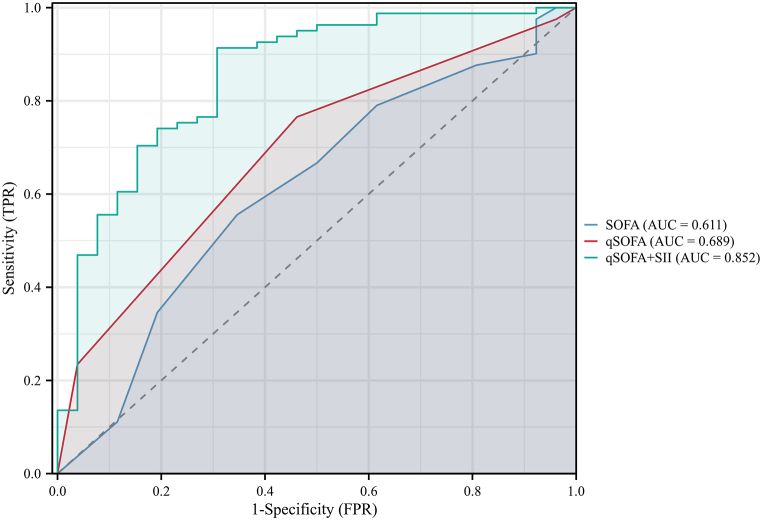
Subsequently, data from the validation set was employed to verify the predictive model, while also being used for the construction and comparison of ROC curves (Fig. 5). The results demonstrated that the qSOFA + SII also exhibited effective predictive performance in the validation set (AUROC = 0.852, 95% CI: 0.764–0.941). The results of the analysis were presented in Table 5 and Table 6. Calibration curves were similarly described using the bootstrap method (Fig. 6).
Fig. 5.
ROC curves for comparing the scoring systems within validation set.
Table 5.
ROC of the different scoring systems within validation set for predicting the mortality of sepsis patients.
| Scoring system | SE | AUROC | Youden index | sensitivity | specificity | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|---|
| qSOFA + SII | 0.046 | 0.852 (0.764–0.941) | 0.606 (0.401–0.734) | 0.692 (0.482–0.857) | 0.914 (0.830–0.965) | 0.720 (0.548–0.845) | 0.902 (0.838–0.943) | 8.011 (3.773–17.011) | 0.337 (0.188–0.602) |
| qSOFA | 0.052 | 0.689 (0.592–0.775) | 0.304 (0.150–0.495) | 0.539 (0.334–0.734) | 0.765 (0.658–0.852) | 0.424 (0.302–0.556) | 0.838 (0.770–0.888) | 2.296 (1.351–3.902) | 0.603 (0.391–0.929) |
| SOFA | 0.065 | 0.611 (0.512–0.703) | 0.209 (0.079–0.348) | 0.654 (0.443–0.828) | 0.556 (0.441–0.666) | 0.321 (0.246–0.406) | 0.833 (0.740–0.898) | 1.471 (1.015–2.132) | 0.623 (0.355–1.094) |
In Brackets: 95% confidence intervals.
Table 6.
Pairwise comparison of ROC curves of the different scoring systems within validation set.
| Scoring system | Difference between AUROC | SE | 95% CI | Z statistic | P value |
|---|---|---|---|---|---|
| qSOFA + SII ∼ qSOFA | 0.163 | 0.0367 | 0.091–0.235 | 4.444 | 0.0001 |
| qSOFA + SII ∼ SOFA | 0.242 | 0.0723 | 0.100–0.383 | 3.343 | 0.0008 |
| qSOFA ∼ SOFA | 0.079 | 0.0656 | −0.050 - 0.207 | 1.198 | 0.2311 |
Fig. 6.
The calibration curves with Bootstrap method for validation set.
The DCA curve was plotted to illustrate the application of qSOFA + SII in the clinical setting, and to compared it with other scoring systems. In the training dataset, guidance of clinical intervention by this predictive model yielded a superior net benefit when the threshold probability ranged between 0.11 and 0.77, as illustrated in Fig. 7. Similarly, the analysis of the validation dataset demonstrated that, within a threshold probability interval of 0.08–0.88, employment of this model to prognosticate the 28-day mortality rate in sepsis patients conferred a greater net benefit than that offered by the SOFA and qSOFA systems, as depicted in Fig. 8.
Fig. 7.
Decision curve analysis of the qSOFA + SII, qSOFA, and SOFA for training set.
Fig. 8.
