Summary
Background
Despite recent improvements in the treatment of cancer, little is known about the long-term survival in patients with cancer and venous thromboembolism. We aimed to examine the five-year mortality of venous thromboembolism in cancer patients in a large population-based cohort study.
Methods
Using Danish healthcare registries from 1995 to 2020, we obtained data on cancer patients with venous thromboembolism and comparison cohorts of cancer patients without venous thromboembolism, matched in terms of cancer type, age, sex, and year of cancer diagnosis, and adjusted for level of comorbidity and frailty using the Charlson Comorbidity Index Score and Hospital Frailty Risk Score, marital status, use of selected medications, and recent surgery (<90 days).
Findings
During the study period, 886,536 patients were diagnosed with cancer. Of 1882 cancer patients diagnosed at the time of their venous thromboembolism, 44.4% (835/1882) had distant metastases. In this cohort, the one- and five-year mortality cumulative incidences were 68% (1284/1882) and 84% (1578/1882), respectively, in contrast to 38% (2135/5549) and 67% (3653/5549) in the comparison cohort. The mortality rate ratio was 4.34 (95% confidence interval [CI], 3.95–4.78) for the first year of follow-up and 3.44 (95% CI 3.17–3.73) for the five-year follow-up period. Of the 23,366 patients diagnosed with venous thromboembolism after cancer diagnosis, 18% (4183/23,366) had distant metastases at the time of cancer diagnosis. The cumulative incidence of death at one year was 45% (10,465/23,366; mortality rate ratio 3.48, 95% CI 3.37–3.60) and at five years 69% (15,669/23,366; mortality rate ratio 2.57, 95% CI 2.50–2.63).
Interpretation
Despite improved cancer treatment, venous thromboembolism in cancer patients is strongly associated with a poor prognosis.
Funding
The study was supported by grants from the Independent Research Fund Denmark (record no. 3101-00102B) and the Karen Elise Jensen Foundation.
Keywords: Cancer, Cohort study, Prognosis, Pulmonary embolism, Venous thrombosis
Research in context.
Evidence before this study
We considered the evidence from cohort studies on the association between venous thromboembolism and survival in cancer patients. We searched MEDLINE on March 23, 2023, with no date or language restriction, using the terms ‘cancer’, ‘venous thromboembolism’, and ‘survival’. Current literature has unequivocally shown that venous thromboembolism is associated with a poor prognosis in cancer patients, independent of the cancer type. Studies have demonstrated a two to three-fold increased risk of death in cancer patients with venous thromboembolism compared to those without venous thromboembolism. However, it is unknown whether the strength of the association has changed over the past decades with the advent of new cancer therapies and overall improved survival.
Added value of this study
This nationwide cohort study provides contemporary evidence that venous thromboembolism remains a strong predictor for a worse prognosis. The mortality rate ratio was 4.34 (95% confidence interval [CI] 3.95–4.78) for the first year of follow-up and 3.44 (95% CI 3.17–3.73) for the five-year follow-up period, adjusted for a broad set of potential confounders. These data demonstrate that cancer associated venous thromboembolism remains a serious complication in cancer patients despite substantial improvements in cancer treatment in recent decades.
Implications of all the available evidence
This study provides clinicians with contemporary estimates on the prognostic significance of cancer-associated venous thromboembolism for mortality in the era of modern cancer therapy, in particular when venous thromboembolism is diagnosed concurrently with cancer. These data can be used for the purpose of prognostication and to inform patients.
Introduction
Venous thromboembolism encompasses deep venous thrombosis and pulmonary embolism. It is predominantly a disease of elderly people. The association between venous thromboembolism and cancer has been known for almost 200 years.1 Specifically, cancer patients have a nine-fold higher venous thromboembolism risk than the general population,2 as well as significantly higher rates of bleeding and recurrence during anticoagulant treatment than venous thromboembolism patients without cancer.3, 4, 5 The occurrence of venous thromboembolism is associated with interruption of cancer treatment, decreased quality of life, and increased morbidity and mortality.6, 7, 8, 9
Cancer patients with venous thromboembolism have shorter survival than cancer patients without venous thromboembolism.10,11 However, the majority of existing studies evaluating mortality associated with venous thromboembolism among cancer patients are relatively old, based on small sample sizes or selected populations, or focused on short-term mortality.10, 11, 12, 13, 14, 15 Cancer therapy has evolved tremendously in recent decades with the advent of targeted therapies and immunotherapy, as well as novel cancer screening tools and better diagnostic modalities.16,17 Concurrently with improved cancer outcomes, the one-year venous thromboembolism incidence among cancer patients has increased from 1% to more than 3% in recent decades, which may be the result of ageing of the oncology population, frequent detection of asymptomatic clots on diagnostic or staging scans, and use of prothrombotic cancer therapies.2 Notably, targeted therapy and immunotherapy have been identified as a strong risk factor for venous thromboembolism in addition to distant metastases and chemotherapy.2
Therefore, data on the relation between venous thromboembolism and cancer are important as they could foster better understanding of both and provide further insight into the clinical course of patients with venous thromboembolism and cancer in the context of new therapies. Accordingly, we undertook a large population-based cohort study to examine the current five-year mortality of venous thromboembolism in cancer patients.
Methods
Study design and setting
We conducted this cohort study based on the entire Danish population including 7,859,209 residents alive between January 1, 1995 and December 31, 2018. The Danish National Health Service provides universal tax-supported health care with free access to general practitioners and hospitals.18 At birth, each Danish citizen (and residents upon immigration) is assigned a unique personal identification number that links them to all health registries.18 We used the Danish Cancer Registry18 to identify all cancer patients with venous thromboembolism during the study period. The Cancer Registry has recorded all incident cases of cancer in Denmark since 1943. In this registry, the extent of tumor spread at the time of diagnosis is classified as localized, regional, metastatic to distant sites, or unknown. Venous thromboembolism cases were identified through the Danish National Patient Registry, which contains records of all discharges and surgical procedures from all Danish hospitals since 1977, and on emergency room and outpatient clinic visits since 1994.18 A description of the registries used in this study can be found in the Supplementary Material.
