Abstract
The project aims to investigate the correlation between obesity, overweight, or low body weight and the risk of mortality in sepsis patients. We performed a rigorous and thorough search of major electronic databases, including PubMed, Web of Science, EMBASE, and Cochrane Library, from the inception of these databases up to March 28, 2023. The data were analyzed with Stata software (version 16.0). Twelve studies incorporating 521,207 individuals were enrolled. The results demonstrated that obesity (OR = 0.82; 95% CI: 0.69–0.97; P < 0.001) or overweight (OR = 0.83; 95% CI: 0.73–0.94; P < 0.001) decreased the risk of mortality in sepsis patients. Instead, the reverse phenomena existed in patients with a low weight (OR = 1.43; 95%CI: 1.16–1.76; P = 0.038).
There is an “obesity paradox” phenomenon in the mortality of obese and overweight patients with sepsis, but low body weight is an independent risk factor for the mortality of sepsis patients. This study demonstrated that the mortality in sepsis patients and obesity or overweight were negatively correlated, but displayed a significant positive relation to low weight.
Keywords: Sepsis, Obesity, Overweight, Meta-analysis, Systematic review
1. Introduction
Sepsis is a clinical syndrome involving physiological, biological, and biochemical abnormalities caused by the body's maladjusted response to infection, sepsis, and subsequent inflammatory reactions that can result in multiple organ dysfunction syndrome (MODS) and death [1]. A retrospective analysis of an international database reported that the global incidence of sepsis between 1995 and 2015 was 437 cases per 100,000 person-years [2], although morbidity and mortality rates varied by region. The global age-standard sepsis incidence decreased by 37% from 1074.7 to 677.5 cases per 100,000 individuals, and the number of deaths decreased by 29.7% from 15.7 million in 1990 to 11 million in 2017, according to the Global Burden of Disease Study [3]. In contrast, Weng et al. revealed the incidence rate increased significantly from 328.25 cases per 100,000 cases in 2017 to 421.85 cases in 2019 [4] through the National Health Service Data Center (NDCMS) and the National Mortality Surveillance System (NMSS) in China. The reasons for these conflicting results were complex, such as updated diagnostic criteria, aging, multi-resistant infections, immune status, and so on. Sepsis was a major contributor to the global burden of disease [5]. Previous studies had shown that risk factors for sepsis include ICU admission [6], bacteremia [7], advanced age (more than 65 years) [8], diabetes and obesity [9], cancer [10], community-acquired pneumonia [11], past hospitalization [12], and genetic factors [13]. Active monitoring of suspected risk factors for sepsis was essential to prevent and treat sepsis. Recent studies have shown that obesity, body weight, and Body Mass Index (BMI) were important factors related to sepsis [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
Obesity refers to the abnormal or excessive accumulation of body fat, which will harm human health. It is a chronic and complex metabolic disease. The obesity rate of adults in the United States reached 42%, and the severe obesity rate (BMI≥40 kg/m2) was 9%, with data from 2017 to 2018 [26]. The incidence of obesity in China increased from 4.2% in 1993 to 15.7% in 2015 and continued to rise at a rate of 0.5% points per year. Based on this prediction, China's obesity rate will exceed 20% within 10 years [27]. Similarly, the incidence in Europe had also risen [28]. Obesity leads to many chronic complications, such as cerebral thrombosis, myocardial infarction, and endometrial carcinoma [21]. However, whether obesity increases the risk of sepsis survival remains unclear. Animal models of sepsis reported that obese mice had a higher risk of death [16]. Previous studies [24] have found that obesity is a risk factor for increased sepsis mortality. In addition, in six studies [15,17,19,20,22,25], obesity was not associated with sepsis mortality. However, these were contradictions in their conclusions. In addition, three different systematic reviews and meta-analyses showed different results. Trivedi, et al. [29] conducted a systematic review, including seven studies published in 2015, showing that clinical evidence of obesity associated with sepsis mortality showed mixed results. After this, Pepper, et al. [30], conducted a meta-analysis, including six observational studies, showing that overweight or obese BMI reduced adjusted mortality in adults admitted to the ICU for sepsis, severe sepsis, or septic shock. However, in 2017, a meta-analysis conducted by Wang, et al. [31] showed that in sepsis cases, overweight, but not obesity or morbid obesity, was associated with lower mortality. These [[29], [30], [31]] studies have shown that sepsis patients with normal BMI have a worse prognosis than obesity, and the phenomenon of obesity paradox in sepsis patients has not been fully supported. Several recent cohorts of studies have been published on the association between obesity and sepsis survival, and these are well-performed cohorts involving more than 100, 000 additional patients [32,33]. Henceforth, we conducted a comprehensive systematic review and meta-analysis to ascertain whether obesity exacerbated the risk of sepsis mortality.