Decision curve analysis of the qSOFA + SII, qSOFA, and SOFA for validation set.
Optimal cut-off values for SII and qSOFA were calculated in the training set, with SII being greater than 1.7668 and qSOFA being greater than 1. Youden's index was utilized to determine these values, resulting in a qSOFA Youden's index of 0.470 (95% CI: 0.349–0.597), sensitivity of 69.6%, and specificity of 77.5%, and a SII Youden's index of 0.555 (95% CI: 0.436–0.644), sensitivity of 85.5%, and specificity of 69.9%. Cases of all the participants that had both SII and qSOFA values greater than these cut-offs were categorized as high-risk group, while others were placed in the low-risk group. Additionally, based on Kaplan-Meier survival curves, a significant difference in 28-day mortality was found between the two groups (log-rank test, HR: 6.942, 95% CI: 3.976–12.121; P < 0.0001) (Fig. 9).
Fig. 9.
The comparison of Kaplan-Meier survival curves between high-risk group and low-risk group.
4. Discussion
In this study, 349 recorded data of sepsis patients were collected and analyzed. From the cohort, we revealed the independent predictor factors relevant to sepsis mortality, compared the different prognostic ability of the qSOFA, qSOFA + SII, SOFA scoring systems. Among these predicting tools, the qSOFA combined with SII shown a better capability than that of SOFA and qSOFA in predicting 28-day mortality in sepsis.
Sepsis is a condition with high mortality. For such patients, the primary concern for medical practitioners is how to save lives and reduce mortality. Previous article has proposed the early detection, risk stratification, and prediction of mortality aided physicians in making key treatment decisions, allowing them to provide more aggressive care for higher-risk patients [23], providing a major impact on the timely management of sepsis, lead to a reduction in the risk of death [24]. Identification of patients with sepsis who have a higher risk of adverse outcome can provide a more efficient framework for therapeutic strategies [16], facilitate hospitals and intensive care units to more effectively distribute their resources, focusing on patients with a higher likelihood of deterioration, enable physicians to stratify the risk of negative outcomes for sepsis patients, furthermore, provided a standardized benchmark to compare the effectiveness of different treatments in clinical trials and helps in guiding future research [25], and provides a certain reference for the psychological expectations of doctors and patients' family members regarding the prognosis.
Numerous researchers have endeavored to identify valuable evaluation and classification systems for sepsis. In the past two decades, the SIRS criteria was employed to score sepsis. Nevertheless, it was deemed to be inadequately specific in gauging and predicting the mortality rate for the sepsis patients. Kaukonen et al. presented evidence that the SIRS criteria were overly restrictive and unable to determine a transition point for the risk of mortality when utilized to predict the mortality rate of patients admitted to intensive care units with severe sepsis [26]. Since introduced in 2016, the SOFA score has been used to identify sepsis patients at risk for death [27]. In contrast, qSOFA score is a more rapid and simple tool in the assessment of multi-organ dysfunction recommended by European Society of Intensive Care Medicine (ESICM) and Society of Critical Care Medicine (SCCM) [28], reported by several studies to be effective in predicting 30-day mortality of the patients with suspected infection and sepsis [29,30]. Freund et al. conducted a multi-center study with 879 patients, found the qSOFA score manifested a superior prognostic accuracy in the in-hospital mortality for sepsis than SIRS, with an AUROC of 0.80 (95% CI: 0.74–0.85) vs 0.65 (95% CI: 0.59–0.70) [10]. Abdullah et al. in their study presented that qSOFA in a score of 2 or 3 point could provide effective prognostic information for patients with sepsis that defined by the SIRS criteria [13]. However, due to the lack of laboratory indicators support [3] and low sensitivity, some performed studies pointed out the poorly prognostic ability of qSOFA for mortality and resultant delayed initiation of intervention for improving outcomes [[31], [32], [33]]. Usman et al. compared the scoring systems among qSOFA, SIRS, and National Early Warning Score (NEWS), found that qSOFA performed a poor sensitivity and ability for the emergency department sepsis screening [34]. Oduncu et al. reported the sensitivity of qSOFA was only 39% for 30-day mortality compared to the SIRS with 82% [35].
