Abstract
In this study, a proper and reliable fluorometric method is introduced for screening acetylcholinesterase (AChE) and its inhibitors, using carbon quantum dots (CQDs) as the signal reporter. Pure, S-doped, and P-doped CQDs, were synthesized and their recoverable fluorescence quenching properties were observed, when exposed to Hg2+, Cu2+, and Fe3+ quenching ions, respectively. The study on the recovery of their emission showed that after the introduction of another guest substance with a stronger affinity to the quenching ions, their fluorescence is restored. The Design Expert software was employed to compare the performance of the three CQDs, as fluorescent probes, based on their quenching efficiency and the percentage of their emission recovery in the presence of AChE and acetylthiocholine (ATCh). Based on the statistical analysis, among the studied CQDs, S-doped CQD was the most suitable candidate for sensor designing. The detection mechanism for the proposed S-doped CQD-based sensor is as follows: The strong binding of Cu2+ ions to carboxyl groups of S-doped CQD quenches the fluorescence signal. Then, hydrolysis of ATCh into thiocholine (TCh) in the presence of AChE causes fluorescence recovery, due to the stronger affinity of Cu2+ to the TCh, rather than the CQD. Finally, in the presence of malathion and chlorpyrifos inhibitors, AChE loses its ability to hydrolyze ATCh to TCh, so the fluorescence emission remains quenched. Based on the proposed detection technique, the designed sensor showed detection limits of 1.70 ppb and 1.50 ppb for malathion and chlorpyrifos, respectively.
Keywords: Optical sensor, Carbon quantum dots, Acetylcholinesterase, Organophosphorus pesticides, Fluorometric assay, Biosensor
Graphical abstract
Fluorescent S-doped carbon quantum dots (CQD) are hydrothermally synthesized from trisodium citrate dihydrate and sodium thiosulfate precursors. In presence of copper ions (Cu2+), CQDs lose their fluorescence. By adding acetylcholinesterase (AChE) and acetylthiocholine (ATCh) to the solution, AChE hydrolyzes ATCh, releasing thiocholine (TCh). The binding of thiol groups to Cu2+ separates them from CQDs, causing the CQD's fluorescence recovery. In the presence of organophosphorus pesticides (OP) as inhibitors, AChE doesn't hydrolyze ATCh and, the CQD fluorescent signal remains off.
Highlights
-
•
Organophosphate (OP) pesticides are considered highly toxic due to AChE inhibition.
-
•
Monitoring of OP concentrations in the environment has become of utmost importance.
-
•
A S-doped CQD/Cu2+/AChE/ATCh platform has been developed to detect the target OP.
-
•
Designed sensor detect malathion and chlorpyrifos with LOD of 1.70 ppb and 1.50 ppb.
-
•
It offers a short processing time (10 min) and making a fast-response biosensor.
1. Introduction
Acetylcholinesterase (AChE) is a vital enzyme in the central nervous system that catalyzes the hydrolysis reaction of the neurotransmitter acetylcholine (ACh) to choline and acetic acid [1], thereby helping to maintain ACh levels [2,3]. Moreover, AChE is present in muscle and peripheral tissues, motor, sensory, cholinergic, and non-cholinergic fibers [[4], [5], [6]]. Its main biological role is to terminate the transmission of neural messages at cholinergic synapses by ACh hydrolysis [7,8]. Inhibition of AChE leads to the accumulation of ACh, which leads to the permanent saturation of its receptors and the creation of a cholinergic crisis throughout the central nervous system. The overstimulation of the cholinergic system can cause many symptoms such as paralysis, seizures, and respiratory failure, which may finally lead to death [9]. Organophosphorus pesticides (OPs) are potent irreversible AChE inhibitors that covalently bind to serine residues at the AChE's active site, thereby inhibiting AChE's activity [[10], [11], [12]]. OP pesticides are classified as extremely dangerous compounds, with a broad spectrum of toxicity types, by the World Health Organization (WHO). Among the various OPs, malathion is a common insecticide that is used due to its low toxicity [13], rapid degradation, and excellent efficacy [14]. It is widely used to increase the storage time of grains by controlling or eradicating diseases caused by arthropods [15], foreign animal parasites, and domestic insects [15,16]. The mechanism of malathion toxicity is through AChE inhibition [17,18]. To eliminate pests, about 1% of the introduced toxic malathion is used [19] and the excess remains there [20], as a pollutant on the soil and water resources, making serious damage to the human nervous, respiratory and cardiovascular systems [14]. It may also develop some other symptoms such as tingling sensation [21], headache [22], dizziness [23], worsening of asthma [24], paralysis [25], or even death [26]. Moreover, the formation of free radicals is one of the main toxicity phenomena observed from OPs [27]. Chlorpyrifos, as another OP insecticide used against agricultural pests, attacks the hydroxyl serine group at the active site of AChE, and inhibits it irreversibly [28,29]. This reaction leads to the accumulation of the neurotransmitter ACh and ultimately to neurotoxicity [30]. These pesticides are absorbed rapidly through the respiratory, gastrointestinal, ocular, and dermal pathways, with absorption after inhalation as the fastest way. Their absorption through the skin is, however, slower, leading to severe poisoning over long-term exposure [31]. Due to the mentioned issues, including the high carcinogenic and genetic toxicity potential, control of the excess OPs on plants, crops, soil, and water is essential and accurate monitoring of their concentrations in the environment and biological sample has become of utmost importance [20].
Hence, various investigations employing a variety of techniques, including colorimetric, electrochemical, interferometric, chemiluminometric, and fluorometric methods have been performed to measure the activity of AChE and its inhibitors, [[32], [33], [34], [35], [36]]. However, the developed techniques are generally lack enough sensitivity. For example, the Elman reagent that used currently in the colorimetric measurement leading to false positive results [33]. In order to increase the sensitivity and decrease the limit of detection, dye molecules were employed in chemiluminometric or fluorometric approaches [[37], [38], [39], [40], [41]]. However, dyes have their own inherent disadvantages, including rapid bleaching, insufficient diffusion intensity, and instability. In recent years, several new fluorescent materials, such as noble metal nanoparticles [[42], [43], [44], [45], [46]], mineral quantum dots [47,48], and fluorescent conjugated polymers or proteins [[49], [50], [51]] have been used to measure the activity of AChE inhibitors. Although these fluorescent materials have properties such as high fluorescence quantum efficiency, strong absorption, and emissions, as well as good performance for measuring AChE activity, there is still high demand for simple, low-cost, unlabeled, and sensitive assays to detect AChE and its inhibitors in biological samples.
Carbon quantum dots (CQDs), as a novel type of carbon-based nanomaterials, have also attracted significant attention, due to their distinctive optical properties and excellent performance in photovoltaic, photocatalytic, and bioimaging applications [[52], [53], [54]]. CQDs have exhibited promising properties such as stable light emission, high quantum efficiency, good light stability, easy modulation, low toxicity, and excellent biocompatibility [55], which makes them excellent fluorophores in fabricating novel fluorescent chemo/biosensors that are, suitable for in vitro and in vivo applications [[56], [57], [58]]. Recent studies have shown that CQDs can be successfully used to create fluorescent assays to evaluate the activity of several enzymes such as protein kinase [59], hyaluronidase [60], alkaline phosphatase [53,61], and AChE [37]. However, the developed techniques are generally time-consuming and showed a long processing time. Based on the current state of knowledge, this study aimed to develop a convenient and highly sensitive fluorescent method for screening AChE and its organophosphate inhibitors of malathion and chlorpyrifos, using CQDs.
