Summary
Not much is known on sex differences in incidence, survival, and treatment characteristics for midline and hemispheric pHGGs. This population-based study confirms previously reported study results that found worse survival outcomes for malignant diffuse gliomas in girls in the age group 0–9 years. Additionally, in our study we pinpoint this difference to girls with midline pHGGs aged 0–4 years. We provide insight in the possible underlying mechanisms contributing to sex survival differences in pHGG patients. With first line treatment having no impact on the higher risk of dying for girls, but age and tumor characteristics having a neutralizing effect. The results of this population-based study serve as a basis for future pre-clinical and clinical studies to further unravel the underlying mechanisms responsible for the survival gap between sexes in midline pHGG.
Subject areas: Pediatrics, Public health, Population, Cancer
Graphical abstract
Highlights
-
•
Hemispheric pHGGs below the age of 18 years are twice as common in boys
-
•
Girls with a midline pHGG have significantly worse survival outcomes than boys
-
•
The sex survival difference is independent of first line treatment
Pediatrics; Public health; Population; Cancer
Introduction
Pediatric high-grade gliomas (pHGGs) are among the most devastating childhood cancers, associated with dismal survival outcomes, and high morbidity.1,2,3 These tumors arise from glial cells or their precursors and are commonly found in the midline structures such as the thalamus, cerebellum, brain stem, and spinal cord, but can also be found in the cerebral hemispheres.
Current practice for the treatment of de novo pHGG is dependent on tumor location and consists of maximal safe neurosurgical resection, radiotherapy, consideration of clinical trial options, and potentially chemotherapy such as temozolomide (TMZ).4,5 Neurosurgical management of midline pHGG is limited and treating these patients with chemotherapy remains a subject of controversy. To date, there arguably remains no standard of care first line therapy for these patients beyond radiation.5 In line with first line therapy, after progression or relapse of pHGG no standardized treatment has been accepted. This has led to a variety of treatment modalities, which may be a contributing factor to differential outcomes in survival.6
Survival of pHGGs is largely dependent on tumor location and biology. In a meta-analysis of 1000 pediatric and young adult HGG patients, in which non-biopsied patients were excluded, superior survival was found for hemispheric pHGG (median 18.5 months) compared to midline pHGG (median 13.5 months).7 Moreover, median survival for a specific group of midline pHGGs located in the pons, formerly known as diffuse intrinsic pontine gliomas (DIPGs), varied between 9.5 and 11 months.8,9,10
Girls aged 0–9 years with a malignant diffuse glioma have been reported to have worse survival outcomes compared to boys.11 This was in contrast to all other age groups where survival for boys was comparable or lower. Several biological hypotheses such as genetic and hormonal differences have been proposed to explain sex difference in survival and treatment response.11,12,13 In addition, clinical aspects like time-to-treatment may play a role.14 However, studies on sex as a determinant for survival of patients with glioma are mainly focused on adults and only a limited number of such studies are available for the pediatric population.
In this population-based cohort study we investigate sex differences in incidence, survival and first line treatment characteristics of pHGG patients <18 years diagnosed between 2003 and 2017 in the Netherlands.
Results
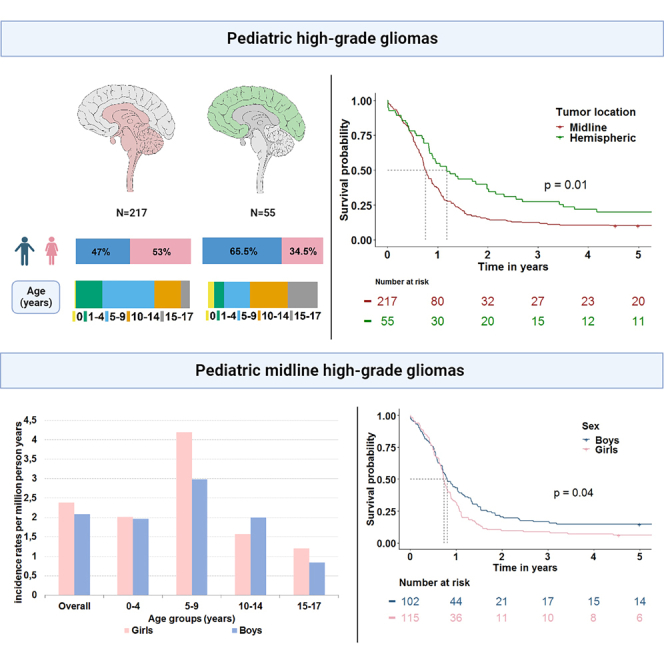
Characteristics of the included pHGG patients are presented in Table 1. In total, 272 children and young adolescents below the age of 18 years were diagnosed with a high-grade glioma during the 14-year time period of 2003–2017. The majority of pHGGs (80%) was found to be located in the midline. Age differed significantly between midline and hemispheric pHGGs with a median age at diagnosis of 7 versus 11 years (p < 0.001), respectively. About half of the midline gliomas were microscopically verified (48%) and this differed significantly from the 100% verified hemispheric pHGGs (p < 0.001). The low percentage of microscopically verified midline pHGGs is reflected by a great amount of malignant glioma, NOS (ICD-O-M9380/3) (56%) and a large number of tumors with unknown grading (47%). More than half of all midline tumors were located in the pons (63%), 19% in the thalamus and 11% in the spinal cord.
Table 1.