Venous thromboembolism and comparison cancer cohorts
We identified two venous thromboembolism cancer cohorts: 1) venous thromboembolism with concurrent cancer diagnosis, and 2) venous thromboembolism after a cancer diagnosis. The cohort with venous thromboembolism with concurrent cancer diagnosis included patients diagnosed with cancer during the venous thromboembolism hospitalization (median length of hospitalization: 11 days [interquartile range, IQR, 4–23]). The cohort with venous thromboembolism after a cancer diagnosis included patients with venous thromboembolism diagnosed after the cancer date (median time between cancer diagnosis and venous thromboembolism: 2.6 years (IQR, 0.5–8.1). Two comparison cohorts of cancer patients without a venous thromboembolism diagnosis were randomly sampled in a ratio as high as 3:1, matched on year of birth (ten-year intervals), sex, year of cancer diagnosis (ten-year intervals), cancer types (23 subgroups according to the papers by Mulder et al.2,19), and cancer stage (localized, regional, metastatic to distant sites, or unknown). Information on cancer stage was missing for 18% of the patients but they were included in the analysis, and unknown stage was considered a separate stage category during the matching process. We defined the index date as the venous thromboembolism admission date in the venous thromboembolism cohort and as the matching date in the comparison cancer cohorts.
Outcome
We obtained data on all-cause mortality from the Danish Civil Registration System, which has recorded all changes in vital status and immigration since 1968.18
Covariates
Information on comorbidities was obtained using the Charlson Comorbidity Index, which includes 19 major medical conditions, all surgical procedures, use of central venous catheter, and frailty according to the Hospital Frailty Risk Score, which is based on 109 frailty-related diagnostic codes20 from the National Patient Registry.21 Cancer treatment in the first four months after cancer diagnosis, including chemotherapy, radiotherapy, surgical procedures, hormonal therapy, and targeted therapy, was defined from the Danish Cancer Registry until 2004, and from the Danish National Patient Registry and the Danish National Prescription Registry thereafter.2 Recent surgery was defined as a surgical procedure within 90 days prior to the index date in the National Patient Registry. Marital status was defined from the Danish Civil Registration System. We also obtained information on the use of other selected medications with potential impact on the coagulation system (vitamin K antagonists, direct oral anticoagulants, low-molecular-weight heparins, statins, aspirin, opioids, glucocorticoids, antihypertensive agents, and loop diuretics) within 60 days before the index date. Supplementary Table S1 lists all diagnostic, medication, and treatment codes used in the study.
Statistical analysis
Members of the four cohorts were categorized by cancer type and stage, cancer treatment determined at time of cancer diagnosis, age group, sex, calendar period, marital status, surgery, central venous catheter, use of selected other medications, Charlson Comorbidity Index Score and the Hospital Frailty Risk Score measured at time of venous thromboembolism/index date. The four cohorts were followed from the index date until five-years of follow up or December 31st, 2020, death, or emigration, whichever came first. We used the Kaplan–Meier method to compute mortality incidences. Mortality rates per 1000 person-years were also calculated. We used Cox proportional hazards regression analysis to compute hazard ratio as a measure of mortality rate ratio when comparing the venous thromboembolism cohort with the comparison cohort. No major violation of the proportional hazards assumption was observed by visual inspection of log-minus-log plots, although the initially higher differences appeared to decline over time. Therefore, we present a series of average hazard ratios for increasingly longer periods of follow-up (0–1 month, 0–3 months, 0–1 year, and 0–5 years).22
We included the matching factors, age (as a continuous variable), marital status, Charlson Comorbidity Index Score (0, 1, 2, 3+), Hospital Frailty Risk Score (0, 1–5, 6+), recent surgery, and use of selected other medications within 60 days prior to the index date in the regression model. The matching factors were controlled by the study design. We also performed stratified analyses by venous thromboembolism type (pulmonary embolism, deep venous thrombosis), by cancer stage and site, and by calendar period of venous thromboembolism diagnosis.
In a sensitivity analysis, we started the follow-up one month after the index date, using the landmark approach to ascertain the robustness of our estimates. This analysis excluded the initial follow-up period with higher mortality and therefore ensured better fulfillment of the proportionality of hazards assumption.
All analyses were conducted using SAS version 9.4 (SAS Institute, INC, Cary, NC, USA). The study was reported to the Danish Data Protection Agency (record number 2016-051-000001-811). We followed the reporting guidelines recommended by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (please see the STROBE statement).
Role of the funding source
None of the funding organizations had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit.
Results
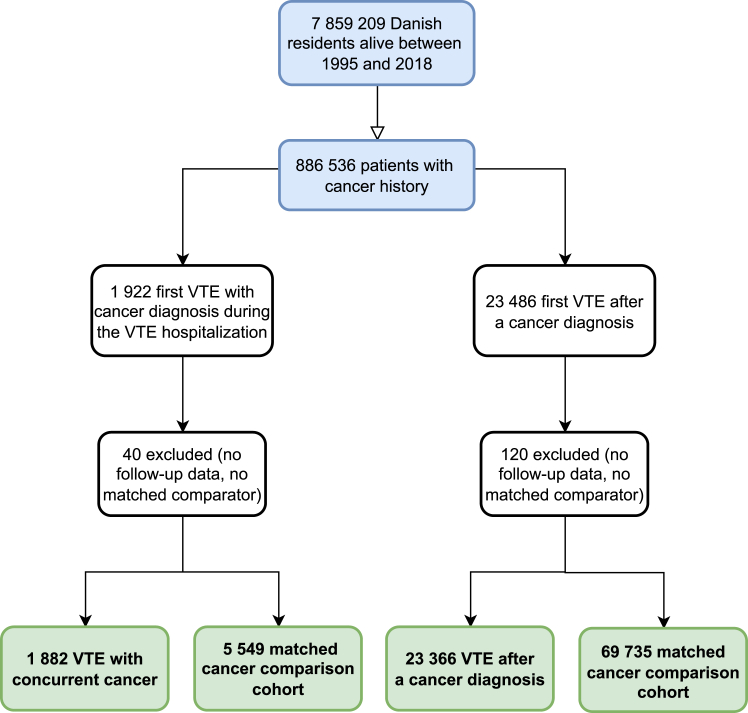
During the study period, 886,536 patients were diagnosed with cancer. A total of 100,532 patients with a cancer diagnosis were included in the analysis, 25,248 (25.1%) of whom had venous thromboembolism, and 75,284 (74.9%) were included in the comparison cohort. Venous thromboembolism was diagnosed concurrently with cancer in 1882 patients (7.5%), while venous thromboembolism was diagnosed after cancer diagnosis in 23,366 patients (92.5%) (Fig. 1). In the group with venous thromboembolism after cancer diagnosis, the median time between cancer diagnosis and venous thromboembolism was 2.6 years (interquartile range [IQR], 0.5–8.1).
