Abstract
This study aims to evaluate the safety, biocompatibility, and functionality of a new accommodative intraocular lens (IOL) (LUZ, patent PCT/ES2016/070,813) after implantation in rabbit eyes. LUZ (Study) and EyeCee® plus a capsular ring (Control) were implanted in rabbits (n = 8 each) after phacoemulsification. Intraoperative follow-up, long-term clinical follow-up, and functional IOL studies were carried out periodically for up to 180 days. A macroscopic examination of the eyeballs to reveal abnormalities and determine the implant centering and a microscopic examination to semi-quantify cell and tissue response were performed. Statistical analysis of the collected data was finally achieved. During follow-up, no significant changes in the general condition nor the clinical evaluation were observed between both groups. However, Study IOL remained centered throughout the study and did not present severe complications as observed in the Control group. Functional studies did not reveal significant differences between both materials. Study showed better centering, fewer adhesions, and maintenance of an opening capsular bag compared to the Control. Local biological effects caused by Study implantation are minimal and comparable to the Control. Therefore, LUZ showed no clinical signs or histological response of adverse reaction to the implanted material, according to UNE-EN ISO 11979-5 and 10993-6. Functionality must be confirmed in another animal species with greater lens accommodation capacity than the rabbit. LUZ keeps the capsular bag open, favoring its centering and avoiding fibrosis and adherence to the bag; this allows potential accommodation of this IOL and theoretically enables the patient to focus dynamically.
Keywords: Presbyopia, Accommodative lens, Intraocular lens, Preclinical test, Biocompatibility
1. Introduction
Since the implantation of the first intraocular lens (IOL) [1], the industry has been improving both its quality and refractive capabilities. Lens intervention is one of the most common ocular surgeries in the world [2]. However, extracting the lens from its capsule and replacing it with an IOL does not restore the ideal functions of the young-adult lens, such as its accommodative capacity. Accommodation is the dioptric change in the power of the eye when focusing on different distances. This fact led to the continuous evolution of the IOL, first from monofocal optics to refractive or diffractive multifocal ones and, more recently, to the EDOF [Extended-Depth-Of-Focus], with extended focus, and the accommodative lenses [[3], [4], [5]]. Some examples of these accommodative or pseudo-accommodative lenses, which have shown favorable but limited results, are the simple optics accommodative lenses, such as Crystalens AT-45 and AT-50 (Bausch-Lomb, Rochester, New York), 1CU (HumanOptics, Erlangen, Germany), Biocomfold 43E lens (Morcher, Stuttgart, Germany), C-Well (Acuity Ltd., OrYehuda, Israel); WIOL-CF (Gelmed, Praha, Czech Republic), OPAL (Bausch-Lomb), Tek-Clear (Tekia, Irvine, California), Tetatraflex KH-3500 (Lenstec Inc, St. Petersburg, Florida), or Fluidvision (Alcon, Fort Worth, Texas); and the dual optics lens, such as Synchrony (Visiogen, Irvine, California) [2]. However, these IOLs have not yet been accommodated, so their use has not been extended in routine clinical practice. Therefore, recovering the accommodative capacity of the presbyopic eye is a demand of today's society and represents a considerable scientific challenge.
Restoring accommodation does not simply mean providing the presbyopic eye with static and functional near vision, such as that obtained with multifocal IOL, EDOF, or monovision, but restoring the eye's dynamics and natural continuous focusing ability [2,[4], [5], [6], [7], [8]]. These types of lenses provide a functional distance and near vision. However, the active change in the dioptric power of the eye is not restored as it occurs during the accommodation of a young-adult eye [5,8]. Multifocal IOL are pseudo-accommodative because they provide near-functional vision without using the eye's accommodative mechanisms. Optical multifocality increases the focus with multiple simultaneous foci for different distances. However, the quality of near and far images is compromised, resulting in decreased contrast sensitivity and visual acuity for all distances [[3], [4], [5],8]. Other factors that can increase the eye's depth of focus include small pupils and optical aberrations, such as spherical aberration or astigmatism [2]. Therefore, restoring the natural accommodation of the presbyopic eye, providing a range of clear vision at all distances, just like the emmetropic young-adult eye, would be ideal. Understanding the accommodative anatomy and mechanisms and the causes of presbyopia are necessary [3,6,[9], [10], [11]]. In this sense, presbyopia is due mainly to the increased rigidity of the lens [5]. Thus, restoring the accommodative capacity of the presbyopic eye by using an accommodating artificial IOL would be the key to presbyopia treatment [3,6,8,10,11].
This study aimed to evaluate the safety, biocompatibility, and potential functionality of a new accommodative lens designed by ophthalmologists for presbyopic eyes after implantation in rabbit eyes. Specifically, this lens works using the accommodative instruments of the eye itself, such as the elasticity of its capsule and the ciliary muscle, which facilitate the posteroanterior displacement of the optic and changes in its curvature. Therefore, theoretically, the lens focuses dynamically, both far and near.
2. Results
2.1. Unexpected events throughout the study
The day before surgery, articular problems in the left hind leg of one animal (Rabbit 7, Control) were detected during the general evaluation and was excluded from the study (Table 1). The rest of the animals had normal findings upon complete general and ophthalmic examinations and underwent the surgical implantation procedure.
Table 1.
Animal groups (Study: Accommodative LUZ IOL; or Control: EyeCee® IOL) and randomization of the rabbits and implanted eyes. *Rabbit 7 (Control) was excluded from the study before implantation. **Rabbits 2 (Study) and 11 (Control) were lost by undetermined causes unrelated to the implanted material. ***Rabbit 8 (Control) was sacrificed at 60 days follow-up due to scleral rupture by IOL incarceration. IOL: intraocular lens.
| Randomization |
||
|---|---|---|
| Rabbit number | Implanted eye | |
| Study (n = 8) | 1 | Left |
| 2** | Left | |
| 3 | Left | |
| 5 | Left | |
| 6 | Left | |
| 9 | Right | |
| 14 | Right | |
| 16 | Left | |
| Control (n = 8) | 4 | Right |
| 7* | Right | |
| 8*** | Left | |
| 10 | Right | |
| 11** | Right | |
| 12 | Left | |
| 13 | Left | |
| 15 | Left | |
Two animals died during follow-up, one of them (Rabbit 2, Study group) for undetermined causes unrelated to the implanted material and another (Rabbit 11, Control group) associated with a persistent diarrheal process not associated with the implanted material (Table 1).
