Abstract
This work aimed to evaluate the antibacterial activity and mechanism of cinnamon essential oil nanoemulsion (CON) against Pseudomonas deceptionensis CM2. The results revealed that CON could effectively inhibit the proliferation of P. deceptionensis CM2 cells in a time- and concentration-dependent manner. After 4 h of incubation with CON at the minimum inhibitory concentration (0.125 mg/mL), the relative fluorescence intensity of propidium iodide and 1-N-phenylnapthylamine (NPN) was increased by 32.0% and 351.4%, respectively. The membrane permeability of P. deceptionensis CM2 cells was significantly disrupted after CON treatment, resulting in the leakage of intracellular substances (such as proteins and electrolytes). CON also caused significant increases in the DiBAC4(3) fluorescence intensity of P. deceptionensis CM2 cells. These results demonstrate that CON induced inactivation of P. deceptionensis CM2 by destroying the integrity and function of bacterial membrane. A higher level of intracellular reactive oxygen species (ROS) was observed in CON-treated cells (p < 0.05), compared with control cells. Moreover, the addition of glutathione to the growth medium remarkably decreased the antimicrobial activity of CON against P. deceptionensis CM2, further confirming that oxidative stress played an important role in the antimicrobial activity of CON. Overall, CON may exhibit antibacterial effects by causing damage to the bacterial membranes and oxidative stress.
Keywords: Cinnamon essential oil, Nanoemulsions, Pseudomonas deceptionensis CM2, Antibacterial, Mechanism
1. Introduction
Currently, microbial contamination of food is one of the greatest global public health risks and can occur at one or several steps in the food supply chain. Microorganisms not only cause undesirable changes in the physicochemical, nutritional, and sensory attributes of foods, but also constitute a grave threat to the health of humans [1,2]. At present, synthetic preservatives are widely used to retard the growth of or kill microbes present in food products, such as benzoic acid, benzoates, sorbates, sulfites, nitrites, and nitrates. However, in recent decades, excessive consumption of artificial preservatives has been a great concern for food safety and public health [3,4]. In this regard, the application of natural antimicrobials and preservatives in the food industry has recently drawn great research attention, mainly including essential oils, plant extracts, bacteriocins, and lactic acid bacteria [[5], [6], [7]].
Essential oils (EOs) are complex mixtures of natural volatile compounds extracted from aromatic and medicinal plants. EOs are the main secondary metabolites synthesized by plants and consist of various volatile compounds, such as terpenes, phenolics, alcohols, aldehydes, ketones, esters, amines, amides, and alkaloids [8,9]. Although EOs show remarkable antioxidant and antimicrobial properties in vitro, the practical application of EOs is limited by their lower solubility in aqueous formulations, uncontrolled volatility and poor stability in ambient conditions [10,11]. On the other hand, EOs can interact with some food matrix components, such as lipids, proteins, and carbohydrates, thereby causing negative impacts on sensory attributes of food products [12,13]. Nanoemulsion formulations of EOs are one of the best strategies to solve the above-mentioned issues. Nanoemulsions are kinetically stable liquid-in-liquid dispersions with a mean droplet size smaller than 200 nm [14]. Nanoemulsions are mainly composed of two immiscible liquid phases, the oil phase and the water phase, in combination with surfactant molecules [15]. The incorporation of EOs in nanoemulsions can greatly improve their dispersibility in aqueous solutions, physico-chemical stability, bioactivity, sustained release, absorption, and bioavailability [10,15]. Several studies have shown that nanoemulsions effectively enhance the antibacterial activity of EOs [16,17]. For instance, Li et al. [18] discovered that emulsion encapsulation could effectively improve the antibacterial properties of eugenol (the most abundant phenolic component in clove oil) against Staphylococcus aureus and Escherichia coli. Similarly, peppermint oil nanoemulsions exhibited prolonged antibacterial activities against Listeria monocytogenes Scott A and S. aureus compared with bulk peppermint oil [19].
Cinnamon essential oil (CO) is one of the most common EOs used in food and other applications and is obtained from the bark and leaves of cinnamon trees of the genus Cinnamomum, such as Cinnamomum zeylanicum and Cinnamomum burmannii [20,21]. Our previous work indicated that cinnamon essential oil nanoemulsion (CON) showed strong antibacterial effects against L. monocytogenes and Pseudomonas deceptionensis CM2 [22]. In addition, CON also effectively inhibited the growth of bacteria and extended the shelf-life of chicken fillets during cold storage at 4 °C [22]. However, the mechanism of action for the antibacterial effects of CON is not completely understood. Therefore, the present study aimed to reveal the antibacterial mechanisms of CON against P. deceptionensis CM2 with respect to the potential disruption in the membrane and oxidative stress.