Fig. 1.
Study flowchart illustrating thecohorts.
Table 1 summarizes the demographics, characteristics, comorbidities, surgery, other treatments, and types of cancer in the four cohorts. More than half of the patients were older than 70 years, and the majority was female. Overall, the most common cancer types were lung (25.2%), colorectal (18.7%), pancreatic (13.4%), and ovarian (7.2%) in the venous thromboembolism with concurrent cancer diagnosis and comparison cohorts, and 44.4% had distant metastases.
Table 1.
Baseline characteristics of the venous thromboembolism and comparison cancer cohorts, Denmark, 1995–2018.
| Venous thromboembolism concurrent with a cancer diagnosis |
Venous thromboembolism after a cancer diagnosis |
|||
|---|---|---|---|---|
| Venous thromboembolism cohort N = 1882 |
Comparison cancer cohort N = 5549 |
Venous thromboembolism cohort N = 23,366 |
Comparison cancer cohort N = 69,735 |
|
| Female, n (%) | 1006 (53.5) | 2955 (53.3) | 12,666 (54.2) | 37,797 (54.2) |
| Age at VTE/index date, median (IQR), years | 71.2 (62.5–79.1) | 71.3 (62.7–78.4) | 71.8 (63.9–79.4) | 71.6 (63.6–79.1) |
| Age at VTE/index date, years | ||||
| <65 | 584 (31.0) | 1729 (31.2) | 6533 (28.0) | 19,954 (28.6) |
| 65–69 | 288 (15.3) | 838 (15.1) | 3652 (15.6) | 10,935 (15.7) |
| 70–79 | 614 (32.6) | 1878 (33.8) | 7778 (33.3) | 23,203 (33.3) |
| 80+ | 396 (21.0) | 1104 (19.9) | 5403 (23.1) | 15,643 (22.4) |
| Year of VTE diagnosis/index date, n (%) | ||||
| 1995–1999 | 316 (16.8) | 930 (16.8) | 1814 (7.8) | 5414 (7.8) |
| 2000–2004 | 417 (22.2) | 1223 (22.0) | 2856 (12.2) | 8505 (12.2) |
| 2005–2009 | 359 (19.1) | 1067 (19.2) | 4611 (19.7) | 13,766 (19.7) |
| 2010–2014 | 464 (24.7) | 1362 (24.5) | 7106 (30.4) | 21,217 (30.4) |
| 2015–2018 | 326 (17.3) | 967 (17.4) | 6979 (29.9) | 20,833 (29.9) |
| Overall follow-up time, median (IQR), years | 0.3 (0.1–1.8) | 1.9 (0.5–5.0) | 1.5 (0.2–4.7) | 4.3 (1.9–5.0) |
| Marital status at VTE/index date | ||||
| Married/registered partnership | 919 (48.8) | 3076 (55.4) | 12,624 (54.0) | 37,050 (53.1) |
| Single/divorced | 273 (14.5) | 732 (13.2) | 3055 (13.1) | 9563 (13.7) |
| Widow | 457 (24.3) | 1286 (23.2) | 5591 (23.9) | 16,260 (23.3) |
| Unknown | 233 (12.4) | 455 (8.2) | 2096 (9.0) | 6862 (9.8) |
| Cancer type, n (%) | ||||
| Esophageal | 27 (1.4) | 77 (1.4) | 253 (1.1) | 737 (1.1) |
| Stomach | 56 (3.0) | 159 (2.9) | 408 (1.7) | 1205 (1.7) |
| Colon | 268 (14.2) | 798 (14.4) | 2519 (10.8) | 7543 (10.8) |
| Rectal | 82 (4.4) | 244 (4.4) | 1451 (6.2) | 4345 (6.2) |
| Liver | 48 (2.6) | 124 (2.2) | 111 (0.5) | 310 (0.4) |
| Pancreas | 254 (13.5) | 741 (13.4) | 723 (3.1) | 2140 (3.1) |
| Non-small cell lung | 443 (23.5) | 1324 (23.9) | 2449 (10.5) | 7321 (10.5) |
| Breast | 69 (3.7) | 206 (3.7) | 4288 (18.4) | 12,847 (18.4) |
| Cervix | 23 (1.2) | 65 (1.2) | 551 (2.4) | 1642 (2.4) |
| Uterus | 39 (2.1) | 117 (2.1) | 1136 (4.9) | 3395 (4.9) |
| Ovary | 135 (7.2) | 401 (7.2) | 794 (3.4) | 2371 (3.4) |
| Prostate | 104 (5.5) | 312 (5.6) | 2752 (11.8) | 8251 (11.8) |
| Kidney | 61 (3.2) | 180 (3.2) | 537 (2.3) | 1600 (2.3) |
| Bladder | 33 (1.8) | 99 (1.8) | 943 (4.0) | 2810 (4.0) |
| Brain | 14 (0.7) | 40 (0.7) | 383 (1.6) | 1144 (1.6) |
| Hodgkin’s lymphoma | <5 | 150 (0.6) | 423 (0.6) | |
| Non-Hodgkin’s lymphoma | 79 (4.2) | 235 (4.2) | 1035 (4.4) | 3093 (4.4) |
| Leukemia | 61 (3.2) | 183 (3.3) | 692 (3.0) | 2068 (3.0) |
| Melanoma | <10 | 1089 (4.7) | 3257 (4.7) | |
| Multiple myeloma | 23 (1.2) | 68 (1.2) | 474 (2.0) | 1404 (2.0) |
| Testicular | 8 (0.4) | 23 (0.4) | 255 (1.1) | 762 (1.1) |
| Biliary | 17 (0.9) | 40 (0.7) | 93 (0.4) | 248 (0.4) |
| Small cell lung | 27 (1.4) | 80 (1.4) | 280 (1.2) | 819 (1.2) |