2.2. Implantation procedure
During the surgical process for implantation of the IOL, no contacts between the test material and the corneal endothelium, collapse of the anterior chamber, bleeding in the anterior chamber, or damage to the iris were detected. In general, the haptics' location and the location/centering of the IOL's optic were adequate. However, the Study group showed significantly better values than the Control (p-value 0.0256; Supplementary 1, Table 1). Regarding intraocular pressure during the implantation procedure, the Study group showed lower levels (Supplementary 1, Tables 2–4).
2.3. General condition follow-up
The weight of the animals increased progressively throughout the study, with the weight of the animals in the Study group being higher at 180 days (Supplementary 2, Tables 1–4). Throughout the study, none of the animals presented alterations in their general condition regarding the variables evaluated: attitude inside and outside the cage, mucous membranes, hair, and skin. No significant differences were observed between groups or study times.
2.4. Ophthalmologic follow-up
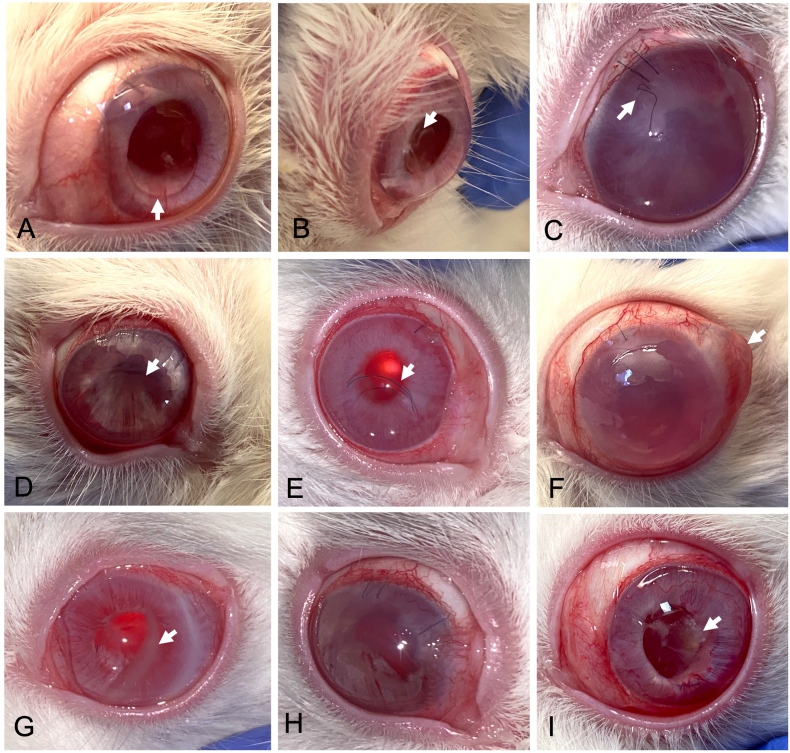
The results obtained in the ophthalmological examination throughout the study and after their statistical analysis are similar between the Control and Study groups. In-depth statistical analysis of the ophthalmologic follow-up data is described in Supplementary 3, specifically: intraocular pressure (S3, Tables 1–3 and Fig. 1); conjunctival congestion (S3, Tables 4–7 and Fig. 2); conjunctival discharge (S3, Tables 8–11 and Fig. 3); conjunctival swelling (S3, Tables 12–15 and Fig. 4); aqueous flare (S3, Tables 16–19 and Fig. 5); light reflex (S3, Tables 20–23 and Fig. 6); iris involvement (S3, Tables 24–27 and Fig. 7); cornea (S3, Tables 28–31 and Fig. 8); cornea cloudiness (S3, Tables 32 and 33, and Fig. 9); pannus (S3, Tables 34–37 and Fig. 10); material clearness (S3, Tables 38 and 39, and Fig. 11); IOL centering (S3, Tables 40 and 41, and Fig. 12); and haptics location (S3, Tables 42 and 43, and Fig. 13). In brief, higher values were observed in the appearance of aqueous flare and corneal opacity in the Study group without becoming statistically significant. The Study IOL remains centered throughout the entire follow-up; however, the IOL of the Control group appeared displaced in various animals. Among complications, we can highlight conjunctivitis, corneal edema, transient increases in IOP that resolve with medical treatment, and posterior synechiae (iris-IOL). Furthermore, the Control group showed displacement of the IOL into the anterior chamber (n = 3, Rabbits 4, 8 and 13), marked buphthalmia (n = 2, Rabbits 1 and 6), and scleral rupture (n = 1, Rabbit 8) due to incarceration of the IOL leading to the execution of the clinical endpoint at 60 days follow-up. Noteworthy findings are illustrated in Fig. 1(A–I).
Fig. 1.
Ophthalmological follow-up, noteworthy findings. (A, arrow) Ring protruding into the anterior chamber in the ventral region (Day 7; Rabbit 8, Control). (B, arrow) Decentered IOL (Day 7; Rabbit 12, Control). (C) Considerable increase in intraocular pressure causing dehiscence of the corneal suture, conjunctival congestion, and corneal cloudiness (Day 15; Rabbit 1, Study). (D) Iris's deformity compatible with posterior synechiae (Day 30; Rabbit 8, Control). (E) IOL in the anterior chamber and mucous secretion (Day 45; Rabbit 4, Control). (F) Buphthalmia and a soft oval-shaped mass in the sclero-conjunctival region prevent complete palpebral occlusion (Day 45; Rabbit 8, Control). (G) Corneal opacity (Day 90; Rabbit 15, Control). (H) Mild conjunctivitis and marked iris deformation (Day 120; Rabbit 1, Study). (I) Yellowish residue on the IOL (Day 150; Rabbit 12, Control). Study: Accommodative LUZ IOL; Control: EyeCee® IOL.
Regarding the functional studies, no significant differences were observed between the Control and Study groups throughout the follow-up.