2. Experimental
2.1. Materials and chemicals
Citric acid monohydrate, sodium hydroxide, phosphoric acid, copper sulfate (CuSo4), iron chloride (FeCl3), mercury chloride (HgCl2), trisodium citrate dihydrate, sodium thiosulfate, acetylcholinesterase and acetylthiocholine, bovine serum albumin (BSA), alkaline phosphatase (ALP), DNA, and sodium chloride (NaCl) were purchased from Merck, Germany and Sigma-Aldrich, Netherlands, and used without further purification.
2.2. Synthesis of CQDs
2.2.1. Synthesis of pure CQD
An aliquot amount of 0.2 g citric acid monohydrate was dissolved in 20 mL of distilled water and then, the solution was transferred to a hydrothermal reactor, being heated in an oven for 5 h at 180 °C. After cooling down to room temperature, 2.0 M sodium hydroxide was used to neutralize the solution. The synthesized pure CQDs were kept at 4 °C before freeze-drying.
2.2.2. Synthesis of S-doped CQD
1.2 g of trisodium citrate dihydrate and 2.3 g of sodium thiosulfate were dissolved in 50 mL distilled water and the solution was transferred to a hydrothermal reactor for synthesis of S-doped CQDS at 200 °C for 6 h. At the end of the chemical reaction, the hydrothermal reactor was left to cool down to 25 °C [62]. The synthesized S-doped CQDs were kept at 4 °C before freeze-drying.
2.2.3. Synthesis of P-doped CQD
For P-doped CQD synthesis, 1.5 g of sucrose was dissolved in 30 mL of distilled water and 2 mL of 1.0 M phosphoric acid, under stirring to obtain a clear solution. Afterward, the solution was transferred to a hydrothermal reactor and heated at 200 °C for 5 h. Thereafter it was left to cool down to room temperature and then centrifuged at 10 k rpm for 30 min to separate large insoluble particles. Finally, pH adjustment was done with 1.0 M sodium hydroxide [63]. The synthesized P-doped CQDs were kept at 4 °C before freeze-drying.
2.3. Characterization of the CQDs
The particle size and its distribution were determined using transmission electron microscopy (TEM, JEOL 2200FS, Tokyo, Japan) and dynamic light scattering (DLS, Nanoparticle Analyzer HORIBA SZ-100, Kyoto, Japan). The zeta potential was also measured in three replications, using the later instrument. The photoluminescence (PL) and x-ray photoelectron spectroscopy (XPS) measurements were performed using spectrophotometer (PerkinElmer LS45, Massachusetts, USA) and (XPS, PHI 5000 Versaprobe, Physical Electronics, USA), respectively. An x-ray powder diffraction (XRD, Rigaku Ultima IV Tokyo, Japan) was used to study of crystal structures and crystalline interlayer distances, and the functional groups of each CQD were obtained using Thermo Nicolet, NEXUS 470 (Illinois, USA) Fourier Transform Infrared (FTIR) spectroscopy.
2.4. Experimental design
Design Expert software (version 7.0.0) was used over the central composite design (CCD) algorithm to compare the intensity variation of CQD's emission in terms of maximum emission and quenching rates, under AChE and quenching ion treatments. For that, two quantitative factors; namely, the interaction time duration (A, in min) and the volume ratio of CQD to AChE enzyme (CQD/En ratio) (B, in %), and one qualitative factor, i.e. the CQD type (C), were considered. The influence of the interactions on CQD performance was monitored using the enzyme-linked immunosorbent assay (ELISA, BioTek Cytation 5, USA) as a response (Table 1).
Table 1.
Factors together with theis notations and levels for the central composite design.
| Factor names | -α* | −1 | 0 | +1 | +αa | |
|---|---|---|---|---|---|---|
| A | Interaction time (min) | 0.3 | 3 | 9.5 | 16 | 18.7 |
| B | CQD/En ratio (%) | 1.5 | 4 | 10 | 16 | 18.5 |
| C | CQD Type | Pure | S-doped | P-doped | ||
α = 0.2.
A total of 33 experiments, containing three center points and eight non-center points (overall 11 experiments) for each CQD was performed as presented in Table 2. The concentration of stock solution for AChE and ATCh in each excrement was 100 U/μL and 0.5 mM, respectively. The pure, S-doped, and P-doped CQD solutions had initial concentrations of 17.5 mg/mL, 11 mg/mL, and 2.5 mg/mL, respectively. The final concentrations were adjusted according to Table 2, using phosphate-buffered saline (PBS) for pure, S-doped, and P-doped CQDs and excited at 360 nm wavelength. Thereafter, the CQD's maximum emissions wavelengths were monitored to be at 446, 436, and 417 nm in the cases of pure, S-doped, and P-doped, respectively. In the next step, the quenching percentage was obtained for each CQD, using aliquot amounts of identified quenching ions. Finally, 50 μL of AChE and 1.5 μL of ATCh were added and after the appropriate designed time, the emission intensity variation was monitored in each CQD. The effect of these treatment steps was studied based on two responses of emission and quenching percentages. Since both responses had equal importance, the desirability function (Df) was defined and calculated according to (Eq. (1)). In this way, the values of both responses are evaluated equally and simultaneously. In this equation, Q is the percentage of quenching, and I is the percentage of CQD emission intensity.
| (1) |
Table 2.
Designed experiments to study the influence of CQD on the detection of AChE and its inhibitors.
| Std* | Run order | Time (min) | CQD/En ratio (%) | CQD type | Df %** |
|---|---|---|---|---|---|
| 14 | 1 | 3 | 16 | S-doped | 34.01884 |
| 8 | 2 | 9.5 | 18.5 | Pure | 29.73972 |
| 15 | 3 | 16 | 16 | S-doped | 29.04908 |
| 24 | 4 | 16 | 4 | P-doped | 27.19716 |
| 10 | 5 | 9.5 | 10 | Pure | 32.89456 |
| 27 | 6 | 0.3 | 10 | P-doped | 31.78781 |
| 6 | 7 | 18.7 | 10 | Pure | 27.96525 |
| 29 | 8 | 9.5 | 1.5 | P-doped | 29.45529 |
| 25 | 9 | 3 | 16 | P-doped | 31.10656 |
| 12 | 10 | 3 | 4 | S-doped | 33.52306 |
| 19 | 11 | 9.5 | 18.5 | S-doped | 31.17059 |
| 1 | 12 | 3 | 4 | Pure | 37.05767 |
| 18 | 13 | 9.5 | 1.5 | S-doped | 32.66461 |
| 26 | 14 | 16 | 16 | P-doped | 26.05196 |
| 9 | 15 | 9.5 | 10 | Pure | 29.05567 |
| 28 | 16 | 18.7 | 10 | P-doped | 25.49794 |
| 3 | 17 | 3 | 16 | Pure | 30.24026 |
| 17 | 18 | 18.7 | 10 | S-doped | 31.26231 |
| 22 | 19 | 9.5 | 10 | S-doped | 29.20168 |
| 20 | 20 | 9.5 | 10 | S-doped | 32.02089 |
| 33 | 21 | 9.5 | 10 | P-doped | 27.97358 |
| 30 | 22 | 9.5 | 18.5 | P-doped | 25.51604 |
| 16 | 23 | 0.3 | 10 | S-doped | 33.58546 |
| 31 | 24 | 9.5 | 10 | P-doped | 32.56716 |
| 21 | 25 | 9.5 | 10 | S-doped | 31.25895 |
| 5 | 26 | 0.3 | 10 | Pure | 33.61139 |
| 7 | 27 | 9.5 | 1.5 | Pure | 35.37244 |
| 23 | 28 | 3 | 4 | P-doped | 28.97916 |
| 4 | 29 | 16 | 16 | Pure | 29.18125 |
| 32 | 30 | 9.5 | 10 | P-doped | 30.17559 |
| 2 | 31 | 16 | 4 | Pure | 29.4359 |
| 13 | 32 | 16 | 4 | S-doped | 31.98053 |
| 11 | 33 | 9.5 | 10 | Pure | 30.63094 |
Std: Standard deviation **Df: Desirability function.