| Cancer stage at diagnosis, n (%)a | ||||
| Localized | 253 (13.4) | 755 (13.6) | 8538 (36.5) | 25,548 (36.6) |
| Regional | 375 (19.9) | 1116 (20.1) | 6421 (27.5) | 19,178 (27.5) |
| Distant | 835 (44.4) | 2439 (44.0) | 4183 (17.9) | 12,425 (17.8) |
| Missing | 419 (22.3) | 1239 (22.3) | 4224 (18.1) | 12,584 (18.0) |
| Cancer treatment, n (%)b | ||||
| No treatment | 783 (41.6) | 1114 (20.1) | 3008 (12.9) | 9720 (13.9) |
| Hormone therapy | 76 (4.0) | 223 (4.0) | 1928 (8.3) | 5457 (7.8) |
| Surgery | 701 (37.2) | 2986 (53.8) | 15,476 (66.2) | 46,848 (67.2) |
| Radiotherapy | 164 (8.7) | 730 (13.2) | 4335 (18.6) | 12,201 (17.5) |
| Chemotherapy | 466 (24.8) | 2099 (37.8) | 7698 (32.9) | 20,537 (29.5) |
| Targeted therapy | 77 (4.1) | 244 (4.4) | 1373 (5.9) | 2942 (4.2) |
| Surgical procedure, n (%)c | 271 (14.4) | 1114 (20.1) | 6330 (27.1) | 8964 (12.9) |
| Central venous catheter, n (%)d | 34 (1.8) | 285 (5.1) | 1914 (8.2) | 3980 (5.7) |
| Charlson Comorbidity Index at VTE/index date, n (%) | ||||
| 0 | 1215 (64.6) | 3239 (58.4) | 12,758 (54.6) | 42,568 (61.0) |
| 1 | 392 (20.8) | 1311 (23.6) | 5727 (24.5) | 15,381 (22.1) |
| 2 | 169 (9.0) | 566 (10.2) | 2676 (11.5) | 6752 (9.7) |
| 3+ | 106 (5.6) | 433 (7.8) | 2205 (9.4) | 5034 (7.2) |
| Hospital Frailty Risk Score at VTE/index date, n (%) | 946 (50.3) | 2204 (39.7) | 6508 (27.9) | 24,478 (35.1) |
| No comorbidity burden (0) | ||||
| Low comorbidity burden (1–5) | 728 (38.7) | 2563 (46.2) | 11,572 (49.5) | 33,198 (47.6) |
| Moderate/severe comorbidity burden (≥6) | 208 (11.1) | 782 (14.1) | 5286 (22.6) | 12,059 (17.3) |
| Use of selected medications, n (%)e | ||||
| Antihypertensives | 560 (29.8) | 1522 (27.4) | 7568 (32.4) | 21,646 (31.0) |
| Vitamin K antagonist | 71 (3.8) | 99 (1.8) | 706 (3.0) | 1930 (2.8) |
| Direct oral anticoagulants | 13 (0.7) | 44 (0.8) | 321 (1.4) | 883 (1.3) |
| Low-molecular-weight heparin | 12 (0.6) | 42 (0.8) | 355 (1.5) | 265 (0.4) |
| Statins | 160 (8.5) | 480 (8.7) | 2276 (9.7) | 8220 (11.8) |
| Aspirin | 210 (11.2) | 542 (9.8) | 2458 (10.5) | 7661 (11.0) |
| Opioids | 356 (18.9) | 1497 (27.0) | 7289 (31.2) | 10,909 (15.6) |
| Glucocorticoids | 116 (6.2) | 556 (10.0) | 3557 (15.2) | 3613 (5.2) |
| Loop diuretics | 216 (11.5) | 573 (10.3) | 3389 (14.5) | 5324 (7.6) |
Abbreviations: VTE, venous thromboembolism; IQR, interquartile range.
For solid cancers and lymphoma.
Treatments received during the first 4 months after cancer diagnosis. Treatments were not mutually exclusive. Targeted therapy treatments available from 2004 to onwards.
Within three months prior VTE date/index date.
Central venous catheter data available from 1999 to onwards.
Within 60 days prior to VTE date/index date.
In the venous thromboembolism after cancer diagnosis and comparison cohorts, the most common cancer types were breast (18.4%), colorectal (17.0%), prostate (11.8%), and lung (11.7%), and 17.9% had distant metastases.
Venous thromboembolism with a concurrent cancer diagnosis
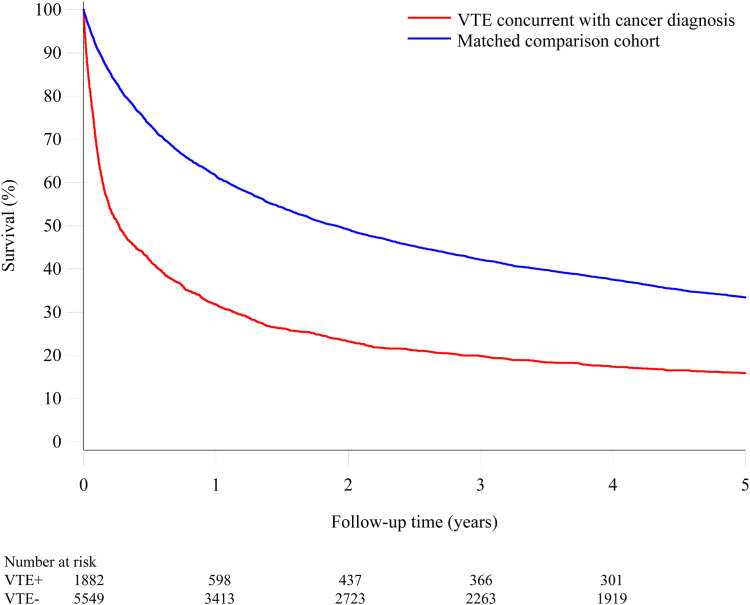
Fig. 2 shows the survival curves for patients in whom cancer was diagnosed at the time of an episode of venous thromboembolism and the comparison cohort. In the venous thromboembolism cohort, the cumulative mortality incidence after one month was 27.7% in contrast to 7.5% in the comparison cancer cohort (mortality rate ratio 6.30, 95% confidence interval [CI], 5.31–7.48), after three months 48.7% in contrast to 17% (mortality rate ratio 5.80, 95% CI 5.12–6.57), at one year 68.2% in contrast to 38.5% (mortality rate ratio 4.34, 95% CI 3.95–4.78), and 84.1% after 5 years in contrast to 66.6% (mortality rate ratio 3.44, 95% CI 3.17–3.73) (Table 2).
