Abstract
Agricultural application is the primary method of recycling sewage sludge. It is an alternative for recycling this residue, providing nutrients and organic matter to crops and soil. However, sewage treatment and management issues may impact its quality. The main objective of the research was to determine the quality of sewage sludge generated at the Kumasi Wastewater Treatment Plant (KWTP). Understanding the effects of using sludge on soil and plants is critical. To overcome this constraint, the soil microbial biomass was used to quantify the growth of microorganisms. The levels of potentially toxic elements in the sludge using atomic absorption spectrometry (AAS) are based on US EPA part 503 regulations for the disposal and management of biosolids. This study found that trace metal concentrations in the biosolids were lower than the referenced background standards threshold. Although the microbial biomass, nutrients and bacteria levels were within the accepted values for their possible use as soil fertilizer. The ecological risk index (135.10) indicated that the level of arsenic was high in the sludge. The salinity in the sludge was low, with electrical conductivity (EC) being high (60.80–436.00 μS/cm) and pH decreasing with age (6.73–7.69). The sludge produced at KWTP is of good quality and meets international standards with only a high concentration of As. This can be used for soil amendment when As is reduced in the sludge.
Keywords: Fertilizer, Recycling, Agriculture, Fecal waste, Biosolids
1. Introduction
Sewage sludge is an organic by-product of global wastewater treatment [1]. With global development and population growth, the production of waste and sewage rises year after year. Besides the massive production of sewage sludge worldwide, it goes through a series of processes that reduce the concentrations of readily decomposable organic materials. Sludge production in China has grown at a rate of 13% since 2007 with a total production of dry biosoilds of 6.25 million tonnes in 2013 [2]. Most wastewater treatment facilities generate sludges that contain the excess solids generated in either physical (i.e. settling) or biological wastewater treatment processes [3]. While approximately 6 million tons are reported in the United States annually, approximately 60% of this amount is applied to agricultural soil and land [4]. Following treatment, the insoluble solid residue is known as biosolids, domestic wastewater residue, or sewage sludge and this organic waste must be properly managed [5,6]. Regulators of WWTPs could employ this information for improved policy making related to biosolids disposition [7]. Organic matter and nutrients comprise most of the sludge composition [8]. The latter varies greatly depending on the source of the sewage, the treatment process, and the season [9]. The large volume of generated sewage sludge makes its disposal difficult. The wastewater treatment plant is growing and needs to ensure that its operations do not cause any environmental impact. Because a large volume of organic waste material is continuously produced, this could create a major waste problem when not properly managed [10]. Managing the biosolids or sludge, the treatment process produces is difficult [11]. In Ireland, authorities are-still changing sewage sludge disposal practices [12]. The primary benefit of using recycled organic waste is that it provides free or low-cost fertilizers and lime [12]. Long-term studies of soil amendments with sewage sludge in Spain, the United Kingdom, and the Mediterranean revealed an improvement in soil properties proportional to the doses and frequency of application. The sludge is also used as fertilizer in forestry areas [13]. Several crops were reported to grow efficiently on sewage sludge-amended substrate/soil, such as mushrooms [14], Sesbania bispinosa [15] and marigold [16], thus, outlining a great fertilization potential of this under-rated valuable waste. Only 7% of developing countries are connected to sewers, and only 1% of waste is treated [17]. Sludge characterization involves the description of sludge behaviour in treatment processes and disposal. Appropriate characterization methods facilitate the understanding and predicting sludge properties [18]. According to global urbanization projections, the generation of fecal sludge in West Africa is estimated to be 100–1000 L per capita per year, while wastewater generation is estimated to be 20–150 L per capita per day [19]. Farmers in Ghana, Benin, and Mali have been known to bribe septic truck drivers to dump feces in their fields [20]. Sludge disposal is becoming more of an issue in developed and developing countries as the volume of waste treated increases [21].
Historically, sewage sludge was regarded as waste that should be disposed of as cheaply as possible [22]. Local sludge is discarded randomly, causing environmental contamination, even though it could be used in agriculture as an alternative to intensive mineral fertilizer [23]. In Ghana, Kumasi, the second-largest city and its a developing commercial and industrial center with an estimated population of 1.5 million inhabitants. The metropolitan area exceeds 2 million and is growing rapidly each year (WHO/UNICE joint monitoring program) [24]. The sanitation service in Kumasi is improving, but only 7–10% of the collected septage is adequately treated [25]. Due to a lack of capacity to collect and process waste in the Ashanti Region of Ghana, liquid waste is discharged into streams in Dompoase and its environs. A wastewater treatment plant has been established to aid in the management of liquid waste generated in Kumasi and other parts of the Ashanti Region.
The Kumasi Wastewater Treatment Plant (KWTP) is a customized wastewater treatment plant designed and built using Septopure Technology, a proprietary wastewater treatment solution developed specifically for the treatment of Kumasi city's wastewater by Pureco. The KWTP is located in Adagya, in the Ashanti Region's Bosomtwe Districts. At the facility, sludge from the final phase of the wastewater treatment plant (WTP) is treated using Profikomp Biological Waste Treatment Technology. This generates sewage sludge, which can be used as an organic fertilizer for agricultural applications; however, the quality of the sludge must be tested before it can be used significantly, and the ecological effects on the health of both plants and animals, as well as human health, must be reduced. Some site-specific factors make sewage sludge unique; hence this specificity must be considered to predict the outcome of its treatment [26]. A careful assessment of the characteristics of sewage sludge is required prior to its soil application to improve the soil health without causing environmental hazards [27]. Hence, the aim of the research is to determine the quality of sewage sludge generated at the Kumasi Wastewater Treatment Plant.
2. Materials and methods
2.1. Study area
Kumasi Wastewater Treatment Plant (KWTP) is located in Adagya, in the Bosomotwe districts, and lies at latitudes 6 35′54″ N and 1 35′03″ N in the Ashanti Region of Ghana (Fig. 1). The district falls within the equatorial zone with a rainfall regime typical of the moist semi-deciduous forest zone of the country. There are two well-defined rainfall seasons. The main season occurs from March to July, peaking in June. The minor season starts from September to November, peaking in October. August is usually cool and dry. The main dry season occurs from December to March, during which the desiccating harmattan winds blow over the area. The area's temperature seems to be uniformly high throughout the year, with a mean average of around 24 °C. The highest mean occurs just before the major wet season in February, as observed in Kumasi at 27.8 °C. The mean minimum occurs during the minor wet season. Kumasi has a population of 3,490,000, according to the 2021 Population and Housing Census. The Kumasi Wastewater Treatment Plant has a capacity of 1000 cubic meters per day and treats fecal waste generated within the city. The wastewater is treated by biological treatment method.
