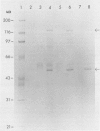
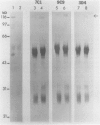
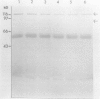
Abstract
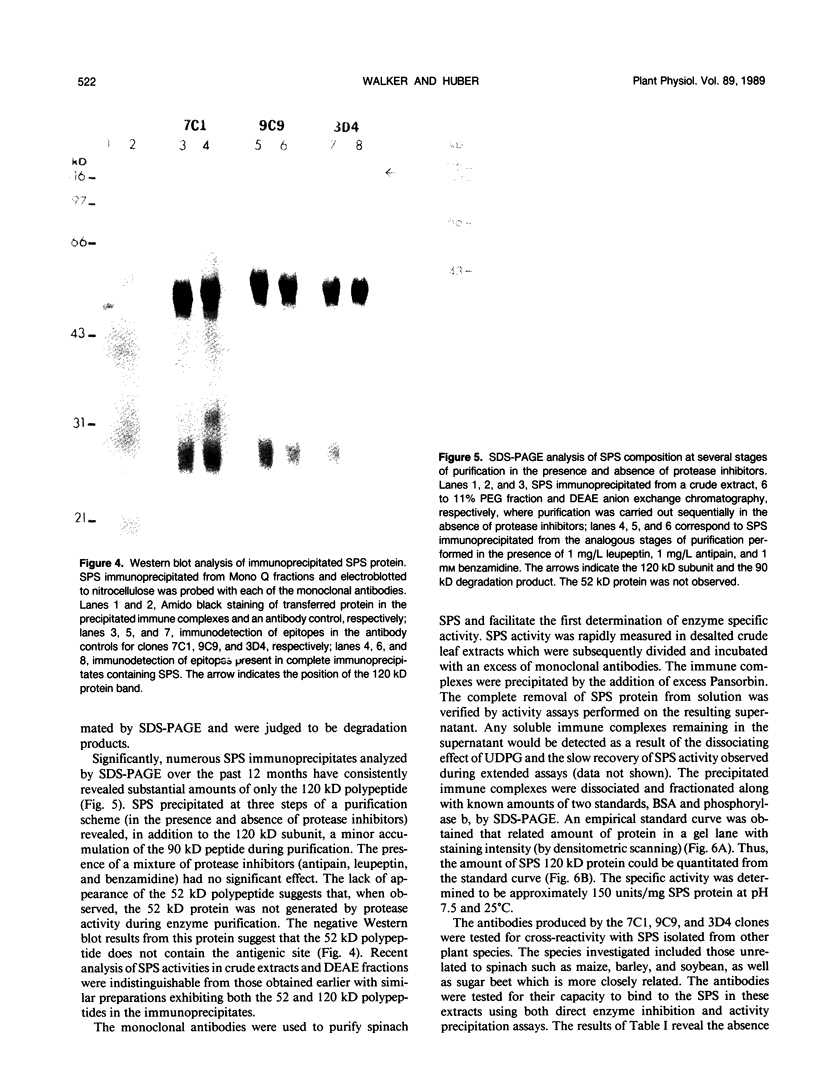
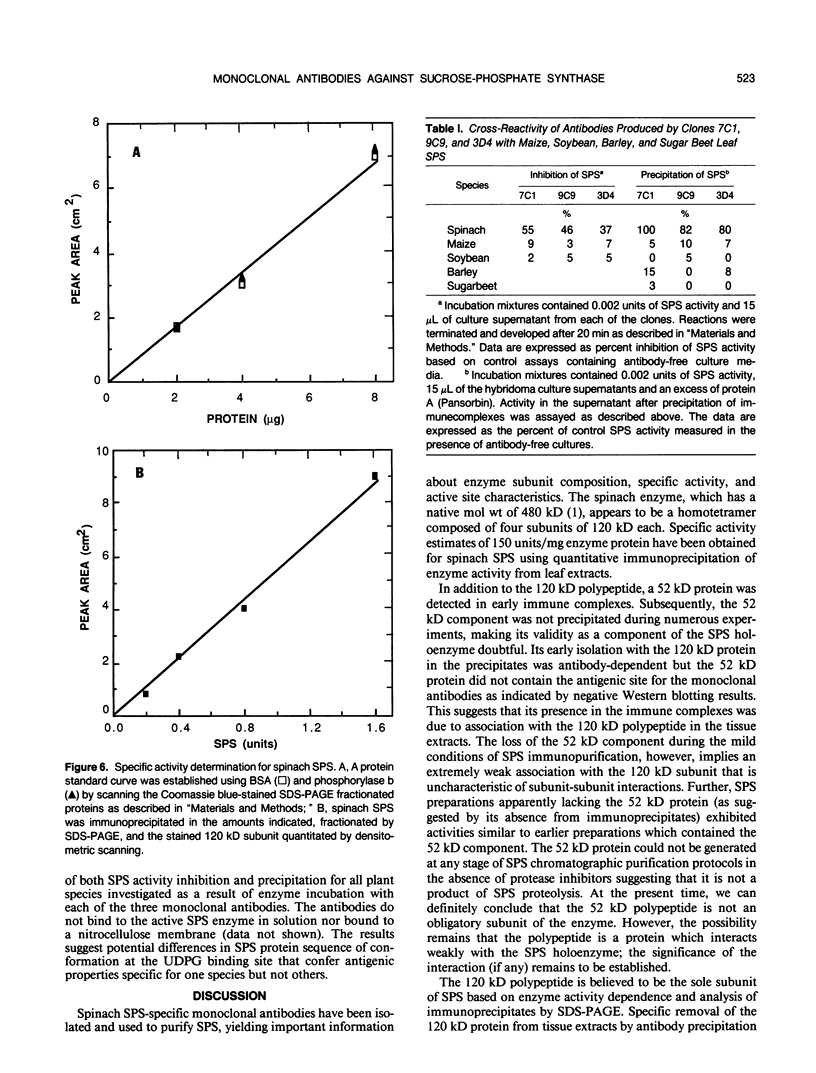
Monoclonal antibodies specific for sucrose phosphate synthase (SPS; EC 2.4.1.14) have been obtained for the first time. Three independent clones have been isolated which inhibited spinach (Spinacia oleracea L.) leaf SPS activity and facilitated the enzyme purification by immunoprecipitation. All three clones were specific for the spinach enzyme but neither inhibited nor precipitated the SPS present in tissue extracts of maize (Zea mays L.), barley (Hordeum vulgare L.), soybean (Glycine max L.), and sugar beet (Beta vulgaris L.). The inhibition of SPS activity by all three clones was reversible in the presence of UDPG, suggesting the presence of an epitope at the substrate-binding site. Immunoprecipitates of active enzyme preparations consistently revealed the presence of a 120 kilodalton polypeptide, indicating that the enzyme may be a homotetramer with a native molecular weight of about 