2. Methods
This study was conducted strictly in accordance with the requirements and standards outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [34]. The project was pre-registered on the platform of PROSPERO and received approval with the assigned registration number CRD42023411884.
2.1. Data sources
We conducted a systematic and comprehensive search of major databases, including PubMed, Web of Science, EMBASE, and Cochrane Library, from inception to March 28, 2023. The search was conducted using medical subject headings (MeSH) and keywords, with a focus on English language publications and no restrictions on other conditions. The keywords used included obesity, overweight, sepsis, bloodstream infection, and pyohemia, among others. The complete search strategies for each major database and the number of relevant documents identified are presented in Supplementary Tables 1–4. In addition, we carefully browsed relevant prior research to establish their relevance to our study.
2.2. Eligibility criteria
The eligible studies strictly adhered to the following criteria: (1) retrospective or prospective cohort study and case-control study design; (2) inclusion of patients with obesity or overweight as exposed groups and normal weight patients as controls; (3) risk of mortality of sepsis patients as outcome and reporting of study result with 95% confidence intervals (CI) and odds ratios (OR). Exclusion criteria: duplicate literature, literature with incomplete information, conference abstracts, and comments.
2.3. Research selection
Once the objectives of this study and the criteria for inclusion and exclusion were established, L Gao and JJ Liu conducted a thorough screening of relevant records, independently excluding duplicate documents, conference abstracts, and articles deemed unrelated to the research topic. After re-evaluating potentially relevant texts, the researchers reached a final consensus on the appropriate materials. Any conflicting opinions or ideas were subject to review by the third author, QC Fang.
2.4. Data extraction
Relevant and crucial data from each study, including but not limited to, the first author, year of publication, study type, sepsis outcome, and adjusted confounding factors were extracted independently by L Gao and J Liu. Any differences in opinions or ideas encountered during the process were referred to a third researcher (Q C Fang) for resolution.
2.5. Risk of-bias
The Newcastle-Ottawa Quality Assessment Scale (NOS) was utilized to systematically score the included studies. The cohort study was evaluated on a maximum score of 9 stars, with a higher number of stars indicating superior literature quality. The scores were then graded into three levels: high, medium, and low quality, with scores ranging from 7 to 9, 4 to 6, and 0 to 3, respectively.
2.6. Statistical analysis
In our analysis, Stata software (version 16.0) was utilized for data processing and statistical evaluation. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were derived from the original studies to assess the risk of mortality in patients with sepsis across diverse BMI. Based on the findings from the analyses, we assessed the presence of heterogeneity and selected a fixed or random effects model accordingly. A heterogeneity value of I2 > 50% and a significance level of P < 0.1 indicated substantial heterogeneity, warranting the use of a random effects model. Sensitivity analyses employing pooled or one-on-one elimination models were conducted to ensure the stability and dependability of the results. Additionally, funnel plots were employed to investigate potential bias, while statistical analysis of possible bias was performed utilizing Egger's regression test.
3. Results
3.1. Literature search
A total of 3865 records were identified via a systematic search of databases published before March 28, 2023, and screened for inclusion based on eligibility criteria. After reviewing the titles and abstracts, 12 studies [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]] were selected for inclusion in this study. Specifically, these studies reported on the association between obesity and sepsis prognosis, with 8 [14,[16], [17], [18], [19], [20], [21],23] of them focusing on overweight patients and 6 [14,16,18,20,21,35] of them restricting the analysis to patients with low weight. The details of the screening process are presented in Fig. 1.
Fig. 1.
| Literature screening flow chart.
3.2. Study characteristics and quality assessment
This meta-analysis comprised 12 studies [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]] encompassing data from multiple countries or regions with a total of 521,207 individuals, and two of which were prospective cohort studies. The follow-up time varied across the studies. Upon review of the included literature, significant differences were identified in the adjusted correctional factors. Their average score was 7.25 in all the studies based on NOS criteria. The characteristics and quality scores of each study are shown in Table 1.
Table 1.