Fig. 1.
Map of the study area.
3. Sample collection
Biosolids were sampled using the grid sampling method [28]. Grids were used to partition the region, and samples were taken at specified positions on the pattern. Using a soil auger, 21 samples were obtained from the depth and surface of each dewatered sludge from ages 6 months, 3 months, 1 month and fresh sludge. The samples were collected from the surface (S) and the bottom (D) of the dewatered sludge (6MS, 6MD, 3MS, 3MD, 1MS and 1MD). The samples were placed in a stainless-steel bucket and mixed well for homogeneity before being transferred to a PET container (Ziploc bag).
3.1. Preparation and transfer
Twenty-one (21) sample containers were clearly labeled for simple identification in the laboratory for examination. The composite samples in the stainless-steel buckets were transferred into sample containers. The containers were then sealed and placed in an ice chest for transit to the laboratory for analysis at 4 °C. This was done to prevent microbial contamination, biochemical activities and to reduce errors during analysis.
3.2. Analytical procedure
Sewage sludge has a diverse composition, is high in organic matter, and has highly variable physical and chemical properties. Analytical techniques determined the sludge's physiochemical characteristics, nutritional content, and risk evaluation. Each sample was replicated to improve the significance of an experimental result.
3.3. Physiochemical parameters
The physicochemical parameters determined were pH, salinity, electrical conductivity (EC), microbial analysis, and the analytical sample's potential toxic elements (PTE).
The 9045D method was used to measure the pH of the samples. The samples were mixed with reagent water, and the pH of the resulting aqueous solution was measured. The pH electrode was calibrated at pH 7 and 4 before each test. In a 50 mL beaker, 20 g of the sample were weighed, and 20 mL of reagent water were added and swirled continuously for 5 min. The aqueous phase was filtered for pH measurement.
The salinity and conductivity of the samples were determined using the OHAUS ST3100M − N multifunctional equipment. The EC and salinity were measured after immersing the electrodes in the samples.
Potentially toxic elements in the samples were analyzed using analytical techniques, Method 3050 (Sw-846, 3rd ed.) was used for acid digestion of the sludge for analysis. To avoid interference in the extraction, digestion tubes were rinsed and immersed in weak HNO3 for 24 h to eliminate absorbed metals. After being rinsed with distilled water and placed on a rack, the tubes were placed in an oven for 15 min to dry 1 g of material was weighed and placed in tubes with 1 mL of distilled water, 6 mL of 1:1HNO3 and HClO4 pipetted into each test tube, and 5 mL of concentrate. H2SO4 was pipetted into each test tube. In a fume chamber, the rack was placed on a hot plate and heated at 20 °C until there was no brown fume and a clear solution. After cooling for 30 min, the solution was diluted with 50 mL of distilled water. The contents were then filtered and placed in an acid-soaked and dried PET container. The resultant solution was stored at 4 °C for spectrophotometric analysis.
Atomic absorption spectrometry (AAS) was used to determine a sample's concentration of specific elements. Method 7000 was used in atomic absorption spectroscopy (Sw-846, 2009). Total As, Cd, Cr, Cu, Ni, Pb, and Zn concentrations in digested samples were determined using the novAA 400P model.
3.3.1. Nutritive content analysis
The nitrogen in ammonia plus the percentage of nitrogen that can be catalytically reduced to ammonia in a concentrated sulfuric acid solution is referred to as Kjeldahl-Nitrogen [Hicks, Muñoz-Huerta]. 1g of the sample was oven dried, crushed and sieved through a 0.5 mm sieve before being weighed into a 500 mL long-necked Kjeldahl flask. To this Kjeldahl catalyst, a mixture of one component selenium, ten parts CuSO4, and one hundred parts Na2SO4 and 10 mL of concentrate H2SO4 were added and digested until a clear, light-greenish solution was seen. After cooling, the digest was transferred to a volumetric flask and filled to the mark with reagent water. Using a pipette 10 mL of the aliquot was transferred into the Kjeldahl distillation with 90 mL of distilled water. In a 500 mL conical flask, 20 mL of 40% NaOH were added to the distillate along with 10 mL of 4% boric acid and three (3) drops of mixed indicator for 5 min. The distillate's bright blue tint suggested the presence of nitrogen.
The phosphorus content of a sample is defined as the total phosphorus in the sample given as a weight percentage. The vanadate-molybdate method (Procedure 4500 -Pc) is based on the APHA Standard method [29]. 5 mL of the digest were transferred to a 50 mL volumetric flask. 10 mL of vanado-molybdate reagent were put to the mark and filled with distilled water. The contents were violently shaken for 5 min before being allowed to stand for 25 min. On the colorimeter, a yellow color appeared and phosphorus was detected at 430 nm.
Potassium content is defined as the proportion of the corresponding metal in the sample expressed as a weight percentage. Iron and potassium concentrations were determined by spectrophotometry with a DR/3900 spectrophotometer [29].
4. Microbial analysis
Escherichia coli is a marker for several human infections that may be found in feces. Mac Conkey and peptone media were produced and sterilized in test tubes in an autoclave at 120 °C at 15 lbs. pressure for 15 min with distilled water. After 10 g of materials were weighed and pulsifyed for 15 s with a peptone, serial dilutions were prepared. The contents were then placed in an incubator at 44 °C for 24 h. Three drops of Kovac's reagents into the test tubes revealed a crimson ring, indicating the presence of E. coli. The most probable number (MPN) calculation was adapted from Fecal Sludge MPN Calculation Guidance [30].
Microbial biomass is a key component of soil organic matter that controls nutrient transformation and storage. Changes in soil microbial biomass can be used to track the toxicity of pollutants and the decomposition of organic molecules (pesticides and industrial chemicals). Microbial carbon, nitrogen, and phosphorus were determined using indirect methods [31].
4.1. Fumigation and extraction
Ten (10) g of sample were weighed and deposited in 14 Petri plates, seven of which served as controls. For five days, 50 mL of chloroform was placed in a desiccator with seven samples and the control in another desiccator without fumigation. This was done to eliminate all microorganisms prior to testing. The soil was extracted with 0.5 mol/L of K2SO4 in both fumigated and unfumigated conditions. The content was shaken for 10 min before being filtered with filter paper. The Kjeldahl method was used to determine the nitrogen biomass [31,32].