Fig. 2.
Survival curves for patients with venous thromboembolism concurrent with a cancer diagnosis and a matched comparison cancercohort.
Table 2.
Mortality risks, rates, and mortality rate ratios overall and by follow-up time.
| Venous thromboembolism concurrent with a cancer diagnosis |
Venous thromboembolism after a cancer diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Follow-up time | No. of persons | Mortality risk (95% CI) | Mortality rate per 1000 person-years (95% CI) | Mortality rate ratio (95% CI) | No. of persons | Mortality risk (95% CI) | Mortality rate per 1000 person-years (95% CI) | Mortality rate ratio (95% CI) |
| 0–1 month | ||||||||
| Comparison cohort | 5549 | 7.5 (6.9–8.3) | 939 (849–1029) | 69,735 | 2.2 (2.1–2.3) | 262 (248–275) | ||
| VTE cohort | 1882 | 27.7 (25.7–29.8) | 3944 (3605–4282) | 6.30 (5.31–7.48) | 23,366 | 13.8 (13.3–14.2) | 1790 (1728–1852) | 6.08 (5.66–6.54) |
| 0–3 months | ||||||||
| Comparison cohort | 5549 | 17.0 (16.0–18.0) | 762 (713–810) | 69,735 | 5.7 (5.6–5.9) | 240 (233–248) | ||
| VTE cohort | 1882 | 48.7 (46.5–51.0) | 2935 (2745–3125) | 5.80 (5.12–6.57) | 23,366 | 25.4 (24.8–26.0) | 1229 (1197–1260) | 4.72 (4.49–4.95) |
| 0–1 year | ||||||||
| Comparison cohort | 5549 | 38.5 (37.2–39.8) | 510 (488–532) | 69,735 | 16.8 (16.5–17.1) | 186 (182–189) | ||
| VTE cohort | 1882 | 68.2 (66.1–70.3) | 1472 (1391–1552) | 4.34 (3.95–4.78) | 23,366 | 44.8 (44.2–45.4) | 657 (644–670) | 3.48 (3.37–3.60) |
| 0–5 years | ||||||||
| Comparison cohort | 5549 | 66.6 (65.3–67.8) | 270 (261–278) | 69,735 | 42.3 (41.9–42.7) | 118 (117–120) | ||
| VTE cohort | 1882 | 84.1 (82.4–85.8) | 662 (629–694) | 3.44 (3.17–3.73) | 23,366 | 68.6 (68.0–69.2) | 308 (303–313) | 2.57 (2.50–2.63) |
Venous thromboembolism after a cancer diagnosis
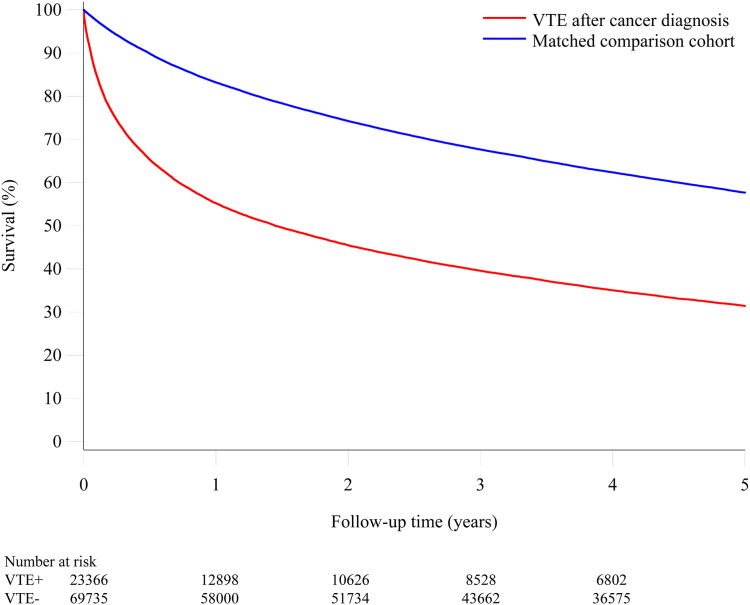
Fig. 3 shows the survival curves for patients in whom venous thromboembolism was diagnosed after the cancer diagnosis and the corresponding comparison cohort. In the venous thromboembolism cohort, the mortality after one month was 13.8% in contrast to 2.2% in the comparison cohort (mortality rate ratio 6.08, 95% CI 5.66–6.54), after three months 25.4% in contrast to 5.7% (mortality rate ratio 4.72, 95% CI 4.49–4.95), one year 44.8% in contrast to 16.8% (mortality rate ratio 3.48, 95% CI 3.37–3.60) and 68.6% after five years in contrast to 42.3% (mortality rate ratio 2.57, 95% CI 2.50–2.63) (Table 2).
Fig. 3.
Survival curves for patients with venous thromboembolism after a cancer diagnosis and a matched comparison cancercohort.
The mortality was much higher in patients with pulmonary embolism than patients with deep venous thrombosis (Supplementary Table S3), for patients with pulmonary embolism concurrent with a cancer diagnosis or with pulmonary embolism after a cancer diagnosis. In the pulmonary embolism cohort, the mortality after one year in patients with pulmonary embolism concurrent with a cancer diagnosis was 73%, in contrast to 39.3% in the comparison cohort (mortality rate ratio 5.10, 95% CI 4.51–5.78). In patients with pulmonary embolism after a cancer diagnosis, the one-year mortality was 49.3%, in contrast to 17.3% in the comparison cohort (mortality rate ratio 4.00, 95% CI 3.82–4.18). In the deep venous thrombosis cohort, the one-year mortality was 60.9%, in contrast to 37.3% in the comparison cohort (mortality rate ratio 3.50, 95% CI 3.00–4.10). In the cohort with deep venous thrombosis after a cancer diagnosis, the one-year mortality was 39.6%, in contrast to 16.2% in the comparison cohort (mortality rate ratio 2.93, 95% CI 2.78–3.07).
The sensitivity analysis showed that the results were consistent with those of our main analyses when we excluded the first month of follow-up after the index date (Supplementary Table S5).