2.5. Detection of AChE and OP pesticides
The results of fluorescence emission and quenching of CQDs were recorded. According to the results, the experiment was selected to be continued using the S-doped CQD as the fluorescent probe for identifying malathion and chlorpyrifos OPs. According to the regulations of the WHO, the maximum acceptable and permissible amount of malathion and chlorpyrifos on the environmental resources are 50 ppb and 10 ppb, respectively. However, much lower concentrations of 0.000, 0.005, 0.030, 0.050, 0.090, 0.150, 0.220, 0.300 ppb were selected for malathion and 0.000, 0.002, 0.020, 0.070, 0.100, 0.150, 0.200, 0.250 ppb for chlorpyrifos. The experiments were performed in three replications for both OPs, giving the sensing system 30 min for the interactions of the components. Limit of detection (LOD) is one of the results that obtained from the calibration curves using following equation:
| (2) |
where Sb is the standard deviation for blank and m is the slope of calibration curve.
3. Results and discussions
3.1. Characterization of CQDs
3.1.1. Characterization of Pure CQDs
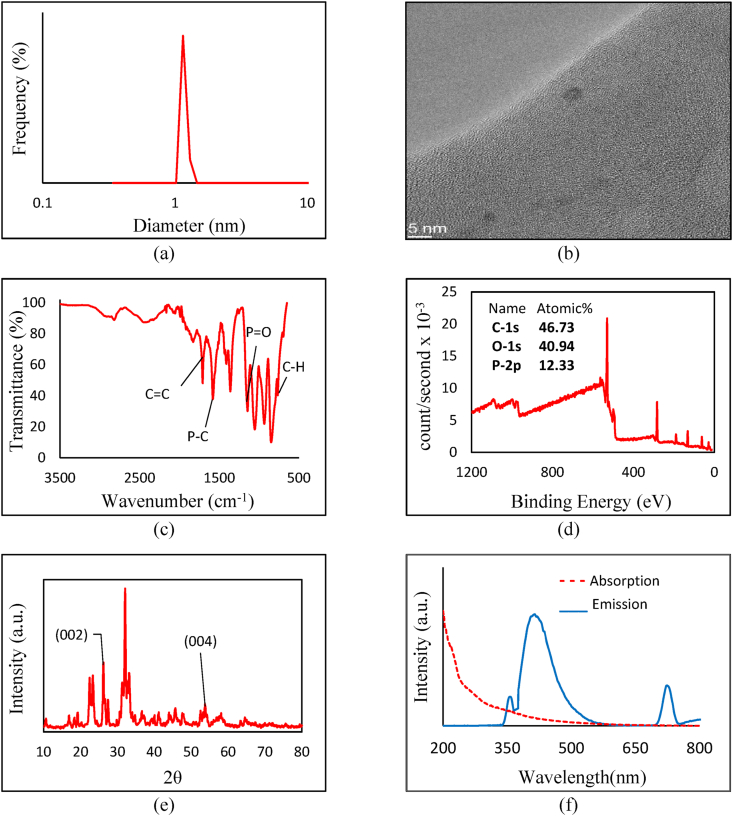
The average particle size of pure CQDs was obtained using TEM and the zeta potential using DLS instrumentations. The results show a relatively uniform distribution of particles of 4.9 ± 2.7 nm with a surface charge of −19.1 ± 2.3 mV (Fig. 1a and b). Based on the XPS and FTIR results, the high negative charge is related to the negatively charged C–O groups. FTIR spectrum reveals the stretching vibrations of C–O (1310 and 1250 cm−1), aromatic C C bond's stretching, and bending frequencies (1560 and 842 cm−1) and C–H bending (1377 cm−1) (Fig. 1c). The XPS study data on the pure CQD composition shows a major peak with 50.9% of atomic percentage at around 283.30 which corresponds to C1s (C C aromatic) and another one at 530.69 eV, showing 49.1% of O1s (C–O) (Fig. 1d). XRD results (Fig. 1e) show that the crystal plates (002) are detectable at angles of 2θ between 20 and 26° with 3.14 nm d-spacing between the crystal plates, as observed in TEM image. The pure CQDs have a maximum PL absorption at 365 nm and a visible emission at 446 nm (Fig. 1f).
Fig. 1.
Characterization of Pure CQD: (a) DLS, (b) TEM, (c) FTIR, (d) XPS, (e) XRD, and (f) PL absorption/emission diagrams.
3.1.2. Characterization of S-doped CQDs
According to the TEM, DLS, and Zeta potential results, the average particle size of S-doped CQDs is 1.30 ± 0.6 nm (Fig. 2a and b), and the surface charge is −2.6 ± 1.1 mV. In the case of S-doped CQDs, a rather inhomogeneous distribution of size is observed. FTIR spectra were used to identify the functional groups of the S-doped CQD. As shown in Fig. 2c, the O–S bond of organic sulfate is significant between 1370 and 1420 cm−1 and C–O bending 1150-1085 cm−1. The XPS spectrum (Fig. 2d) shows a relatively 19.3% of sulfur, which is relatively high. The C1s spectrum shows that the C C sp2 bonds, covering 79.4% of the spectrum, are the most dominant bindings, observed at 283.55 eV. The S bonds are revealed in the high-resolution S2p XPS results, as the S O bond at the binding energy of 168.89 eV and the C–S–C bond at 162.68 eV. The XRD (Fig. 2e) shows the (002) crystalline plate. The (102) graphitic carbon crystalline phases are remarkable between 40 and 50°, and (004) is placed between 50 and 55°. The distance between the crystal plates is equal to 3.18 nm in S-doped CQDs. The optical properties of water-dispersed S-doped CQDs are shown in Fig. 2f. Maximum absorption is observed at 356 nm and two PL emission wavelengths are recorded at 436 and 732 nm.
Fig. 2.
(a) DLS, (b) TEM, (c) FTIR, (d) XPS, (e) XRD, and (f) PL absorption/emission of S-doped CQD.