Characteristics of midline and hemispheric pediatric high-grade gliomas (pHGGs) overall and differentiated by sex during the period 2003–2017 in the Netherlands
| Midline |
Hemispheric |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall |
Boys |
Girls |
Pa | Overall |
Boys |
Girls |
Pa | |
| (N=217) | (N=102) | (N=115) | (N=55) | (N=36) | (N=19) | |||
| Period of diagnosis | ||||||||
| 2003–2006 | 63 (29.0%) | 38 (37.3%) | 25 (21.7%) | 0.08 | 16 (29.1%) | 11 (30.6%) | 5 (26.3%) | 0.94 |
| 2007–2010 | 66 (30.4%) | 27 (26.5%) | 39 (33.9%) | 13 (23.6%) | 9 (25.0%) | 4 (21.1%) | ||
| 2011–2014 | 58 (26.7%) | 26 (25.5%) | 32 (27.8%) | 13 (23.6%) | 8 (22.2%) | 5 (26.3%) | ||
| 2015–2017 | 30 (13.8%) | 11 (10.8%) | 19 (16.5%) | 13 (23.6%) | 8 (22.2%) | 5 (26.3%) | ||
| Age groups (years) | ||||||||
| 0–4 | 52 (24.0%) | 26 (25.5%) | 26 (22.6%) | 0.38 | 8 (14.5%) | 6 (16.7%) | 2 (10.5%) | 0.92 |
| 5–9 | 98 (45.2%) | 41 (40.2%) | 57 (49.6%) | 13 (23.6%) | 9 (25.0%) | 4 (21.1%) | ||
| 10–14 | 50 (23.0%) | 28 (27.5%) | 22 (19.1%) | 19 (34.5%) | 12 (33.3%) | 7 (36.8%) | ||
| 15–17 | 17 (7.8%) | 7 (6.9%) | 10 (8.7%) | 15 (27.3%) | 9 (25.0%) | 6 (31.6%) | ||
| Age, median years (IQR) | 7 (5–10) | 7 (4–11) | 7 (5–10) | 0.57 | 11 (7–15) | 11 (7–14) | 12 (8–15) | 0.7 |
| WHO grade | ||||||||
| Grade II | 16 (7.4%) | 10 (9.8%) | 6 (5.2%) | 0.44 | – | – | – | 0.16 |
| Grade III | 42 (19.4%) | 22 (21.6%) | 20 (17.4%) | 16 (29.1%) | 13 (36.1%) | 3 (15.8%) | ||
| Grade IV | 57 (26.3%) | 24 (23.5%) | 33 (28.7%) | 37 (67.3%) | 21 (58.3%) | 16 (84.2%) | ||
| Unknown | 102 (47.0%) | 46 (45.1%) | 56 (48.7%) | 2 (3.6%) | 2 (5.6%) | 0 (0%) | ||
| ICCC-3 subgroup | ||||||||
| (b) Astrocytomas | 81 (37.3%) | 37 (36.3%) | 44 (38.3%) | 0.64 | 46 (83.6%) | 30 (83.3%) | 16 (84.2%) | 0.9 |
| (d.1) Oligodendrogliomas | 3 (1.4%) | 1 (1.0%) | 2 (1.7%) | 1 (1.8%) | 1 (2.8%) | 0 (0%) | ||
| (d.2) Mixed and unspecified gliomas | 131 (60.4%) | 62 (60.8%) | 69 (60.0%) | 4 (7.3%) | 3 (8.3%) | 1 (5.3%) | ||
| (d.3) Neuroepithelial glial tumors of uncertain origin | 1 (0.5%) | 1 (1.0%) | 0 (0%) | 4 (7.3%) | 2 (5.6%) | 2 (10.5%) | ||
| (f) Unspecified intracranial and intraspinal neoplasms | 1 (0.5%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Detailed tumor location midline | ||||||||
| Pons | 136 (62.7%) | 68 (66.7%) | 68 (59.1%) | 0.24 | – | – | – | 1 |
| Thalamus | 42 (19.4%) | 13 (12.7%) | 10 (8.7%) | 0 (0%) | – | – | ||
| Spinal | 23 (10.6%) | 5 (4.9%) | 11 (9.6%) | 0 (0%) | – | – | ||
| Other | 16 (7.4%) | 16 (15.7%) | 26 (22.6%) | 0 (0%) | – | – | ||
| Hemispheric | 0 (0%) | – | – | 55 (100%) | 36 (100%) | 19 (100%) | ||
| Microscopic verification | ||||||||
| No | 113 (52.1%) | 50 (49.0%) | 63 (54.8%) | 0.48 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Yes | 104 (47.9%) | 52 (51.0%) | 52 (45.2%) | 55 (100%) | 36 (100%) | 19 (100%) | ||
ICCC-3, International Classification of Childhood Cancer, Third edition; IQR, interquartile range.
Pearson’s Χ2 test or Fisher’s Exact test (when N ≤ 5 in one or more categories). For continuous variables the Wilcoxon rank test was used.
Incidence of pHGG
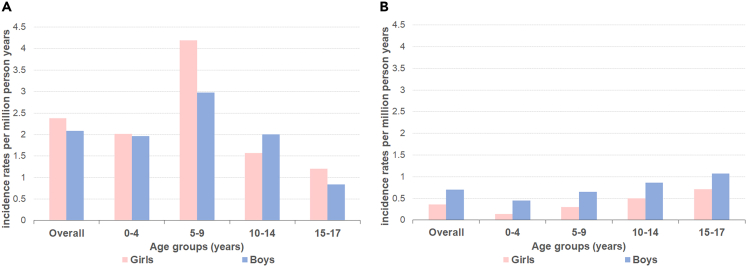
On average, 19 patients were diagnosed with a pHGG annually in the Netherlands. The world standardized incidence rate for patients aged 0–17 years was 5.5 per million person-years. Midline pHGG had an average incidence rate per million person-years of 4.5, while hemispheric pHGG had an incidence rate of 1.1. Overall, incidence of midline pHGGs was comparable in boys and girls (2.1 and 2.3 per million person-years, SRR 0.9 (95% CI 0.7–1.1)). Regarding age, the largest incidence difference between girls and boys was seen in the age group 5–9 (SRR 0.7 (95% CI 0.5–1.1)), and 15–17 years (SRR 0.7 (95%CI 0.3–1.8)) (Figure 1A). For the age group 0–4 years, incidence was comparable (SRR 1.0 (95% CI 0.6–1.7)) and for the age group 10–14 years boys were more commonly diagnosed (SRR 1.3 (95% CI 0.7–2.2)). In contrast, hemispheric tumors were twice as common in boys (SRR 2.0 (95% CI 1.1–3.4)) with an average incidence rate per million person-years of 0.7 for boys compared to 0.3 for girls (Figure 1B). The overrepresentation of boys for hemispheric pHGG was consistent for all age groups but highest in the younger age groups with SRRs of 3.2 (95% CI 0.7–16.1), 2.2 (95% CI 0.7–7.2), 1.7 (95% CI 0.7–4.4), and 1.5 (95% CI 0.5–4.2) for the age groups 0–4, 5–9, 10–14 and 15–17, respectively.
Figure 1.
Incidence rates for pediatric high-grade gliomas (pHGGs)
Average incidence rate per million person-years for (A) midline and (B) hemispheric pediatric high-grade gliomas (pHGGs) stratified to sex and age.
First line treatment of pHGG
Table 2 presents the symptom duration and first line treatment characteristics for midline and hemispheric pHGGs differentiated by sex during the period 2003–2017.
Table 2.