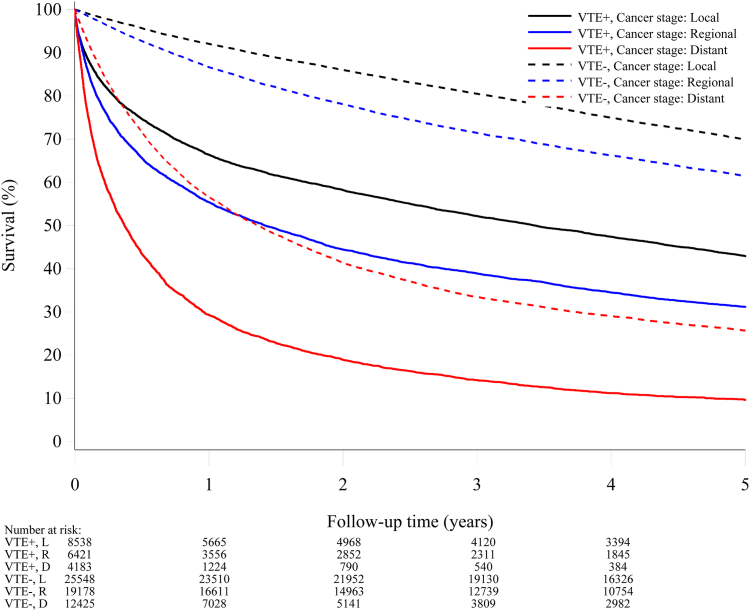
Venous thromboembolism, cancer stage, and cancer site
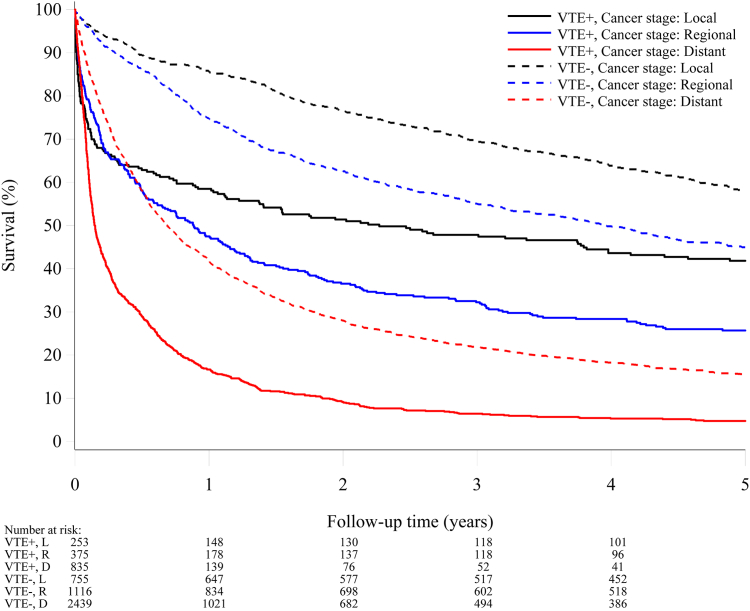
Figs. 4 and 5 show the survival curves stratified according to the cancer stage for the venous thromboembolism and comparison cohorts. The prognosis was, as expected, better for patients with local and regional cancer stages compared to those with distant metastases, where very few patients were alive after two years of follow-up. Notably, within all strata of cancer stage, venous thromboembolism was a strong predictor for poor survival (Supplementary Figures S1 and S2).
Fig. 4.
Survival curves for patients with venous thromboembolism concurrent with a cancer diagnosis and a matched comparison cancer cohort, by cancerstage.
Fig. 5.
Survival curves for patients with venous thromboembolism after a cancer diagnosis and a matched comparison cancer cohort, by cancerstage.
For patients with venous thromboembolism concurrent with a cancer diagnosis and the comparison cohort, the five-year adjusted mortality rate ratio was 3.17 (95% CI 2.45–4.10) for localized disease, 2.84 (95% CI 2.36–3.41) for regional spread, and 3.39 (95% CI 3.01–3.82) for distant metastases (Supplementary Figure S1).
The corresponding mortality rate ratios for patients with venous thromboembolism after a cancer diagnosis and the comparison cohorts were 2.69 (95% CI 2.57–2.82) for no spread, 2.84 (95% CI 2.70–2.99) for regional spread, and 2.14 (95% CI 2.04–2.26) for distant metastases (Supplementary Figure S2).
The mortality risk for the major individual cancer sites can be found in Supplementary Table S4. The highest one-year mortality was 97.6% for patients with pancreatic cancer and lowest for non-Hodgkin’s lymphoma (44.3%) in the cohort with venous thromboembolism concurrent with a cancer diagnosis. The one-year mortality risks in the venous thromboembolism after a cancer diagnosis cohort were 88.7% for pancreatic cancer and 32.0% for breast cancer with the lowest risk.
Stratified analysis by calendar period of venous thromboembolism
In an analysis of venous thromboembolism diagnosis stratified by calendar period, we observed that the mortality risk slightly decreased in recent years in patients in whom venous thromboembolism was diagnosed after the cancer diagnosis. The five-year cumulative mortality incidence was 72.0% between 1995 and 1999, and 65.6% between 2015 and 2018. In the matched comparison cancer cohort, the five-year mortality risk was 46.8% between 1995 and 1999, and 39.5% between 2015 and 2018. This trend was less pronounced in patients with venous thromboembolism diagnosed concurrently with cancer (five-year mortality 85.4% between 1995 and 1999 vs. 81.3% between 2015 and 2018). However, regardless of the absolute risks, venous thromboembolism remained a strong and stable predictor of five-year mortality in both the cohort with venous thromboembolism after cancer diagnosis (mortality rate ratio 2.63 [95% CI 2.41–2.87] between 1995 and 1999 vs. 2.55 [95% CI 2.43–2.68] between 2015 and 2018) and the cohort with venous thromboembolism diagnosed concurrently with cancer (mortality rate ratio 4.02 [95% CI 3.27–4.93] between 1995 and 1999 vs. 3.26 [95% CI 2.66–3.99] between 2015 and 2018) (Supplementary Table S2).
Discussion
In this large population-based study of more than 25,000 patients with cancer-associated venous thromboembolism, venous thromboembolism was associated with a poor prognosis compared to cancer patients without venous thromboembolism. The mortality risk was particularly high when cancer was diagnosed concurrently with a thromboembolic event; only approximately 32% and 16% of these patients were alive after one and five years, respectively. Furthermore, within strata of cancer stage, venous thromboembolism was a strong predictor for an adverse prognosis. Pulmonary embolism was associated with poorer prognosis than venous thrombosis. Some cancers such as pancreatic cancer and lung cancer had a particularly poor prognosis. Our findings could not be explained by age, gender, marital status, major comorbidities, frailty, or recent surgery or co-medications. Although survival improved during the study period, venous thromboembolism remained associated with a poor prognosis in recent years.
