Summary
Electrocatalytic reduction of nitrite to ammonia (NO2RR) is considered as an appealing route to simultaneously achieve sustainable ammonia production and abate hazardous nitrite pollution. Herein, atomically Nb-doped NiO nanoflowers are designed as a high-performance NO2RR catalyst, which exhibits the highest NH3-Faradaic efficiency of 92.4% with an NH3 yield rate of 200.5 μmol h−1 cm−2 at −0.6 V RHE. Theoretical calculations unravel that Nb dopants can act as Lewis acid sites to render effective NO2− activation, decreased protonation energy barriers, and restricted hydrogen evolution, ultimately leading to a high NO2RR selectivity and activity.
Subject areas: Electrochemistry, Applied chemistry, Energy materials
Graphical abstract
Highlights
-
•
Nb-doped NiO (Nb-NiO) is explored for electrochemical NO2−-to-NH3 reduction (NO2RR)
-
•
Nb-NiO exhibits the FENH3 of 92.4% with an NH3 yield rate of 200.5 μmol h−1 cm−2
-
•
Nb-NiO can promote the NO2− activation and decrease the protonation energy barriers
Electrochemistry; Applied chemistry; Energy materials
Introduction
Ammonia (NH3) serves as a crucial chemical for fertilizer production and also as a storage medium for renewable energy.1,2,3 Electrochemical N2-to-NH3 reduction (NRR) in aqueous media is regarded as a prospective method for green NH3 production,4 but the NRR remains far from practical application, arising from the high dissociation energy of N≡N bond (927 kJ mol−1), the competitive hydrogen evolution reaction (HER), and the low N2 solubility.5,6,7,8,9,10 Nitrite (NO2−), a widespread N− pollutant, is extremely harmful to human health and ecological environment.11,12,13,14 Since NO2− possesses a weaker N=O bond dissociation energy (204 kJ mol−1) and a higher water solubility, electrocatalytic NO2−-to-NH3 reduction (NO2RR) via a direct six-electron transfer process is considered as an attractive approach to simultaneously achieve effective ammonia production and abate hazardous nitrite pollution.15,16,17,18,19 However, developing NO2RR catalysts with high selectivity and activity remains a grand challenge.
As electron-deficient centers, Lewis acid sites possess empty orbitals capable of interacting with the electron lone pair of Lewis base NO2− species,20 facilitating the activation and dissociation of NO2−. Besides, owing to the strong electrostatic repulsion effect, the adsorption of H atoms can be effectively impeded on Lewis acid sites,21 leading to an inhibited HER process. Therefore, the construction of Lewis acid sites on catalysts offers an efficient method for potentially achieving active and selective NO2RR. Extensive research has indicated that incorporating metal dopants into transition metal oxides is an effective method to create Lewis acid sites because their strong electronic interactions lead to charge redistribution on different metal atoms.20,21,22,23,24,25 Particularly, when metal dopants exist in isolated single-atom form, abundant Lewis acid sites would be generated,26,27,28 which are greatly favorable for the maximized Lewis acidity and largely expedited NO2RR activity.
Nb is known to possess a prominent Lewis acidity due to the existence of holes in its d orbitals.29 Nb-based catalysts have also been demonstrated to show high catalytic activity in N-cycle electrocatalytic reactions toward the ammonia electrosynthesis.30,31,32 Therefore, in this work, atomically Nb doped in NiO (Nb−NiO) nanoflowers are designed as a high-performance NO2RR catalyst, which exhibits a fascinating NO2RR performance with the highest FENH3 of 92.4% and NH3 yield rate of 200. 5 μmol h−1 cm−2 at −0.6 V, outperforming pristine NiO and many other reported NO2RR catalysts. Theoretical calculations reveal the pivotal role of Lewis acid Nb dopants in facilitating the activity and selectivity of NO2RR process.
Results and discussion
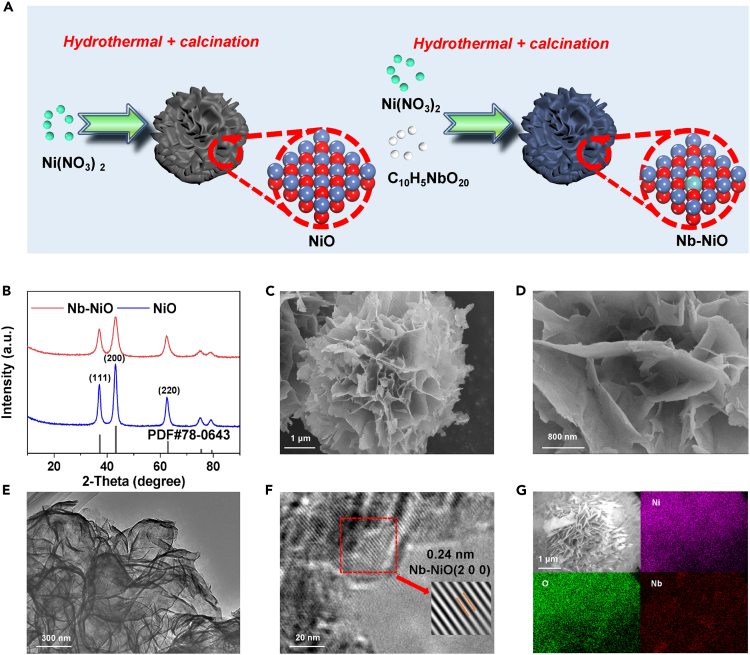
The synthesis of Nb−NiO nanoflowers is conducted by the combined hydrothermal and calcination methods (Figure 1A). The X-ray diffraction (XRD) patterns of both pristine NiO and Nb−NiO (Figure 1B) show the characteristic diffraction peaks of cubic NiO (No. 78−0643).33 A detailed inspection reveals that Nb−NiO delivers a slightly lower peak intensity and wider full width at half maximum compared to pristine NiO, arising from the incorporation of Nb dopants in Nb−NiO (Figure S1). Representative scanning electron microscopy (SEM) (Figures 1C and 1D) image of Nb−NiO shows a typical nanoflower structure comprising many vertically aligned nanosheets, similar to that of original NiO (Figure S2A). The thin nanosheet feature of Nb−NiO (Figure 1E) and NiO (Figure S2B) can be further revealed by the transmission electron microscopy (TEM) image showing clear wrinkles and corrugations. In addition, the high-resolution transmission electron microscopy (HRTEM) image exhibits a clear lattice fringe of 0.24 nm, correlating well with (200) crystallographic plane of cubic NiO (Figure 1F). Elemental mapping images (Figure 1G) unveil that Nb dopants are uniformly dispersed over the whole surface of Nb−NiO nanoflowers.