2.5. Histological evaluation
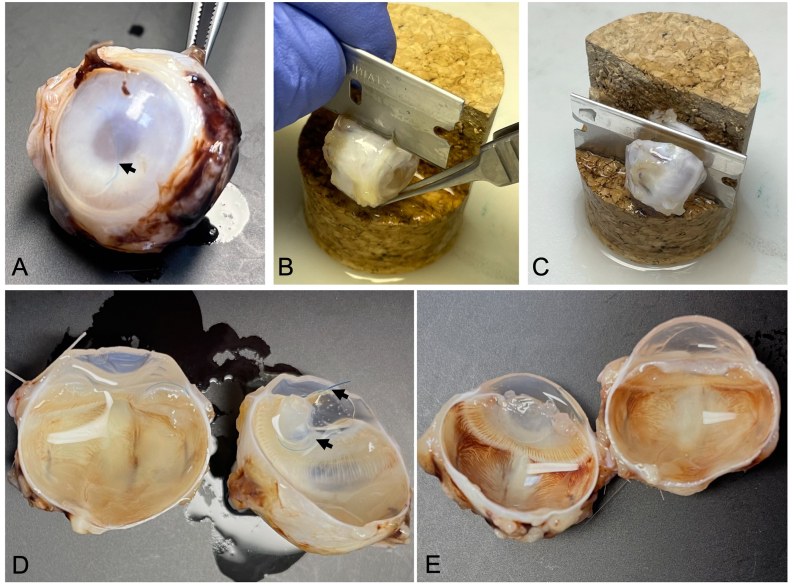
In the Control group (Fig. 2A–D), the hemidissection of the eyeballs and their macroscopic examination after 180 days of follow-up revealed dislocations of the IOL and the capsular ring and adhesions of these elements with the capsular bag and the iris, while in the Study group adhesions were not observed (Fig. 2E). The Study IOL remained centered in the capsular bag (Fig. 2E).
Fig. 2.
Recovery of study material. (A) Eyeball after fixation showing the IOL dislocated in the anterior chamber (arrows, Rabbit 4, Control); (B and C, Rabbit 12, Control) Eyeball hemidissection process; (D, Rabbit 12, Control; and E, Rabbit 9, Study) Dissected eyeballs, the IOL location (D, arrows) and the open lens bag are appreciated. Study: Accommodative LUZ IOL; Control: EyeCee® IOL. IOL: intraocular lens.
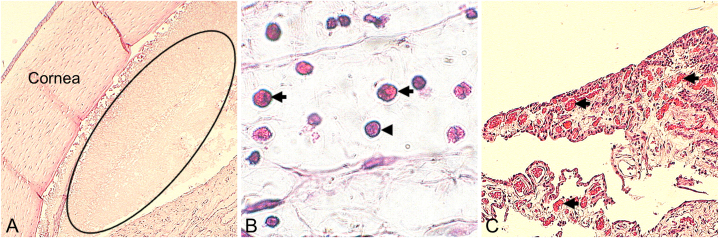
Among the noteworthy histological findings were the presence of fibrin in the anterior chamber, infiltrates of inflammatory cells in the anterior uvea, or neovascularization in the ciliary body, both in the Control and Study groups (Fig. 3A–C). In-depth semi-quantitative evaluation of the local biological effects after implantation is described in Table 2. Thus, under the conditions of this study, the tissue response of the Study material was considered minimal/no response (2.73) compared to the reaction to the Control sample.
Fig. 3.
Remarkable histological findings. (A, Rabbit 1, Study. Magnification: 4×) presence of fibrin in the anterior chamber (ellipse). (B, Rabbit 2, Study. Magnification: 60×) Inflammatory cell infiltrates anterior uvea (arrows: heterophils; arrowhead: lymphocyte). (C, Rabbit 12, Control. Magnification: 10×) neovascularization in the ciliary body (arrows). Study: Accommodative LUZ IOL; Control: EyeCee® IOL.
Table 2.
Semi-quantitative assessment of histological findings according to UNE-EN ISO 10993-6 Annex E. Study: Accommodative LUZ IOL; Control: EyeCee® IOL. L: left eye implanted. R: right eye implanted.
| Test sample | LUZ sterile accommodative intraocular lens (LUZ Global, Valencia, Spain) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Implantation interval | 180 days | |||||||||||
| Control sample | EyeCee® 3-piece preloaded intraocular lens (Bausch + Lomb, NY, USA) and rigid PMMA capsular ring (AJL Ophthalmics S.A., Álava, Spain) | |||||||||||
| Rabbit number/Implanted eye | Study | Control | ||||||||||
| 1/L | 3/L | 5/L | 6/L | 9/R | 14/R | 16/L | 4/R | 10/R | 12/L | 13/L | 15/L | |
| F1. Inflammation | ||||||||||||
| Lymphocytes | 0.42 | 0.08 | 0.08 | 1.00 | 0.00 | 0.50 | 0.83 | 0.33 | 0.08 | 0.17 | 0.58 | 0.25 |
| Plasmatocytes | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Polymorphonuclear (Heterophilic) | 2.25 | 0.08 | 0.33 | 3.17 | 0.67 | 2.83 | 0.42 | 0.17 | 1.08 | 0.08 | 0.25 | 0.50 |
| Macrophages | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Giant cells | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Necrosis | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SUB-TOTAL (x2) | 5.67 | 0.33 | 0.83 | 8.33 | 1.33 | 6.67 | 2.50 | 1.00 | 2.33 | 0.50 | 1.67 | 1.50 |
| F2. Neovascularization | 0.17 | 1.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 |
| Fibrin deposits | 1.75 | 0.00 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Adipose infiltrate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SUB-TOTAL | 1.92 | 1.00 | 0.17 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 |
| TOTAL (F1 + F2) | 7.58 | 1.33 | 1.00 | 8.67 | 1.33 | 6.67 | 2.50 | 1.00 | 2.33 | 0.50 | 1.75 | 1.50 |
| Group total | 29.08 | 7.08 | ||||||||||
| AVERAGE ** | 4.15 | 1.42 = 2,73 | ||||||||||
| Traumatic necrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Strange detritus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of histological sections examined*** | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
*It is worth mentioning that the “group total” value was calculated from n = 7 “test samples” and n = 5 “control samples” at 180 days after implantation.
**It is used to determine the irritant rating shown below as the conclusion. A negative difference is recorded as zero.
***The histological evaluation rating represents the averaged value for the given animal, considering the number of histological sections examined.
3. Discussion
The possibility of restoring the accommodative capacity of the eye after lens intervention using accommodating IOL is a demand of today's society and still a goal to be achieved [12,13]. In this sense, restoring the natural accommodation of the presbyopic eye, providing a range of clear vision at all distances, as occurs in the emmetropic eye of the young adult, would be ideal. This study demonstrated the safety and biocompatibility of a new accommodative lens (LUZ; LUZ Global S.L., Valencia, Spain) by surgical and clinical follow-up and histological analysis after implantation in rabbit eyes and compared with commercially available EyeCee® IOL plus intraocular ring (Control). This study is the preliminary step to evaluate the LUZ accommodative lens before its implantation in the human eye since the cataract extraction model in rabbits is considered a valuable tool for extrapolating biocompatibility results to the human being [[14], [15], [16], [17]].