This study shows that cancer associated venous thromboembolism remains a serious complication in cancer patients despite substantial improvements in cancer treatment in recent decades. Our data are consistent with the limited data available on the short-term and long-term prognoses of patients with both cancer and venous thromboembolism. A slightly higher survival was reported by Ording et al. in a Danish study focusing on patients who developed venous thromboembolism one year after cancer diagnosis with a one-year mortality of 48%.12 Small studies have shown that venous thromboembolism is a common cause of death among patients with cancer,23,24 but it is unclear what proportion of deaths in our study were directly caused by venous thromboembolism.
Our data are also consistent with those from a recently published analysis from the Scandinavian Thrombosis and Cancer (STAC) cohort, which has indicated a 3.4-fold greater mortality risk in cancer patients with than without venous thromboembolism.15 However, the study did not report data beyond one year, trends over time, or analyses stratified by timing of venous thromboembolism (i.e., concurrently with a cancer diagnosis or thereafter).
The current findings extend our earlier comprehensive analysis, which showed that only 12% of patients with a cancer diagnosis at the time of venous thromboembolism were alive one year after being diagnosed with cancer before 1993.11 There might be several explanations for this increase in survival from 12% to 32% over the last three decades. First and most importantly, cancer treatment has improved significantly due to the introduction of several novel classes of drugs, including targeted therapies and immunotherapy.16 Secondly, unlike our earlier analysis, our study also included outpatients diagnosed with venous thrombosis. Thirdly, the prognosis of venous thromboembolism itself may have improved due to better treatment and management of pulmonary embolism and its sequelae. Fourthly, increased diagnostic sensitivity of imaging techniques has led to detection of incidental and less extensive cases of venous thromboembolism, which may be associated with a better prognosis.25,26
Despite improvements in the survival of patients with venous thromboembolism over the past three decades, venous thromboembolism remained a strong and stable predictor of mortality. The higher mortality risk in the cohort of patients with venous thromboembolism and a concurrent cancer diagnosis is likely to be explained by a higher proportion of aggressive cancers (e.g., pancreatic and non-small cell lung cancer), a higher proportion of patients with metastatic disease, and a higher proportion of patients identified in the early time period (1995–1999), when novel cancer treatments were not yet available. Our cancer site-specific analysis showed a particularly poor prognosis for these cancer sites.
The poor prognosis of venous thromboembolism in cancer patients is incompletely understood. The highest risk of venous thromboembolism is associated with aggressive types of cancer such as those originating from the pancreas, ovary, stomach, and lung.2 Our cancer site-specific analysis also showed a particularly poor prognosis for these cancer sites. An activated coagulation system can help tumors metastasize by evading the immune system.27 Venous thromboembolism is also more frequent in patients with advanced disease than in those with localized disease.2 Therefore, venous thromboembolism may be merely an epiphenomenon mediated by biologically aggressive cancers rather than a direct cause of poor survival. This is supported by the notion that clinical risk assessment tools for venous thromboembolism and hemostatic biomarkers also identify patients with poor survival.28 Patient-related risk factors for venous thromboembolism, such as surgery, immobilization, other complications and comorbidities, and co-medications, might also contribute to poor prognosis.2 Nonetheless, the association between venous thromboembolism and all-cause mortality persisted despite matching and comprehensive adjusting for these factors and was stronger among patients with pulmonary embolism compared to deep venous thrombosis. This observation suggests that at least some deaths may be attributed to venous thromboembolism itself, interruption or cessation of cancer therapy, or the complications of anticoagulant treatment.
Several guidelines now recommend using reduced-dose apixaban or rivaroxaban or prophylactic-dose low-molecular-weight heparin in ambulatory cancer patients judged to be at high risk of venous thromboembolism.29, 30, 31, 32, 33 Although such an approach may mitigate the burden of venous thromboembolism and its sequelae, no studies have yet demonstrated a benefit in survival associated with prophylactic anticoagulation therapy.34,35 The lack of clinical detail and the inability to validly ascertain causes of death in the present study precludes conclusions about a potential causal relation between venous thromboembolism and mortality.
Our study has several strengths and limitations. Our cohort study was conducted in a setting in which health care services are provided free of charge. The study had the strength of being large and population-based with virtually no loss to long-term follow-up. The validity of our findings is dependent on the quality of the registry data. The study included all patients with a hospital diagnosis of cancer, venous thromboembolism, comorbidities, and procedures, thereby preventing distortion of selection, referral, and diagnostic biases. The clinical diagnosis of venous thromboembolism can be difficult, and the recorded venous thromboembolism diagnosis that we used might have included some misclassification. However, the validity of the venous thromboembolism diagnosis in the Danish National Patient Registry has been confirmed to be 88–90% of cases.36 The same applies to procedure, comorbidity, and prescription data.21,37,38 The validity of the cancer diagnosis is high, and the completeness and positive predictive value of cancer diagnoses in the Cancer Registry have been shown to be as high as 95–98% in validation against medical records.39,40 All these biases would tend to be conservative and result in underestimation of the strength of the associations among cancer, venous thromboembolism, and mortality.
In conclusion, our contemporary data show that venous thromboembolism is associated with a poor prognosis in cancer patients, particularly when it is diagnosed concomitantly with cancer. Despite improvements in cancer therapy, venous thromboembolism remains a strong and consistent predictor of all-cause mortality.
Contributors
HTS had the study idea. HTS, LAP, and EHP developed the concept of the study. HTS, LAP, NVE, HRB, and EHP contributed to the design of the study. HTS and EHP acquired the data. HTS directed the analyses, which were carried out by EHP. HTS wrote the initial draft. All authors contributed to the discussion and interpretation of the results, which secured the intellectual content of the manuscript. All authors accepted the final version for submission.
Data sharing statement
No additional data will be made available due to data security issues. According to Danish legislation, informed consent and approval from an ethics committee are not required for registry-based studies.
Declaration of interests
The Department of Clinical Epidemiology, Aarhus University, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study.