Figure 1.
Morphology characteristics of Nb−NiO
(A) Schematic diagram of the preparation route of NiO and Nb−NiO.
(B−G) Characterizations of as-prepared Nb−NiO: (B) XRD patterns, (C and D) SEM images, (E) TEM image, (F) HRTEM image, (G) Elemental mapping images.
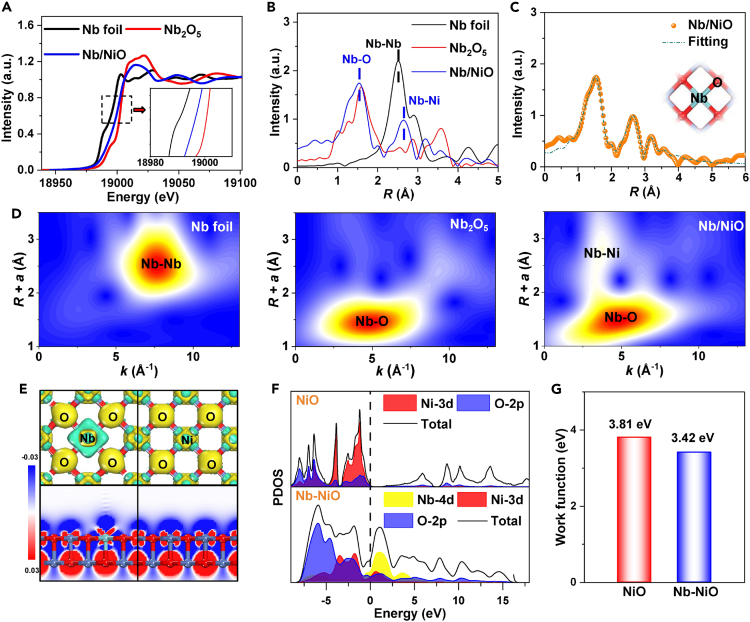
The X-ray absorption spectroscopy (XAS) characterizations are conducted to evaluate the coordination environment of Nb−NiO. The Nb K−edge X-ray absorption near edge structure (XANES) spectra (Figure 2A) show that the absorption edge of Nb-NiO is situated between Nb foil and Nb2O5, indicating that Nb dopants are in oxidation state. Linear XANES fitting result reveals that the average Nb valence state is +3.4 (Figure S3). The Nb K−edge extended X-ray absorption fine-structure (EXAFS) spectrum of Nb−NiO (Figure 2B) reveals a dominant peak at 1.54 Å, which is assigned to Nb−O first coordination shell. The absence of Ni−Ni coordination bond confirms the automatically dispersed Nb dopants in Nb−NiO.34,35,36 Besides, the 2.65 Å peak is assigned to Nb−Ni second coordination shell. Similarly, the wavelet-transformed (WT, Figure 2D) profiles display that Nb−NiO exhibits two Nb−O and Nb−Ni intensity maxima. EXAFS fitting analysis shows that the isolated Nb atom coordinates with five adjacent O atoms to form geometric Nb1−O5 moiety (Figure 2C; Table S1).
Figure 2.
Structural characteristics of Nb−NiO
(A, B, and D) (A) Nb K−edge XANES, (B) EXAFS spectra, and (D) WT profiles of Nb−NiO, Nb foil and Nb2O5.
(C) EXAFS fitting analysis of Nb−NiO.
(E) Charge density difference (top half) and electron location function (bottom half), yellow and red: charge accumulation, cyan and blue: charge depletion.
(F and G) (F) PDOS profiles and (G) calculated work functions of NiO and Nb−NiO.