3.1.3. Characterization of P-doped CQDs
As shown in Fig. 3a and b, a uniform distribution of P-doped CQD particles with an average diameter of 1.15 ± 0.05 nm is observed, with a surface charge of −19.3 ± 2.0 mV. The FTIR spectrum (Fig. 3c), shows an apparent absorption peak at about 1146 cm−1, attributed to P O vibration, and at 1581 cm−1 to P–C vibration. Simultaneously, the C C tensile vibration absorption band appeared at 2119 cm−1. The XPS analysis of P-doped CQDs (Fig. 3d), confirms the existence of 46.7% of carbon and 40.9% of oxygen as the most dominant atoms, accompanied by a high content of phosphorous doping (12.3%) in the structure of the CQD. As also admitted by notable vibrations in the FTIR results, the P heteroatom doping (P–O bond) is observed at 284.74 eV in O1s, and at 132.8 eV in P2p diagrams. The C O bond on the other hand is observed in the C1s, at 286.70 eV, and in the O1s at 529.89 eV binding energies. The C–C bond can be seen as sp2 configuration at 283.48 eV in the C1s diagram. The X-ray powder diffraction (Fig. 3e) shows that in the range between 20 and 30°, the (002) crystalline plate can be seen and the (004) is placed between 50 and 55°. Furthermore, the distance between the crystalline plates is about 2.80 nm in P-doped CQD. A UV–vis absorption at 360 nm and two strong fluorescence emission peaks at 417 and 727 nm are observed, indicating that P-doped CQDs are capable of emitting visible light when excited by UV electromagnetic waves (Fig. 3f).
Fig. 3.
(a) DLS, (b) TEM, (c) FTIR, (d) XPS, (e) XRD, and (f) PL absorption/emission of P-doped CQD.
3.1.4. Fluorescence quenching of the CQDs
Metal ions are known fluorescence quenchers of CQDs, making them candidates for designing various optical sensors based on visual observation of PL turn-off and/or turn-on. Fluorescence quenching of 17.5 mg/mL pure, 11 mg/mL S-doped and 2.5 mg/mL P-doped CQDs was monitored against CuSo4, CaCl2, ZnCl2, HgCl2, FeCl3, PbCl2, FeSo4 ions solutions respectively (Fig. 4a, b and 4c). As a result, the fluorescent emission of pure, S-doped and P-doped CQDs were quenched by 6.6 mM Hg2+ ions, 12 mM Cu2+ ions and 4 mM Fe3+ ions, respectively (Fig. 4). The fluorescence quenching mechanism occurs due to the electron, charge or energy transfer resulting from CQD-metal ions interactions. The mechanism of this interaction is based on the fact that the metal ions introduced to the system, selectively interact with the surface hydroxyl and carboxyl functional groups of CQDs. Due to this interaction, the electronic structure of CQDs changes and the distribution of excitons alters, which subsequently leads to a non-radiative charge or energy transfer through the recombination of the electrons and excitons on the surface areas, and quenching of the fluorescence emission [64].
Fig. 4.
The change of fluorescent emission of (a) pure, (b) S-doped and (c) P-doped CQDs in the presence of various metal ions.
3.2. Study the CQDs and enzyme interaction
In order to study the CQD/enzyme interaction, a CCD-based analysis was performed. So that the Df % of the 33 designed experiments were calculated (Table 2).
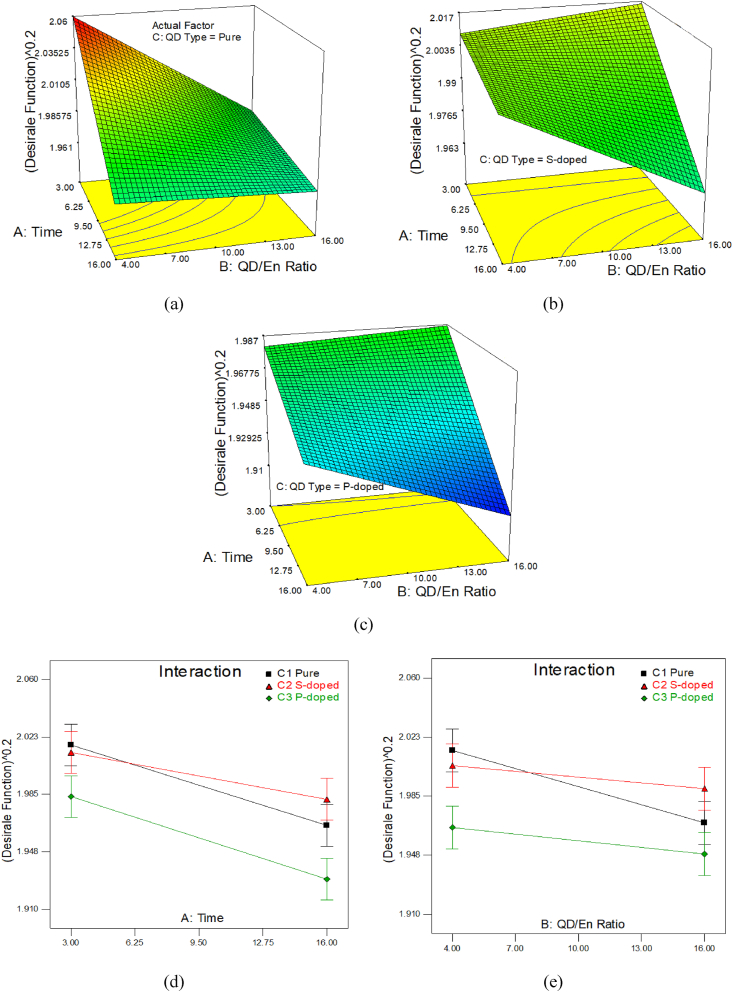
As shown in the data variance analysis table (Table 3), the designed model is valid with a value of 0.0001. Among the individual factors, the time (A) and the type of the CQD (C) are the most effective factors. Among the two-way factors, the simultaneous effect of CQD-type and the CQD/En ratio (BC) are the most influential factors. The value of Lack of Fit, which is an indicator of test error, is insignificant and indicates the absence of any systematic and clear error in this study. In order to understand the synergy and variational influences of the factors on the response, the response surface diagrams based on Df % were traced for each CQD (Fig. 5).
Table 3.
ANOVA for the study of the CQDs interaction Enzyme.
| Source | Sum of Squares | df | Mean Square |
F Value |
p-value Prob > F |
|
|---|---|---|---|---|---|---|
| Model | 0.031419882 | 11 | 0.002856 | 7.170653 | <0.0001 | significant |
| A-Time | 0.012526379 | 1 | 0.012526 | 31.44651 | <0.0001 | |
| B-CQD/En Ratio | 0.003999856 | 1 | 0.004 | 10.04133 | 0.0046 | |
| C-CQD Type | 0.01046129 | 2 | 0.005231 | 13.13113 | 0.0002 | |
| AB | 8.2271E-06 | 1 | 8.23E-06 | 0.020653 | 0.8871 | |
| AC | 0.000682597 | 2 | 0.000341 | 0.856803 | 0.4388 | |
| BC | 0.001222463 | 2 | 0.000611 | 1.534449 | 0.2388 | |
| ABC | 0.00251907 | 2 | 0.00126 | 3.161966 | 0.0630 | |
| Residual | 0.008365125 | 21 | 0.000398 | |||
| Lack of Fit | 0.004616148 | 15 | 0.000308 | 0.492524 | 0.8754 | not significant |
| Pure Error | 0.003748977 | 6 | 0.000625 | |||
| Cor Total | 0.039785007 | 32 | ||||
| R-Squared | 0.8797 | Adj R-Squared | 0.7969 | |||
Fig. 5.
Comparison of influential factors on Df, versus A: time and B: CQD/En ratio for C: CQD-types (a) pure, (b) S-doped, and (c) P-doped; and (d) factor AC (time and CQD type); and (e) factor BC (CQD/AChE ratio and CQD type).