Symptom duration and first line treatment characteristics of midline and hemispheric pediatric high-grade gliomas (pHGGs) overall and differentiated by sex during the period 2003–2017 in the Netherlands
| Midline |
Hemispheric |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall |
Boys |
Girls |
Pa | Overall |
Boys |
Girls |
Pa | |
| (N=217) | (N=102) | (N=115) | (N=55) | (N=36) | (N=19) | |||
| Symptom duration, days (IQR) | 21 (14–61) | 21 (14–30) | 21 (14–61) | 0.62 | 21 (7–30) | 14 (4–46) | 21 (14–30) | 0.6 |
| Care setting | ||||||||
| Pediatric oncology | 213 (98.2%) | 100 (98.0%) | 113 (98.3%) | 1 | 50 (90.9%) | 33 (91.7%) | 17 (89.5%) | 1 |
| Adult | 4 (1.8%) | 2 (2.0%) | 2 (1.7%) | 5 (9.1%) | 3 (8.3%) | 2 (10.5%) | ||
| No Therapy | 21 (9.7%) | 12 (11.7%) | 9 (7.9%) | 3 (5.5%) | 3 (8.3%) | 0 (0%) | ||
| Died quickly after diagnosis | 2 (0.9%) | 1 (1.0%) | 1 (0.9%) | 0.4 | 1 (1.8%) | 1 (2.8%) | 0 (0%) | 0.7 |
| High tumor load/Extensive disease | 7 (3.2%) | 5 (4.9%) | 2 (1.7%) | 2 (3.6%) | 2 (5.6%) | 0 (0%) | ||
| Comorbidity | 1 (0.5%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Functional status | 2 (0.9%) | 1 (1.0%) | 1 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Wish/Refusal patient or family | 7 (3.2%) | 2 (2.0%) | 5 (4.3%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Not defined | 2 (0.9%) | 2 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Neurosurgery | ||||||||
| GTR | 7 (3.2%) | 3 (2.9%) | 4 (3.5%) | 0.94 | 25 (45.5%) | 13 (36.1%) | 12 (63.2%) | 0.02 |
| None | 128 (59.0%) | 62 (60.8%) | 66 (57.4%) | 1 (1.8%) | 1 (2.8%) | 0 (0%) | ||
| Biopsy | 40 (18.4%) | 19 (18.6%) | 21 (18.3%) | 11 (20.0%) | 11 (30.6%) | 0 (0%) | ||
| STR | 42 (19.4%) | 18 (17.6%) | 24 (20.9%) | 18 (32.7%) | 11 (30.6%) | 7 (36.8%) | ||
| Radiotherapy | ||||||||
| No | 47 (21.7%) | 25 (24.5%) | 22 (19.1%) | 0.43 | 13 (23.6%) | 9 (25.0%) | 4 (21.1%) | 1 |
| Yes | 170 (78.3%) | 77 (75.5%) | 93 (80.9%) | 42 (76.4%) | 27 (75.0%) | 15 (78.9%) | ||
| Radiotherapy, median total dose in Gy (IQR) | 45.0 (39.0–54.0) | 54.0 (39.0–54.0) | 44.8 (39.0–54.0) | 0.09 | 59.4 (54.0–59.9) | 59.4 (54.0–59.6) | 59.4 (59.4–59.9) | 0.29 |
| Radiotherapy, median fractions (IQR) | 17.0 13.0–30.0) | 28.0 (13.0–30.0) | 16.0 (13.0–30.0) | 0.26 | 30.0 (30.0–33.0) | 30.0 (30.0–33.0) | 30.0 (30.0–33.0) | 0.88 |
| Systemic therapy | ||||||||
| None | 148 (68.2%) | 69 (67.6%) | 79 (68.7%) | 1 | 16 (29.1%) | 11 (30.6%) | 5 (26.3%) | 0.85 |
| Chemo | 63 (29.0%) | 30 (29.4%) | 33 (28.7%) | 38 (69.1%) | 24 (66.7%) | 14 (73.7%) | ||
| Chemo + Target | 6 (2.8%) | 3 (2.9%) | 3 (2.6%) | 1 (1.8%) | 1 (2.8%) | 0 (0%) | ||
| Temozolomide | ||||||||
| No | 174 (80.2%) | 82 (80.4%) | 92 (80.0%) | 1 | 21 (38.2%) | 14 (38.9%) | 7 (36.8%) | 1 |
| Yes | 43 (19.8%) | 20 (19.6%) | 23 (20.0%) | 34 (61.8%) | 22 (61.1%) | 12 (63.2%) | ||
| Dexamethasone | ||||||||
| No | 81 (37.3%) | 46 (45.1%) | 35 (30.4%) | 0.04 | 25 (45.5%) | 17 (47.2%) | 8 (42.1%) | 0.94 |
| Yes | 136 (62.7%) | 56 (54.9%) | 80 (69.6%) | 30 (54.5%) | 19 (52.8%) | 11 (57.9%) | ||
IQR, interquartile range; Gy, gray.
Pearson’s Χ2 test or Fisher’s Exact test (when N ≤ 5 in one or more categories). For continuous variables the Wilcoxon rank test was used.
Median symptom duration before first consult with a (pediatric) oncologist was 21 days, and this was comparable between sexes for both, midline (p = 0.62) and hemispheric pHGG (p = 0.6). Less than 2% of midline and 9% of hemispheric glioma patients were diagnosed and treated in an adult care setting. No significant sex differences were found for children treated at a pediatric oncology site compared to children treated in an adult care setting. Due to several disease related reasons (e.g., death quickly after diagnosis), 10% of midline and 5% of hemispheric tumor patients did not start any treatment. Determination to start treatment was comparable between boys and girls for both diagnostic groups.
During first line treatment of midline pHGGs dexamethasone use was higher in girls (70%) than boys (55%, p = 0.04). No sex differences were found in neurosurgical interventions, radiotherapy, systemic therapy and, more specifically, treatment with TMZ use for midline pHGG patients. However, for radiotherapy the median total dose tended to be lower in girls compared to boys (44.8Gy vs. 54 Gy, p = 0.09).
For hemispheric pHGG a significant difference in neurosurgical intervention was seen between sexes with girls receiving a gross total resection (GTR) (63%) or subtotal resection (STR) (37%) while one-third of the boys got only a biopsy (31%) or no intervention (3%) (p = 0.02). We did not find a sex difference in radiotherapy, systemic therapy, or use of TMZ or dexamethasone.
Survival of patients with pHGG
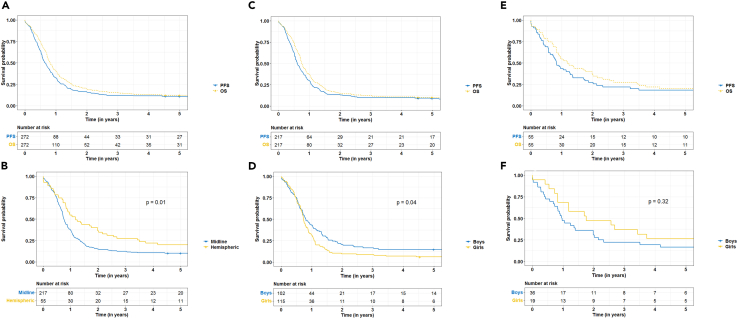
Median PFS for pHGG patients overall during the period 2003–2017 was 7.7 months and median OS was 9.7 months (OS-PFS = 2 months, Figure 2A). Median OS for hemispheric pHGGs (14.2 months) significantly differed from midline pHGGs (9 months, p = 0.01, Figure 2B).We found no significant differences between boys and girls in median PFS (7.5 months versus 7.9 months) and OS (10.6 months versus 9.2 months) for pHGG patients overall.
Figure 2.
Progression Free Survival (PFS) and Overall Survival (OS) for pediatric high-grade gliomas (pHGGs)
(A) pHGG OS and PFS.
(B) pHGG OS stratified to midline pHGG and hemispheric pHGG.
(C) Midline pHGG OS and PFS.
(D) Midline pHGG OS stratified to boys and girls.
(E) Hemispheric pHGG OS and PFS.
(F) Hemisphiric pHGG OS stratified to boys and girls.
Midline pHGGs had the poorest outcomes with a median PFS of 7.2 months and an OS of 9 months (OS-PFS = 1.8 months, Figure 2C). Comparable PFS outcomes were seen for boys and girls (median 7.1 versus 7.5 months, p = 0.1), but different outcomes in median OS (9.7 months versus 8.8 months, p = 0.04) were found (Figure 2D).
Median OS for boys and girls with a midline pHGG differed significantly within the age group 0–4 years (13.5 months versus 8.8 months, p = 0.01) with dismal outcomes for girls. Differences in median OS were found non-significant for the age group 5–9 (9.7 months versus 8.8 months), 10–14 (9.3 months versus 10.7 months) and 15–17 (9.4 months versus 8.1 months) for boys and girls respectively.
Hemispheric pHGGs had a median PFS of 10 months and median OS of 14.2 months (OS-PFS = 4.2 months, Figure 2E). For boys and girls with a hemispheric pHGG median PFS (9.7 versus 8.8 months) and OS (11.5 and 21.1 months, Figure 2F) were comparable.