Inflammatory activity can be assessed through a series of indicators derived from peripheral blood tests, such as the white blood cells, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reaction protein that have been previously reported by scholars [36,37]. The SII was developed and combined three biomarkers of peripheral blood, including platelet, neutrophil, and lymphocyte count, presents as a comprehensive and robust indicator, which thoroughly sum up the balance of the host inflammatory and immunological status. The varied roles that platelets, lymphocytes, and neutrophils function during the immune response explain the usefulness of SII in identifying the risk of serious infections. In addition, the complete blood count is inexpensive, easy to perform, that facilitates implementation for clinician. However, since developed in 2014, the application of SII in infectious diseases was only be studied in few researches and has not yet been fully explored. Fois et al. in their retrospective study with 119 patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), found the SII on admission is an independent predictor of in-hospital mortality [38]. Mangalesh et al. retrospectively evaluated 267 patients with diagnosed sepsis [39]. In that study, they found the SII was independently predicted the sepsis mortality. In a recent retrospective study conducted by Pricop et al. a group of 108 patients with acute odontogenic infections were observed. The study revealed that the ratio of patients developing sepsis was accurately predicted by the SII score, which also demonstrated the potential to predict the development of systemic inflammatory response syndrome [37].
The advantage of qSOFA is its simplicity as opposed to other clinical scoring systems (e.g., SIRS, NEWS). Therefore, it would be beneficial to develop a novel scoring system that maintains this simplicity while augmenting the predictive capability of qSOFA [40]. As a result of our finding, the combination of SII with qSOFA score manifested a distinguishing incremental effect on the prognostic ability in the sepsis, Furthermore, when compared to the qSOFA scoring system, there was a substantial enhancement in terms of sensitivity. Recently, researchers have explored the role of biochemical markers in the prognosis of sepsis [41,42], while some studies have attempted combining laboratory test biomarker and qSOFA score to improve the prognostic accuracy. Shetty et al. found that combined with lactate ≥2 mmol/L and qSOFA performed a better identification ability for the suspected sepsis patients with an adverse outcome compared to qSOFA ≥2 [43]. Liu et al. retrospective observed a total of 821 sepsis patients, used the ROC curves analysis and shown the lactate in addition to qSOFA is superior to SIRS, modified early warning score (MEDS), qSOFA, and mortality in emergency department sepsis (MEWS) in predicting in-hospital mortality of sepsis patients [16]. Yu et al. combined the procalcitonin with the qSOFA and found a great improvement in the mortality prediction capability of sepsis [44]. Xiao et al. investigated the increasing predictor ability of conjugated bilirubin and creatinine levels combine with qSOFA score for sepsis progression and prognosis [42]. However, conflicting results were also raised by some other scholars. Freund et al. in their study found the addition of lactate to qSOFA could not elevate the discriminative performance for in-hospital mortality compared with using qSOFA alone [10]. Mellhammar et al. found in their study that the effect of adding biomarker lactate to qSOFA shown a slightly increasing AUROC from 0.62 (95% CI: 0.55–0.68) to 0.64 (95% CI: 0.58–0.70), was not significantly improved (p = 0.66) in the performance for predicting a composite outcome of sepsis [45]. Therefore, the pursuit of a robust predictive system still necessitates ongoing efforts from researchers.
To our knowledge, this study is the first attempt to utilize a combination of SII and qSOFA as a predictor for sepsis mortality. The performance of our model in both the training and validation sets suggested its solid generalizability and robustness. It is particularly noteworthy that the model's performance in the validation set, as indicated by an AUC of 0.852, indeed exceeded that of the training set, which might result in the validation set consisting of cases was exceptionally adept at predicting. On the other side, the validation set may embody a patient population slightly distinct from the training set, thus enhancing the efficacy of the model. Taken as a whole, we propose that our study introduced and evaluated a novel combination of predictors that contribute to the existing body of knowledge in sepsis research and could potentially facilitate clinical decision-making.
We conducted a preliminary assessment of the prognostic impact of combining the SII with the qSOFA score, and demonstrated the potential of this combined approach to predict mortality in patients with sepsis. Nevertheless, the relatively small sample size and retrospective nature could not be neglected, which may influence results and cause bias. Moreover, larger sample sizes, along with more sophisticated grouping and dataset arrangements, and more robust external validation, would lend greater stability and persuasiveness to the constructed model. Additionally, this study focuses solely on mortality as a prognostic indicator, and unable to analyze dynamic changes in the SII and the qSOFA score during the course of disease, thereby not reflecting the full progression of a sepsis patient's condition. Consequently, it is necessary for future research to investigate the dynamic variations of SII and qSOFA throughout the progression of sepsis, and to establish a broader set of outcome measures. This would aid in evaluating the predictive ability of SII in conjunction with qSOFA for sepsis prognosis from a more comprehensive perspective.