As can be seen in Fig. 5a, in pure CQD, the response value is obtained in a shorter interaction time and a lower percentage of CQD/En ratio. Meanwhile, the response decreases with the increase of interaction time interval and CQD/En ratio. In Fig. 5b and c, similar behavior can be seen in the case of S-doped and P-doped nanoparticles. In both cases, the greatest response is related to the application of a higher percentage of CQD/En ratio, in a short period of time. In this type, as the duration of the interaction increases, the response drops sharply. Whereas reducing the CQD/En ratio in a short time does not have a significant effect on the response. As can be seen in Fig. 5d and e, with the increase of interaction time and the CQD/En ratio, respectively, the Df % decreases in all three CQD types. The reduction in Df % on CQD-pure is much higher than the other types.
3.3. AChE screening based on S-doped CQDs
After examining the Df % for different CQDs two experiments were suggested by Design Expert software as the most influential conditions. The proposed software experiments include the interaction of S-doped in 4% ratio with enzyme for 3 min and the interaction of enzyme and pure CQD at 16% ratio for 3 min. The results demonstrate that S-doped had the highest emission intensity and was quenched in the shortest time in the presence of Cu2+ ions. The proposed experiments were performed as follows: In the first step, the S-doped CQD's PL emission intensity was recorded at 450 nm. Then, Cu2+ was added, so that the carboxyl group of S-doped CQDs bound to Cu2+ ions, and the emission intensity drops dramatically. After that, ATCh was added to the solution. Finally, by adding AChE, ATCh was hydrolyzed to TCh and acetate, forming a Cu-TCh complex. As a result, Cu2+ separates from the carboxyl groups on the surface of the S-doped CQD, ceasing the non-radiative recombination of the electrons and excitons on the surface areas and leading to recovery of the PL emission of the CQD. The strength of copper-thiol complexes, including Cu-TCh complex provides a critical insight into how they might affect Cu chemistry in a CQD based sensors. First, TCh is a mercaptan substance which reacts with Cu2+ to form the Cu-TCh complex by the well-known reaction of copper with mercaptans. Furthermore, the strong affinity between thiol and copper can be influenced by the functional groups present in the larger peptide ligands. Walsh and Ahner reported the stability constants for Cu-thiol complexes, using dissociation constant, as the reciprocal of the stability constant. They demonstrated that the addition of a coordinating thiol, increases the affinity value, as reported in Table 4 for some dicysteinal and tripodal cysteine derivatives [65]. In the S-doped CQD/Cu2+/AChE/ATCh system, due to the stronger affinity of Cu2+ to form the Cu-TCh complex, PL emission recovery is observed. (Fig. 6a). The increase in the emission intensity after adding ATCh was observed to be time-dependent. However, as shown in Figs. 6b and 10 min was suitable for the catalytic hydrolysis of ATCh by AChE, after which the emission intensity reached a stable state.
Table 4.
Stability constants for various copper-binding ligands.
| Copper binding ligand | Thiol complexation | Technique | Stability constant (K′) |
|---|---|---|---|
| GSH | Monothiol | EMF Titration | 13.9 |
| Cystein | Monothiol | PGSK Titration | 11.1 |
| Arg-Cys | Monothiol | PGSK Titration | 11 |
| Gln-Cys | Monothiol | PGSK Titration | 11.4 |
| DTT | Dithiol | BCS Titration | 15.3 |
| Synthetic dicysteinal peptides | Dithiol | BCS Titration | 15.5–16.7 |
| Tripodal-cysteine | Trithiol | BCS Titration | 18.8–19.2 |
| GSH3 | Trithiol | BCS Titration | 18.8 |
Fig. 6.
Variations on PL intensity of CODs (a) in the presence of different compositions, and (b) at various incubation periods.
3.4. AChE inhibitor screening
For further demonstration of the potential applications of this method, the malathion and chlorpyrifos, known as AChE inhibitors, were selected as samples for proof of concept. The emission intensity of 11 mg/mL S-doped CQD was quenched in the presence of 12 mM Cu2+ ions. By addition of ATCh and AChE to the system, a significant fluorescence recovery was achieved. However, when 0.005 ppb malathion and 0.002 ppb chlorpyrifos were added to the sensor system, in presence of ATCh and AChE, no apparent fluorescence increase was induced, which clearly demonstrates the inhibitory effect of malathion and chlorpyrifos on AChE. The emission intensity of the sensor system gradually decreases over the increasing inhibitor's concentrations, which further confirms the correlation between AChE inhibition and the concentration of the inhibitors. However, in the presence of AChE inhibitors, ATCh would no longer hydrolyze to TCh and acetate, which in turn leaves Cu2+ in interaction with S-doped CQD and the PL emission of the CQD would not be recovered. Therefore, the activity of the AChE and its inhibitor can be well reflected by observing the fluorescence intensity change of S-doped CQD. (Fig. 7a and b).
Fig. 7.
Change of fluorescence spectra versus various concentrations of (a) malathion and (b) chlorpyrifos.
3.4.1. Proof of concept
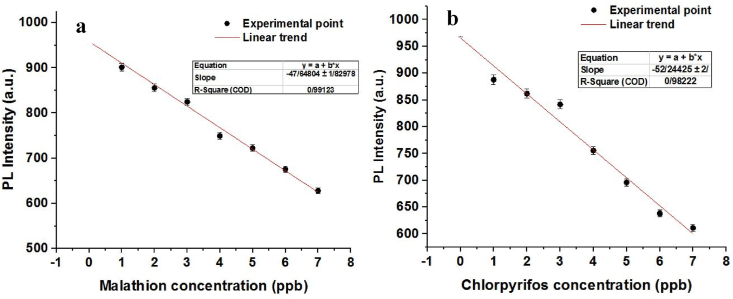
Our results indicate that the designated AChE monitoring system can be used to screen the existence of AChE inhibitors, such as malathion and chlorpyrifos, as provided in Fig. 7a and b, respectively, where various concentrations of the mentioned OPs were introduced to the designed sensing system. The calibration plot derived from AChE inhibitor screening plots is presented in Fig. 8. Linear behavior of designed biosensor through various concentrations of inhibitors indicated the reliable performance of developed platform for detection of malathion and chlorpyrifos. The magnitude of LOD using Eq. (2) for malathion and chlorpyrifos were obtained as 1.70 ppb and 1.50 ppb, respectively (Fig. 8a and b). The proposed biosensor achieved a wide linear dynamic range from 5 to 300 ppb in 10 min analysis time, which demonstrates reliable and significant performance.
Fig. 8.
Calibration plot of CQD designed sensor versus various concentrations of (a) malathion and (b) chlorpyrifos.
3.4.2. Selectivity for OPs
Biosensor selectivity was assessed by examining the S-doped CQD/Cu2 +/ATCh system in the presence of 50 μL AChE, 0.300 ppb malathion, 0.250 ppb chlorpyrifos, 1.000 ppb alkaline phosphatase, 2.500 ppb NaCl, 0.5 μL DNA, and 0.066 ppb BSA. As shown in Fig. 9, emission intensity increased in the presence of AChE, due to the hydrolysis of ATCh to thiocholine and the binding of Cu2+ ions to thiols groups. Moreover, by addition of each mentioned substances, the emission intensity didn't show a significant change. Consequently, the designed biosensor system is specific to detect the AChE inhibitors through the AChE enzyme.