5. Biomass carbon
An Erlenmeyer flask was filled with 10 mL of the extractant. After adding 5 mL of 1.0 eq/l K2Cr2O7 and 10 mL of H2SO4, the solution was allowed to cool for 30 min and the background C for the control and fumigated samples was determined by digesting the blank filtrate [33,34]. At 600 nm, absorbance values for both the control and the samples were measured.
6. Biomass phosphorus
2 mL of extractant was put into an Erlenmeyer flask along with 1.0 mL of ammonium molybdate and ascorbic acid 6 mL of distilled water was added and the mixture was allowed to stand for 15 min. The absorbance at 600 nm wavelength was then measured.
6.1. Risk assessment
6.1.1. Enrichment factor (EF)
The Enrichment Factor is used to quantify the presence and severity of anthropogenic pollutant deposition on soil surfaces. It is computed by comparing the concentration of one potential toxic element in topsoil to that of a reference element. The reference metal utilized in the study was Fe [35], which is defined in equation (1):
| EF = [Ca/Caref] sample/ [Cb/Cbref] background | (1) |
Where Ca is the measured “concentration of the element and Cb is the background concentration of the measured element. Caref is the measured concentration of the reference element, whereas Cbref is the background concentration of the reference element [36]. The categories are as follows: deficiency to minimum enrichment (EF < 2), moderate enrichment (2< EF < 5), significant enrichment (5< EF < 20), very high enrichment (20< EF < 40), and extremely high enrichment (EF > 40) [36].
6.1.2. Contamination factor (CF)
The contamination factor is the ratio of each element's measured concentration to its background concentration, and it shows the specific impact of each element on the sediments. As shown in equation (2) below [36].
| CF Cs/Cb | (2) |
Where Cs is the measured concentration of the element and Cb is the background concentration of the same element. It varies between Cf < 1(low contamination), 1≤ Cf < 3 (moderate contamination), 3< Cf < 6 (considerate contamination) and Cf > 6 (very high contamination”) [36].
Pollution Index (PI)
The PI is a summative indicator of metal toxicity in a given sample, calculating how much the metal level in the soil exceeds the normal and natural background concentration. The PI of the research region was calculated by taking the n-root of all metals' n-CFs as shown in equation (3) as follows [36].
| PI (CF1 × CF2 × CF3 × CF4 × CF5 … … … … …. CFn) 1/n | (3) |
Where n = the number of metals analyzed. The severity of PLI was determined by PLI < (the study area is free from contamination), PLI = 1 (baseline level of contamination), PLI >1 (deterioration of the area quality).
6.1.3. Potential ecological risk index (RI)
The potential ecological risk index (RI) is derived as the sum of risk factors for all heavy metals in sediments. It depicts the biological community's susceptibility to the toxic chemical, demonstrating the potential ecological risk caused by heavy metal contamination in general. RI was calculated as shown in equation (4). 5 and 6 below [35]:
| (4) |
| (5) |
| (6) |
Where CIf is a single-metal pollution factor; Cn is the metal concentration in samples; Cnr is a reference value for the metal. Eir is the potential ecological risk index of individual metals and n the metals analyzed. Tir is the toxic factor of an individual metal. The RI value was classified as: low (RI ≤ 50), moderate (50 < RI ≤ 100), considerable (100 < RI ≤ 200); and high contamination (RI > 200) [35].
6.2. Data analysis
The Statistical Package for Social Sciences (SPSS version 26) was used to analyze the laboratory data: Pearson's correlation coefficient was utilized to establish a significant association between PTE and physicochemical parameters. The one-way ANOVA method was used to investigate the significance in the mean concentrations of heavy metals, and the t-test at the 0.05 level of significance (p < 0.05) was employed to compare mean values and standard deviations. Microsoft Excel program 2016 for statistical value analysis; Tables, line graphs (trend analysis), and error bar charts were used to illustrate the data. Data on heavy metals in sludge were compared to the South African Limits (Snyman, n.d.) for metals introduced to agricultural land by sewage sludge and USA regulation 40 cfr part 503 [37].
7. Results and discusssion
7.1. Heavy metals in sludge
The mean concentrations of heavy metals shown in Table 2 are high in 6 MD, 3 MS, and Fresh sludge sampled. But the fresh sludge has the highest concentrations of heavy metals in all the sludge samples analyzed. The heavy metals concentrations were within the permissible levels when compared to USA regulation for sludge and only As is above the permissible level in the fresh sludge to the South Africa guidelines for sludge management (Table 1&2). Potential toxic element levels were inconsistent in the samples in relation to the age of the sludge. The potential toxic elements concentration were Iron > zinc (Zn) > arsenic (As) > copper (Cu) > Lead (Pb), Nickel (Ni) > Cadmium (Cd). A high concentration of Zn was also identified in nine treatment plants in China [38]. Generally, there were more heavy metals at the surface of the various sludge ranging between 26 and 0.02 mg/kg compared to the samples collected from the bottom ranging between 25 and 0.01 mg/kg. This could be due to the high number of microorganisms at the bottom because of moisture and temperature. The concentration of heavy metals within the sludge decreased with age (Table 2). Compared to the relevant norms, the values obtained for the sludge at KWTP were low (Table 1). One-way ANOVA revealed significant differences in heavy metal concentrations between samples; As (F = 103.079), Cd (F = 27.955), Cr (F = 5.046), Cu (F = 53.361), Ni (F = 1.004), Pb (F = 0.688), and Zn (F = 434.703) at (P < 0.05).
Table 2.
Potentially toxic elements concentration with mean and standard deviations.
| Sample | Parameters (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| As |
Cd |
Cr |
Cu |
Pb |
Zn |
Ni |
Fe |
|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Fresh | 55.61 | 0.33 | 0.69 | 10.86 | 0.03 | 55.61 | 0.18 | 9.1 |
| 1MS | 26 | 0.25 | 0.67 | 5.55 | 0.04 | 58.73 | 0.02 | 9.1 |
| 1 MB | 21.63 | 0.18 | 0.79 | 7.45 | 0.02 | 48.75 | 0.1 | 14.95 |
| 3MS | 31.62 | 0.2 | 0.68 | 5.52 | 0.03 | 59.62 | 0.19 | 7.8 |
| 3 MB | 25.3 | 0.28 | 0.83 | 1.97 | 0.01 | 51.48 | 0.03 | 9.1 |
| 6MS | 22.93 | 0.3 | 1.02 | 6.35 | 0.02 | 43 | 0.18 | 26.65 |
| 6 MB | 19.74 | 0.21 | 0.52 | 1.3 | 0.01 | 33.78 | 0.04 | 88.4 |
| P-value | 0.01 | 0.01 | 0.01 | 0.3 | 0.01 | 0.01 | 0.01 | 0.1 |
Table 1.