Table 3 provides median OS in months together with 1-, 2-, and 5-year OS rates for the different age groups according to sex and their interaction for midline pHGG and hemispheric pHGG. OS rates for midline and hemispheric tumors steadily decrease over time after diagnosis with 5-year OS rates of 10% for midline pHGG and 20% for hemispheric pHGG. For midline pHGG, girls have consistently worse survival outcomes in the younger age groups compared to boys. This is especially notable for the age group 0–4 with no girl surviving at 5 years, contrasting the 31% 5-year OS rate for boys aged 0–4.
Table 3.
Median OS in months, 1-, 2- and 5-year OS rates of midline and hemispheric pediatric high-grade gliomas (pHGGs), overall and stratified to sex, age and the interaction of sex and age
| Midline |
Hemispheric |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median |
1-year |
2-year |
5-year |
N | Median |
1-year |
2-year |
5-year |
|
| OS (months) | OS (95%CI) | OS (95%CI) | OS (95%CI) | OS (months) | OS (95%CI) | OS (95%CI) | OS (95%CI) | |||
| Overall | 217 | 9.0 | 37 (31–44) | 15 (11-203 | 10 (7–15) | 55 | 14.2 | 55 (43–69) | 36 (26–52) | 20 (12–34) |
| Sex | ||||||||||
| Boys | 102 | 9.7 | 43 (35–54) | 21 (14–30) | 15 (9–24) | 36 | 11.5 | 47 (33–67) | 31 (19–50) | 17 (8–35) |
| Girls | 115 | 8.8 | 31 (24–41) | 10 (5–17) | 6 (3–13) | 19 | 21.1 | 68 (50–93) | 47 (30–76) | 26 (12–56) |
| Age groups (years) | ||||||||||
| 0–4 | 52 | 9.5 | 39 (27–54) | 21 (13–36) | 15 (8–29) | 8 | 3.5 | 25 (8–83) | 25 (8–83) | 25 (8–83) |
| 5–9 | 98 | 8.8 | 35 (26–46) | 8 (4–16) | 4 (2–11) | 13 | 14.9 | 62 (40–95) | 39 (19–77) | 15 (4–55) |
| 10–14 | 50 | 9.5 | 40 (28–56) | 18 (10–33) | 16 (8–30) | 19 | 12.6 | 53 (34–81) | 37 (20–66) | 26 (12–56) |
| 15–17 | 17 | 8.7 | 35 (19–67) | 24 (10–55) | 12 (3–43) | 15 | 23.8 | 67 (47–95) | 40 (22–74) | 13 (4–48) |
| Boys ∗ Age groups (years) | ||||||||||
| 0–4 | 26 | 13.5 | 54 (38–77) | 35 (20–59) | 31 (17–55) | 6 | 3.5 | 17 (3–100) | 17 (3–100) | 17 (3–100) |
| 5–9 | 41 | 9.7 | 39 (27–57) | 12 (5–28) | 5 (1–19) | 9 | 14.9 | 56 (31–100) | 44 (21–92) | 22 (7–75) |
| 10–14 | 28 | 9.3 | 39 (25–62) | 18 (8–40) | 14 (6–35) | 12 | 11.5 | 42 (21–81) | 25 (9–67) | 17 (5–59) |
| 15–17 | 7 | 9.4 | 43 (18–100) | 29 (9–92) | 14 (2–88) | 9 | 23.8 | 67 (42–100) | 33 (13–84) | 11 (18–71) |
| Girls∗ Age groups (years) | ||||||||||
| 0–4 | 26 | 8.8 | 23 (11–47) | 8 (2–29) | 0 | 2 | 106.3 | 50 (13–100) | 50 (13–100) | 50 (13–100) |
| 5–9 | 57 | 8.8 | 32 (22–46) | 5 (2–16) | 4 (0–14) | 4 | 17.3 | 75 (43–100) | 25 (5–100) | 0 |
| 10–14 | 22 | 10.7 | 41 (25–68) | 18 (7–44) | 18 (7–44) | 7 | 32.2 | 71 (45–100) | 57 (30–100) | 43 (18–100) |
| 15–17 | 10 | 8.1 | 30 (12–77) | 20 (6–69) | 10 (2–64) | 6 | 25.9 | 67 (38–100) | 50 (22–100) | 17 (3–100) |
N, number of patients; OS, Overall survival; CI, confidence interval.
Determinants for risk of death
Univariable and multivariable OS cox regression models for midline and univariable OS cox regression models for hemispheric pHGGs are presented in Table 4. For midline tumors, the risk of dying was significantly higher among girls compared to boys (HR 1.3 (95% CI 1–1.8)) in univariable analyses. WHO grade was also found to be associated with OS: WHO grade II (HR0.1 (95%CI0-0.3)) and IV (HR1.6 (95%CI 1–2.4)) being significant using WHO grade III as reference. Furthermore, a significant interaction between age and gender was found: Taking the age group 0–4 as a reference, girls aged 10–14 had a lower risk of dying (HR0.4 95%CI 0.2–0.9)).
Table 4.
Univariable and multivariable associations of sex with overall survival (OS) in midline pHGG corrected for A) first line treatment and B) first line treatment, patient and tumor characteristics and univariable associations in hemispheric pHGG
| Midline |
Midline |
Midline |
Hemispheric |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Univariable HR (95%CI) |
p | Adjusted 1a HR (95%CI) |
p | Adjusted 2b HR (95%CI) |
p | N | Univariable HR (95%CI) |
p | |
| Sex | ||||||||||
| Boys | 102 | REF | REF | REF | 36 | REF | ||||
| Girls | 115 | 1.3 (1–1.8) | 0.04 | 1.4 (1–1.8) | 0.03 | 1.2 (0.9–1.6) | 0.24 | 19 | 0.7 (0.4–1.3) | 0.32 |
| Neurosurgery | ||||||||||
| GTR | 7 | REF | REF | REF | 25 | REF | ||||
| None | 128 | 1.1 (0.5–2.4) | 0.8 | 1.2 (0.5–2.6) | 0.73 | 1.6 (0.6–4.5) | 0.35 | 1 | N < 5 | |
| Biopsy | 40 | 1.4 (0.6–3.2) | 0.38 | 1.5 (0.7–3.4) | 0.33 | 2.3 (0.9–5.7) | 0.08 | 11 | 4.2 (1.8–9.4) | <0.001 |
| STR | 42 | 1.1 (0.5–2.4) | 0.84 | 1 (0.5–2.3) | 0.96 | 1 (0.4–2.3) | 0.97 | 18 | 1.1 (0.6–2.1) | 0.81 |
| Radiotherapy | ||||||||||
| No | 47 | REF | REF | REF | 13 | REF | ||||
| Yes | 170 | 0.9 (0.6–1.3) | 0.67 | 0.9 (0.6–1.3) | 0.46 | 0.3 (0.2–0.4) | <0.001 | 42 | 0.7 (0.3–1.3) | 0.24 |
| Systemic therapy | ||||||||||
| No | 148 | REF | REF | REF | 16 | REF | ||||
| Yes | 69 | 1 (0.8–1.5) | 0.6 | 1.1 (0.8–1.7) | 0.52 | 0.7 (0.4–1) | 0.05 | 39 | 1.5 (0.8–3.0) | 0.22 |
| Age groups | ||||||||||
| 0–4 | 52 | REF | REF | 8 | REF | |||||
| 5–9 | 98 | 1.3 (0.9–1.9) | 0.15 | 1.7 (1.2–2.6) | 0.008 | 13 | 0.9 (0.3–2.5) | 0.89 | ||
| 10–14 | 50 | 1 (0.6–1.5) | 0.85 | 1.3 (0.8–2.1) | 0.27 | 19 | 0.8 (0.3–2.2) | 0.73 | ||
| 15–17 | 17 | 1 (0.6–1.8) | 0.97 | 1.5 (0.8–2.8) | 0.26 | 15 | 0.8 (0.3–2.0) | 0.57 | ||
| Tumor location | ||||||||||
| Pons | 136 | REF | REF | |||||||
| Thalamus | 23 | 1.2 (0.8–2) | 0.37 | 1.8 (1–3.5) | 0.07 | |||||
| Spinal | 16 | 1.6 (0.9–2.7) | 0.1 | 1.4 (0.7–2.9) | 0.3 | |||||
| Other | 42 | 1.1 (0.8–1.6) | 0.53 | 2 (1.2–3.2) | 0.004 | |||||
| WHO grade | ||||||||||
| Grade III | 42 | REF | REF | 16 | ||||||
| Grade II | 16 | 0.1 (0–0.3) | <0.001 | 0.05 (0–0.1) | <0.001 | 0 | N < 5 | |||
| Grade IV | 57 | 1.6 (1–2.4) | 0.03 | 1.8 (1.1–2.8) | 0.01 | 37 | 1.9 (0.9–3.8) | 0.07 | ||
| Unknown | 102 | 1.2 (0.8–1.7) | 0.42 | 1.2 (0.7–2.2) | 0.5 | 2 | N < 5 | |||
Deaths within 5 years from diagnosis are considered here.