Density functional theory (DFT) computations are performed to investigate the electronic structures of Nb−NiO. On the basis of XRD and HRTEM results, we select (200) plane of NiO slab for Nb−NiO structural modeling. As seen in Figure S4, by substituting a surface-exposed Ni atom with an Nb dopant, the resulting Nb−NiO shows a rather negative formation energy of −2.46 eV, suggesting that Nb dopant incorporated in NiO lattice is thermodynamically feasible.37 Charge density difference and electron location function (ELF, Figure 2E) maps of Nb−NiO exhibit the noticeable electron-deficient regions around Nb dopant. This can be further verified by the detailed charge analysis (Figure S5), in which Nb dopant (+1.12 |e|) is more positively charged than Ni (+0.77 |e|) and thus Nb dopants can serve as Lewis acid sites to activate and polarize NO2− during the NO2RR process.38 The projected density of states (PDOS, Figure 2F) analysis displays that NiO possesses a distinct band gap, indicating its semiconducting nature. In stark contrast, introducing Nb dopant in NiO generates significant electronic states crossing the Fermi level, suggesting the metallic character and improved conductivity of Nb−NiO.39 Meanwhile, as shown in Figure 2G (Figure S6), the calculated work function (Φ) value of Nb−NiO is 3.42 eV, which is lower than that of NiO (3.81 eV). Thus, the proton-coupled electron transfer process and the electrocatalytic NO2RR kinetics can be significantly facilitated on Nb−NiO.40 Moreover, AIMD simulations of Nb−NiO display the equilibrium states of energy and temperature (Figure S7), signifying the high thermodynamic stability of Nb−NiO.40
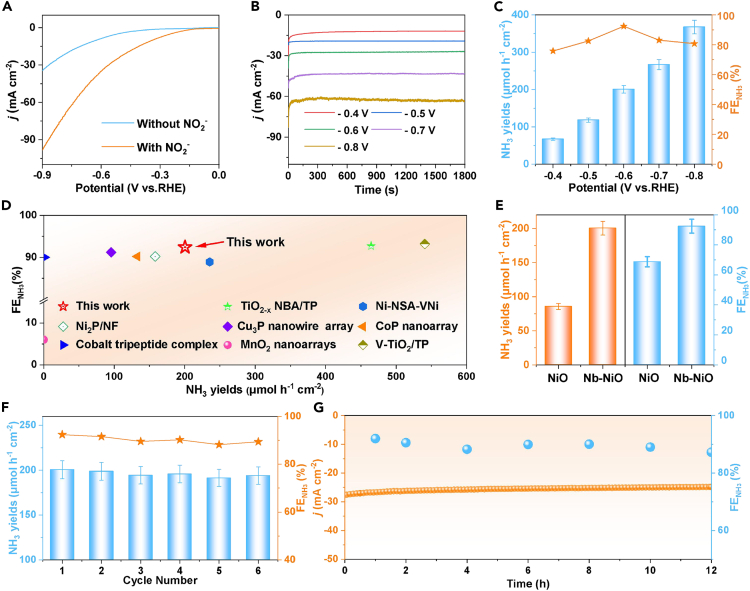
Electrochemical NO2RR performance of Nb−NiO is evaluated in 0.5 M Na2SO4 + 0.1 M NaNO2 solution using an H cell based on a standard procedure flow chart (Figure S8).14 The produced liquid and gas products after NO2RR electrolysis are determined by colorimetric and gas chromatography methods (Figures S9−S11),41,42,43,44 respectively. The linear sweep voltammetry (LSV) curves of Nb−NiO are measured firstly (Figure 3A), and a significant increase in current density (j) is observed for NO2−-containing electrolyte compared to NO2−-free electrolyte, signifying the high NO2RR activity of Nb−NiO. Subsequently, the NO2RR performance of Nb−NiO is quantitatively evaluated at various potentials using the combined chronoamperometry (Figure 3B) and colorimetric tests. Figure 3C shows that Nb−NiO shows a maximum FENH3 of 92.4% at −0.6 V, with the corresponding NH3 yield rate reaching 200.5 μmol h−1 cm−2. Such NO2RR performance is better than that of most reported NO2RR catalysts as depicted in Figure 3D and Table S2. The controlled colorimetric measurements (Figure S12) and alternating experiments (Figure S13) attest that the generated NH3 is derived from the NO2RR electrolysis on Nb−NiO.45,46,47,48
Figure 3.
Electrochemical NO2RR tsts
(A) LSV curves of Nb−NiO in various electrolytes.
(B and C) (B) Chronoamperometry test of Nb−NiO at different potentials after 0.5 h electrolysis and (C) obtained NH3 yield rates and FENH3.
(D) Comparison of NH3 yield rates and FENH3 between Nb−NiO and reported NO2RR catalysts.
(E) Comparison of the NO2RR performance between NiO and Nb−NiO at −0.6 V.
(F and G) (F) Cycling and (G) long-term stability tests of Nb−NiO at −0.6 V.