The implantation procedure and clinical and histological evaluations were performed following the standards UNE-EN ISO 11979-5 and 10993-6 [18,19]. We selected the rabbit species and a duration of 180 days for the experiment, as the ISO standard reads “the rabbit is the first to consider due to its long history of use in ophthalmic studies and its availability” and “If the rabbit is chosen for ocular implantation, the duration of the study is six months. The rabbit is subject to fibrin formation and rapid cell regrowth, making long-term biocompatibility difficult to assess. Since the rabbit eye is known to be more reactive, a six-month study is considered appropriate” [18]. Furthermore, observation of the inflammatory response to lens extraction and lens implantation in the rabbit model at six months postoperatively would be comparable to that seen at two years in the human eye, as it is known that lens extraction in the rabbit induces earlier inflammatory responses compared to the human being [[14], [15], [16], [17]]. The choice of the number of animals follows the principles of the 3Rs and is similar to previous studies [15,[20], [21], [22], [23]]. Furthermore, the number of animals (n = 16) is determined by UNE-EN ISO 11979-5 Annex G [18], as it reads, “Based on the estimated abandonment rate and other health and welfare considerations of the chosen species, use a sufficient number of animals so that a minimum of six test eyes and six control eyes are available at the end of the follow-up period”. Although there were only five animals in the Control group at the end of the study, the detailed and in-depth statistical analysis allowed us to obtain results with enough statistical power despite losses during follow-up.
The selection of the EyeCee® IOL plus an intraocular ring under clinical use as Control is based on the combination of materials with which these elements are made. LUZ and EyeCee® are made of two materials: hydrophobic acrylic and PMMA. These materials have different hardness, thus eliciting a different intraocular response in the human eye [24,25]. However, by combining them with specific designs, a better adaption to the capsular bag and transmission of the eye's accommodative effort is sought. Furthermore, the implantation procedure in the capsular bag is similar for both IOL, through a corneal incision less than 3 mm. Therefore, both IOLs are equivalent to a safety and biocompatibility study.
Study and Control materials were implanted in all the rabbits without significant complications. Regarding the excellent location of the lens after the implantation procedure, the centering of the optics is considered equal between groups; however, the Control tended to be displaced, and the location of the haptics was better in the Study group. No remarkable modifications of the general condition of the rabbits were reported for 180 days follow-up. Results from the clinical evaluation are similar between the Control and Study groups. However, Study IOL remained centered throughout the study and did not show severe complications, such as the buphthalmia and scleral rupture observed in the Control group. Macroscopic evaluation after enucleation and hemidissection of the eyes showed better centration, lesser adhesions, and opened capsular bag in Study compared with the Control group, which allowed LUZ to be easily removed, unlike the Control. Semi-quantitative microscopic evaluation showed that local biological effects originating from the ocular implantation of Study are minimal and comparable to that of the Control, an IOL plus capsular ring under clinical use.
The separation effect of the anterior and posterior capsules and the ring's tension on the capsular bag reduce the capsule's fibrosis [15,17]. Less fibrosis and the maintenance of capsule elasticity are elements directly related to the accommodative capacity of the eye [13]. One of the disadvantages of accommodative lenses is the more significant capsular fibrosis compared to other lenses [12,26]. In this sense, Study IOL presents a significant difference since it theoretically acts by tensioning and separating the anterior and posterior capsule, factors that will lead to less fibrosis [15,17,27]. In this experiment, histological evaluation of LUZ-implanted eyes did not reveal posterior capsule fibrosis. However, histological evaluation could not corroborate the lens bag opening maintenance since, according to the ISO standards [18], the IOLs were extracted from the lens bag before their histological processing. However, capsular rings reduce capsular fibrosis and keep the sac open, thus maintaining the capsule's elasticity and favoring the lens's movement in accommodation [7].
Although functional studies did not reveal significant differences between Study and Control, we were aware that the rabbit model is not the ideal one to obtain valuable information on the potential accommodative functionality of LUZ. However, the main objective of this work has been to evaluate the safety and biocompatibility of the materials under study. According to ISO standards, the best species is the rabbit [18]. Based on the lesser fibrosis of the capsule and more excellent capsular elasticity obtained with LUZ, future functional studies with other animal species will be performed to elucidate the accommodative potential of LUZ before implantation in human subjects. Furthermore, maintaining an open intrasaccular space allows the lens and its optic to remain centered and unobstructed for accommodative movement [28]. Other authors have reported that using rings decreases fibrosis and capsular opacification [29,30]. Therefore, those new functional tests would help us understand how the accommodating capacity is maintained or improved compared to other IOL without the LUZ characteristics [26].
4. Conclusions
LUZ accommodative IOL after implantation in the rabbit capsular bag, clinical follow-up of 180 days, and histological evaluation did not reveal clinical signs or histological response of adverse reaction against the implanted material, in comparison with an IOL plus intracapsular ring under clinical use and following the UNE-EN ISO 11979-5 and 10,993-6 standards. Future experiments are necessary for an animal species with greater accommodation capacity than the rabbit to confirm the functionality of the LUZ lens. Due to its design, LUZ keeps the capsular bag open, favoring its centering and preventing its fibrosis and adherence to the capsular bag, which tends to the potential accommodation capacity of this IOL that theoretically will allow the patient to focus dynamically, both far and near.
5. Materials and methods
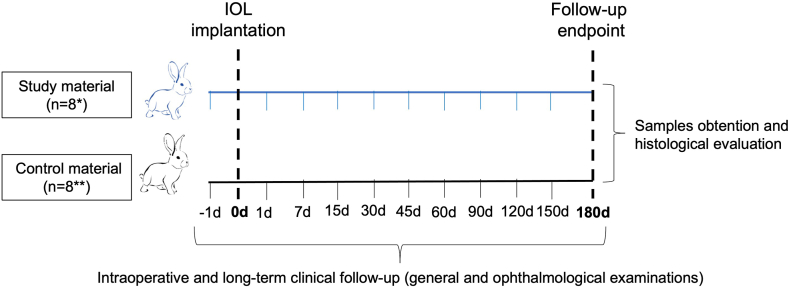
5.1. Study design
This study has been designed (Fig. 4) following the standards UNE-EN ISO 11979-5:2020 Ophthalmic implants. Intraocular lens - Part 5: biocompatibility [18]; and ISO 10993-6:2016 Biological evaluation of medical devices - Part 6: Tests for local effects after implantation [19].