Potentially toxic elements compared with standard values.
| Pollutant | USA regulation for sludge (mg/kg) | South Africa guidelines for sludge management (mg/kg) |
|---|---|---|
| As | 75 | <40 |
| Cd | 85 | <40 |
| Cr | N/A | <1200 |
| Cu | 4300 | <1500 |
| Pb | 840 | <300 |
| Hg | 57 | <15 |
| Ni | 420 | <420 |
| Zn | 7500 | <2800 |
A similar study conducted at Accra Sewage Treatment Plants (ASTP), the heavy metal concentrations were greater [39], with Cd (0.60 mg/kg), Cr (42.3 mg/kg), Cu (125 mg/kg), Ni (25.12 mg/kg), Pb (6.69 mg/kg), and Zn (694 mg/kg) (Table 2). Except for As (10.82 mg/kg) being lower than the results obtained from KWTP. According to Zhang et al. [38] reported that high heavy metal concentrations were identified in nine treatment plants in China. This is due to the varied dewatered sludge treatment practices in both plants and the effect of the climate, as Kumasi receives more rainfall than Accra. The KWTP stockpiled the sludge in an open-spaced at the landfill site, where the Kumasi Compost and Recycling Company also disposed of its residues after compost had been produced. In contrast to ASTP, the plant includes mechanical drying beds that retain metal concentrations while also controlling environmental conditions that can cause metal accumulation and reduction. Plant phyto-mining caused low Cr, Cd, Cu, Pb, Ni, and Zn levels. Organic and inorganic compounds in plants can be affected in different directions by the complex interplay between plants, herbivores, and heavy metal contamination of soil [39]. The purpose of testing trace metal levels in sludge was to provide an understanding of the state of the sludge and its potential use for land application. According to Tchounwou et al. [40], heavy metals are classified into two groups: (a) essential metals (Zn, Cu, Cr, and Ni), which are required in minute amounts by plants, humans, and animals in ecological processes. The necessary metals can be harmful in high doses. (b) non-essential metals (Cd, Pb, and As) are poisonous and are not required by humans, animals, or plants. Except for arsenic, the content of toxic metals in the sludge were generally low compared to the Limits for South Africa (Table 1). The general profile of the heavy metals concentration in all the samples indicates that applying fresh sludge could cause more harm than when the sludge is allowed to dry up for some time. Also, in other to reduce the heavy metal concentration, the sludge condition must be maintained to promote microbial growth.
8. Physicochemical parameters
The pH of the samples ranged from 6.73 to 7.69, with conductivity ranging from 60.80 to 436.00 μS/cm, and the salinity from 0.03 to 0.19 μS/cm (Table 3). Sewage sludge from Gaza has a pH of 6.78 and electrical conductivity (2.49 ± 0.04) mS/cm by El-Nahhal et al. [41]. The samples have very high conductivity, with 3MD being the highest and 3MS being the lowest. The fresh sample's pH was higher than the aged samples, with 3MD being the lowest. The samples have extremely low salinity, with 3MS having the lowest.
Table 3.
Physicochemical properties of sewage sludge from KWTP.
| Sample | pH | Salinity μS/cm | Electric conductivity μS/cm |
|---|---|---|---|
| Fresh | 7.69 | 0.07 | 143.8 |
| 1MS | 7.26 | 0.1 | 199 |
| 1MD | 7.15 | 0.09 | 173.4 |
| 3MS | 7.03 | 0.03 | 60.8 |
| 3 MB | 6.39 | 0.21 | 436 |
| 6MS | 6.53 | 0.06 | 119.4 |
| 6 MB | 6.73 | 0.19 | 401 |
| P-values | 0.03 | 0.01 | 0.01 |
Salinity and conductivity exhibited a positive association EC/S(r = 0.999) as observed in Table 4. Because dissolved salts carry electrical currents, as the salinity increased, so did the conductivity. However, at (p < 0.05), pH showed a positive association with As, Cu, and Pb (r = 0.721, 0.782, and 0.723). However, these correlations were statistically insignificant, though increasing physical parameters may cause metal concentrations to rise. Furthermore, a strong negative correlation between pH/Cr–Fe, EC/As–Cd–Cr–Cu–Ni–Pb–Zn, and S/Cd–Cr–Cu–Ni–Pb–Zn–Fe indicates that the relationship is statistically insignificant.
Table 4.
Correlations between physicochemical parameters and Potentially toxic elements of sludge.
| As | Cd | Cr | Cu | Ni | Pb | Zn | Fe | pH | Conductivity | Salinity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Pearson Correlation | 1 | ||||||||||
| Cd | Pearson Correlation | 0.596 | 1 | |||||||||
| Cr | Pearson Correlation | −0.141 | 0.398 | 1 | ||||||||
| Cu | Pearson Correlation | 0.724a | 0.375 | 0.223 | 1 | |||||||
| Ni | Pearson Correlation | .515 | 0.240 | 0.349 | 0.645 | 1 | ||||||
| Pb | Pearson Correlation | 0.440 | 0.107 | −0.120 | 0.593 | 0.250 | 1 | |||||
| Zn | Pearson Correlation | 0.502 | 0.128 | 0.024 | 0.457 | 0.215 | 0.762a | 1 | ||||
| Fe | Pearson Correlation | −0.407 | −0.268 | −0.450 | −0.541 | −0.299 | −0.559 | −0.884** | 1 | |||
| pH | Pearson Correlation | 0.721a | 0.080 | −0.407 | 0.782a | 0.286 | 0.723a | 0.518 | −0.322 | 1 | ||
| Conductivity | Pearson Correlation | −0.368 | −0.014 | −0.266 | −0.740a | −0.796a | −0.703a | −0.501 | 0.500 | −0.523 | 1 | |
| Salinity | Pearson Correlation | −0.381 | −0.022 | −0.252 | −0.733a | −0.811a | −0.697a | −0.493 | 0.483 | −0.521 | 0.999** | 1 |
| Sample(N) | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
P > 0.05 and **P > 0.0.