Regarding the NO2RR selectivity, Nb−NiO exhibits fairly low Faradaic efficiencies (FEs) for H2, NH2OH, and N2H4 by-products relative to FENH3 (Figure S14), confirming a high NO2RR selectivity of Nb−NiO toward the NH3 generation. This finding can be further confirmed by the time-dependent NO2RR electrolysis (Figure S15), which shows a considerably decreased NO2−−N concentration coupled with a significantly increased NH3−N concentration as the electrolysis time increases. As a comparison, we evaluate the NO2RR performance of pristine NiO (Figure 3E), which exhibits much lower NO2RR activity and selectivity than Nb−NiO. Specifically, Nb−NiO outperforms pristine NiO by 2.3 and 1.3 times in NH3 yield rate and FENH3 at −0.6 V, respectively. Besides, Nb−NiO displays a higher electrochemical active surface area (ECSA, Figure S16) than NiO, while the catalyst performance normalized by ECSA (Figure S17) exhibits the same trend with Figure 3E, suggesting the high intrinsic activity of Nb−NiO toward the NO2RR. As for the electrocatalytic stability of Nb−NiO, slight changes in NH3 yield rates and FENH3 over six consecutive cycles can be seen, indicating an excellent cycling stability of Nb−NiO (Figure 3F). The long-term chronoamperometric experiment shows a negligible decline in current density and calculated FENH3 over 12 h continuous electrolysis (Figure 3G), substantiating the excellent long-term durability of Nb−NiO.49,50,51 After stability tests, Nb−NiO retains its original phase, morphology, and coordination structure (Figure S18), evidencing the high structural stability of Nb−NiO.52,53,54
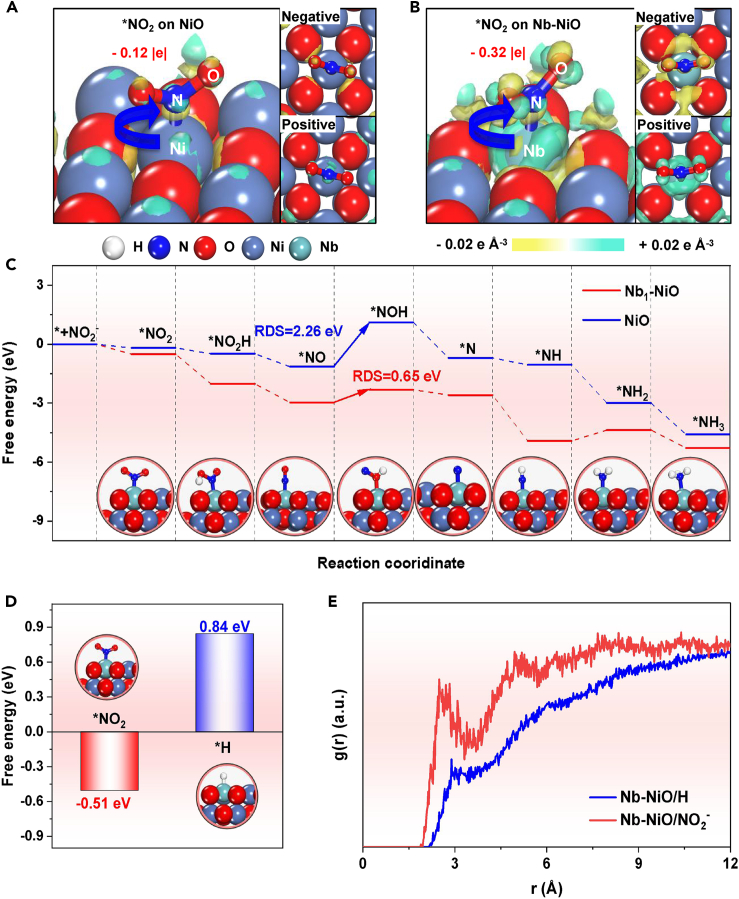
Theoretical calculations are conducted to elucidate the mechanism for the Nb-doping-induced enhanced NO2RR performance of Nb−NiO. Upon the NO2− adsorption on Nb−NiO (Figure S19), the electron-deficient Nb dopant, as previously determined in Figure 2E, can serve as Lewis acid site to favorably absorb Lewis base NO2−, resulting in enhanced NO2− activation and N=O bond dissociation.55,56,57,58 Charge density difference analysis (Figures 4A and 4B) reveals a remarkable Nb−∗NO2 electronic coupling where Nb dopant donates −0.32 |e| to ∗NO2, in stark contrast to −0.12 |e| for Ni−to−∗NO2 charge transfer. In addition, the free energy diagram (Figure 4C; Figure S20) presents that both Nb dopant of Nb−NiO and Ni site of NiO exhibit the same rate determining step (RDS) of ∗NO → ∗NHO.59 Nonetheless, Nb dopant exhibits a much reduced RDS energy barrier compared to Ni site (−2.26 eV). Besides, Nb dopant presents much lower free energies of all protonation intermediates than Ni site. Both findings demonstrate that Lewis acid Nb dopant serve as active site to significantly enhance the protonation energetics to boost the NO2−−to−NH3 conversion process on Nb−NiO.
Figure 4.
Theoretical analysis
(A and B) Charge density difference plots of ∗NO2 on (A) NiO and (B) Nb−NiO. Yellow: charge accumulation, cyan: charge depletion.
(C) Free energy profiles of NO2RR process on NiO and Nb−NiO.
(D) Free energies of absorbed H and NO2− on Nb-dopant site of Nb−NiO.
(E) RDF curves of the interactions between Nb−dopant and NO2−/H+.
Considering that HER is the main competing reaction of NO2RR,60 the HER activity of NO2RR-active Nb-dopant site is further investigated. As displayed in Figure 4D, the binding free energy of ∗H on Nb dopant of Nb−NiO is calculated as 0.84 eV, which is much positive than that of ∗NO2 (−0.51 eV), confirming an unfavorable HER performance of Nb−NiO, which is attributed to the Lewis acidity of Nb dopant capable of repelling the binding of positively charged H. Additionally, molecular dynamics (MD) simulations (Figure 4E) reveal that the snapshot after simulation (Figure S21) shows the aggregation of evident NO2− on Nb−NiO, and the calculated radial distribution function (RDF) curves (Figure 4E) present a more intense Nb−NiO/∗NO2− interaction compared to Nb−NiO/∗H interaction,61,62,63 further corroborating that Nb−NiO is able to selectively adsorb NO2− and suppress H coverage, thus facilitating the boosted NO2RR and inhibited HER. These theoretical results reveal that the Lewis acid Nb dopant of Nb−NiO plays a crucial role in enhancing the efficient adsorption and activation of NO2−, boosting the protonation energetics and suppressing the HER, eventually leading to the high catalytic activity and selectivity of Nb−NiO for the NO2RR.