2. Materials and methods
2.1. Materials
Chitosan (deacetylation >90%, <200 mPa • s), Tween 80, CO, pectin, and glutathione (GSH) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Propidium iodide (PI), 2′7′-dichlorofluorescein diacetate (H2DCFDA), and 1-N-phenylnapthylamine (NPN) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Nutrient broth (NB) medium and plate count agar (PCA) were provided by Beijing Land Bridge Technology Co., Ltd. (Beijing, China).
2.2. Antibacterial activity and time-kill assay
P. deceptionensis CM2 isolated from spoiled chicken meat was used in this study. CON was prepared with chitosan (0.5%, w/v), pectin (0.5%, w/v), CO (1.0%, w/v), and Tween 80 (0.25%, w/v) according to our previous publication [22]. The minimum inhibitory concentration (MIC) and minimum bactericide concentration (MBC) of CON against P. deceptionensis CM2 were 0.125 and 0.25 μL/mL, respectively, according to the broth microdilution method described previously [22]. The mean droplet size, polydispersity index (PDI), and zeta potential of CON were 144.27 ± 13.10 nm, 0.38 ± 0.12, and 32.50 ± 3.46 mV, respectively [22]. P. deceptionensis CM2 cells were incubated in NB medium at 25 °C for 12 h, and then centrifuged and resuspended in sterile phosphate-buffered saline (PBS, 0.1 mol/L, pH 7.0) to approximately 108 CFU/mL [23]. Bacterial suspensions were incubated with CON at indicated concentrations (0, 1 × MIC, 2 × MIC, 4 × MIC, and 6 × MIC) at 25 °C with constant shaking at 140 rpm, and 1.0 mL of bacterial suspensions were withdrawn for determination of bacterial counts at 4 h. The obtained suspension was serially diluted and 0.1 mL of suspension of each dilution was plated onto PCA plates. Bacterial populations were counted after incubation at 25 °C for 48 h, and the results are given as log10 CFU/mL.
2.3. Time-kill assay
The time-kill test was performed using the method described previously [24]. Briefly, the diluted bacterial suspension (approximately 104 CFU/mL) in sterile PBS was mixed with CON at 0, 1 × MIC, and 1 × MBC, respectively. The resulting inoculums were mixed and incubated at 25 °C with constant shaking at 180 rpm. At 0, 2, 4, 8, and 10 h, 1.0 mL of the inoculum was taken and the population of live bacterial cells was counted by the plate counting method described in Section 2.2.
2.4. Scanning electron microscopy analysis
After CON was incubated in PBS at 0, 1 × MIC and 2 × MIC at 25 °C for 4 h, P. deceptionensis CM2 cells were harvested by centrifugation at 4000×g for 2 min. The obtained cells were fixed with 2.5% glutaraldehyde solution for 12 h at 4 °C. After being rinsed three times with PBS, the cells were dehydrated with graded ethanol solutions (30%, 50%, 70%, 80%, 90%, and 100%) for 15 min each. The obtained cell pellets were dried twice with 100% isoamyl acetate for 15 min each time and then dried. Thereafter, the samples were sputter-coated with gold and observed with a high-resolution field emission scanning electron microscope (FE-SEM) Regulus 8100 (Hitachi, Tokyo, Japan) in high vacuum mode with an accelerating voltage of 3.0 kV [25].
2.5. Analysis of cell membrane damage
P. deceptionensis CM2 cells were treated with CON at 0, 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC for 4 h at room temperature, respectively. After each treatment, P. deceptionensis CM2 cells were harvested by centrifugation (4000×g, 25 °C, 7 min) and then resuspended in sterile saline.
2.5.1. Inner membrane permeability assay
The fluorescent probe PI was used to measure the changes in the inner membrane permeabilization of P. deceptionensis CM2 cells [26]. Briefly, the obtained bacterial suspensions were incubated with PI solution (3 μmol/L in sterile saline) at 25 °C for 15 min in the dark. Thereafter, the cells were harvested by centrifugation, washed twice, and then resuspended in sterile saline. Finally, the fluorescence intensity was measured using a Spark 20 M multimode microplate reader (Tecan Group Ltd., Männedorf, Switzerland) with λex = 493 nm and λem = 630 nm, respectively [27]. The relative mean fluorescence intensity was expressed as a percentage of the control group:
| (1) |
where F1 and F0 represent the fluorescence intensity of CON-treated cells and control cells, respectively.
2.5.2. Outer membrane permeability assay
The outer membrane permeability of viable bacteria was assessed through the NPN uptake assay by the method described in our previous report [26]. Briefly, the bacterial suspensions were mixed with NPN to reach a final concentration of 10 μmol/L and were incubated for at least 15 min at room temperature in the dark. After that, the cells were collected by centrifugation, washed twice, and then resuspended in sterile saline. Finally, the fluorescence was measured at an excitation wavelength of 350 nm and an emission wavelength of 408 nm. The results were expressed as the mean relative fluorescence intensity to the average of the control group.