* Correlation is significant at the 0.05 level (1-tailed). ** Correlation is significant at the 0.01 level (1-tailed).
The sludge's alkalinity dropped with age from 7.69-6.53 and samples 3 MB, 6MS and 6MD were acidic. This indicates that with age the sludge becomes acidic and this could be due to nitrification of ammonia which generates a significant amount of acidity. The relationship between microbial biomass and pH clearly shows that the growth and number of microorganisms in the sludge, as measured by MBC, MBN, and MBP, decreased as the pH increased (Table 3, Table 4). In the samples, conductivity and salinity had a positive relationship. Indicating that any change in conductivity in the samples will have an equal change in salinity. The pH of the biosolids changed from alkaline to acidic as they age [42]. This can also impact the biochemical activities within the biosolids. The conductivity increases as biosolids age, indicating the movement of ions in sludge as it ages, hence influencing biochemical reactions [43]. The negative association between physical parameters (Table 4) and heavy metals demonstrates that there is no direct increase or decrease in heavy metal levels with changes in physical parameter levels. The link between salinity and conductivity suggests that changes in EC levels may cause changes in salinity.
8.1. Microbial analysis
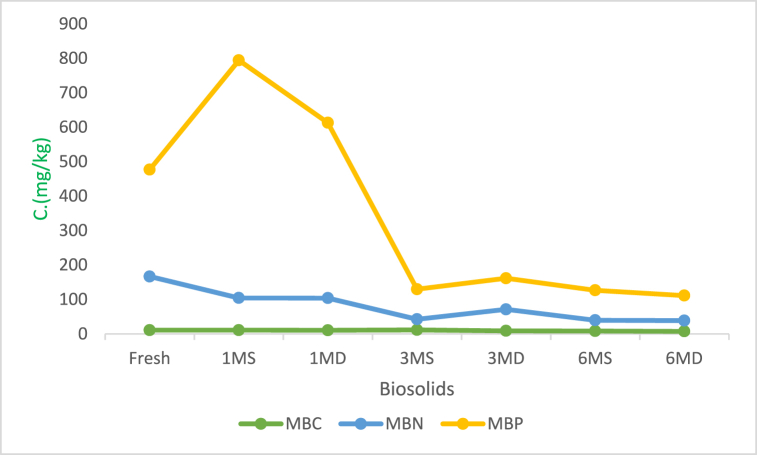
Carbon, nitrogen, and phosphorus were used to calculate the mass of microorganisms in the sludge. The characteristics in Fig. 2 increased initially and then dropped with time. The sample's phosphorus (P) mass revealed many microorganisms in the 1MS but decreased as the sludge aged.
Fig. 2.
Mass of living components showing the minimum bactericidal concentration (MBC), microbial biomass nitrogen (MBN) and maltose-binding protein (MBP).
8.2. Escherichia coli
E. coli levels in the samples were low, increasing from 1MS, 3MD, and 6MD. The Total Coliform and Fecal Coliform ranged from 0.00 to 9.50 and 1.60–10.00, respectively. They met the exceptional quality criteria set by the [44] biosolids rules E. coli <1000MPN/100 mL.
Microbe biomass in biosolids declined over time, even though MBN and MBP boosted microbe growth for 3 MD at one point in time. The general decline recorded in the microbial mass could be due to the absence of nutrients needed for the microbes to grow and poor environmental factors such as oxygen, temperature, and moisture [45]. The decline of microorganisms revealed by MBN and MBP over time could also be caused by rainwater creating runoff and leachate during rains. According to de Amorim Júnior [46] Zn tends to increase MBC, which explains the low amounts of it accumulated in the sludge, as Zn was found in high concentrations in the samples, with a mean range of 33.78–59.62 mg/kg (Table 5). E. coli met the standards outlined in the US EPA Part 503 rule for classifying biosolids as Class A exceptional biosolids quality. Except for the fresh sample, Total Coliforms and Fecal Coliform levels decreased at the surface more than at the depth with time. This could be related to the reduction in pH, which promotes microbial growth. Kokina et al. [47] also reported that there are slight changes in sludge microbial mass in both acidic and alkaline pH. The acidic medium promoted growth, while alkaline's one inhibited microbial growth.
Table 5.
Level of Escherichia coli in sludge.
| Sample |
E. coli MPN/100 mL |
Total Coliforms MPN/100 mL |
Fecal Coliforms MPN/100 mL |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | MPN/100 mL | Mean ± SD | MPN/100 mL | Mean ± SD | MPN/100 mL | |
| Fresh | – | – | 12.4 | 1.04 | 6.5 | 5.49 |
| 1MS | 7.8 | 6.59 | 6.9 | 5.83 | 5.3 | 4.48 |
| 1MD | – | – | 9.25 | 7.82 | 8.25 | 6.97 |
| 3MS | – | – | 6.5 | 5.49 | 9.25 | 7.82 |
| 3MD | 42.9 | 3.6 | 12.4 | 1.04 | 9.5 | 8.03 |
| 6MS | – | – | 6.5 | 5.49 | 4.4 | 3.72 |
| 6MD | 12.40 ± 6.50 | 1.04 × 10−3 | 24 | 2.03 | 9.25 | 7.82 |
| P-value | 0.01 | 0.01 | ||||
9. Nutritive content
The percentage of nutrients in the samples revealed that nitrogen and phosphorus declined at the surface and increased in depth as sludge aged (Table 6). However, potassium was relatively low. Nitrogen had the highest nutrient content. Generally, the nutrient decreased in the third month and then increased again in the sixth month. The increment in the sixth month was observed at the sample collected from the bottom. Characterization of sewage sludge from 26 sewage treatment plants in India indicated that nitrogen has the highest nutrient content and potassium the lowest in the dry sludge [48].
Table 6.