Conclusion
Nb−NiO has been proved to be an efficient and robust NO2RR catalyst. Theoretical computations suggest that the enhanced NO2RR performance of Nb−NiO originates from the key role of Lewis acid Nb dopant in suppressing the HER and enhancing NO2− activation and protonation. This work not only offers an in-depth understanding of the Lewis acid dopant-catalyzed NO2RR mechanism but also implies the great potential of constructing Lewis acid dopants in catalysts to achieve exceptional NO2− electroreduction and beyond.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| NaNO2 | Aladdin Co., Ltd. | 7632-00-0 |
| NaClO | Aladdin Co., Ltd. | 7681-52-9 |
| C5FeN6Na2O·2H2O | Aladdin Co., Ltd. | 13755-38-9 |
| H2O2 | Beijing Chemical Corporation | 7722-84-1 |
| H2SO4 | Beijing Chemical Corporation | 7664-93-9 |
| HCl | Beijing Chemical Corporation | 7647-01-0 |
| C2H5OH | Beijing Chemical Corporation | 64-17-5 |
| C10H5NbO20 | Mclean Co., Ltd. | 21348-59-4 |
| Ni(NO3)2·6H2O | Sinopharm Chemical Reagent Co., Ltd. | 13478-00-7 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Dr. Ke Chu (chuk630@mail.lzjtu.cn).
Materials availability
This study did not generate new unique reagents. All chemicals were obtained from commercial resources and used as received.
Method details
Synthesis of Nb−NiO
0.3 g Ni(NO3)2·6H2O and 0.32 g C10H5NbO20 were dispersed in 30 mL ethanol solution under stirring to form a transparent solution. Afterward, the solution was transferred into a 50 mL autoclave. After treatment at 150°C for 6 h, the light green precipitates were collected by centrifuging, washed with deionized water/ethanol and dried under vacuum overnight. The obtained precipitates were ground in an agate mortar and then transferred to a muffle furnace for calcination at 300°C for 4 h to obtain Nb−NiO. Pristine NiO was prepared by the same method as Nb−NiO by without addition of C10H5NbO20.
Electrochemical experiments
Electrochemical measurements were investigated with a CHI−760E electrochemical workstation using a conventional three−electrode cell. Nb−NiO coated on carbon cloth (1 × 1 cm2, 0.5 mg cm−2) was used as the working electrode, Ag/AgCl (saturated KCl) electrode was used as the reference electrode, and Pt foil was used as the counter electrode. All potentials were referenced to reversible hydrogen electrode (RHE) by following equation: E (V vs. RHE) = E (V vs. Ag/AgCl) + 0.198V + 0.059 × pH. The NO2RR measurements were carried out in 0.5 M Na2SO4 + 0.1 M NaNO2 electrolyte using an H−type two−compartment electrochemical cell separated by a Nafion 211 membrane. After each chronoamperometry test for 0.5 h, the produced NH3 and other possible by-product (N2H4) were analyzed by various colorimetric methods using UV-vis absorbance spectrophotometer (MAPADA P5), while the gas products (H2, NH2OH) were analyzed by gas chromatography (Shimadzu GC2010). The detailed determination procedures are given in our previous publication.46
Faradaic efficiency (FE) of NH3 generation was calculated by the following equation:
| (Equation 1) |
NH3 yield rate is calculated using the following equation:
| (Equation 2) |
where c (μg mL−1) is the measured NH3 concentration, V (mL) is the volume of the electrolyte in the cathode chamber (35 mL), t (s) is the electrolysis time, A(cm−2) is the surface area of CC (1 × 1 cm2), F (96500 C mol−1) is the Faraday constant, Q (C) is the total quantity of applied electricity.
Characterizations
X−ray diffraction (XRD) was conducted on a Rigaku D/max 2400 diffractometer. Scanning electron microscopy (SEM) was carried out on a ZEISS GeminiSEM−500 microscope. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) were performed on a Tecnai G2 F20 microscope at an acceleration voltage of 200 kV.
Acknowledgments
This work is supported by The First Batch of University Level Scientific Research Projects of Hebei North University in 2022 (E2022405002).
Author contributions
Ying Zhang: Investigation, Writing-original draft. Yuying Wan: Investigation.Xiaoxu Liu: Software, Investigation.Kai Chen: Investigation.
Declaration of interests
The authors declare no competing interests.
Published: September 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107944.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon reasonable request.