Fig. 4.
Study design and timeline. This experiment followed UNE-EN ISO 11979-5:2020 Ophthalmic implants. Intraocular lens and ISO 10993-6:2016 Biological evaluation of medical devices. Animals were randomly divided into two groups (n = 8 each) according to the implant materials (Study: Accommodative LUZ IOL; or Control: EyeCee® IOL). During the implantation procedure, intraoperative follow-up was performed. Long-term clinical follow-up consisted of examining the animal's general condition and eyes on the day before surgery and periodically until the end of the study at 180 days. Finally, the eyes were enucleated and evaluated macro/microscopically. d: days; IOL: intraocular lens. *7 animals completed the follow-up. **5 animals completed the follow-up.
This study has been approved by the Ethics Committee on Animal Experimentation and Welfare (CEEBA) of the University of Valladolid and authorized by Junta de Castilla y León regional government before the start of the experiments (Registration code: 9202866), in agreement with the European (Council Directive 2010/63/EU) and Spanish regulations (RD 53/2013). In addition, experimental animals in this study followed the Association for Research in Vision and Ophthalmology (ARVO) recommendations for the benefit of animals in research in ophthalmology and vision sciences. Animal housing followed the European regulation with free access to food and water during the experiment.
Sixteen (n = 16; numbered 1 to 16) female rabbits (New Zealand White) weighing around 3 Kg at the implantation procedure time were used. Homogeneity between animals was ensured during the procedure. Animals were randomly divided into two groups (n = 8 each) according to the implant materials (Study or Control), and its assignment to a particular animal eye was provided by the online tool “Research Randomizer” (https://www.randomizer.org/) (Table 1). Each of the selected eyes only underwent a single procedure in this study.
5.2. Study material and control
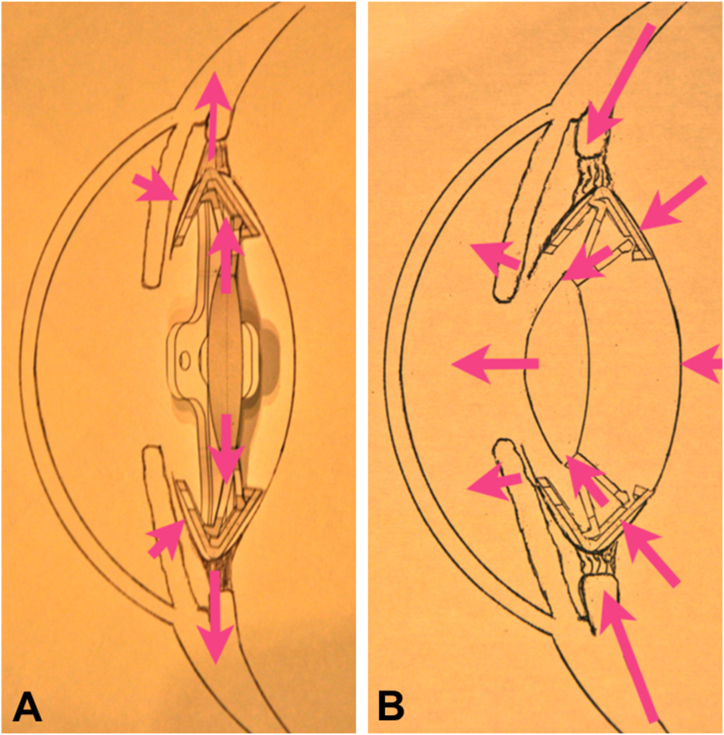
Study material: Accommodative LUZ IOL (Lens Undergone Zonula Global S.L., Valencia, Spain), patented (PCT/ES2016/070,813) [31]. LUZ is an accommodative IOL with two parts: one IOL of hydrophobic acrylic material and polymethylmethacrylate (PMMA) and one ring of silicone and PMMA. The ring is incomplete and consists of a rigid and a soft part. The rigid one is also deformable, engages the haptics of the IOL, and transmits the accommodation movement to the lens optic while keeping it stable. Due to its shape, the soft silicone part allows adaptation to the capsular bag, keeping it open and elastic, which allows the IOL to move freely within the ring. The IOL has a 5 mm diameter optic and consists of four soft and four rigid haptics. Both types of haptics keep the lens stable within the capsular bag. The rigid ones fit the IOL in the fixed ring and thus allow the optic to be deformed and moved forward, and the soft ones give tension to the capsular bag while preventing anterior dislocation of the optic. LUZ IOL is implanted through less than 3 mm corneal incisions with a routine surgical technique, like that required by any other ring or lens on the market. Theoretically, accommodation occurs when the optic moves posteroanterior within the capsular bag that the ring opens. Furthermore, the ring allows modification of the optic curvature and provides stability to the lens within the capsular bag during the accommodation mechanisms [9,12,13]. Thus, when implanting the LUZ IOL, it is fixed to the intrasaccular ring, which makes it possible to keep the capsular bag open and in tension, and therefore, its adaptation to the movements of the capsule during accommodation (Fig. 5 A-B and Supplementary 4 and 5).
Fig. 5.
Schematic representation of the Study intraocular lens (LUZ) inside the lens sac (intrasaccular). (A) LUZ lens resting. (B) LUZ lens accommodating.
Control animals' were implanted with EyeCee® IOL (Bausch + Lomb, NY, USA) and capsular ring (AJL Ophthalmics S.A., Álava, Spain). EyeCee® is a hydrophobic acrylic monofocal lens with PMMA haptics, and PMMA also makes the capsular ring. Both elements are implanted in the capsular bag through a less than 3 mm corneal incision.
5.3. Surgical technique
On the day of implantation, animals were administered, after a 12-h fasting period, analgesia, and anesthesia by intramuscular administration of buprenorphine (0.02 mg/kg; Buprecare Multidosis 0.3 mg/ml, Ecuphar NV, Belgium) and the combination of medetomidine (0.5 mg/kg; Sedator 1 mg/ml, Eurovet Animal Health BV, The Netherlands) and ketamine (25 mg/kg; Anesketin 100 mg/ml, Eurovet Animal Health BV). The absence of reflexes was used to monitor the level of anesthesia. As antibiotic and analgesic prophylaxis, benzathine/procaine benzylpenicillin (7 IU/kg; Benzatard 150,000 IU/ml; Laboratorios SYVA S.A.U., León, Spain) and butorphanol (0.1 mg/kg, Torphadine 10 mg/ml, Le Vet Beheer B.V., The Netherlands) were used by intramuscular and subcutaneous injection, respectively.