Percentages of total nitrogen (TN), phosphorous (TP) and potassium (TK) in sludge.
| Nutrients (%) | |||
|---|---|---|---|
| Sample | Nitrogen (%) | Phosphorous (%) | Potassium (%) |
| Fresh | 4.05 | 2.89 | 0.19 |
| 1MS | 4.12 | 2.71 | 0.20 |
| 1MD | 4.33 | 2.88 | 0.17 |
| 3MS | 2.45 | 2.66 | 0.13 |
| 3MD | 2.80 | 3.90 | 0.17 |
| 6MS | 2.38 | 3.36 | 0.14 |
| 6MD | 3.41 | 4.03 | 0.10 |
The nutrients in the samples ranged from (2.38–4.33%), (2.60–4.03%) and (0.10–0.2%) for nitrogen, phosphorous and potassium, respectively. According to How et al. [49], to safeguard surface and groundwater bodies, NPK for biosolids land application must comply with the US EPA Part 503 rule, ranging from 2.0 to 8.0% for nitrogen, 1.5–3.0% for phosphorus, and 0.10–0.2% for potassium. The high nitrogen and phosphorus concentrations in the fresh sludge were mostly due to the inability of the treatment facility to remove all the nitrogen and phosphorus in the fecal waste. This was reduced with age due to environmental factors as they become exposed to oxygen. The reduction measured at the surface with age could be due to the nitrogen and phosphorus reacting with oxygen to produce oxides [50]. More of these nutrients were found in the middle and the bottom because there were mostly fewer chemical reactions to convert the elements to their oxides. The results in Table 5 showed that the sludge samples met the regulatory limits, with only phosphorus exhibiting an increase and this could be due to the inability of the microbes within the sludge to ingest it.
9.1. Risk assessments
9.1.1. Enrichment factor (EF)
As, Cd, Cr, Cu, Ni, Pb, and Zn enrichment factors were 2.13, 0.02, 0.0005, 0.12, 0.0006, 0.0004, 0.16 respectively (Table 6). In the order of Pb < Cr < Ni < Cd < Cu < Zn < As E.F gives an indicator of its presence, but in the permissible limit. As was moderate in the samples, followed by Zn and Cu, with the E.F of Pb, Cr, Ni, and Cd being deficient to minimal enrichment. Prominent heavy metals present in the sewage sludge from industrial sites were Cd, Ni, and Cr, with maximum values of 2.83, 1449.0, and 3918.5 mg/kg, respectively which was higher than the samples from KWTP [51].
9.1.2. Contamination factor (Cf)
The mean contamination factors of the biosolids obtained were 1.93, 0.01, 0.0004, 0.11, 0.0006, 0.0004 and 0.14 for AS, Cd, Cr, Cu, Ni, Pb, and Zn, respectively (Table 7). The level of contamination of the heavy metals investigated increased in the order of Pb < Cr < Ni < Cd < Cu < Zn < As. The ratio of the metals loadings in the sludge was moderate for As, and low for Zn, Cu, Cd, Ni, Cr, and Pb. Nkeshita et al. [52] reported that Cf shows a decreasing trend of Cd > Ni > Zn > Pb > Cu with sludge sample varying from moderately polluted to severely polluted.
Table 7.
Results of Potential Ecological risk assessments.
| Heavy Metals | Mean ± SD | Enrichment Factor (EF) | Contamination Factor (CF) | Potential ecological risk Index (RI) |
||
|---|---|---|---|---|---|---|
| Cif | Eir | RI | ||||
| As | 28.98 ± 12.35 | 2.13 | 1.93 | 1.93 | 19.30 | 135.10 |
| Cd | 0.25 ± 0.06 | 0.02 | 0.01 | 0.01 | 0.60 | 4.20 |
| Cr | 0.74 ± 0.16 | 0.0005 | 0.0004 | 0.0004 | 0.0008 | 0.006 |
| Cu | 5.53 ± 3.26 | 0.12 | 0.11 | 0.11 | 0.55 | 3.85 |
| Ni | 0.11 ± 0.08 | 0.0006 | 0.0006 | 0.0006 | 0.001 | 0.008 |
| Pb | 0.02 ± 0.01 | 0.0004 | 0.0004 | 0.0004 | 0.002 | 0.01 |
| Zn | 50.14 ± 9.27 | 0.16 | 0.14 | 0.14 | 0.14 | 1.00 |
The contamination assessment results obtained from EF, CF, and PL revealed that arsenic in the sludge posed a significant ecological risk to the sludge, with the remaining metals displaying moderate contamination.
10. Pollution index (PI)
The overall PI in the sludge for As, Cd, Cr, Cu, Ni, Pb, and Zn was 0.02, indicating the samples are free from contamination. So, the heavy metals in the sludge cannot cause any negative effect when applied to the soil or for any agricultural practices. The pollution index of heavy metals of the sludge in treatment plants in Beijing assessed by Sheng et al. [53] reported a comprehensive pollution index of heavy metals of 0.798, indicating no contaminated sludge.
10.1. Potential ecological risk assessment index (RI)
The RI shown in Table 7, indicated that As has a high contamination rate due to its ability to easily react with environmental elements. The rest of the metals shows moderate contamination. But contrary to their research, Sheng et al. [53] reported that Cu and Zn's potential ecological risk assessment was higher. The comprehensive risk level of sludge indicated a higher ecological risk.
11. Conclusion
The application of sludge for agricultural practices has proven very beneficial. But its benefit to the soil depends on the quality of the sludge. Therefore, the need to determine the quality of the sludge produced at the Kumasi Wastewater Treatment Plant (KWTP) to ascertain its level of quality to help understand how it can be used as a soil amendment. It can be concluded from the analysis that the sludge produced at KWTP is of good quality and meets international standards with only a high concentration of As, which was even within the acceptable limit to be applied on the soil according to the US EPA Part 503 rule. However, the ecological risk index indicated that the level of arsenic was high in the sludge; hence it could be harmful to the environment over time. Therefore, the application of sludge for agricultural activities must be made in a way to reduce its impact on the environment. Also, the local community must be encouraged to use sludge as a soil amendment in their farming activities and also in their gardens to reduce the impact of chemical fertilizers on human health and the environment.
Author contribution statement
Conceived and designed the experiments; LNAS, PK.
Performed the experiments; JK, RK, AAH.
Analyzed and interpreted the data; LNAS, JK, RK, AAH, PK.
Contributed reagents, materials, analysis tools or data; LNAS, JK, RK, AAH, PK.