Characteristics of studies included in the review.
| Author | Year | Country | Study Type | Age (Mean ± SD) | Follow-up years | No. Of participants | Confounders adjusted | NOS scores |
|---|---|---|---|---|---|---|---|---|
| Lin et al. | 2020 | China | Retrospective Cohort Study |
Underweight 67.02 ± 17.6 | 28-day | 7967 | age,sex, SOFA, mechanical ventilation on the first day, renal replacement therapy on the first day, congestive heart failure, cardiac arrhythmias, valvular disease, peripheral vascular disease, hypertension, other neurological diseases, chronic pulmonary disease, liver disease, renal failure, AIDS, lymphoma, metastatic cancer, solid tumor, diabetes, fluid and electrolyte disorders, alcohol abuse, drug abuse, and depression. | 8 |
| Normal 67.56 ± 16.88 | ||||||||
| Overweight 66.51 ± 16.21 | ||||||||
| Obese 63.71 ± 14.36 | ||||||||
| Weng et al. | 2020 | China | Prospective Cohort Study |
50.9 (±10.3) | 10 years | 440,763 | age, sex, study area、smoking status | 7 |
| Pepper et al. | 2019 | America | Retrospective Cohort Study |
NA | NA | 55,038 | patient, infection, and hospital-level factors | 7 |
| Zhou et al. | 2018 | China | Prospective Cohort Study | Survivors n = 104 | 90-day | 178 | NA | 7 |
| Non-survivors, n = 74 | ||||||||
| Wurzinger et al. | 2010 | Austria | Retrospective cohort study | Survivors 68 ± 15 |
NA | 301 | admission year, sex, age, presence of heart disease or chronic renal insufficiency, the number of pre-morbidities, origin of sepsis, and the simplified acute physiology score II | 7 |
| Non- survivors 76 ± 10 | ||||||||
| Zhang et al. | 2023 | China | Retrospective cohort study | Overweight 65.9 ± 17.0 |
1- year | 3145 | age, gender, SAPS, SOFA, diabetes, hypertension, coronary artery disease, congestive heart failure, atrial fibrillation, malignancy cancer, stroke, chronic obstructive pulmonary disease, renal disease, liver disease, MAP, creatinine, hemoglobin | 8 |
| Obese 62.9 ± 15.3 | ||||||||
| Tay-Lasso et al. | 2022 | America | Retrospective Cohort Study |
BMI<30 60 (64–85) |
1-year | 1246 | age, alcohol use, hypertension, congestive heart failure, diabetes mellitus, injury severity score, respiratory rate, pulse rate, systolic blood pressure, intensive care unit days, Glasgow coma scale. | 6 |
| BMI≥30 54 (60–80) | ||||||||
| Li et al. | 2019 | China | Retrospective Cohort Study |
Overweight,67.4 | 1-year | 5563 | Gender, Age, Marriage status | 8 |
| Obese,64.2 | ||||||||
| Gaulton et al. | 2014 | America | Retrospective Cohort Study |
Obese 60.6 (51.8–69) |
28-day | 1779 | ICU location and administration of a vasopressor | 7 |
| Non-obese 60.9 (49.2–71.9) | ||||||||
| Prescott et al. | 2014 | America | Prospective Cohort Study |
Overweight 78.7 ± 8.6 |
1-year | 1404 | age, race, gender, marital status, wealth, acute organ dysfunctions, ICU use, mechanical ventilation use, diabetes, other comorbidities, baseline cognitive status, and functional limitations. | 8 |
| Obese,75.3 ± 8.1 | ||||||||
| Huttunen et al. | 2007 | Finland | Retrospective Cohort Study |
NA | 30-day | 149 | alcohol abuse, age (continuous variable), sex, and causative organism | 7 |
| Arabi et al. | 2013 | Saudi Arabia | Retrospective Cohort Study |
Overweight,63.5 ± 15.9 | 12-year | 2882 | baseline characteristics and sepsis interventions | 7 |
| Obese,62.2 ± 14.6 |
3.3. Obesity and risk of mortality in sepsis
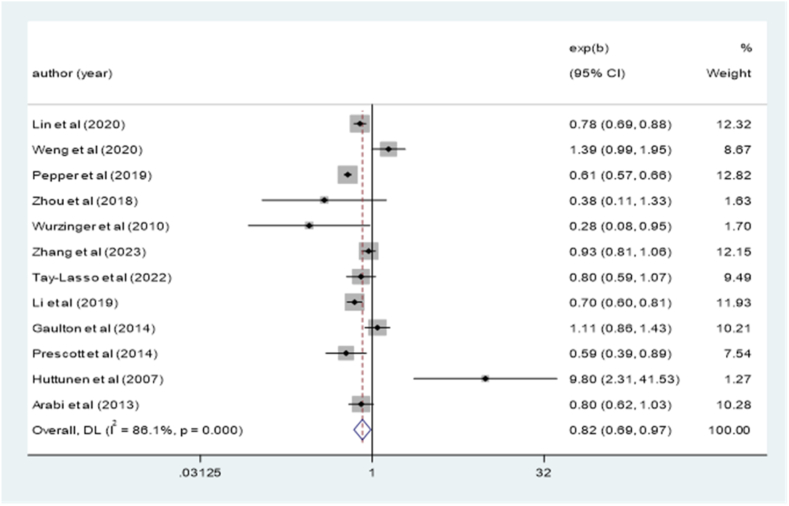
Twelve cohort studies [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]] were included in this meta-analysis to explore the associations between obesity and the risk of mortality in sepsis (OR = 0.82; 95% CI: 0.69–0.97; I2 = 86.1%, P < 0.001; Fig. 2). Sensitivity analysis revealed that no single study had a substantial impact on the overall findings.