Fig. 9.
Emission intensity of S-doped CQD/Cu2 +/ATCh system with some interfering substances.
Comparison of the proposed biosensor, based on the S-doped CQD/Cu2+/AChE/ATCh system to previous fluorometric assay biosensors, reported for AChE's inhibitors is presented in Table 5. Compared to previous works, this study developed the biosensor that can provide a significantly lower LOD for quantitative analysis of malathion and chlorpyrifos. Furthermore, the designed sensor offers a shorter processing time, making a fast-response biosensor.
Table 5.
Analytical comparison of the performance of the designed sensor.
| Main examine reagent | Activity/Detection limit (as reported) | Time | Type of OP | Reference |
|---|---|---|---|---|
| CQD | 1.70 ppb 1.50 ppb |
10 min | Malathion Chlorpyrifos |
This study |
| CQD | 3 ng/mL | 15 min | Chlorpyrifos | [66] |
| CQD | 0.21 ng/mL 0.46 ng/mL |
25 min | Paraoxon Chlorpyrifos |
[67] |
| CQD | 0.21 ± 0.021 μg/L 0.44 ± 0.069 μg/L 0.32 ± 0.033 μg/L |
120 min | Methyl parathion Chlorpyrifos Trichlorfon |
[68] |
| CQD | 0.05 nM 0.10 nM 0.12 nM 0.13 nM |
20 min | Paraoxon Malathion Methamidophos Carbaryl |
[69] |
| CdTe QD | 1.62 × 10−15 M 75.3 × 10−15 M 0.23 × 10−9 M 10.6 × 10−12 M |
15 min | Paraoxon Dichlorvos Malathion Triazophos |
[1] |
4. Conclusions
In summary, our study has successfully demonstrated a carbon quantum dot-based sensor for the detection of AChE and its inhibitors. The designed sensor took the advantage of the competitive coordination between the affinity of Cu2+ ions to the carboxyl groups on the surface of the carbon quantum dots and the thiocholine. The detection strategy was employed for AChE, by emitting a fluorescence signal in combination with its catalytic hydrolysis ability to ATCh. Upon the addition of malathion and chlorpyrifos, the OPs react with AChE to inhibit its activity, resulting in a very poor fluorescence response. This biosensor highlighted a sensitive, specific and rapid detection of malathion and chlorpyrifos, with the LOD of 1.70 ppb and 1.50 ppb, respectively, that was lower than the previously published investigations. Based on our state of knowledge, the organophosphate malathion and chlorpyrifos have not been previously reported to be detected with this state of achievement in terms of analysis processing time and LOD, which is crucial in environmental assessments. This study provides a mid-stage platform to develop a convenient, low-cost and ultrasensitive fluorometric device using S-doped CQD/Cu2+/AChE/ATCh system for detection of target OP pesticides residues in agricultural products.
Author contribution statement
Niloofar Mahmoudi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Fataneh Fatemi, Moones Rahmandost: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials; analysis tools or data; Wrote the paper.
Fateme Mirzajani: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Seyed Omid Ranaei Siadat: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data associated with this study has been deposited at Social Science Research Network (SSRN)https://ssrn.com/abstract%20=%204401576 https://doi.org/10.2139/ssrn.4401576.
Additional information
No additional information is available for this paper.
Ethical approval
This article does not contain any studies with human or animal subjects.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial support from research council of Shahid Beheshti University is gratefully acknowledged. This article is presented the results of Ms. Niloofar Mahmoudi MSc research and educations.
Contributor Information
Fataneh Fatemi, Email: f_fatemi@sbu.ac.ir.
Moones Rahmandoust, Email: m_rahmandoust@sbu.ac.ir.
References
- 1.Korram J., Dewangan L., Karbhal I., Nagwanshi R., Vaishanav S.K., Ghosh K.K., Satnami M.L. CdTe QD-based inhibition and reactivation assay of acetylcholinesterase for the detection of organophosphorus pesticides. RSC Adv. 2020;10:24190–24202. doi: 10.1039/d0ra03055d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X., Cen Y., Xu G., Wei F., Shi M., Hu Q. A ratiometric fluorescence probe based on carbon dots for discriminative and highly sensitive detection of acetylcholinesterase and butyrylcholinesterase in human whole blood. Biosens. Bioelectron. 2019;131:232–236. doi: 10.1016/j.bios.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Xuereb J.H., Perry E.K., Candy J.M., Bonham J.R., Perry R.H., Marshall E. Parameters of cholinergic neurotransmission in the thalamus in Parkinson's disease and Alzheimer's disease. J. Neurol. Sci. 1990;99:185–197. doi: 10.1016/0022-510X(90)90155-G. [DOI] [PubMed] [Google Scholar]
- 4.Colovic M.B., Krstic D.Z., Lazarevic-Pasti T.D., Bondzic A.M., Vasic V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F.M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993;41:31–91. doi: 10.1016/0301-0082(93)90040-Y. [DOI] [PubMed] [Google Scholar]
- 6.Koelle G.B. The histochemical localization of cholinesterases in the central nervous system of the rat. J. Comp. Neurol. 1954;100:211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- 7.Daniel M.Q. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem. Rev. 1987;87:955–979. [Google Scholar]
- 8.Taylor Palmer, Radii Zoran. The CHOLINESTERASES: from genes to proteins. J. Biol. Chem. 1991;266:4025–4028. doi: 10.1016/s0021-9258(20)64277-6. [DOI] [PubMed] [Google Scholar]
- 9.Mercey G., Verdelet T., Renou J., Kliachyna M., Baati R., Nachon F., Jean L., Renard P.Y. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- 10.Vinotha Alex A., Mukherjee A. Review of recent developments (2018–2020) on acetylcholinesterase inhibition based biosensors for organophosphorus pesticides detection. Microchem. J. 2021;161 doi: 10.1016/j.microc.2020.105779. [DOI] [Google Scholar]
- 11.Dvir H., Silman I., Harel M., Rosenberry T.L., Sussman J.L. Acetylcholinesterase: from 3D structure to function. Chem. Biol. Interact. 2010;187:10–22. doi: 10.1016/j.cbi.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suo Z., Liu X., Hou X., Liu Y., Lu J., Xing F., Chen Y., Feng L. Ratiometric assays for acetylcholinesterase activity and organo-phosphorous pesticide based on superior carbon quantum dots and BLGF-protected gold nanoclusters FRET process. ChemistrySelect. 2020;5:9254–9260. doi: 10.1002/slct.202002042. [DOI] [Google Scholar]
- 13.Badr A.M. Organophosphate toxicity: updates of malathion potential toxic effects in mammals and potential treatments. Environ. Sci. Pollut. Res. 2020;27:26036–26057. doi: 10.1007/s11356-020-08937-4. [DOI] [PubMed] [Google Scholar]