Wrote the paper. LNAS, JK, RK, AAH, PK.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Binh N.T. A study of sewage sludge composting, utilization of compost and nitrogen dynamics in plant? Soil System. 2017 [Google Scholar]
- 2.Yang G., Zhang G., Wang H. Current state of sludge production, management, treatment and disposal in China. Water Research. 2015;78:60–73. doi: 10.1016/j.watres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Burton F.L., et al. 2014. Wastewater Engineering: Treatment and Resource Recovery. [Google Scholar]
- 4.Goldstein N. 2020. Available us Biosolids Data. [Google Scholar]
- 5.Collivignarelli M.C., Abbà A., Frattarola A., Carnevale Miino M., Padovani S., Katsoyiannis I., Torretta V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability. 2019;11(21):6015. [Google Scholar]
- 6.Collivignarelli M.C., Abbà A., Benigna I. The reuse of biosolids on agricultural land: Critical issues and perspective. Water Environ. Res. 2020;92(1):11–25. doi: 10.1002/wer.1196. [DOI] [PubMed] [Google Scholar]
- 7.Mininni G., Blanch A.R., Lucena F., Berselli S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. 2015;22:7361–7374. doi: 10.1007/s11356-014-3132-0. [DOI] [PubMed] [Google Scholar]
- 8.Moretti S.M.L., Bertoncini E.I., Abreu-Junior C.H. Composting sewage sludge with green waste from tree pruning. Sci. Agric. 2015;72:432–439. [Google Scholar]
- 9.Wyrwicka A., Urbaniak M. The biochemical response of willow plants (Salix viminalis L.) to the use of sewage sludge from various sizes of wastewater treatment plant. Sci. Total Environ. 2018;615:882–894. doi: 10.1016/j.scitotenv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Odlare M., Lindmark J., Ericsson A., Pell M. Use of organic wastes in agriculture. Energy Proc. 2015;75:2472–2476. [Google Scholar]
- 11.Minari G.D., Rosalen D.L., da Cruz M.C.P., de Melo W.J., Alves L.M.C., Saran L.M. Agricultural management of an Oxisol affects accumulation of heavy metals. Chemosphere. 2017;185:344–350. doi: 10.1016/j.chemosphere.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Romanos D., Nemer N., Khairallah Y., Abi Saab M.T. Assessing the quality of sewage sludge as an agricultural soil amendment in Mediterranean habitats. Int. J. Recycl. Org. Waste Agric. 2019;8(1):377–383. [Google Scholar]
- 13.Kilbride C. 2006. Application of Sewage Sludge and Composts. Best Practice Guidance for Land. [Google Scholar]
- 14.Kumar P., Kumar V., Adelodun B., Bedeković D., Kos I., Širić I., Alamri S.A.M., Alrumman S.A., Eid E.M., Abou Fayssal S., et al. Sustainable use of sewage sludge as a casing material for button mushroom (Agaricus bisporus) cultivation: Experimental and prediction modeling studies for uptake of metal elements. J. Fungi. 2022;8(2):112. doi: 10.3390/jof8020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Širić I., AL-Huqail A.A., Kumar P., Goala M., Abou Fayssal S., Adelodun B., Ajibade F.O., Alrumman S.A., Alamri S.A.M., Taher M.A., et al. (2023) Sustainable management of sewage sludge using Dhaincha (Sesbania bispinosa (Jacq.) W.Wight) cultivation: Studies on heavy metal uptake and characterization of fibers. Agronomy. 2023;13:1066. doi: 10.3390/agronomy13041066. [DOI] [Google Scholar]
- 16.AL-Huqail A.A., Kumar P., Abou Fayssal S., Adelodun B., Širić I., Goala M., Choi K.S., Taher M.A., El-Kholy A.S., Eid E.M. Sustainable use of sewage sludge for marigold (Tagetes erecta L.) cultivation: Experimental and predictive modeling studies on heavy metal accumulation. Horticulturae. 2023;9:447. doi: 10.3390/horticulturae9040447. [DOI] [Google Scholar]
- 17.Berendes D.M., Sumner T.A., Brown J.M. Safely managed sanitation for all means fecal sludge management for at least 1.8 billion people in low and middle income countries. Environ. Sci. Technol. 2017;51(5):3074–3083. doi: 10.1021/acs.est.6b06019. [DOI] [PubMed] [Google Scholar]
- 18.Haugan B.E., Mininni G. In: Characterization, Treatment and Use of Sewage Sludge. L’Hermite P., Ott H., editors. Springer; Dordrecht: 1981. Characterization of Sewage Sludges. [Google Scholar]
- 19.Dorling D. The Struggle for Social Sustainability. Policy Press; 2021. World population prospects at the UN: our numbers are not our problem? pp. 129–154. [Google Scholar]
- 20.Cofie O.O., Kranjac-Berisavljevic G., Drechsel P. The use of human waste for peri-urban agriculture in Northern Ghana. Renew. Agric. Food Syst. 2005;20(2):73–80. [Google Scholar]
- 21.Romanos D., Nemer N., Khairallah Y., Saab M.T.A. Assessing the quality of sewage sludge as an agricultural soil amendment in Mediterranean habitats. Int. J. Recycl. Org. Waste Agric. 2019;8:S377–S383. [Google Scholar]
- 22.Jiménez B., Drechsel P., Koné D., Bahri A. Wastewater Irrigation and Health; 2009. pp. 29–54. (Wastewater, sludge and excreta use in developing countries: an overview). [Google Scholar]
- 23.Abdel-Shafy H.I., Mansour M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egyptian Journal of Petroleum. 2018;27(4):1275–1290. [Google Scholar]
- 24.Ghana Statistical Service . 2014. Population and Housing Census Report (2010) Ghana. [Google Scholar]
- 25.Owusu-Nimo F., Oduro-Kwarteng S., Essandoh Hellen., Wayo Farida., Shamudeen Mohammed. Characteristics and management of landfill solid waste in Kumasi, Ghana. Sci. African. 2019;3 [Google Scholar]
- 26.Muter O., Dubova L., Kassien O., Cakane J., Alsina I. IntechOpen; 2022. Application of the Sewage Sludge in Agriculture: Soil Fertility, Technoeconomic, and Life-Cycle Assessment. [Google Scholar]
- 27.Jatav S.S., Singh S.K., Patra A., Jatav H.S., Mohapatra K.K., Singh P. Characterization of Sewage Sludge for Quality Assessment and Its Safe Utilization in Agriculture. Curr. J. Appl. Sci. Technol. 2021;40(25):28–35. [Google Scholar]