Fig. 2.
| Meta-analysis of the risk of sepsis by obesity.
3.4. Overweight and risk of mortality in sepsis
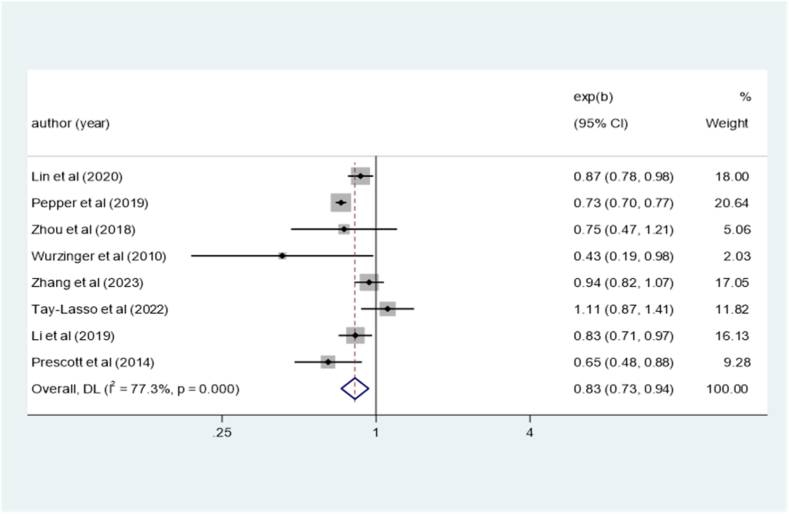
Eight cohort studies [14,[16], [17], [18], [19], [20], [21],23] were included in this investigation to examine the association between overweight status and the risk of mortality in sepsis (OR = 0.83; 95% CI: 0.73–0.94; I2 = 77.3%, P < 0.001; Fig. 3). Sensitivity analysis confirmed that none of the included studies significantly influenced the overall findings.
Fig. 3.
Meta-analysis of the risk of sepsis by overweight.
3.5. Underweight and risk of mortality in sepsis
Six studies [14,16,18,20,21,35] were conducted to investigate the association between being underweight and the risk of mortality in sepsis. The pooled results indicated that being underweight was significantly associated with an increased risk of mortality in sepsis (OR = 1.43; 95%CI: 1.16–1.76; I2 = 57.5%, P = 0.038; Fig. 4).
Fig. 4.
Meta-analysis of the risk of sepsis by underweight.
3.6. Subgroup analysis
Subgroup analysis in terms of location showed that the prognosis of sepsis and obesity was not related, whether in the ICU ward (OR = 0.79; 95%CI: 0.70–0.89; I2 = 50.8%, P = 0.058, Table 2), or in the Non-ICU ward (OR = 1.03; 95%CI: 0.64–1.66; I2 = 92.4%, P = 0.000, Table 2). In the retrospective study design, obesity was a protective factor in patients with sepsis (OR = 0.81; 95%CI: 0.68–0.97; I2 = 87.2%, P = 0.000, Table 2).
Table 2.
Subgroup analysis for the risk of sepsis in patients with obesity.
| Subgroups | Included |
OR |
Heterogeneity |
|
|---|---|---|---|---|
| studies | (95%CI) | I 2 (%) | P-values | |
| Study type | ||||
| Retrospective cohort | 9 | 0.81 (0.68,0.97) | 87.2% | 0.000 |
| Prospective cohort | 3 | 0.77 (0.36,1.62) | 83.5% | 0.002 |
| Inpatient Ward | ||||
| ICU | 7 | 0.79 (0.70,0.89) | 50.8% | 0.058 |
| Non-ICU | 5 | 1.03 (0.64,1.66) | 92.4% | 0.000 |
3.7. Publication bias
The funnel plot was approximately symmetrical, suggesting that there was less possibility of publication bias. Meanwhile, Egger's regression test (P = 0.150) yielded the same result, with no further evidence of publication bias (Fig. 5).
Fig. 5.
| Publication bias of the risk of sepsis by obesity.