All original code is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
References
- 1.Liang J., Li Z., Zhang L., He X., Luo Y., Zheng D., Wang Y., Li T., Yan H., Ying B., et al. Advances in ammonia electrosynthesis from ambient nitrate/nitrite reduction. Chem. 2023;9:1768–1827. [Google Scholar]
- 2.Liang J., Liu Q., Alshehri A.A., Sun X. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy. 2022;1 [Google Scholar]
- 3.Qi D., Lv F., Wei T., Jin M., Meng G., Zhang S., Liu Q., Liu W., Ma D., Hamdy M.S., et al. High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN. Nano Res. Energy. 2022;1 [Google Scholar]
- 4.Liu Q., Xu T., Luo Y., Kong Q., Li T., Lu S., Alshehri A.A., Alzahrani K.A., Sun X. Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem. 2021;29 [Google Scholar]
- 5.Luo Y., Shen P., Li X., Guo Y., Chu K. Sulfur-deficient Bi2S3−x synergistically coupling Ti3C2Tx-MXene for boosting electrocatalytic N2 reduction. Nano Res. 2022;15:3991–3999. [Google Scholar]
- 6.Chu K., Luo Y., Shen P., Li X., Li Q., Guo Y. Unveiling the synergy of O-vacancy and heterostructure over MoO3-x/MXene for N2 electroreduction to NH3. Adv. Energy Mater. 2022;12 [Google Scholar]
- 7.Qing G., Ghazfar R., Jackowski S.T., Habibzadeh F., Ashtiani M.M., Chen C.-P., Smith M.R., Hamann T.W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020;120:5437–5516. doi: 10.1021/acs.chemrev.9b00659. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Shen P., Luo Y., Li Y., Guo Y., Zhang H., Chu K. PdFe single-atom alloy metallene for N2 electroreduction. Angew. Chem. Int. Ed. Engl. 2022;61 doi: 10.1002/anie.202205923. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Chen C., Cao X., Wang Z., Zhang N., Liu T. Recent advances in defect and interface engineering for electroreduction of CO2 and N2. Acta Phys. Chim. Sin. 2023;39 [Google Scholar]
- 10.Chu K., Li X., Li Q., Guo Y., Zhang H. Synergistic enhancement of electrocatalytic nitrogen reduction over boron nitride quantum dots decorated Nb2CTx-MXene. Small. 2021;17 doi: 10.1002/smll.202102363. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Zhang F., Jin M., Zhao D., Fan X., Li Z., Luo Y., Zheng D., Li T., Wang Y., et al. V-doped TiO2 nanobelt array for high-efficiency electrocatalytic nitrite reduction to ammonia. Mater. Today Phys. 2023;30 [Google Scholar]
- 12.He X., Li Z., Yao J., Dong K., Li X., Hu L., Sun S., Cai Z., Zheng D., Luo Y., et al. High-efficiency electrocatalytic nitrite reduction toward ammonia synthesis on CoP@TiO2 nanoribbon array. iScience. 2023;26 doi: 10.1016/j.isci.2023.107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X., Hu L., Xie L., Li Z., Chen J., Li X., Li J., Zhang L., Fang X., Zheng D., et al. Ambient ammonia synthesis via nitrite electroreduction over NiS2 nanoparticles-decorated TiO2 nanoribbon array. J. Colloid Interface Sci. 2023;634:86–92. doi: 10.1016/j.jcis.2022.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z., Zhao D., Fan X., Zhang L., Liang J., Li Z., Li J., Luo Y., Zheng D., Wang Y., et al. Rational construction of heterostructured Cu3P@TiO2 nanoarray for high-efficiency electrochemical nitrite reduction to ammonia. Small. 2023;19 doi: 10.1002/smll.202300620. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X., Fan X., Lin H., Li S., Zhai Q., Jiang Y., Chen Y. Highly efficient electroenzymatic cascade reduction reaction for the conversion of nitrite to ammonia. Adv. Energy Mater. 2023;13 [Google Scholar]
- 16.Zhang Y., Wang Y., Han L., Wang S., Cui T., Yan Y., Xu M., Duan H., Kuang Y., Sun X. Nitrite electroreduction to ammonia promoted by molecular carbon dioxide with near-unity Faradaic efficiency. Angew. Chem. Int. Ed. Engl. 2023;62 doi: 10.1002/anie.202213711. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Wen G., Zhao D., Xie L., Sun S., Zhang L., Luo Y., Ali Alshehri A., Hamdy M.S., Kong Q., Sun X. Nitrite reduction over Ag nanoarray electrocatalyst for ammonia synthesis. J. Colloid Interface Sci. 2022;623:513–519. doi: 10.1016/j.jcis.2022.04.173. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q., Liu Q., Xie L., Yue L., Li T., Luo Y., Li N., Tang B., Yu L., Sun X. A 3D FeOOH nanotube array: an efficient catalyst for ammonia electrosynthesis by nitrite reduction. Chem. Commun. 2022;58:5160–5163. doi: 10.1039/d2cc00611a. [DOI] [PubMed] [Google Scholar]
- 19.Li S., Liang J., Wei P., Liu Q., Xie L., Luo Y., Sun X. ITO@TiO2 nanoarray: An efficient and robust nitrite reduction reaction electrocatalyst toward NH3 production under ambient conditions. eScience. 2022;2:382–388. [Google Scholar]
- 20.Lang R., Du X., Huang Y., Jiang X., Zhang Q., Guo Y., Liu K., Qiao B., Wang A., Zhang T. Single-atom catalysts based on the metal–oxide interaction. Chem. Rev. 2020;120:11986–12043. doi: 10.1021/acs.chemrev.0c00797. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Kumar A., Kim M.G., Yang T., Shao X., Liu X., Liu Y., Hong Y., Jadhav A.R., Liang M., et al. Single-metal-atom dopants increase the Lewis acidity of metal oxides and promote nitrogen fixation. ACS Energy Lett. 2021;6:4299–4308. [Google Scholar]
