Abstract
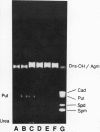
The high performance liquid chromatographic (HPLC) method of Flores and Galston (1982 Plant Physiol 69: 701) for the separation and quantitation of benzoylated polyamines in plant tissues has been widely adopted by other workers. However, due to previously unrecognized problems associated with the derivatization of agmatine, this important intermediate in plant polyamine metabolism cannot be quantitated using this method. Also, two polyamines, putrescine and diaminopropane, also are not well resolved using this method. A simple modification of the original HPLC procedure greatly improves the separation and quantitation of these amines, and further allows the simulation analysis of phenethylamine and tyramine, which are major monoamine constituents of tobacco and other plant tissues. We have used this modified HPLC method to characterize amine titers in suspension cultured carrot (Daucus carota L.) cells and tobacco (Nicotiana tabacum L.) leaf tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Cohen S. S. Dicyclohexylamine-induced shift of biosynthesis from spermidine to spermine in plant protoplasts. Plant Physiol. 1985 Jul;78(3):568–575. doi: 10.1104/pp.78.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Shih L. M., Galston A. W. Relation of polyamine biosynthesis to the initiation of sprouting in potato tubers. Plant Physiol. 1982 Feb;69(2):411–415. doi: 10.1104/pp.69.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. K., Lai C. C. Chromophoric determination of putrescine, spermidine and spermine with dabsyl chloride by high-performance liquid chromatography and thin-layer chromatography. J Chromatogr. 1982 Feb 12;227(2):369–377. doi: 10.1016/s0378-4347(00)80390-4. [DOI] [PubMed] [Google Scholar]
- Seiler N., Deckandt K. Determination of amines and amino acids in sugar-containing samples by dansylation. J Chromatogr. 1975 Apr 9;107(1):227–229. doi: 10.1016/s0021-9673(00)82771-5. [DOI] [PubMed] [Google Scholar]
- Seiler N. Identification and quantitation of amines by thin-layer chromatography. J Chromatogr. 1971 Dec 9;63(1):97–112. doi: 10.1016/s0021-9673(01)85620-x. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. Determination of di-and polyamines by high-performance liquid chromatographic separation of their 5-dimethylaminonaphthalene-1-sulfonyl derivatives. J Chromatogr. 1978 Jan 1;145(1):29–39. doi: 10.1016/s0378-4347(00)81665-5. [DOI] [PubMed] [Google Scholar]
- Seiler N. Liquid chromatographic methods for assaying polyamines using prechromatographic derivatization. Methods Enzymol. 1983;94:10–25. doi: 10.1016/s0076-6879(83)94004-1. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Galston A. W. In vivo inhibition of polyamine biosynthesis and growth in tobacco ovary tissues. Plant Cell Physiol. 1985;26(8):1519–1526. [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Davies P. J. Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol. 1985 May;78(1):89–91. doi: 10.1104/pp.78.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Danzin C., Mamont P. Reversed-phase ion-pair liquid chromatographic procedure for the simultaneous analysis of S-adenosylmethionine, its metabolites and the natural polyamines. J Chromatogr. 1982 Feb 12;227(2):349–368. doi: 10.1016/s0378-4347(00)80389-8. [DOI] [PubMed] [Google Scholar]
- Walter H. J., Geuns J. M. High speed HPLC analysis of polyamines in plant tissues. Plant Physiol. 1987 Feb;83(2):232–234. doi: 10.1104/pp.83.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]