4. Discussion
4.1. Main findings
The meta-analysis incorporated 12 studies encompassing a total of 521,207 individuals and presented an all-encompassing evaluation regarding the association between varying body mass indices and sepsis-induced mortality. Our findings indicate that individuals with lower body weight have a significantly higher risk of mortality in the context of sepsis, with an approximate 1.43-fold increase in risk. Conversely, those with obesity or overweight exhibit a modestly decreased risk of mortality, with an overall 0.82-fold or 0.83-fold reduction in risk, respectively.
4.2. Interpretation of findings
Previous studies showed the relationships between obesity and sepsis mortality. The results [14,16,18,21,23] showed that obesity did not increase its risk. When increasing the samples by a large amount, we found that BMI was inversely associated with sepsis-related mortality, and obesity or overweight reduced the risk of death of patients with sepsis. This phenomenon was called the obesity paradox, whose underlying physiological and pathological mechanisms have not been confirmed. This was an unexpected result from the perspective of many clinicians. Obesity is a chronic inflammatory state, which is closely related to the increase of oxidative stress [29]. Fat cells secrete factors that regulate the body's inflammatory response, including leptin, soluble tumor necrosis factor receptor 2, interleukin-10 (IL-10), and leptin [[36], [37], [38]]. Leptin plays an important role in the regulation of cell-mediated immunity, endothelial cell activation, and cytokine production [29]. Serum leptin levels are high in sepsis survivors and human obesity [39]. As we all know, high-density lipoprotein (HDL) plays a positive role in cardiovascular disease. Similarly, it can also be combined with internal toxins in the bacteria to reduce the inflammatory response [40]. Obese patients with septicemia have a lower need to receive fluids, either crystalline or colloidal, during initial resuscitation [25], activating the renal angiotensin system may increase the hemodynamic advantage of sepsis [14]. Obesity means higher nutrient reserves and provides enough energy for the body to produce antibodies, which are essential for the survival of an acute life-threatening illness. However, most of these possible mechanisms were the result of basic research, which not cleared up our doubts. Earlier studies showed that increasing BMI improved survival in patients with chronic renal insufficiency [16]. Of course, some scholars have suggested that increasing BMI can improve the survival rate of sepsis to some extent [16,41].
Low body weight was an independent risk factor for sepsis. Researchers [16,42] have found there was an increased incidence of sepsis and severe sepsis in underweight people. In Asian countries, they were more concerned with underweight populations and believe that a low body mass index harms the prognosis of sepsis [43,44]. According to incomplete statistics, the possibility of negative bacterial infection in hospitalized patients was higher, and was main pathogenic substance was endotoxin, whose component is lipopolysaccharide (LPS). It may be sequestered in adipose tissue via very low-density lipoprotein (VLDL) receptors [45,46], and this sequestration may help improve survival. This protective effect is diminished in patients with low body weight. Sepsis was essentially an inflammatory response and low body weight was associated with lower levels of anti-inflammatory interleukins, which may not suppress excessive immune responses [47]. However, there was a trend towards high blood IL-6 levels in patients with low BMI. High or low blood IL-6 values for assessing the severity of sepsis [47]. Underweight patients were often deficient in essential nutrients, such as vitamins and micronutrients [48]. Many of them were involved in the metabolism of cellular substances.
4.3. Advantages and limitations
Our meta-analysis summarized the correlation between different body mass indexes and the risk of sepsis death and showed that low body weight was an independent risk factor. In addition, compared to the meta-analysis on similar topics published [49], our study has three prominent advantages. Firstly, previous studies have only focused on overweight and obesity as protective factors for sepsis death. However, our study found a negative correlation between low body weight and the risk of sepsis death, which is not deeply explored in the latest meta-analysis [49]. Secondly, our study focuses on the mortality rate of sepsis, without considering the shock caused by sepsis, reducing potential clinical, methodological, and other biases, making the results more robust and closer to clinical reality. Thirdly, our subgroup analysis shows that the obesity paradox only exists in sepsis patients in ICU and sepsis patients in a retrospective cohort study, which has not been explored in previous meta analysis [49].
Of course, this study also had its limitations. Firstly, the meta-analysis included patients with extensive sepsis caused by heterogeneous diseases. The comparison between different populations with sepsis may affect the reliability of the results. Secondly, although we use BMI to determine obesity standards, the grading and classification of obesity in the original study were not entirely consistent, and there may be clinical heterogeneity. BMI cannot accurately represent obesity and may not accurately represent fat content, muscle to fat ratio, or overall body composition. In addition, although the original study controlled for confounding variables and reduced the impact of confounding factors on the results, we also used adjusted data for meta-analysis. However, the included studies were mostly retrospective studies, inevitably leading to issues such as recall bias. Finally, the original study included no mention of mortality risk data for ICU patients with severe sepsis and obesity, making it impossible to conduct a more comprehensive subgroup analysis of disease severity.