GTR = Gross total resection, STR = Subtotal resection, HR = hazard rate, CI = confidence interval, N < 5 = in case the number of patients is lower than 5 no results are reported.
Neurosurgery, radiotherapy, systemic therapy.
Neurosurgery, radiotherapy, systemic therapy, age, tumor location, WHO grade.
When adjusting for treatment characteristics (i.e., neurosurgical intervention, radiotherapy and systemic therapy) the risk of dying for girls slightly increased (HR 1.4 (95% CI 1–1.8)). When also adding age, tumor location and WHO grade to the adjusted treatment characteristic model, the significance disappeared (HR 1.2 (95% CI 0.9–1.6). In this model, an association with worse survival outcomes was found for patients in the age group 5–9 years (HR 1.7 (95% CI 1.2–2.6)), WHO CNS grade IV tumors (HR 1.8 (95% CI 1.1–2.8)) and tumors located outside the pons, thalamus or spinal cord (HR 2 (95% CI 1.2–3.2)). A lower risk of dying was found for patients with a WHO CNS grade II tumor (HR 0.05 (95% CI 0–0.1)), receiving radiotherapy (HR 0.3 (95% CI 0.2–0.4)) and systemic therapy (HR 0.7 (95% CI 0.4–1)) during first line treatment.
Additional analysis (data not presented) showed that in univariable analysis use of dexamethasone during first line treatment was associated with worse survival outcomes compared to patients not receiving dexamethasone (HR 1.7 (95%CI 1.2–2.2)). However, when adding the use of dexamethasone to model 2 there was no effect on the risk of dying for girls (HR 1.2 (95%CI 0.9–1.6)) and the association of dexamethasone with worse survival became non-significant (HR 1.4 (95%CI 1–1.9)).
For hemispheric tumors we found no significant sex difference in univariable analysis (HR 0.7 (95%CI 0.4–1.3)). The only significant association for hemispheric tumors in univariable analyses was a higher risk of dying for patients receiving a biopsy (HR 4.2 (95%CI 1.8–9.4)) using the gross total resection group as a reference (Table 4).
Discussion
This is the first population-based study describing sex differences in incidence and survival for pHGG subtypes in the Netherlands. Incidence varied across pHGG subtype and sex with an overrepresentation of girls in midline pHGGs in the age group 5–9 and 15–17 years. In contrast, boys were more commonly diagnosed with a hemispheric pHGG across all age groups. Survival was worse for girls with midline pHGGs compared to boys. When adjusting for first line treatment worse outcomes for girls remained intact. However, after adding age and tumor characteristics the sex difference became less pronounced.
Incidence
We found that midline tumors were diagnosed four times more often (80%) compared to their hemispheric counterparts in children and young adolescents <18 years with average incidence rates of 4.5 and 1.1 per million respectively. This is in line with previous reports showing that midline tumors are more commonly diagnosed in children than hemispheric tumors.7 However, this is to our knowledge the first study providing insight into this 4:1 distribution of midline and hemispheric pHGGs for children and young adolescents below the age of 18 years.
Incidence rates for boys and girls with a midline pHGG were comparable overall, but incidence varied by age group with girls being more affected than boys in the age groups of 5–9 years and 15–17 years, while boys were more common in the age group of 10–14 years. Incidence of most childhood cancers is strongly correlated with confined developmental periods resulting in differential incidence by sex and age.15 Underlying reasons can be found in gene expression which varies across age, human tissue and sex. For example, polycomb repressive complex 2 (PRC2) and trimethylation of histone H3 at Lys16 (H3K27me3) are highly enriched genes in females across multiple tissue types including the brain.17 These differences are of importance as most midline pHGG harbor a H3K27M alteration leading to loss of H3 trimethylation affecting PRC2 which in turn can result in glioma tumorigenesis.18 Additionally, for midline pHGG, recurrent mutations in the gene encoding H3.1 histone variants are more common at a younger age and in girls.2,7
Moreover, it has been shown that cancer stem cell-like oligodendrocyte precursor cells (OPCs) are overrepresented in H3K27M DMGs and play an important role in tumor initiation.19 Interestingly, OPCs and their function tends to vary across age and sex.20,21,22 These differences further support the notion that differential incidence may be related to the variability in developmental periods across ages between sexes.
For hemispheric pHGGs, a predominance of boys was reported that was consistent across all age groups. These results are in line with previously reported sex differences in incidence for glioblastoma in adults.23 Glioblastoma in adults is 1.6 times more common in males compared to females. Contributing factors to the difference may be found in metabolic factors such as cerebral glucose metabolism and sex differences in immune response.23,24 For example, in case-control studies an inverse relationship between pre-diagnostic immunoglobulin E (IgE) levels and risk for HGG among females has been reported.24 Other possible contributing factors can be found in hormonal differences, with higher testosterone levels and androgen receptors contributing to an increased incidence for males and estrogens having a protective effect on developing glioblastoma for females.25,26 However, as hormonal differences are less pronounced at younger ages the higher incidence for male hemispheric pHGG seems to indicate an underlying genetic mechanism. This hypothesis is supported in the adult population with sex specific methylation patterns and sex-specific risk loci (7p11.2, 8q24.21, 3p21.31) for gliomas.16,27,28
Survival
In line with literature, survival outcomes of midline pHGGs were inferior compared to hemispheric pHGGs.7 However, median survival was lower than observed in other clinical and biological studies.7,8 Possible reasons for this difference in survival are data type (hospital/trial-based versus population-based data), completeness of non-microscopically verified cases and differences in treatment regimen. In this population-based study, all pHGG patients were included regardless their hospital of diagnosis or trial participation which probably resulted in a higher percentage of patients with a poor prognosis.
Additionally, we found worse survival outcomes for girls with a midline pHGG compared to boys. This result is in line with a previous report by Wang et al.11 that found that girls below <10 years diagnosed with a malignant diffuse glioma had a significantly higher risk of dying compared to boys. Interestingly, when we stratified our analysis by age we found that the difference in median OS was only significant for the age group 0–4 years. When adjusting the sex survival difference for treatment, that is neurosurgery, radiotherapy and systemic therapy, the effect remained significant, but after adding age at diagnosis and tumor characteristics to the multivariate survival model the significant effect diminished, indicative that patient and tumor characteristics are partly explaining the survival difference.