The exposed skin area and conjunctival sacs were disinfected with 5% povidone-iodine (Betadine®; Meda Manufacturing, Bordeaux, France). Then, one drop of topical anesthetic (Oxybuprocaine/Tetracaine, Colircusí Anestésico Doble®; Alcon Cusí S.A., Barcelona, Spain) and one drop of cycloplegic (Cyclopentolate hydrochloride, Colircusí Cycloplegic; Alcon Cusí S.A.) was instilled in the eye to be operated on.
The surgical procedure was performed by an experienced ophthalmologist expert in cataract surgery and IOL implantation techniques (LIO). A blepharostat was placed to keep the animal's eye open. Under a surgical microscope (Leica M220 F12 surgical; Leica Microsystems, Wetzlar, Germany), a 2.75 mm incision was made in the clear cornea, followed by viscoelastic injection (AJL VISC; AJL Ophthalmic S.A., Vitoria, Spain) in the anterior chamber and continuous circular capsulorhexis with the aid of a cistitome and capsulorhexis forceps. Hydrodissection was performed, and phacoemulsification removed the lens nucleus and cortical material entirely. 0.5 ml of epinephrine (1 mg/ml; B. Braun Medical S.A., Barcelona, Spain) was added per 500 ml irrigation solution to maintain pupillary dilation during the surgical procedure. The capsular bag was filled with viscoelastic (AJL VISC). An EyeCee® IOL and a capsular ring or an accommodative LUZ IOL were implanted in one eye of each randomly selected animal of the Control or Study (LUZ) group, respectively. Finally, the corneal incision was sutured with 9–0 nylon using a simple stitch pattern, and the anterior chamber was made watertight.
At the end of the surgical procedure, an intracameral injection of 0.1 ml of reconstituted cefuroxime (1 mg; Prokam 50 mg, Laboratoires Théa, Clermont-Ferrand, France) and subconjunctival injection of 0.25 ml of triamcinolone acetonide (Trigon Depot; Bristol-Myers Squibb, Anagni, Italy) were performed. Postoperative ocular treatment was performed with 1% ophthalmic solution of atropine sulfate (Colircusi Atropine; Alcon Cusi S.A.) daily for one week, and ophthalmic ointment polymyxin B sulfate, neomycin, and dexamethasone (Maxitrol; Alcon) every two days for three weeks. Furthermore, the animals were given intramuscular antibiotic therapy and subcutaneous analgesia for three days with benzathine/procaine benzylpenicillin (7 IU/kg) and butorphanol (0.1 mg/kg), respectively.
5.4. Clinical evaluation
During the implantation procedure, intraoperative follow-up was performed to assess possible contacts between the test material and the corneal endothelium, anterior chamber collapse, anterior chamber bleeding, iris damage, lens haptics placement, optic location/centering, or unusual surgical problems.
Long-term clinical follow-up consisted of an examination of the animal's general condition and eyes on the day before surgery and periodically until the end of the study at 180 days; thus, the animals were examined on days 0, 1, 7, 15, 30, 45, 60, 90, 120, 150, and 180.
Specifically, the attitude of the animals was evaluated, both inside and outside the cage; the condition of the mucous membranes (color, humidity, and capillary refill time); the state of the hair (alopecia and dirtiness); and the condition of the skin (wounds and erythema); and they were semi-quantified as the absence of alterations (0), presence of mild alterations (1), presence of moderate alterations (2) or presence of severe alterations (3). The weight of the animals was also recorded at the experiments' beginning and end.
A complete ophthalmologic examination was also performed. Changes in the conjunctiva, cornea, anterior chamber, iris, lens, vitreous, fundus, and intraocular pressure were evaluated according to the method described by Hackett and McDonald [32], which allows the grading of possible ophthalmic clinical findings in New Zealand White rabbits. Observations included evaluating fibrin, increased optical density in the aqueous humor (flare), cells; adhesions, neovascularization; corneal edema; clarity of material; haptic location; and lens centration. Evaluation of the anterior pole was performed by portable biomicroscope (Kowa SL-15; Kowa Optimed Inc., CA, USA); of the posterior pole by Panoptic™ ophthalmoscope (Welch Allyn Inc, Skaneateles Falls, NY, USA); and IOP was quantified by contact tonometry (Tono Pen Vet™ Tonometer; Reichert Inc. Depew, NY, USA). Macroscopic photography was used to represent the main ophthalmological findings.
Finally, functional IOL studies, refraction with and without the constriction of the ciliary muscle and the pupil (Colircusi Pilocarpina 2%; Alcon Cusi S.A.) to evaluate the potential refractive change in the rabbit's eye, were performed on days 30 and 150 after implantation. The spherical equivalents obtained before and after applying pilocarpine were compared. At least three measurements were performed on all operated eyes, both Control and Study groups, with an auto refractometer (AR-800; Nidek Co., LTD, Gamagori, Japan).
5.5. Histological evaluation
As previously described, the animals were sedated 180 days after the surgical procedure and euthanized by an intravenous overdose of sodium pentobarbital (200 mg/kg, Dolethal®, Vétoquinol, Cedex, France). The animals' death was confirmed by cessation of heartbeat and respiration. A conjunctival suture was conducted in the upper central region to facilitate sample orientation during tissue processing, and enucleation was performed.
The samples were fixed for at least 24 h in 10% formalin. The fixed eyeballs with the implanted IOL were then hemidisected anteroposteriorly, making a central superoventral cut so that two halves of the implanted materials could be entirely extracted.
An internal examination of the eyeball was performed to reveal any macroscopic abnormalities, the location of the implant, and its centering. The support and contact areas between the IOL and surrounding tissues were also examined. The study material was extracted, and the two halves of the eyeballs were processed and embedded in paraffin using an automatic tissue processor (Leica ASP300, Leica Microsystems, Wetzlar, Germany). Subsequently, serial slices of 4 μm thick were made at different levels. The samples were stained with hematoxylin-eosin (HE) and examined by standard light microscopy. Histological findings were assessed according to each sample's cellular and tissular components. Specifically, the presence of inflammation (such as lymphocytes, macrophages, and giant cells), neovascularization (fibrosis and adipose infiltrate), necrosis, and detritus, particularly at the materials contact point, were evaluated semi-quantitatively according to UNE-EN ISO 10993-6 Biological evaluation of medical devices - Part 6: Tests for local effects after implantation, Annex E [19]. Finally, values were totaled, and the average score was calculated for each Study and Control material. According to this ISO standard, it is considered that, under the conditions of the study, the Study sample (LUZ), in comparison with the reaction produced by the Control material, demonstrates minimal response or no response (0.0–2.9); slight reaction (3.0–8.9); moderate reaction (9.0–15.0); or severe reaction (above 15.1). Images were acquired with a Leica DM4000B light microscope and a Leica DFC490 digital camera (Leica Microsystems). The final processing and composition of the figures were performed with Pixelmator 3.8.2 Phoenix Software (Pixelmator Team, Vilnius, Lithuania).