5. Conclusions
There is an “obesity paradox” phenomenon in the mortality of obese and overweight with sepsis patients, but low body weight is an independent risk factor for the mortality of sepsis patients. Our findings should be interpreted cautiously as they do not exhibit this obesity paradox in sepsis patients in ICU and prospective cohorts. Nevertheless, medical staff's emphasis on the weight of hospitalized patients is still beneficial for early identification of high-risk groups for sepsis patients.
Author contribution statement
Liang Gao; Hai bo Ding; Jun Jin Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qi Chao Fan; Li ting Ling: Contributed materials, analysis tools or data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Liang Gao, Hai bo Ding reports statistical analysis was provided by the First Affiliated Hospital, Fujian Medical University, Fuzhou 350,005, China. Liang Gao, Hai bo Ding reports a relationship with the First Affiliated Hospital, Fujian Medical University, Fuzhou 350,005, China. That includes: employment. L G and J L contributed to the selection of the paper, collection of data, analysis of data, and interpretation of results. Q C F, L T Land H B D revised the manuscript. All authors reviewed and approved the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19556.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., Schorr C., Simpson S., Wiersinga W.J., Alshamsi F., Angus D.C., Arabi Y., Azevedo L., Beale R., Beilman G., Belley-Cote E., Burry L., Cecconi M., Centofanti J., Coz Y.A., De Waele J., Dellinger R.P., Doi K., Du B., Estenssoro E., Ferrer R., Gomersall C., Hodgson C., Møller M.H., Iwashyna T., Jacob S., Kleinpell R., Klompas M., Koh Y., Kumar A., Kwizera A., Lobo S., Masur H., Mcgloughlin S., Mehta S., Mehta Y., Mer M., Nunnally M., Oczkowski S., Osborn T., Papathanassoglou E., Perner A., Puskarich M., Roberts J., Schweickert W., Seckel M., Sevransky J., Sprung C.L., Welte T., Zimmerman J., Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., Fleischmann-Struzek C., Machado F.R., Reinhart K.K., Rowan K., Seymour C.W., Watson R.S., West T.E., Marinho F., Hay S.I., Lozano R., Lopez A.D., Angus D.C., Murray C., Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng L., Xu Y., Yin P., Wang Y., Chen Y., Liu W., Li S., Peng J.M., Dong R., Hu X.Y., Jiang W., Wang C.Y., Gao P., Zhou M.G., Du B. National incidence and mortality of hospitalized sepsis in China. Crit. Care. 2023;27(1):84. doi: 10.1186/s13054-023-04385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd K.E., Kissoon N., Limmathurotsakul D., Bory S., Mutahunga B., Seymour C.W., Angus D.C., West T.E. The global burden of sepsis: barriers and potential solutions. Crit. Care. 2018;22(1):232. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent J.L., Bihari D.J., Suter P.M., Bruining H.A., White J., Nicolas-Chanoin M.H., Wolff M., Spencer R.C., Hemmer M. The prevalence of nosocomial infection in intensive care units in europe. Results of the european prevalence of infection in intensive care (epic) study. Epic international advisory committee. JAMA, J. Am. Med. Assoc. 1995;274(8):639–644. [PubMed] [Google Scholar]
- 7.Jones G.R., Lowes J.A. The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. QJM-An Int. J. Med. 1996;89(7):515–522. doi: 10.1093/qjmed/89.7.515. [DOI] [PubMed] [Google Scholar]
- 8.Martin G.S., Mannino D.M., Moss M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 9.Falagas M.E., Kompoti M. Obesity and infection. Lancet Infect. Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 10.Williams M.D., Braun L.A., Cooper L.M., Johnston J., Weiss R.V., Qualy R.L., Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit. Care. 2004;8(5):R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dremsizov T., Clermont G., Kellum J.A., Kalassian K.G., Fine M.J., Angus D.C. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129(4):968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 12.Prescott H.C., Dickson R.P., Rogers M.A., Langa K.M., Iwashyna T.J. Hospitalization type and subsequent severe sepsis. Am. J. Respir. Crit. Care Med. 2015;192(5):581–588. doi: 10.1164/rccm.201503-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea M.G., van der Meer J.W. Immunodeficiency and genetic defects of pattern-recognition receptors. N. Engl. J. Med. 2011;364(1):60–70. doi: 10.1056/NEJMra1001976. [DOI] [PubMed] [Google Scholar]