- 22.Chang Q.-Y., Wang K.-Q., Sui Z.-J., Zhou X.-G., Chen D., Yuan W.-K., Zhu Y.-A. Rational design of single-atom-doped Ga2O3 catalysts for propane dehydrogenation: breaking through volcano plot by Lewis acid–base interactions. ACS Catal. 2021;11:5135–5147. [Google Scholar]
- 23.Zhang J., Zhou R.-J., Chang Q.-Y., Sui Z.-J., Zhou X.-G., Chen D., Zhu Y.-A. Tailoring catalytic properties of V2O3 to propane dehydrogenation through single-atom doping: A DFT study. Catal. Today. 2021;368:46–57. [Google Scholar]
- 24.Ma F., Chang Q.-Y., Yin Q., Sui Z.-J., Zhou X.-G., Chen D., Zhu Y.-A. Rational screening of single-atom-doped ZnO catalysts for propane dehydrogenation from microkinetic analysis. Catal. Sci. Technol. 2020;10:4938–4951. [Google Scholar]
- 25.Chu K., Liu Y.p., Li Y.b., Guo Y.l., Tian Y., Zhang H. Multi-functional Mo-doping in MnO2 nanoflowers toward efficient and robust electrocatalytic nitrogen fixation. Appl. Catal., B. 2020;264 [Google Scholar]
- 26.Huang H., Gong F., Wang Y., Wang H., Wu X., Lu W., Zhao R., Chen H., Shi X., Asiri A.M., et al. Mn3O4 nanoparticles@reduced graphene oxide composite: An efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions. Nano Res. 2019;12:1093–1098. [Google Scholar]
- 27.Zhang Y., Qiu W., Ma Y., Luo Y., Tian Z., Cui G., Xie F., Chen L., Li T., Sun X. High-performance electrohydrogenation of N2 to NH3 catalyzed by multishelled hollow Cr2O3 microspheres under ambient conditions. ACS Catal. 2018;8:8540–8544. [Google Scholar]
- 28.Zhang L., Ji X., Ren X., Ma Y., Shi X., Tian Z., Asiri A.M., Chen L., Tang B., Sun X. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies. Adv. Mater. 2018;30 doi: 10.1002/adma.201800191. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L., Zhong Z., Xue J., Xu Y., Wang C., Wang L. NH3-SCR performance and the resistance to SO2 for Nb doped vanadium based catalyst at low temperatures. J. Environ. Sci. 2018;65:306–316. doi: 10.1016/j.jes.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Kong W., Liu Z., Han J., Xia L., Wang Y., Liu Q., Shi X., Wu Y., Xu Y., Sun X. Ambient electrochemical N2-to-NH3 fixation enabled by Nb2O5 nanowire array. Inorg. Chem. Front. 2019;6:423–427. [Google Scholar]
- 31.Huang L., Wu J., Han P., Al-Enizi A.M., Almutairi T.M., Zhang L., Zheng G. NbO2 electrocatalyst toward 32% Faradaic efficiency for N2 fixation. Small Methods. 2019;3 [Google Scholar]
- 32.Han J., Liu Z., Ma Y., Cui G., Xie F., Wang F., Wu Y., Gao S., Xu Y., Sun X. Ambient N2 fixation to NH3 at ambient conditions: Using Nb2O5 nanofiber as a high-performance electrocatalyst. Nano Energy. 2018;52:264–270. [Google Scholar]
- 33.Wang X., Wang J., Li Y., Chu K. Nitrogen-doped NiO nanosheet array for boosted electrocatalytic N2 reduction. ChemCatChem. 2019;11:4529–4536. [Google Scholar]
- 34.Chen K., Zhang Y., Xiang J., Zhao X., Li X., Chu K. p-block antimony single-atom catalysts for nitric oxide electroreduction to ammonia. ACS Energy Lett. 2023;8:1281–1288. [Google Scholar]
- 35.Wang J., Cao G., Duan R., Li X., Li X. Advances in single metal atom catalysts enhancing kinetics of sulfur cathode. Acta Phys. Chim. Sin. 2023;39:2212005. [Google Scholar]
- 36.Chen K., Wang J., Zhang H., Ma D., Chu K. Self-tandem electrocatalytic NO reduction to NH3 on a W single-atom catalyst. Nano Lett. 2023;23:1735–1742. doi: 10.1021/acs.nanolett.2c04444. [DOI] [PubMed] [Google Scholar]
- 37.Chen K., Wang J., Kang J., Lu X., Zhao X., Chu K. Atomically Fe-doped MoS2−x with Fe-Mo dual sites for efficient electrocatalytic NO reduction to NH3. Appl. Catal., B. 2023;324 [Google Scholar]
- 38.Zhang N., Zhang G., Shen P., Zhang H., Ma D., Chu K. Lewis acid Fe-V pairs promote nitrate electroreduction to ammonia. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 39.Zhang G., Zhang N., Chen K., Zhao X., Chu K. Atomically Mo-doped SnO2-x for efficient nitrate electroreduction to ammonia. J. Colloid Interface Sci. 2023;649:724–730. doi: 10.1016/j.jcis.2023.06.160. [DOI] [PubMed] [Google Scholar]
- 40.Li X., Chen K., Lu X., Ma D., Chu K. Atomically dispersed Co catalyst for electrocatalytic NO reduction to NH3. Chem. Eng. J. 2023;454 [Google Scholar]
- 41.Xu Y., Wen Y., Ren T., Yu H., Deng K., Wang Z., Li X., Wang L., Wang H. Engineering the surface chemical microenvironment over CuO nanowire arrays by polyaniline modification for efficient ammonia electrosynthesis from nitrate. Appl. Catal., B. 2023;320 [Google Scholar]
- 42.Ren T., Yu Z., Yu H., Deng K., Wang Z., Li X., Wang H., Wang L., Xu Y. Sustainable ammonia electrosynthesis from nitrate wastewater coupled to electrocatalytic upcycling of polyethylene terephthalate plastic waste. ACS Nano. 2023;17:12422–12432. doi: 10.1021/acsnano.3c01862. [DOI] [PubMed] [Google Scholar]