In conclusion, the SII combined with qSOFA score shows an effective predicting value in the adverse outcome of sepsis. Based on the easily measurement and inexpensive cost, it may facilitate the condition assessment and treatment strategies development in clinical practice for improving clinical outcomes. Further explores and studies with larger samples and heterogeneous populations are necessary for more generalizable findings.
Declarations
Ethics statement
The study involving human participants was reviewed and approved by the Medical Ethics Committee of LongHua Hospital, Shanghai University of Traditional Chinese Medicine (No.2019LCSY012), and was confirmed to comply with all relevant ethical regulations. The requirement of informed consent was waived.
Author contribution statement
Changya Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Xinxin Wu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rou Deng; Xiangru Xu; Caiyu Chen; Hongqiang Yang: Performed the experiments.
Linguangjin Wu; Wen Zhang: Contributed reagents, materials, analysis tools or data.
Yuerong Fei; Yuting Sun: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shuang Zhou; Bangjiang Fang: Conceived and designed the experiments.
Data availability statement
Data included in article/supp. material/referenced in article.
Funding
This study was supported by the National Key Research and Development Program of China (2018YFC1705900), Shanghai Key Clinical Specialty Project (shslczdzk04401), Yangtze River Delta Specialist Disease Alliance - Chinese Medicine Emergency Department Project ZY (2021–2023)-0302.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19526.
Contributor Information
Shuang Zhou, Email: zhoushuang8008@163.com.
Bangjiang Fang, Email: fangbji@163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Raith E.P., et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 2.Rudd K.E., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C., et al. SOFA score in relation to sepsis: clinical implications in diagnosis, treatment, and prognostic assessment. Comput. Math. Methods Med. 2022;2022 doi: 10.1155/2022/7870434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Goulden R., et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg. Med. J. 2018;35(6):345–349. doi: 10.1136/emermed-2017-207120. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y., et al. SAPS III is superior to SOFA for predicting 28-day mortality in sepsis patients based on Sepsis 3.0 criteria. Int. J. Infect. Dis. 2022;114:135–141. doi: 10.1016/j.ijid.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., et al. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019;217:49–56. doi: 10.1016/j.lfs.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Shankar-Hari M., et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour C.W., et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui S., et al. A comparison of pre ICU admission SIRS, EWS and q SOFA scores for predicting mortality and length of stay in ICU. J. Crit. Care. 2017;41:191–193. doi: 10.1016/j.jcrc.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Freund Y., et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301–308. doi: 10.1001/jama.2016.20329. [DOI] [PubMed] [Google Scholar]
- 11.Finkelsztein E.J., et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit. Care. 2017;21(1):73. doi: 10.1186/s13054-017-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J.U., et al. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Crit. Care. 2018;22(1):28. doi: 10.1186/s13054-018-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah S., et al. qSOFA is a useful prognostic factor for 30-day mortality in infected patients fulfilling the SIRS criteria for sepsis. Am. J. Emerg. Med. 2020;38(3):512–516. doi: 10.1016/j.ajem.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Ling H., et al. Evaluation of qSOFA combined with inflammatory mediators for diagnosing sepsis and predicting mortality among emergency department. Clin. Chim. Acta. 2023;544 doi: 10.1016/j.cca.2023.117352. [DOI] [PubMed] [Google Scholar]
- 15.Zacharakis A., et al. Combining C-reactive protein and quick sequential organ failure assessment (qSOFA) to improve prognostic accuracy for sepsis and mortality in adult inpatients: a systematic review. Health Sci Rep. 2023;6(4):e1229. doi: 10.1002/hsr2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., et al. Lactate-enhanced-qSOFA (LqSOFA) score is superior to the other four rapid scoring tools in predicting in-hospital mortality rate of the sepsis patients. Ann. Transl. Med. 2020;8(16):1013. doi: 10.21037/atm-20-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B., et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 18.Aziz M.H., et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann. Surg. 2019;270(1):139–146. doi: 10.1097/SLA.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 19.Ye Z., et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.933913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trifan G., Testai F.D. Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105057. [DOI] [PubMed] [Google Scholar]