In this full model we found that radiotherapy was associated with better survival outcomes (HR 0.3 (95% CI 0.2–0.4)). Interestingly, we found a trend toward hypofractionated radiotherapy in girls (median total dose Gy 44.9, and median fractions 16). Not much is known on differential responses to treatment in brain tumors in children. However, several underlying biological differences may play a role. For example, it has been reported that the frequency of TP53 mutations differs according to sex.29 The p53 protein plays an important role in apoptosis as a response to radiotherapy. Differences in mutational status between sexes can therefore be a contributing factor to differential survival outcomes. Although hormonal differences are commonly suggested as playing a role in response to therapy for adult GBM, this is less well investigated at younger ages.24 Meanwhile, the pharmacological response to therapy may differ as sex can act as a genetic modifier.13
Dexamethasone use during first line treatment was 15% higher in girls compared to boys. Although dexamethasone use was associated with worse survival outcomes in univariable analysis, the effect diminished when correcting for treatment, age at diagnosis, tumor location and WHO grade (HR 1.4 (95%CI 1–1.9)). Corticosteroids like dexamethasone are not part of the tumoricidal treatment of pHGGs but are commonly used for symptom relief.30 A higher usage of dexamethasone may therefore be indicative for worse clinical symptoms or cultural aspects with girls receiving corticosteroids more easily compared to boys.
Although the underlying pathobiology needs further elucidation, one can speculate that as dexamethasone induces cytochrome P450 CYP3A activity, it potentially leads to faster clearance of a large number of systemic anti-cancer treatments, making the drug less effective. In addition, sex difference has been reported in CYP3A expression, with higher levels of CYP3A4 expression in the liver for females. This difference may strengthen the clearance of drugs that are catalyzed by CYP3A even further making it less effective.
Lastly, recent developments in the field of single cell sequencing seem to hint at a bigger role for the immune system in disease development and progression of midline pHGGs.19 High proportions of microglia were found in pediatric H3K27M DMGs contrasting adults who had higher proportions of macrophages. Microglia are multifunctional cells and can play a role in phagocytosis, brain homeostasis and neuroimmunological defense mechanisms.31 Differences in morphology, quantity, phenotype and transcriptome of microglia have been reported between sexes leading to different functions in the brain.24 Additionally, myeloid-derived suppressor cells (MDSCs) inhibit anti-tumor immune response and MDSCs have been reported to drive immune suppression in a sex-specific manner.32 The aforementioned sex differences may play a role in tumor progression, response to therapy and survival. Therefore, future studies should aim to further differentiate their results based on sex.
Conclusion
Our study confirms previously reported sex differences in incidence and survival and further specify these in a clinically relevant manner, with hemispheric pHGG being twice as common in boys, and girls having worse survival outcomes in midline pHGGs. The latter was especially notable in girls below the age of 5 years. First line treatment did not alter the risk of dying for girls. However, taking age at diagnosis and tumor characteristics into account led to a diminished risk, indicating that patient and tumor characteristics are partly explaining the survival difference. Future studies should focus on integrating disease characteristics and clinical data of pediatric midline gliomas to further unravel the underlying mechanisms responsible for the difference in survival between boys and girls for midline pHGGs.
Limitations of the study
The main strength of our study is the use of population-based data covering a 14-year period with no restriction regarding hospital or treatment, which resulted in a relatively large sample size considering the rarity of the disease. Moreover, detailed clinical data were gathered for all pHGG patients providing a unique opportunity to perform detailed analyses on first line treatment, age, and tumor characteristics in relation to survival outcomes of pHGG in the Netherlands. Another strength of this study is the inclusion of radiologically diagnosed tumors. However, due to the inclusion of non-microscopically verified tumors (i.e., half of the midline pHGGs was not biopsied) there is the risk of classifying lower grade tumors (e.g., pilocytic astrocytomas) as midline pHGG. This may result in an overestimation of survival. However, the gathered radiological conclusions made it possible to exclude most midline tumors for which the radiology was interpreted as most consistent with whom CNS grade I and II, (n = 56). Additionally, as the survival outcomes in our study are lower than previously reported, our results seem to reflect real-world survival outcomes with minimal bias through contamination of lower grade tumors. Of note, patients with radiologically a seemingly lower grade diffuse infiltrative tumor located in the pons considered as prototype DIPG were included in this study as it has been shown that the vast majority of these tumors are H3 K27-altered and should indeed be considered as WHO grade 4.
Unfortunately, it was not possible to provide exact information on the state-of-the-art ‘histomolecular’ diagnoses of the included tumors following the fifth edition of the WHO CNS tumor classification due to the lack of molecular information.33 Most of the included pHGGs were diagnosed during an era in which diagnoses were histology based and additional molecular testing was rare and tumor material for midline pHGG was scarce. Nonetheless, we were able to review the full pathology report or the conclusion of the radiology report for non-biopsied patients, making it possible to group tumors to a clinically relevant tumor location (i.e., midline or hemispheric) increasing the value of the presented results. Future studies will be initiated to unravel the molecular biology background of these historical cases.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Clinical data | This study | N/A |
| Software and algorithms | ||
| R (version 4.2.2) | R foundation | https://www.r-project.org/ |
| tidyverse | Wickham et al.34 | https://www.tidyverse.org/ |
| survminer | N/A | https://github.com/kassambara/survminer |
| survival | N/A | https://github.com/therneau/survival |
| SAS/STAT® software | SAS Institute Inc. | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Raoull Hoogendijk (r.hoogendijk@prinsesmaximacentrum.nl).
Materials availability
This study did not generate new unique materials.
Experimental model and study participant details
Patient selection
Data on pHGG patients <18 years diagnosed in 2003-2017 were derived from the population-based Netherlands Cancer Registry (NCR). We included the International Classification of Diseases for Oncology (ICD-O) morphology codes: M9380/3 (malignant glioma, NOS), M9381/3 (gliomatosis cerebri), M9382/3 (anaplastic oligoastrocytic tumors), M9385/3 (H3K27M-mutant diffuse midline glioma), M9400/3 (diffuse astrocytoma), M9401/3 (anaplastic astrocytoma), M9420/3 (fibrillary astrocytoma), M9440/3 (glioblastoma and variants), M9441/3 (giant cell glioblastoma), M9442/3 (gliosarcoma), M9445/3 (glioblastoma IDH-mutant), M9451/3 (anaplastic oligodendroglioma) and M8000/3-M8002/3 (malignant, unspecified tumors) located in the brain stem (C71.7).
Unspecified tumors like malignant gliomas, NOS (9380/3) were checked for their diagnoses based on imaging. If there was a high probability of a low-grade tumor (WHO grade I-II) we removed the tumor from the dataset (n=54). However, radiologically seemingly low grade, diffuse infiltrative tumors located in the pons were included in the dataset as the vast majority of these are nowadays known to represent the highly malignant diffuse midline gliomas, H3 K27-altered (n=16).