Acknowledgements
Funding: The study was supported by grants from the Independent Research Fund Denmark (record no. 3101-00102B) and the Karen Elise Jensen Foundation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100739.
Appendix A. Supplementary data
References
- 1.Bouillaud S., Bouillaud J. De l’obliteration des veines et de son influence sur la formation des hydropisies partielles: consideration sur la hydropisies passive et general. Arch Gen Med. 1823;1:188–204. [Google Scholar]
- 2.Mulder F.I., Horvath-Puho E., van Es N., et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 3.Chee C.E., Ashrani A.A., Marks R.S., et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. 2014;123(25):3972–3978. doi: 10.1182/blood-2014-01-549733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prandoni P., Lensing A.W., Piccioli A., et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J.I., Haas S., Ageno W., et al. Cancer associated thrombosis in everyday practice: perspectives from GARFIELD-VTE. J Thromb Thrombolysis. 2020;50(2):267–277. doi: 10.1007/s11239-020-02180-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee A.Y., Levine M.N., Baker R.I., et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 7.Lee A.Y.Y., Kamphuisen P.W., Meyer G., et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 8.Khorana A.A., Yannicelli D., McCrae K.R., et al. Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. doi: 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Kahn S.R., Springmann V., Schulman S., et al. Management and adherence to VTE treatment guidelines in a national prospective cohort study in the Canadian outpatient setting. The recovery study. Thromb Haemost. 2012;108(3):493–498. doi: 10.1160/TH12-03-0169. [DOI] [PubMed] [Google Scholar]
- 10.Chew H.K., Wun T., Harvey D., Zhou H., White R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 11.Sørensen H.T., Mellemkjaer L., Olsen J.H., Baron J.A. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 12.Ording A.G., Skjøth F., Søgaard M., et al. Increasing incidence and declining mortality after cancer-associated venous thromboembolism: a nationwide cohort study. Am J Med. 2021;134(7):868–876.e5. doi: 10.1016/j.amjmed.2021.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Heit J.A., Silverstein M.D., Mohr D.N., Petterson T.M., O'Fallon W.M., Melton L.J., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999;159(5):445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber S.Z., Visani L., De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 15.Crobach M.J., Anijs R.J., Brækkan S.K., et al. Survival after cancer-related venous thrombosis: the Scandinavian thrombosis and cancer study. Blood Adv. 2023;7(15):4072–4079. doi: 10.1182/bloodadvances.2022009577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro C.L. Cancer survivorship. N Engl J Med. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 17.Quaresma M., Coleman M.P., Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385(9974):1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M., Schmidt S.A.J., Adelborg K., et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulder F.I., Horváth-Puhó E., van Es N., et al. Arterial thromboembolism in cancer patients: a Danish population-based cohort study. JACC CardioOncol. 2021;3(2):205–218. doi: 10.1016/j.jaccao.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert T., Neuburger J., Kraindler J., et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen S.K., Christiansen C.F., Christensen S., Lash T.L., Sørensen H.T. The predictive value of ICD-10 diagnostic coding used to assess Charlson Comorbidity Index conditions in the population-based Danish national registry of patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernán M.A. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 24.Gimbel I.A., Mulder F.I., Bosch F.T.M., et al. Pulmonary embolism at autopsy in cancer patients. J Thromb Haemost. 2021;19(5):1228–1235. doi: 10.1111/jth.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder F.I., Di Nisio M., Ay C., et al. Clinical implications of incidental venous thromboembolism in cancer patients. Eur Respir J. 2020;55(2) doi: 10.1183/13993003.01697-2019. [DOI] [PubMed] [Google Scholar]
- 26.Le Gal G., Kovacs M.J., Bertoletti L., et al. Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation: a multicenter prospective cohort study. Ann Intern Med. 2022;175(1):29–35. doi: 10.7326/M21-2981. [DOI] [PubMed] [Google Scholar]
- 27.Ward M.P., Kane L.E., Norris L.A., et al. Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Mol Cancer. 2021;20(1):59. doi: 10.1186/s12943-021-01347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moik F., Ay C. Hemostasis and cancer: impact of haemostatic biomarkers for the prediction of clinical outcomes in patients with cancer. J Thromb Haemost. 2022;20(12):2733–2745. doi: 10.1111/jth.15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyman G.H., Carrier M., Ay C., et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falanga A., Brenner B., Khorana A.A., Francis C.W. Thrombotic complications in patients with cancer: advances in pathogenesis, prevention, and treatment-a report from ICTHIC 2021. Res Pract Thromb Haemost. 2022;6(5) doi: 10.1002/rth2.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Key N.S., Khorana A.A., Kuderer N.M., et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO guideline update. J Clin Oncol. 2023;41(16):3063–3071. doi: 10.1200/JCO.23.00294. [DOI] [PubMed] [Google Scholar]
- 32.Streiff M.B., Holmstrom B., Angelini D., et al. Cancer-associated venous thromboembolic disease, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(10):1181–1201. doi: 10.6004/jnccn.2021.0047. [DOI] [PubMed] [Google Scholar]
- 33.Farge D., Frere C., Connors J.M., et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23(7):e334–e347. doi: 10.1016/S1470-2045(22)00160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch F.T.M., Mulder F.I., Kamphuisen P.W., et al. Primary thromboprophylaxis in ambulatory cancer patients with a high Khorana score: a systematic review and meta-analysis. Blood Adv. 2020;4(20):5215–5225. doi: 10.1182/bloodadvances.2020003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schünemann H.J., Ventresca M., Crowther M., et al. Evaluating prophylactic heparin in ambulatory patients with solid tumours: a systematic review and individual participant data meta-analysis. Lancet Haematol. 2020;7(10):e746–e755. doi: 10.1016/S2352-3026(20)30293-3. [DOI] [PubMed] [Google Scholar]
- 36.Sundbøll J., Adelborg K., Munch T., et al. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottegard A., Schmidt S.A.J., Wallach-Kildemoes H., Sørensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46(3) doi: 10.1093/ije/dyw213. 798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storm H.H. Completeness of cancer registration in Denmark 1943-1966 and efficacy of record linkage procedures. Int J Epidemiol. 1988;17(1):44–49. doi: 10.1093/ije/17.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Storm H.H., Michelsen E.V., Clemmensen I.H., Pihl J. The Danish cancer registry--history, content, quality and use. Dan Med Bull. 1997;44(5):535–539. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.