- 14.Dan X., Ruiyi L., Qinsheng W., Yongqiang Y., Haiyan Z., Zaijun L. A NiAg-graphene quantum dot-graphene hybrid with high oxidase-like catalytic activity for sensitive colorimetric detection of malathion. New J. Chem. 2021;45:7129–7137. doi: 10.1039/d1nj00621e. [DOI] [Google Scholar]
- 15.Coban F.K., Ince S., Kucukkurt I., Demirel H.H., Hazman O. Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem. Toxicol. 2015;38:391–399. doi: 10.3109/01480545.2014.974109. [DOI] [PubMed] [Google Scholar]
- 16.Suresh Babu N., Malik J.K., Rao G.S., Aggarwal M., Ranganathan V. Effects of subchronic malathion exposure on the pharmacokinetic disposition of pefloxacin. Environ. Toxicol. Pharmacol. 2006;22:167–171. doi: 10.1016/j.etap.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz-Delgado J.B., Funes V., Sarasquete C. The organophosphate pesticide -OP- malathion inducing thyroidal disruptions and failures in the metamorphosis of the Senegalese sole, Solea senegalensis. BMC Vet. Res. 2019;15:1–21. doi: 10.1186/s12917-019-1786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLuca T.H., Gao S. Use of biochar in organic farming. Org. Farming. 2019:25–49. doi: 10.1007/978-3-030-04657-6_3. [DOI] [Google Scholar]
- 19.Capoferri D., Della Pelle F., Del Carlo M., Compagnone D. Affinity sensing strategies for the detection of pesticides in food. Foods. 2018:7. doi: 10.3390/foods7090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Hong P.T., Jang C.H. Sensitive and label-free liquid crystal-based optical sensor for the detection of malathion. Anal. Biochem. 2020;593 doi: 10.1016/j.ab.2020.113589. [DOI] [PubMed] [Google Scholar]
- 21.Igbedioh S.O. Effects of agricultural pesticides on humans, animals, and higher plants in developing countries. Arch. Environ. Health. 1991;46:218–224. doi: 10.1080/00039896.1991.9937452. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Salam O., Sleem A., Youness E., Morsy F. Preventive effects of cannabis on neurotoxic and hepatotoxic activities of malathion in rat. Asian Pac. J. Trop. Med. 2018;11:272–279. doi: 10.4103/1995-7645.231467. [DOI] [Google Scholar]
- 23.Kumar S., Sharma J.G. Effect of malathion on seed germination and photosynthetic pigments in wheat (Triticum aestivum L.) Asian J. Appl. Sci. Technol. 2017;1:158–167. [Google Scholar]
- 24.Ki-Hyun Kim S.A.J. 2017. Ehsanul Kabir, Exposure to Pesticides and the Associated Human Health Effects. [DOI] [PubMed] [Google Scholar]
- 25.Tchounwou P.B., Patlolla A.K., Yedjou C.G., Moore P.D. Environmental exposure and Health effects associated with malathion toxicity. Toxic. Hazard Agrochem. 2015 doi: 10.5772/60911. [DOI] [Google Scholar]
- 26.The Structural and Biochemical Impact of Monomerizing Human Acetylcholinesterase, (n.d.). [DOI] [PMC free article] [PubMed]
- 27.Wu H., Zhang R., Liu J., Guo Y., Ma E. Effects of malathion and chlorpyrifos on acetylcholinesterase and antioxidant defense system in Oxya chinensis (Thunberg) (Orthoptera: acrididae) Chemosphere. 2011;83:599–604. doi: 10.1016/j.chemosphere.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Gaviria M.I., Barrientos K., Arango J.P., Cano J.B., Peñuela G.A. Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots–graphene oxide quenching test for analytical and commercial organophosphate pesticide detection. Front. Environ. Sci. 2022;10 doi: 10.3389/fenvs.2022.825112. [DOI] [Google Scholar]
- 29.Ubaid ur Rahman H., Asghar W., Nazir W., Sandhu M.A., Ahmed A., Khalid N. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: evidence of mechanisms, exposures and mitigation strategies. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142649. [DOI] [PubMed] [Google Scholar]
- 30.Topal A., Şişecioʇlu M., Atamanalp M., Işik A., Yilmaz B. The in vitro and in vivo effects of chlorpyrifos on acetylcholinesterase activity of rainbow trout brain. J. Appl. Anim. Res. 2016;44:243–247. doi: 10.1080/09712119.2015.1031776. [DOI] [Google Scholar]
- 31.Kwong T.C. Organophosphate pesticides: biochemistry and clinical toxicology. Ther. Drug Monit. 2002;24:144–149. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Miao Y., He N., Zhu J.J. History and new developments of assays for cholinesterase activity and inhibition. Chem. Rev. 2010;110:5216–5234. doi: 10.1021/cr900214c. [DOI] [PubMed] [Google Scholar]
- 33.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Guo Y., Xiao L., Chen B. A fluorometric biosensor based on H2O2-sensitive nanoclusters for the detection of acetylcholine. Biosens. Bioelectron. 2014;59:289–292. doi: 10.1016/j.bios.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Hou S., Ou Z., Chen Q., Wu B. Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol-gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens. Bioelectron. 2012;33:44–49. doi: 10.1016/j.bios.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z., Ren X., Meng X., Tan L., Chen D., Tang F. Quantum dots-based fluorescent probes for turn-on and turn-off sensing of butyrylcholinesterase. Biosens. Bioelectron. 2013;44:204–209. doi: 10.1016/j.bios.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Qian Z., Chai L., Tang C., Huang Y., Chen J., Feng H. A fluorometric assay for acetylcholinesterase activity and inhibitor screening with carbon quantum dots. Sensors Actuators, B Chem. 2016;222:879–886. doi: 10.1016/j.snb.2015.09.023. [DOI] [Google Scholar]
- 38.Liao D., Chen J., Zhou H., Wang Y., Li Y., Yu C. In situ formation of metal coordination polymer: a strategy for fluorescence turn-on assay of acetylcholinesterase activity and inhibitor screening. Anal. Chem. 2013;85:2667–2672. doi: 10.1021/ac302971x. [DOI] [PubMed] [Google Scholar]
- 39.Peng L., Zhang G., Zhang D., Xiang J., Zhao R., Wang Y., Zhu D. A fluorescence “turn-on” ensemble for acetylcholinesterase activity assay and inhibitor screening. Org. Lett. 2009;11:4014–4017. doi: 10.1021/ol9016723. [DOI] [PubMed] [Google Scholar]
- 40.Sabelle S., Renard P.Y., Pecorella K., De Suzzoni-Dézard S., Créminon C., Grassi J., Mioskowski C. Design and synthesis of chemiluminescent probes for the detection of cholinesterase activity. J. Am. Chem. Soc. 2002;124:4874–4880. doi: 10.1021/ja0171299. [DOI] [PubMed] [Google Scholar]
- 41.Wang M., Gu X., Zhang G., Zhang D., Zhu D. Convenient and continuous fluorometric assay method for acetylcholinesterase and inhibitor screening based on the aggregation-induced emission. Anal. Chem. 2009;81:4444–4449. doi: 10.1021/ac9002722. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Cai Y., Qi Z., Lu L., Qian Y. DNA-templated silver nanoclusters for fluorescence turn-on assay of acetylcholinesterase activity. Anal. Chem. 2013;85:8455–8461. doi: 10.1021/ac401966d. [DOI] [PubMed] [Google Scholar]