- 14.Lin S., Ge S., He W., Zeng M. Association between body mass index and short-term clinical outcomes in critically ill patients with sepsis: a real-world study. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/5781913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng L., Fan J., Yu C., Guo Y., Bian Z., Wei Y., Yang L., Chen Y., Du H., Chang L., Gong W., Chen J., Chen Z., Du B., Lv J., Li L. Body-mass index and long-term risk of sepsis-related mortality: a population-based cohort study of 0.5 million Chinese adults. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-03229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepper D.J., Demirkale C.Y., Sun J., Rhee C., Fram D., Eichacker P., Klompas M., Suffredini A.F., Kadri S.S. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit. Care Med. 2019;47(5):643–650. doi: 10.1097/CCM.0000000000003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q., Wang M., Li S., Zhang J., Ma Q., Ding Y., Ge H., Shen N., Zheng Y., Sun Y. Impact of body mass index on survival of medical patients with sepsis: a prospective cohort study in a university hospital in China. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-021979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wurzinger B., Dünser M.W., Wohlmuth C., Deutinger M.C., Ulmer H., Torgersen C., Schmittinger C.A., Grander W., Hasibeder W.R. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin. Wochenschr. 2010;122(1–2):31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Fang L., Lihua H., Li C. Association between obesity and 1-year mortality in septic patients: a retrospective cohort study. BMJ Open. 2023;13(2) doi: 10.1136/bmjopen-2022-066526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay-Lasso E., Grigorian A., Lekawa M., Dolich M., Schubl S., Barrios C., Nguyen N., Nahmias J. Obesity does not increase risk for mortality in severe sepsis trauma patients. Am. Surg. 2022 doi: 10.1177/00031348221078986. [DOI] [PubMed] [Google Scholar]
- 21.Li S., Hu X., Xu J., Huang F., Guo Z., Tong L., Lui K.Y., Cao L., Zhu Y., Yao J., Lin X., Guan X., Cai C. Increased body mass index linked to greater short- and long-term survival in sepsis patients: a retrospective analysis of a large clinical database. Int. J. Infect. Dis. 2019;87:109–116. doi: 10.1016/j.ijid.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Gaulton T.G., Weiner M.G., Morales K.H., Gaieski D.F., Mehta J., Lautenbach E. The effect of obesity on clinical outcomes in presumed sepsis: a retrospective cohort study. Intern. Emerg. Med. 2014;9(2):213–221. doi: 10.1007/s11739-013-1002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott H.C., Chang V.W., O'Brien J.M., Langa K.M., Iwashyna T.J. Obesity and 1-year outcomes in older americans with severe sepsis. Crit. Care Med. 2014;42(8):1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huttunen R., Laine J., Lumio J., Vuento R., Syrjänen J. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect. Dis. 2007;7 doi: 10.1186/1471-2334-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arabi Y.M., Dara S.I., Tamim H.M., Rishu A.H., Bouchama A., Khedr M.K., Feinstein D., Parrillo J.E., Wood K.E., Keenan S.P., Zanotti S., Martinka G., Kumar A., Kumar A. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit. Care. 2013;17(2) doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 27.Hemmingsson E. The unparalleled rise of obesity in China: a call to action. Int. J. Obes. 2021;45(5):921–922. doi: 10.1038/s41366-021-00774-w. [DOI] [PubMed] [Google Scholar]
- 28.Hemmingsson E., Ekblom Ö., Kallings L.V., Andersson G., Wallin P., Söderling J., Blom V., Ekblom B., Ekblom-Bak E. Prevalence and time trends of overweight, obesity and severe obesity in 447,925 Swedish adults, 1995-2017. Scand. J. Publ. Health. 2021;49(4):377–383. doi: 10.1177/1403494820914802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trivedi V., Bavishi C., Jean R. Impact of obesity on sepsis mortality: a systematic review. J. Crit. Care. 2015;30(3):518–524. doi: 10.1016/j.jcrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Pepper D.J., Sun J., Welsh J., Cui X., Suffredini A.F., Eichacker P.Q. Increased body mass index and adjusted mortality in icu patients with sepsis or septic shock: a systematic review and meta-analysis. Crit. Care. 2016;20(1):181. doi: 10.1186/s13054-016-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Liu X., Chen Q., Liu C., Huang C., Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2017;17(1):118. doi: 10.1186/s12871-017-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepper D.J., Demirkale C.Y., Sun J., Rhee C., Fram D., Eichacker P., Klompas M., Suffredini A.F., Kadri S.S. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit. Care Med. 2019;47(5):643–650. doi: 10.1097/CCM.0000000000003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S., Hu X., Xu J., Huang F., Guo Z., Tong L., Lui K.Y., Cao L., Zhu Y., Yao J., Lin X., Guan X., Cai C. Increased body mass index linked to greater short- and long-term survival in sepsis patients: a retrospective analysis of a large clinical database. Int. J. Infect. Dis. 2019;87:109–116. doi: 10.1016/j.ijid.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Page M.J., Mckenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., Mcdonald S., Mcguinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ Br. Med. J. (Clin. Res. Ed.) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuperman E.F., Showalter J.W., Lehman E.B., Leib A.E., Kraschnewski J.L. The impact of obesity on sepsis mortality: a retrospective review. BMC Infect. Dis. 2013;13:377. doi: 10.1186/1471-2334-13-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 37.Winkler G., Kiss S., Keszthelyi L., Sápi Z., Ory I., Salamon F., Kovács M., Vargha P., Szekeres O., Speer G., Karádi I., Sikter M., Kaszás E., Dworak O., Gerö G., Cseh K. Expression of tumor necrosis factor (tnf)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum tnf-alpha, soluble serum tnf-receptor-2 concentrations and c-peptide level. Eur. J. Endocrinol. 2003;149(2):129–135. doi: 10.1530/eje.0.1490129. [DOI] [PubMed] [Google Scholar]
- 38.Rice T.W. Obesity in acute lung injury: the "weight" is over. Chest. 2007;131(2):333–334. doi: 10.1378/chest.06-2584. [DOI] [PubMed] [Google Scholar]
- 39.Bornstein S.R., Licinio J., Tauchnitz R., Engelmann L., Negrão A.B., Gold P., Chrousos G.P. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J. Clin. Endocrinol. Metab. 1998;83(1):280–283. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 40.Rice T.W. Obesity in acute lung injury: the "weight" is over. Chest. 2007;131(2):333–334. doi: 10.1378/chest.06-2584. [DOI] [PubMed] [Google Scholar]
- 41.Wang S., Liu X., Chen Q., Liu C., Huang C., Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2017;17(1) doi: 10.1186/s12871-017-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore L.J., Moore F.A., Jones S.L., Xu J., Bass B.L. Sepsis in general surgery: a deadly complication. Am. J. Surg. 2009;198(6):868–874. doi: 10.1016/j.amjsurg.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Sato T., Kudo D., Kushimoto S., Hasegawa M., Ito F., Yamanouchi S., Honda H., Andoh K., Furukawa H., Yamada Y., Tsujimoto Y., Okuyama M., Kobayashi M. Associations between low body mass index and mortality in patients with sepsis: a retrospective analysis of a cohort study in Japan. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oami T., Karasawa S., Shimada T., Nakada T.A., Abe T., Ogura H., Shiraishi A., Kushimoto S., Saitoh D., Fujishima S., Mayumi T., Shiino Y., Tarui T., Hifumi T., Otomo Y., Okamoto K., Umemura Y., Kotani J., Sakamoto Y., Sasaki J., Shiraishi S.I., Takuma K., Tsuruta R., Hagiwara A., Yamakawa K., Masuno T., Takeyama N., Yamashita N., Ikeda H., Ueyama M., Fujimi S., Gando S. Association between low body mass index and increased 28-day mortality of severe sepsis in Japanese cohorts. Sci. Rep. 2021;11(1):1615. doi: 10.1038/s41598-020-80284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada T., Topchiy E., Leung A., Kong H.J., Genga K.R., Boyd J.H., Russell J.A., Oda S., Nakada T.A., Hirasawa H., Walley K.R. Very low density lipoprotein receptor sequesters lipopolysaccharide into adipose tissue during sepsis. Crit. Care Med. 2020;48(1):41–48. doi: 10.1097/CCM.0000000000004064. [DOI] [PubMed] [Google Scholar]
- 46.Walley K.R., Thain K.R., Russell J.A., Reilly M.P., Meyer N.J., Ferguson J.F., Christie J.D., Nakada T.A., Fjell C.D., Thair S.A., Cirstea M.S., Boyd J.H. Pcsk9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014;6(258):143r–258r. doi: 10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zampieri F.G., Jacob V., Barbeiro H.V., Pinheiro D.S.F., de Souza H.P. Influence of body mass index on inflammatory profile at admission in critically ill septic patients. Int. J. Inflamm. 2015;2015 doi: 10.1155/2015/734857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danninger T., Rezar R., Mamandipoor B., Dankl D., Koköfer A., Jung C., Wernly B., Osmani V. Underweight but not overweight is associated with excess mortality in septic icu patients. Wien Klin. Wochenschr. 2022;134(3–4):139–147. doi: 10.1007/s00508-021-01912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai L., Huang J., Wang D., Zhu D., Zhao Q., Li T., Zhou X., Xu Y. Association of body mass index with mortality of sepsis or septic shock: an updated meta-analysis. J. Intensive Care. 2023;11(1):27. doi: 10.1186/s40560-023-00677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.