Throughout the manuscript we use Roman numericals to reflect the WHO grade assigned to tumors at initial diagnosis. This in contrast to the fifth edition of the World Health Organization Classification of Tumors of the Central Nervous System (WHO CNS5) published in 2021 where Arabic numerals are employed.33
ICD-O-3 topography codes such as cerebrum (71.0) are non-specific as they include tumors in the cerebral cortex but also the thalamus.35 Therefore, a more detailed tumor location was extracted from the diagnoses text field. The diagnoses text field contained a short summary of the pathology and/or radiology diagnoses and were specifically collected for this project. In case of uncertainty on tumor location the full pathology report was reviewed by the first author (RH). Using the detailed tumor location, tumors were classified as midline or hemispheric. Midline tumors were defined as having their primary location in the thalamus, brain stem (e.g. pons, mesencephalon) and spinal cord. Additionally, we’ve included a basket category with the title “other” which includes medulla oblongata and cerebellum.
Details on the final patient selection are presented in the supplemental information (Table S1).
Method details
Data sources
The NCR contains all patients with malignancies in the Netherlands since 1989 and has showed a completeness in capture of at least 96%.36 Case notifications are provided through the Nationwide Network and Registry of Histopathology and Cytopathology (PALGA ), and the National Registry of Hospital Discharges.37 Detailed clinical data for all pediatric patients with a high-grade CNS tumor were collected through retrospective medical records review by dedicated data managers trained in neuro-oncology. In addition, we linked our database with the PALGA database to gather full pathology reports for review purposes.
Information on vital status (i.e., alive, dead or emigrated) is obtained through annual linkage of the NCR with the Nationwide Personal Record Database (BRP) that holds vital statistics on all Dutch residents. The last linkage for this study was on 1 February 2021. This project was submitted to the MREC Utrecht for approval, but as the Medical Research Involving Human Subjects Act (WMO) does not apply, a waiver was provided (reference number MvdLmb/20/500572).
Quantification and statistical analysis
Included patients were analyzed according to three groups - overall, midline and hemispheric pHGGs and stratified by sex. In this study sex is defined as the sex assigned at birth. Differences in characteristics of the study population were tested by using the Chi-squared or Fisher’s exact test for categorical variables and the Wilcoxon rank test for continuous variables.
Incidence rates were calculated as the average annual number of cases per million person-years, using the mid-year population size as obtained from Statistics Netherlands (CBS).38 Rates for the age range 0-17 years were age-adjusted according to the Segi world standard population.39 Age-specific rates were calculated for the age groups 0-4, 5-9, 10-14, and 15-17 years. Differences in incidence rates between boys and girls were expressed as the standardized rate ratio (SRR) using girls as the reference group. SRRs were calculated according to Rothman et al.40
Overall Survival (OS) was calculated instead of Relative Survival (RS) as competing causes of death are rare in childhood.41 OS was defined as the time from date of diagnosis until death from any cause (i.e., event), date of emigration (i.e., censored) or to February 1, 2021 (i.e., study endpoint). Progression Free Survival (PFS) was defined as the time period from date of diagnosis until progression, death from any cause (i.e., event), date of emigration (i.e., censored) or to February 1st, 2021 (i.e., study endpoint). If progression date was not available we used the start date of second line therapy. In cases where no second line therapy was given, we used the date of death from any cause. Median, 1-, 2-, and 5-year OS and PFS were estimated by Kaplan-Meier method. Cox-proportional hazard models were used to test for differences in OS and PFS. Age, tumor location, WHO grade and first line treatment (i.e., neurosurgical intervention, radiotherapy and systemic therapy) were entered in uni- and multivariable cox-proportional hazard models limited to deaths within 5 years from diagnosis to evaluate the effect of sex on OS.
Two-sided tests with α =0.05 level of statistical significance were used throughout the analyses. Descriptive and survival analyses were performed using R: A language and environment for statistical computing using the tidyverse,34 survival and survminer packages. Analyses on incidence rates were performed with SAS/STAT® software.
Acknowledgments
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the NCR and this project.
Author contributions
Conceptualization, R.H., H.K.K., J.v.d.L., D.v.V.; Methodology, R.H., H.K.K., J.v.d.L., and D.v.V.; Formal Analysis, R.H.; Writing – R.H.; Writing – Review and Editing, All authors; Supervision, H.K.K., J.v.d.L., D.v.V., P.W., and E.H.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: September 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107957.
Contributor Information
Raoull Hoogendijk, Email: r.hoogendijk@prinsesmaximacentrum.nl.
Henrike Karim-Kos, Email: h.e.karim-kos@prinsesmaximacentrum.nl.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon reasonable request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Fischer C., Petriccione M., Donzelli M., Pottenger E. Improving Care in Pediatric Neuro-oncology Patients: An Overview of the Unique Needs of Children With Brain Tumors. J. Child Neurol. 2016;31:488–505. doi: 10.1177/0883073815597756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones C., Karajannis M.A., Jones D.T.W., Kieran M.W., Monje M., Baker S.J., Becher O.J., Cho Y.J., Gupta N., Hawkins C., et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19:153–161. doi: 10.1093/neuonc/now101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoogendijk R., van der Lugt J., van Vuurden D., Kremer L., Wesseling P., Hoving E., Karim-Kos H.E. Survival rates of children and young adolescents with CNS tumors improved in the Netherlands since 1990: A population-based study. Neurooncol. Adv. 2022;4:vdab183. doi: 10.1093/noajnl/vdab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker D.A., Liu J., Kieran M., Jabado N., Picton S., Packer R., St Rose C., CPN Paris 2011 Conference Consensus Group A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15:462–468. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen K.J., Pollack I.F., Zhou T., Buxton A., Holmes E.J., Burger P.C., Brat D.J., Rosenblum M.K., Hamilton R.L., Lavey R.S., Heideman R.L. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kline C., Felton E., Allen I.E., Tahir P., Mueller S. Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J. Neuro Oncol. 2018;137:103–110. doi: 10.1007/s11060-017-2701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay A., Burford A., Carvalho D., Izquierdo E., Fazal-Salom J., Taylor K.R., Bjerke L., Clarke M., Vinci M., Nandhabalan M., et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017;32:520–537.e5. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman L.M., Veldhuijzen van Zanten S.E.M., Colditz N., Baugh J., Chaney B., Hoffmann M., Lane A., Fuller C., Miles L., Hawkins C., et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018;36:1963–1972. doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay A., Burford A., Molinari V., Jones D.T.W., Izquierdo E., Brouwer-Visser J., Giangaspero F., Haberler C., Pietsch T., Jacques T.S., et al. Molecular, Pathological, Radiological, and Immune Profiling of Non-brainstem Pediatric High-Grade Glioma from the HERBY Phase II Randomized Trial. Cancer Cell. 2018;33:829–842.e5. doi: 10.1016/j.ccell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldhuijzen van Zanten S.E.M., Jansen M.H.A., Sanchez Aliaga E., van Vuurden D.G., Vandertop W.P., Kaspers G.J.L. A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Expert Rev. Anticancer Ther. 2015;15:157–164. doi: 10.1586/14737140.2015.974563. [DOI] [PubMed] [Google Scholar]