- 43.Wang D.Z.M., Gu X., Zhang G., Zhang D. Continuous colorimetric assay for acetylcholinesterase and inhibitor screening with gold nanoparticles. Langmuir. 2009:2504–2507. doi: 10.1039/c1an15224f. [DOI] [PubMed] [Google Scholar]
- 44.Liu D., Chen W., Tian Y., He S., Zheng W., Sun J., Wang Z., Jiang X. A highly sensitive gold-nanoparticle-based assay for acetylcholinesterase in cerebrospinal fluid of transgenic mice with alzheimer's disease. Adv. Healthc. Mater. 2012;1:90–95. doi: 10.1002/adhm.201100002. [DOI] [PubMed] [Google Scholar]
- 45.Li W., Li W., Hu Y., Xia Y., Shen Q., Nie Z., Huang Y., Yao S. A fluorometric assay for acetylcholinesterase activity and inhibitor detection based on DNA-templated copper/silver nanoclusters. Biosens. Bioelectron. 2013;47:345–349. doi: 10.1016/j.bios.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 46.Li H., Guo Y., Xiao L., Chen B. Selective and sensitive detection of acetylcholinesterase activity using denatured protein-protected gold nanoclusters as a label-free probe. Analyst. 2014;139:285–289. doi: 10.1039/c3an01736b. [DOI] [PubMed] [Google Scholar]
- 47.Gill R., Bahshi L., Freeman R., Willner I. Optical detection of glucose and acetylcholine esterase inhibitors by H2O2‐sensitive CdSe/ZnS quantum dots. Angew. Chemie - Int. Ed. 2008;47:1676–1679. doi: 10.1002/ange.200704794. [DOI] [PubMed] [Google Scholar]
- 48.Saa L., Virel A., Sanchez-Lopez J., Pavlov V. Analytical applications of enzymatic growth of quantum dots. Chem. Eur J. 2010;16:6187–6192. doi: 10.1002/chem.200903373. [DOI] [PubMed] [Google Scholar]
- 49.Feng F., Tang Y., Wang S., Li Y., Zhu D. Continuous fluorometric assays for acetylcholinesterase activity and inhibition with conjugated polyelectrolytes. Angew. Chemie - Int. Ed. 2007;46:7882–7886. doi: 10.1002/anie.200701724. [DOI] [PubMed] [Google Scholar]
- 50.Lei C., Wang Z., Nie Z., Deng H., Hu H., Huang Y., Yao S. Resurfaced fluorescent protein as a sensing platform for label-free detection of copper(II) ion and acetylcholinesterase activity. Anal. Chem. 2015;87:1974–1980. doi: 10.1021/ac504390e. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W., Zhu L., Qin J., Yang C. Novel water-soluble red-emitting poly(p -phenylenevinylene) derivative: synthesis, characterization, and fluorescent acetylcholinesterase assays. J. Phys. Chem. B. 2011;115:12059–12064. doi: 10.1021/jp206930v. [DOI] [PubMed] [Google Scholar]
- 52.Shen J., Zhu Y., Yang X., Li C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012;48:3686–3699. doi: 10.1039/c2cc00110a. [DOI] [PubMed] [Google Scholar]
- 53.Qian Z., Chai L., Tang C., Huang Y., Chen J., Feng H. Carbon quantum dots-based recyclable real-time fluorescence assay for alkaline phosphatase with adenosine triphosphate as substrate. Anal. Chem. 2015;87:2966–2973. doi: 10.1021/ac504519b. [DOI] [PubMed] [Google Scholar]
- 54.Baker S.N., Baker G.A. Luminescent carbon nanodots: emergent nanolights. Angew. Chemie - Int. Ed. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 55.Hoang V.C., Dave K., Gomes V.G. Carbon quantum dot-based composites for energy storage and electrocatalysis: mechanism, applications and future prospects. Nano Energy. 2019;66 doi: 10.1016/j.nanoen.2019.104093. [DOI] [Google Scholar]
- 56.Lim S.Y., Shen W., Gao Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015;44:362–381. doi: 10.1039/c4cs00269e. [DOI] [PubMed] [Google Scholar]
- 57.Zhao A., Chen Z., Qu X., Zhao C., Gao N. Recent advances in bioapplications of C-dots. Carbon N. Y. 2014;85:309–327. doi: 10.1016/j.carbon.2014.12.045. [DOI] [Google Scholar]
- 58.Zheng X.T., Ananthanarayanan A., Luo K.Q., Chen P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small. 2015;11:1620–1636. doi: 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Zhang L., Liang R.P., Bai J.M., Qiu J.D. Using graphene quantum dots as novel photoluminescent probes for protein kinase sensing. Anal. Chem. 2013;85:9148–9155. doi: 10.1021/ac401807b. [DOI] [PubMed] [Google Scholar]
- 60.Liu S., Zhao N., Cheng Z., Liu H. Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale. 2015;7:6836–6842. doi: 10.1039/c5nr00070j. [DOI] [PubMed] [Google Scholar]
- 61.Qian Z.S., Chai L.J., Huang Y.Y., Tang C., Jia Shen J., Chen J.R., Feng H. A real-time fluorescent assay for the detection of alkaline phosphatase activity based on carbon quantum dots. Biosens. Bioelectron. 2015;68:675–680. doi: 10.1016/j.bios.2015.01.068. [DOI] [PubMed] [Google Scholar]
- 62.Xu Q., Pu P., Zhao J., Do C. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(III) detection. Mater. Chem. A. 2014;3:542–546. doi: 10.1039/C4TA05483K. [DOI] [Google Scholar]
- 63.Shi D., Yan F., Wang Y., Zhou X., Chen L. P-doped carbon dots act as nanosensor for trace 2,4,6-trinitrophenol detection and fluorescent reagent for biological imaging. RSC Adv. 2015;5:98492–98499. doi: 10.1039/C5RA18800H. [DOI] [Google Scholar]
- 64.Liang N., Hu X., Li W., Mwakosya A.W., Guo Z., Xu Y., Huang X., Li Z., Zhang X., Zou X., Shi J. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128494. [DOI] [PubMed] [Google Scholar]
- 65.Walsh Michael J., Ahner Beth A. Determination of stability constants of Cu(I), Cd(II) & Zn(II) complexes with thiols using fluorescent probes. J. Inorg. Biochem. 2013;128:112–123. doi: 10.1016/j.jinorgbio.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Lin B., Yan Y., Guo M., Cao Y., Yu Y., Zhang T., Huang Y., Wu D. Modification-free carbon dots as turn-on fluorescence probe for detection of organophosphorus pesticides. Food Chem. 2018;245:1176–1182. doi: 10.1016/j.foodchem.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 67.Reshma B., Gupta R., Sharma K.K., Ghosh Facile and visual detection of acetylcholinesterase inhibitors by carbon quantum dots. New J. Chem. 2019;43:9924–9933. doi: 10.1039/C9NJ02347J. [DOI] [Google Scholar]
- 68.Jiang M., He J., Gong J., Gao H., Xu Z. Development of a quantum dot-labelled biomimetic fluorescence immunoassay for the simultaneous determination of three organophosphorus pesticide residues in agricultural products. Food Agric. Immunol. 2019;30:248–261. doi: 10.1080/09540105.2019.1572714. [DOI] [Google Scholar]
- 69.Jyoti Korrama K.K.G., Dewangana Lakshita, Nagwanshib Rekha, Karbhala Indrapal, Satnamia* M.L. 2019. rCarbon Quantum Dot-Gold Nanoparticle System as Probe for Inhibition and Reactivation of Acetylcholinesterase: Detection of Pesticides. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at Social Science Research Network (SSRN)https://ssrn.com/abstract%20=%204401576 https://doi.org/10.2139/ssrn.4401576.