480 kilodaltons. The occasional appearance of a 52 kilodalton polypeptide in the immunoprecipitates of some enzyme preparations was not the result of proteolysis, was not necessary for enzyme activity, and did not contain an antigenic site as revealed by Western blotting experiments. All three antibodies bind weakly to the SDS denatured 120 kilodalton subunit bound to nitrocellulose. The specific activity of the purified spinach enzyme was determined for the first time to be approximately 150 units per milligram SPS protein (pH 7.5 and 25°C) based on quantitative immunoprecipitation of the enzyme.
Full text
PDF


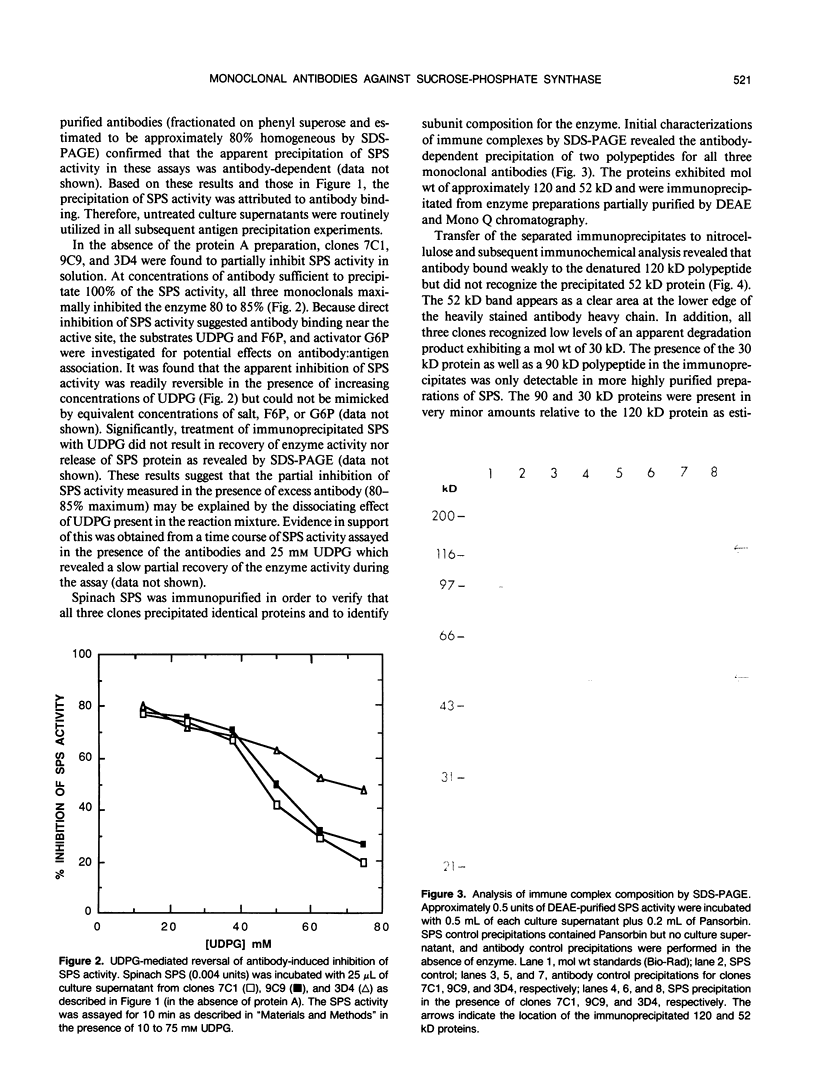

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doehlert D. C., Huber S. C. Regulation of Spinach Leaf Sucrose Phosphate Synthase by Glucose-6-Phosphate, Inorganic Phosphate, and pH. Plant Physiol. 1983 Dec;73(4):989–994. doi: 10.1104/pp.73.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to a positively charged membrane filter. Anal Biochem. 1982 Aug;124(2):396–405. doi: 10.1016/0003-2697(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]