5.6. Statistical analysis
The data generated in this study were collected in paper laboratory notebooks, transferred to Microsoft® Office Excel 2016 software sheets (Microsoft Corporation, Redmond, WA, USA), and subsequently analyzed with SPSS software (IBM SPSS statistic v23, SPSS Inc. Chicago, IL, USA).
5.6.1. Descriptive analysis
For the observed values and according to the nature of the variable, the following descriptive data are presented:
-
-
Quantitative variables: using the mean, together with the standard deviation (SD) and its 95% confidence interval (CI), median, minimum, and maximum. In addition, the normality hypothesis is tested using the Shapiro-Wilk test.
-
-
Ordinal variables: using the percentage of individuals at each level of the variable per visit and group together with their confidence interval. In addition, the following statistics are used: mean, median, 95% CI, 25th and 75th percentiles, interquartile range, minimum and maximum.
-
-
Qualitative variables: summarized using the percentage of individuals at each level of the variable per review and grouped with their CI.
5.6.2. Comparison of groups
According to the nature of the variable, the following descriptive data are presented:
-
-
Quantitative variables: the t-Student contrast for two independent samples are used to test the hypothesis of equality of means between groups. The homogeneity of variances is tested using Levene's test. If it is impossible to assume it, Welch's test is used when it is impossible to assume normality, the Mann-Whitney U test is used, and the nonparametric alternative of the t-Student test.
-
-
Ordinal variables: the Mann-Whitney U test is used to test the hypothesis of equality of means between two independent groups. The homogeneity of variances was tested using Levene's test.
-
-
Qualitative variables: the chi-square test is used to test the hypothesis of independence between the group and the levels of the variable. In some cases, it is impossible to guarantee the convergence of the statistic because the expected frequencies are minimal, in which Fisher's exact test is used. In other cases, the variable is constant (all individuals take the same value), which makes no sense to propose any contrast.
5.6.3. Comparison of animal revisions in each group
According to the nature of the variable, the following descriptive data are presented:
-
-
Quantitative variables: the Pillai trace is used as a multivariate contrast for the hypothesis of equality of visits. Pairwise comparisons are not performed since the number of reviews is high, and the adjustment for multiple comparisons is very restrictive. Instead, the means and their CI are plotted to assess differences.
-
-
Ordinal variables: the Friedman test is used to hypothesize equality of reviews. As in the previous case, pairwise comparisons are not performed since the number of studies is high, and the adjustment for multiple comparisons is very restrictive. Instead, the medians and their CI are plotted to assess the differences.
-
-
Qualitative variables: Cochran's overall Q test is used to test the hypothesis that all reviews are equal in the percentage of individuals with a certain level. As in the previous cases, pairwise comparisons are not performed since the number of studies is high, and the adjustment for multiple comparisons is very restrictive. Instead, the percentages and their CI are plotted to assess the differences.
Author contribution statement
Ivan Fernandez-Bueno; Luis Igancio Olcina: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Cristina Andrés-Iglesias: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kevin Puertas-Neyra; Ricardo Usategui-Martín: Performed the experiments.
Itziar Fernández-Martínez: Analyzed and interpreted the data.
Miguel José Maldonado-López: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding information
This work was supported by the Ministerio de Ciencia e Innovación (Spain) (Neotec Project EXP-00104711/SNEO-20171172). The Junta de Castilla y Leon 2021 predoctoral contract funded KPN.
Data availability statement
Data will be made available on request.
Declaration of competing interest
IFB and CAI are the ISO Technical Committee members involved in ISO 16672:2020 Ophthalmic Implants–Ocular Endotamponades. CAI receives funding from AJL Ophthalmic S.A. LIO is President and Technical Director of Lens Undergone Zonula Global S.L. (Valencia, Spain) and coinventor of the patent PCT/ES2016/070813.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19604.
Abbreviations
- CI
confidence interval
- EDOF
Extended-Depth-Of-Focus
- HE
hematoxylin-eosin
- IOL
intraocular lens
- ISO
International Organization for Standardization
- PMMA
polymethylmethacrylate
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Apple D.J., Sims J. Harold Ridley and the invention of the intraocular lens. Surv. Ophthalmol. 1996;40(4):279–292. doi: 10.1016/S0039-6257(96)82003-0. [DOI] [PubMed] [Google Scholar]
- 2.Glasser A. Restoration of accommodation. Curr. Opin. Ophthalmol. 2006;17(1):12–18. doi: 10.1097/01.ICU.0000193069.32369.E1. [DOI] [PubMed] [Google Scholar]
- 3.De Vries N.E., Nuijts R.M.M.A. Multifocal intraocular lenses in cataract surgery: literature review of benefits and side effects. J. Cataract Refract. Surg. 2013;39(2):268–278. doi: 10.1016/J.JCRS.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y.L., Jia S.B. Clinical application of accommodating intraocular lens. Int. J. Ophthalmol. 2018;11(6):1028–1037. doi: 10.18240/IJO.2018.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodov L., Reitblat O., Levy A., Assia E.I., Kleinmann G. Visual outcomes and patient satisfaction for trifocal, extended depth of focus and monofocal intraocular lenses. J. Refract. Surg. 2019;35(7):434–440. doi: 10.3928/1081597X-20190618-01. [DOI] [PubMed] [Google Scholar]