- 11.Wang G.M., Cioffi G., Patil N., Waite K.A., Lanese R., Ostrom Q.T., Kruchko C., Berens M.E., Connor J.R., Lathia J.D., et al. Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas. Neuro Oncol. 2022;24:302–310. doi: 10.1093/neuonc/noab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W., Warrington N.M., Taylor S.J., Whitmire P., Carrasco E., Singleton K.W., Wu N., Lathia J.D., Berens M.E., Kim A.H., et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019;11:eaao5253. doi: 10.1126/scitranslmed.aao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F., Berthold H.K., Campesi I., Carrero J.J., Dakal S., Franconi F., Gouni-Berthold I., Heiman M.L., Kautzky-Willer A., Klein S.L., et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021;73:730–762. doi: 10.1124/pharmrev.120.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stabellini N., Krebs H., Patil N., Waite K., Barnholtz-Sloan J.S. Sex Differences in Time to Treat and Outcomes for Gliomas. Front. Oncol. 2021;11:630597. doi: 10.3389/fonc.2021.630597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behjati S., Gilbertson R.J., Pfister S.M. Maturation Block in Childhood Cancer. Cancer Discov. 2021;11:542–544. doi: 10.1158/2159-8290.CD-20-0926. [DOI] [PubMed] [Google Scholar]
- 16.Ostrom Q.T., Kinnersley B., Wrensch M.R., Eckel-Passow J.E., Armstrong G., Rice T., Chen Y., Wiencke J.K., McCoy L.S., Hansen H.M., et al. Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Sci. Rep. 2018;8:7352. doi: 10.1038/s41598-018-24580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva M., Muñoz-Aguirre M., Kim-Hellmuth S., Wucher V., Gewirtz A.D.H., Cotter D.J., Parsana P., Kasela S., Balliu B., Viñuela A., et al. The impact of sex on gene expression across human tissues. Science. 2020;369:eaba3066. doi: 10.1126/science.aba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harutyunyan A.S., Krug B., Chen H., Papillon-Cavanagh S., Zeinieh M., De Jay N., Deshmukh S., Chen C.C.L., Belle J., Mikael L.G., et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 2019;10:1262. doi: 10.1038/s41467-019-09140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu I., Jiang L., Samuelsson E.R., Marco Salas S., Beck A., Hack O.A., Jeong D., Shaw M.L., Englinger B., LaBelle J., et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet. 2022;54:1881–1894. doi: 10.1038/s41588-022-01236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buyanova I.S., Arsalidou M. Cerebral White Matter Myelination and Relations to Age, Gender, and Cognition: A Selective Review. Front. Hum. Neurosci. 2021;15:662031. doi: 10.3389/fnhum.2021.662031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalçın B., Monje M. Microenvironmental interactions of oligodendroglial cells. Dev. Cell. 2021;56:1821–1832. doi: 10.1016/j.devcel.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akay L.A., Effenberger A.H., Tsai L.H. Cell of all trades: oligodendrocyte precursor cells in synaptic, vascular, and immune function. Genes Dev. 2021;35:180–198. doi: 10.1101/gad.344218.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrano A., Juarez J.J., Incontri D., Ibarra A., Guerrero Cazares H. Sex-Specific Differences in Glioblastoma. Cells. 2021;10 doi: 10.3390/cells10071783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massey S.C., Whitmire P., Doyle T.E., Ippolito J.E., Mrugala M.M., Hu L.S., Canoll P., Anderson A.R.A., Wilson M.A., Fitzpatrick S.M., et al. Sex differences in health and disease: A review of biological sex differences relevant to cancer with a spotlight on glioma. Cancer Lett. 2021;498:178–187. doi: 10.1016/j.canlet.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plunkett R.J., Lis A., Barone T.A., Fronckowiak M.D., Greenberg S.J. Hormonal effects on glioblastoma multiforme in the nude rat model. J. Neurosurg. 1999;90:1072–1077. doi: 10.3171/jns.1999.90.6.1072. [DOI] [PubMed] [Google Scholar]
- 26.Bao D., Cheng C., Lan X., Xing R., Chen Z., Zhao H., Sun J., Wang Y., Niu C., Zhang B., Fang S. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget. 2017;8:23142–23154. doi: 10.18632/oncotarget.15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen M.L., Stetson L.C., Vadmal V., Waite K., Berens M.E., Connor J.R., Lathia J., Rubin J.B., Barnholtz-Sloan J.S. Gliomas display distinct sex-based differential methylation patterns based on molecular subtype. Neurooncol. Adv. 2020;2:vdaa002. doi: 10.1093/noajnl/vdaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alpen K., Vajdic C.M., MacInnis R.J., Milne R.L., Koh E.-S., Hovey E., Harrup R., Bruinsma F., Nguyen T.L., Li S., et al. Australian genome-wide association study confirms higher female risk for adult glioma associated with variants in the region of CCDC26. Neuro Oncol. 2023;25:1355–1365. doi: 10.1093/neuonc/noac279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockwell N.C., Yang W., Warrington N.M., Staller M.V., Griffith M., Griffith O.L., Gurnett C.A., Cohen B.A., Baldridge D., Rubin J.B. Sex- and mutation-specific p53 gain-of-function activity in gliomagenesis. Cancer Res. Commun. 2021;1:148–163. doi: 10.1158/2767-9764.crc-21-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palombi L., Marchetti P., Salvati M., Osti M.F., Frati L., Frati A. Interventions to Reduce Neurological Symptoms in Patients with GBM Receiving Radiotherapy: From Theory to Clinical Practice. Anticancer Res. 2018;38:2423–2427. doi: 10.21873/anticanres.12494. [DOI] [PubMed] [Google Scholar]
- 31.Prinz M., Jung S., Priller J. Microglia Biology: One Century of Evolving Concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 32.Bayik D., Zhou Y., Park C., Hong C., Vail D., Silver D.J., Lauko A., Roversi G., Watson D.C., Lo A., et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020;10:1210–1225. doi: 10.1158/2159-8290.CD-19-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J., et al. Welcome to the Tidyverse. J. Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 35.Hoogendijk R., van der Lugt J., Hoving E., Kremer L., Visser O., Wesseling P., van Vuurden D., Karim-Kos H. The fifth edition of the World Health Organization Classification of Tumors of the Central Nervous System: Implications for cancer registries. Neuro Oncol. 2022;24:1811–1814. doi: 10.1093/neuonc/noac176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Sanden G.A., Coebergh J.W., Schouten L.J., Visser O., Vanleeuwen F.E. Cancer Incidence in the Netherlands in 1989 and 1990 - First Results of the Nationwide Netherlands Cancer Registry. Eur. J. Cancer. 1995;31a:1822–1829. doi: 10.1016/0959-8049(95)00355-m. [DOI] [PubMed] [Google Scholar]
- 37.Reedijk A.M.J., Klein K., Coebergh J.W.W., Kremer L.C., Dinmohamed A.G., de Haas V., Versluijs A.B., Ossenkoppele G.J., Beverloo H.B., Pieters R., et al. Improved survival for children and young adolescents with acute myeloid leukemia: a Dutch study on incidence, survival and mortality. Leukemia. 2019;33:1349–1359. doi: 10.1038/s41375-018-0314-7. [DOI] [PubMed] [Google Scholar]
- 38.Statline. https://opendata.cbs.nl/#/CBS/nl/
- 39.Ahmad O.B., Boschi-Pinto C., Lopez A.D., Murray C.J.L., Lozano R., Inoue M.I. 2001. Age Standardization of Rates: A New WHO Standard. [Google Scholar]
- 40.Rothman K.J., Greenland S., Lash T.L. 3rd Edition. Lippincott Williams & Wilkin; 2008. Modern Epidemiology. [Google Scholar]
- 41.Sankila R., Martos Jiménez M.C., Miljus D., Pritchard-Jones K., Steliarova-Foucher E., Stiller C. Geographical comparison of cancer survival in European children (1988-1997): report from the Automated Childhood Cancer Information System project. Eur. J. Cancer. 2006;42:1972–1980. doi: 10.1016/j.ejca.2006.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon reasonable request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.