- 21.Singer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraghty J.R., et al. Systemic immune-inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;89(6):1071–1079. doi: 10.1093/neuros/nyab354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rello J., et al. Sepsis: a review of advances in management. Adv. Ther. 2017;34(11):2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones A.E., Saak K., Kline J.A. Performance of the Mortality in Emergency Department Sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am. J. Emerg. Med. 2008;26(6):689–692. doi: 10.1016/j.ajem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleuren L.M., et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46(3):383–400. doi: 10.1007/s00134-019-05872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaukonen K.M., et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015;372(17):1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 27.Granholm A., et al. Predictive performance of the simplified acute physiology score (SAPS) II and the initial sequential organ failure assessment (SOFA) score in acutely ill intensive care patients: post-hoc analyses of the SUP-ICU inception cohort study. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., et al. Prognostic values of SOFA score, qSOFA score, and LODS score for patients with sepsis. Ann. Palliat. Med. 2020;9(3):1037–1044. doi: 10.21037/apm-20-984. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.Y., et al. The qSOFA score combined with the initial red cell distribution width as a useful predictor of 30 day mortality among older adults with infection in an emergency department. Aging Clin. Exp. Res. 2021;33(6):1619–1625. doi: 10.1007/s40520-020-01738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W., et al. Predictive value of qSOFA score for death in emergency department resuscitation room among adult trauma patients:a retrospective study. BMC Emerg. Med. 2021;21(1):103. doi: 10.1186/s12873-021-00498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maitra S., Som A., Bhattacharjee S. Accuracy of quick Sequential Organ Failure Assessment (qSOFA) score and systemic inflammatory response syndrome (SIRS) criteria for predicting mortality in hospitalized patients with suspected infection: a meta-analysis of observational studies. Clin. Microbiol. Infect. 2018;24(11):1123–1129. doi: 10.1016/j.cmi.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Shahsavarinia K., et al. qSOFA score for prediction of sepsis outcome in emergency department. Pakistan J. Med. Sci. 2020;36(4):668–672. doi: 10.12669/pjms.36.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devia Jaramillo G., Ibáñez Pinilla M. Quick sequential organ failure assessment, sequential organ failure assessment, and procalcitonin for early diagnosis and prediction of death in elderly patients with suspicion of sepsis in the emergency department, based on sepsis-3 definition. Gerontology. 2022;68(2):171–180. doi: 10.1159/000515851. [DOI] [PubMed] [Google Scholar]
- 34.Usman O.A., Usman A.A., Ward M.A. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am. J. Emerg. Med. 2019;37(8):1490–1497. doi: 10.1016/j.ajem.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 35.Oduncu A.F., Kıyan G.S., Yalçınlı S. Comparison of qSOFA, SIRS, and NEWS scoring systems for diagnosis, mortality, and morbidity of sepsis in emergency department. Am. J. Emerg. Med. 2021;48:54–59. doi: 10.1016/j.ajem.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Peng J., et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): a multicenter, cross-sectional study. J. Clin. Lab. Anal. 2020;34(10) doi: 10.1002/jcla.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pricop M., et al. The predictive value of systemic immune-inflammation index and symptom severity score for sepsis and systemic inflammatory response syndrome in odontogenic infections. J. Personalized Med. 2022;12(12) doi: 10.3390/jpm12122026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fois A.G., et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23) doi: 10.3390/molecules25235725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangalesh S., Dudani S., Malik A. The systemic immune-inflammation index in predicting sepsis mortality. Postgrad. Med. 2022:1–7. doi: 10.1080/00325481.2022.2140535. [DOI] [PubMed] [Google Scholar]
- 40.Saito A., et al. The prognostic utility of prehospital qSOFA in addition to emergency department qSOFA for sepsis in patients with suspected infection: a retrospective cohort study. PLoS One. 2023;18(2) doi: 10.1371/journal.pone.0282148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun T., et al. Prognostic value of syndecan-1 in the prediction of sepsis-related complications and mortality: a meta-analysis. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.870065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y., et al. Evaluation of qSOFA score, and conjugated bilirubin and creatinine levels for predicting 28-day mortality in patients with sepsis. Exp. Ther. Med. 2022;24(1):447. doi: 10.3892/etm.2022.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shetty A., et al. Lactate ≥2 mmol/L plus qSOFA improves utility over qSOFA alone in emergency department patients presenting with suspected sepsis. Emerg. Med. Australasia (EMA) 2017;29(6):626–634. doi: 10.1111/1742-6723.12894. [DOI] [PubMed] [Google Scholar]
- 44.Yu H., et al. Combining procalcitonin with the qSOFA and sepsis mortality prediction. Medicine (Baltim.) 2019;98(23) doi: 10.1097/MD.0000000000015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellhammar L., et al. NEWS2 is superior to qSOFA in detecting sepsis with organ dysfunction in the emergency department. J. Clin. Med. 2019;8(8) doi: 10.3390/jcm8081128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.