- 43.Ren T., Yu Z., Yu H., Deng K., Wang Z., Li X., Wang H., Wang L., Xu Y. Interfacial polarization in metal-organic framework reconstructed Cu/Pd/CuOx multi-phase heterostructures for electrocatalytic nitrate reduction to ammonia. Appl. Catal., B. 2022;318 [Google Scholar]
- 44.Ren T., Sheng Y., Wang M., Ren K., Wang L., Xu Y. Recent advances of Cu-based materials for electrochemical nitrate reduction to ammonia. Chinese J. Struc. Chem. 2022;41:2212089–2212106. [Google Scholar]
- 45.Wang G., Zhang Y., Chen K., Guo Y., Chu K. PdP2 nanoparticles on reduced graphene oxide: a catalyst for the electrocatalytic reduction of nitrate to ammonia. Inorg. Chem. 2023;62:6570–6575. doi: 10.1021/acs.inorgchem.3c01046. [DOI] [PubMed] [Google Scholar]
- 46.Li X., Shen P., Li X., Ma D., Chu K. Sub-nm RuOx clusters on Pd metallene for synergistically enhanced nitrate electroreduction to ammonia. ACS Nano. 2023;17:1081–1090. doi: 10.1021/acsnano.2c07911. [DOI] [PubMed] [Google Scholar]
- 47.Chen K., Wang F., Lu X., Li Y., Chu K. Atomically dispersed W1-O3 bonded on Pd metallene for cascade NO electroreduction to NH3. ACS Catal. 2023;13:9550–9557. [Google Scholar]
- 48.Zhang G., Li X., Chen K., Guo Y., Ma D., Chu K. Tandem electrocatalytic nitrate reduction to ammonia on MBenes. Angew. Chem. Int. Ed. Engl. 2023;62 doi: 10.1002/anie.202300054. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q., Lian K., Liu Q., Qi G., Zhang S., Luo J., Liu X. High entropy alloy nanoparticles as efficient catalysts for alkaline overall seawater splitting and Zn-air batteries. J. Colloid Interface Sci. 2023;646:844–854. doi: 10.1016/j.jcis.2023.05.074. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Wei T., Qiu Y., Zhang S., Liu Q., Hu G., Luo J., Liu X. Recent progress in metal phosphorous chalcogenides: potential high-performance electrocatalysts. Small. 2023;19 doi: 10.1002/smll.202207249. [DOI] [PubMed] [Google Scholar]
- 51.Wei T., Liu W., Zhang S., Liu Q., Luo J., Liu X. A dual-functional Bi-doped Co3O4 nanosheet array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production. Chem. Commun. 2023;59:442–445. doi: 10.1039/d2cc05722k. [DOI] [PubMed] [Google Scholar]
- 52.Hu P., Hu S., Du H., Liu Q., Guo H., Ma K., Li T. Efficient electrocatalytic reduction of nitrate to ammonia over fibrous SmCoO3 under ambient conditions. Chem. Commun. 2023;59:5697–5700. doi: 10.1039/d3cc00889d. [DOI] [PubMed] [Google Scholar]
- 53.Du H., Guo H., Wang K., Du X., Beshiwork B.A., Sun S., Luo Y., Liu Q., Li T., Sun X. Durable electrocatalytic reduction of nitrate to ammonia over defective pseudobrookite Fe2TiO5 nanofibers with abundant oxygen vacancies. Angew. Chem. Int. Ed. Engl. 2023;62 doi: 10.1002/anie.202215782. [DOI] [PubMed] [Google Scholar]
- 54.Dong S., Niu A., Wang K., Hu P., Guo H., Sun S., Luo Y., Liu Q., Sun X., Li T. Modulation of oxygen vacancy and zero-valent zinc in ZnCr2O4 nanofibers by enriching zinc for efficient nitrate reduction. Appl. Catal., B. 2023;333 [Google Scholar]
- 55.Chen K., Ma Z., Li X., Kang J., Ma D., Chu K. Single-atom Bi alloyed Pd metallene for nitrate electroreduction to ammonia. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 56.Zhang Y., Xiang J., Chen K., Guo Y., Ma D., Chu K. Palladium metallene for nitric oxide electroreduction to ammonia. Chem. Commun. 2023;59:8961–8964. doi: 10.1039/d3cc00131h. [DOI] [PubMed] [Google Scholar]
- 57.Wang G., Shen P., Chen K., Guo Y., Zhao X., Chu K. Rare-earth La-doped VS2-x for electrochemical nitrate reduction to ammonia. Inorg. Chem. Front. 2023;10:2014–2021. [Google Scholar]
- 58.Chen K., Zhang N., Wang F., Kang J., Chu K. Main-group indium single-atom catalysts for efficient electrocatalytic NO reduction to NH3. J. Mater. Chem. A. 2023;11:6814–6819. [Google Scholar]
- 59.Wang J., Liang J., Liu P., Yan Z., Cui L., Yue L., Zhang L., Ren Y., Li T., Luo Y., et al. Biomass Juncus derived carbon decorated with cobalt nanoparticles enables high-efficiency ammonia electrosynthesis by nitrite reduction. J. Mater. Chem. A. 2022;10:2842–2848. [Google Scholar]
- 60.Zhang X., Wang Y., Wang Y., Guo Y., Xie X., Yu Y., Zhang B. Recent advances in electrocatalytic nitrite reduction. Chem. Commun. 2022;58:2777–2787. doi: 10.1039/d1cc06690k. [DOI] [PubMed] [Google Scholar]
- 61.Zhang G., Wan Y., Zhao H., Guo Y., Chu K. A metal-free catalyst for electrocatalytic NO reduction to NH3. Dalton Trans. 2023;52:6248–6253. doi: 10.1039/d3dt00994g. [DOI] [PubMed] [Google Scholar]
- 62.Chen K., Zhang Y., Du W., Guo Y., Chu K. Atomically-isolated and unsaturated Sb sites created on Sb2S3 for highly selective NO electroreduction to NH3. Inorg. Chem. Front. 2023;10:2708–2715. [Google Scholar]
- 63.Chen K., Wang G., Guo Y., Ma D., Chu K. Iridium single-atom catalyst for highly efficient NO electroreduction to NH3. Nano Res. 2023;16:8737–8742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon reasonable request.
All original code is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.