- 28.Gardner W.C., Anne N.M., Broersma K., Chanasyk D.S., Jobson A.M. Influence of biosolids and fertilizer amendments on element concentrations and revegetation of copper mine tailings. Can. J. Soil Sci. 2012;92(1):89–102. doi: 10.4141/cjss2011-005. [DOI] [Google Scholar]
- 29.Bin M., Yu L., Xiaoyuan Li., Zijun F., Lin Z., Zuhua H. A combined approach to evaluate total phosphorus/inorganic phosphate levels in plants. Star Protocols. 2022;3(3) doi: 10.1016/j.xpro.2022.101456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GOV N.C. Fecal Sludge MPN Calculation Guidance. 2019 https://files.nc.gov/ncdeq/Water Quality/Chemistry,Lab/Certification/Policies/Fecal_Sludge_MPN_Calculation_Guidance_Policy_FINAL.pdf [Google Scholar]
- 31.Osman E. In: Microbiological Analysis of Foods and Food Processing Environments. Erkmen Osman., editor. Academic Press; 2022. Practice 4 - Most probable number technique; pp. 31–37. [Google Scholar]
- 32.Hicks T.D., Kuns C.M., Raman C., Bates Z.T., Nagarajan S. Simplified method for the determination of total kjeldahl nitrogen in wastewater. Environments. 2022;9:55. doi: 10.3390/environments9050055. [DOI] [Google Scholar]
- 33.Muñoz-Huerta R.F., Guevara-Gonzalez R.G., Contreras-Medina L.M., Torres-Pacheco I., Prado-Olivarez J., Ocampo-Velazquez R.V. A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances. Sensors (Basel) 2013;13(8):10823–10843. doi: 10.3390/s130810823. 16. PMID: 23959242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokashi K., Shetty V., George S., Sibi G. Sodium bicarbonate as inorganic carbon source for higher biomass and lipid production integrated carbon capture in chlorella vulgaris. Achiev. Life Sci. 2016;10(1) doi: 10.1016/j.als.2016.05.011. [DOI] [Google Scholar]
- 35.Souza D.M., Morais P.A.O., Matsushige I., Rosa L.A. Development of alternative methods for determining soil organic matter. Rev Bras Cienc Solo. 2016;40 [Google Scholar]
- 36.Abrahim G.M.S., Parker R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland. New Zealand. Environ. Monit. Assess. 2008;136(1–3):227–238. doi: 10.1007/s10661-007-9678-2. [DOI] [PubMed] [Google Scholar]
- 37.Wiafe S., Yeboah E.A., Boakye E., Ofosu S. Environmental risk assessment of heavy metals contamination in the catchment of small-scale mining enclave in Prestea Huni-Valley District, Ghana. Sustain. Environ. 2022;8:1. [Google Scholar]
- 38.Stein D.J.W., L . 1995. A Guide to the Biosolids Risk Assesments for the EPA Part 503 Rule. [Google Scholar]
- 39.Zhang X., Wang X., Wang D. Immobilization of heavy metals in sewage sludge during land application process in China: A review. Sustainability. 2017;9:1–19. [Google Scholar]
- 40.Ahmed I., Ofori-Amanfo D., Awuah E., Cobbold F. A Comprehensive Study on the Physicochemical Characteristics of Faecal Sludge in Greater Accra Region and Analysis of Its Potential Use as Feedstock for Green Energy. J. Renew. Energy. 2019;2019 doi: 10.1155/2019/8696058. [DOI] [Google Scholar]
- 41.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Nahhal I.Y., Al-Najar H., El-Nahhal Y. Physicochemical Properties of Sewage Sludge from Gaza. Int. J. Geosci. 2014;5(6) [Google Scholar]
- 43.Hitha S., Vinaya C., Linu M. In: Controlled Release Fertilizers for Sustainable Agriculture. Lewu F.B., Volova Tatiana, Thomas Sabu, Rakhimol K.R., editors. Academic Press; 2021. Organic fertilizers as a route to controlled release of nutrients. [Google Scholar]
- 44.https://www.epa.gov/biosolids/plain-english-guide-epa-part-503-biosolids-rule
- 45.Charlton A., Sakrabani R., Tyrrel S., Rivas M., Mcgrath S.P., Crooks B., Cooper P., Campbell C.D. Long-term impact of sewage sludge application on soil microbial biomass : An evaluation using meta-analysis. Environ. Pollut. 2016:1–15. doi: 10.1016/j.envpol.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 46.de Amorim Júnior S.S., de Souza Pereira M.A., Morishigue M., da Costa R.B., de Oliveira Guilherme D., Magalhães Filho F.J.C. Circular economy in the biosolids management by nexus approach: a view to enhancing safe nutrient recycling—pathogens, metals, and emerging organic pollutants concern. Sustainability. 2022;14 doi: 10.3390/su142214693. [DOI] [Google Scholar]
- 47.Kokina K., Mezule L., Gruskevica K., Neilands R., Golovko K., Juhna T. Impact of rapid pH changes on activated sludge process. Appl. Sci. 2022;12:5754. [Google Scholar]
- 48.Singh V., Phuleria H.C., Chandel M.K. Unlocking the nutrient value of sewage sludge. Water Environ. J. 2022;36(2):321–331. [Google Scholar]
- 49.How S.W., Ting C.X., Yap J.Y., et al. Effect of carbon-to-nitrogen ratio on high-rate nitrate removal in an upflow sludge blanket reactor for polluted raw water pre-treatment application. Sustain. Environ. Res. 2021;31:16. [Google Scholar]
- 50.Edwards J., Othman M., Crossin E., Burn S. Anaerobic co-digestion of municipal food waste and sewage sludge: A comparative life cycle assessment in the context of a waste service provision. Bioresour. Technol. 2017;223:237–249. doi: 10.1016/j.biortech.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 51.Sundha P., Basak N., Rai A.K., Chandra P., Bedwal S., Yadav G., Yadav R.K., Sharma P.C. Characterization and ecotoxicological risk assessment of sewage sludge from industrial and non-industrial cities. Environ Sci Pollut Res. 2022 doi: 10.1007/s11356-022-21648-2. [DOI] [PubMed] [Google Scholar]
- 52.Nkeshita F., Gbadewole O., Adekunle A., Alayak F. Heavy metal pollution and ecological risk assessment of sludge deposits from selected wastewater treatment plants in Lagos, Nigeria. J. Sci. Technol. Educ. 2021;9(2):293–299. [Google Scholar]
- 53.Sheng Q., Zhang H.F., Wang C.Y., Lang C., Guo Y., Dong J.Z., Gao K., Song X.M., Yang Y.S. IOP Conf. Ser.: Earth Environ. Sci. 2018;186 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.