- 6.Glasser A. Restoration of accommodation: surgical options for correction of presbyopia. Clin. Exp. Optom. 2008;91(3):279–295. doi: 10.1111/J.1444-0938.2008.00260.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong H.S., Evans J.R., Allan B.D.S. Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery. Cochrane Database Syst. Rev. 2014;2014(5) doi: 10.1002/14651858.CD009667.PUB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoughi M., Einollahi B., Roshandel D., Sarimohammadli M., Feizi S. Visual and refractive outcomes of phacoemulsification with implantation of accommodating versus standard monofocal intraocular lenses. J. Ophthalmic Vis. Res. 2015;10(4):370–374. doi: 10.4103/2008-322X.176896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krag S., Andreassen T.T. Mechanical properties of the human lens capsule. Prog. Retin. Eye Res. 2003;22(6):749–767. doi: 10.1016/S1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 10.Marchini G., Pedrotti E., Sartori P., Tosi R. Ultrasound biomicroscopic changes during accommodation in eyes with accommodating intraocular lenses: pilot study and hypothesis for the mechanism of accommodation. J. Cataract Refract. Surg. 2004;30(12):2476–2482. doi: 10.1016/J.JCRS.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard T., Maier P., Böhringer D., et al. Comparison of two extended depth of focus intraocular lenses with a monofocal lens: a multi-center randomized trial. Graefes Arch. Clin. Exp. Ophthalmol. 2021;259(2):431–442. doi: 10.1007/S00417-020-04868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick H.B. Accommodative intraocular lenses: current status. Curr. Opin. Ophthalmol. 2005;16(1):8–26. doi: 10.1097/00055735-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Pepose J.S., Burke J., Qazi M.A. Benefits and barriers of accommodating intraocular lenses. Curr. Opin. Ophthalmol. 2017;28(1):3–8. doi: 10.1097/ICU.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 14.Guan J.J., Kramer G.D., MacLean K., et al. Optic replacement in a novel modular intraocular lens system. Clin. Exp. Ophthalmol. 2016;44(9):817–823. doi: 10.1111/CEO.12786. [DOI] [PubMed] [Google Scholar]
- 15.Kohl J.C., Werner L., Ford J.R., et al. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J. Cataract Refract. Surg. 2014;40(12):2113–2119. doi: 10.1016/J.JCRS.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Maclean K.D., Werner L., Kramer G.D., et al. Evaluation of stability and capsular bag opacification of a new foldable adjustable intraocular lens. Clin. Exp. Ophthalmol. 2015;43(7):648–654. doi: 10.1111/CEO.12526. [DOI] [PubMed] [Google Scholar]
- 17.Werner L., Ellis N., Heczko J.B., et al. In vivo evaluation of a new hydrophobic acrylic intraocular lens in the rabbit model. J. Cataract Refract. Surg. 2018;44(12):1497–1502. doi: 10.1016/J.JCRS.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 18.ISO – ISO 11979-5:2020 – ophthalmic implants — intraocular lenses — Part 5: biocompatibility. https://www.iso.org/standard/72602.html. Accessed June 22, 2022.
- 19.ISO – ISO 10993-6:2016 – biological evaluation of medical devices — Part 6: tests for local effects after implantation. https://www.iso.org/standard/61089.html. Accessed June 22, 2022.
- 20.Balendiran V., Werner L., Ellis N., et al. Uveal and capsular biocompatibility of a new hydrophobic acrylic microincision intraocular lens. J. Cataract Refract. Surg. 2020;46(3):459–464. doi: 10.1097/J.JCRS.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 21.Kramer G.D., Werner L., Maclean K., Farukhi A., Gardiner G.L., Mamalis N. Evaluation of stability and capsular bag opacification with a foldable intraocular lens coupled with a protective membrane in the rabbit model. J. Cataract Refract. Surg. 2015;41(8):1738–1744. doi: 10.1016/J.JCRS.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Werner L., Guan J.J., Reiter N., Mamalis N. Evaluation of long-term biocompatibility and capsular bag opacification with a new silicone-polyimide plate-type intraocular lens in the rabbit model. J. Cataract Refract. Surg. 2016;42(7):1066–1072. doi: 10.1016/J.JCRS.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 23.Ludlow J., Nguyen J., Aliancy J., et al. Long-term uveal and capsular biocompatibility of a novel modular intraocular lens system. Acta Ophthalmol. 2018;96(4):e427–e433. doi: 10.1111/AOS.13674. [DOI] [PubMed] [Google Scholar]
- 24.Kulnig W., Menapace R., Skorpik C., Juchem M. Tissue reaction after silicone and poly(methyl methacrylate) intraocular lens implantation: a light and electron microscopy study in a rabbit model. J. Cataract Refract. Surg. 1989;15(5):510–518. doi: 10.1016/S0886-3350(89)80107-5. [DOI] [PubMed] [Google Scholar]
- 25.Yan Q., Perdue N., Sage E.H. Differential responses of human lens epithelial cells to intraocular lenses in vitro: hydrophobic acrylic versus PMMA or silicone discs. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243(12):1253–1262. doi: 10.1007/S00417-005-1181-2. [DOI] [PubMed] [Google Scholar]
- 26.van Kooten T.G., Koopmans S., Terwee T., Norrby S., Hooymans J.M.M., Busscher H.J. Development of an accommodating intra-ocular lens--in vitro prevention of regrowth of pig and rabbit lens capsule epithelial cells. Biomaterials. 2006;27(32):5554–5560. doi: 10.1016/J.BIOMATERIALS.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Katsuki Y., Matsushima H., Mukai K., et al. Open-capsule intraocular lens to prevent posterior capsule opacification. J. Cataract Refract. Surg. 2019;45(7):1007–1012. doi: 10.1016/J.JCRS.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Kim M.H., Hwang H.S., Park K.J., Hwang J.H., Joo C.K. Introduction of lens-angle reconstruction surgery in rabbit eyes. Kor. J. Ophthalmol. 2014;28(6):486–492. doi: 10.3341/KJO.2014.28.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halili I., Mutlu F.M., Cüneyt Erdurman F., Gündogan F.C., Kiliç S. Influence of capsular tension ring on posterior capsule opacification in myopic eyes. Indian J. Ophthalmol. 2014;62(3):311–315. doi: 10.4103/0301-4738.116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara T., Hara T., Hashimoto T., Motoyama Y., Narita M. Posterior capsular opacification in highly myopic eyes with an endocapsular equator ring. Jpn. J. Ophthalmol. 2016;60(5):373–376. doi: 10.1007/S10384-016-0456-Y. [DOI] [PubMed] [Google Scholar]
- 31.Olcina Portilla L.I. 2016. Improved Accomodative Intraocular Lens. WO/2017/085344; PCT/ES2016/070813. [Google Scholar]
- 32.Hackett R., McDonald T.O. In: Advances in Modern Toxicology: Dermatoxicology. fourth ed. Marzulli F., Maibach H., editors. Hemisphere Publishing Corporation; Washington DC: 1991. Eye irritation; pp. 749–815. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.