2.5.3. Analysis of membrane potential
The voltage-sensitive dye DiBAC4(3) was used to determine the changes in the transmembrane potential of P. deceptionensis CM2 cells [28]. P. deceptionensis CM2 cells were treated with CON and resuspended in sterile PBS. Subsequently, the cells were incubated with DiBAC4(3) at a final concentration of 2.5 μg/mL for 15 min at 25 °C in the dark. Thereafter, the cells were harvested, washed twice, and were then resuspended in sterile PBS. Furthermore, the fluorescence intensity of each bacterial suspension was determined at λex = 488 nm and λem = 525 nm. The values were expressed as a percentage of fluorescence intensity relative to the control cells according to Eq. (1).
2.6. Measurement of bacterial proteins and electrolyte leakage
2.6.1. Assay of intracellular proteins leakage
P. deceptionensis CM2 cell suspensions in PBS were incubated with CON (0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC) for 4 h at room temperature. After centrifugation (8000×g, 4 °C, 10 min), the supernatants were recovered. The protein concentrations of the supernatants were determined by the BCA protein assay (#P0012, Beyotime Biotechnology, Shanghai, China) [29].
2.6.2. Measurement of intracellular electrolyte leakage
P. deceptionensis CM2 cell suspensions in PBS were treated with CON at 1 × MIC and 2 × MIC for 0, 2, 4, 6, and 8 h at room temperature. At the indicated time intervals, bacterial suspensions were taken and then centrifuged (8000×g, 4 °C, 10 min). Finally, the conductivity of the supernatant was measured using an electrical conductivity meter (DDS-307A, Leici, Shanghai, China). The bacterial suspension without CON was used as a control.
2.7. Measurement of intracellular ROS levels
P. deceptionensis CM2 cells were treated with CON at 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC for 4 h at room temperature. After each treatment, the collected bacterial cells in sterile PBS were incubated with the redox-sensitive fluorescent dye H2DCFDA at a final concentration of 10 μmol/L at 25 °C for 30 min in the dark. Thereafter, the cells were thoroughly washed with PBS, and the fluorescence intensity was measured at Ex/Em = 488/525 nm [20].
2.8. Effect of GSH on the antibacterial action of CON
The tests were performed with a Bioscreen-C Automated Growth Curves Analysis System (Lab systems, Helsinki, Finland). GSH stock solution in water was filter-sterilized using a 0.22 μm pore filter. A bacterial suspension was prepared in NB at a concentration of approximately 5 × 105 cells/mL. P. deceptionensis CM2 suspensions in NB were mixed with CON at 1 × MIC and GSH (2 mmol/L), alone or in combination. Consequently, the cultures were then transferred to a Bioscreen plate (100 μL/well) and grown at 25 °C under a constant agitation rate. The optical density (OD) of each well was determined photometrically at 560 nm every 2 h for 24 h. Each culture was performed in triplicate.
2.9. Statistical analysis
All experiments were repeated three times, and the data are presented as mean ± standard deviation (SD). Statistical analysis was conducted using one-way analysis of variance (ANOVA), and the differences were evaluated using Fisher's least significant difference (LSD) post-hoc comparison at p < 0.05. Statistical analysis was performed using SPSS Statistics version 26.0 (IBM SPSS Inc., New York, NY, USA).
3. Results
3.1. Antimicrobial activity of CON against P. deceptionensis CM2
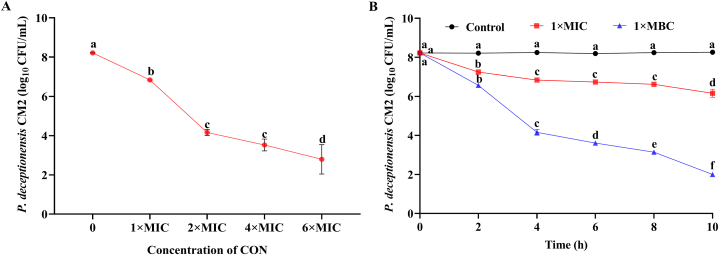
As shown in Fig. 1A, the initial population of P. deceptionensis CM2 was 8.21 log10 CFU/mL. The bacterial population decreased significantly by 1.38, 4.05, 4.69, and 5.42 log units after exposure to CON at 1 × MIC, 2 × MIC, 4 × MIC, and 6 × MIC for 4 h, respectively (p < 0.05). The results obtained for the time-killing curves are presented in Fig. 1B. There were no significant changes in P. deceptionensis CM2 populations in the control group during incubation at 25 °C for up to 10 h. After exposure to CON at 1 × MIC or 1 × MBC, the bacterial count significantly decreased in a time-dependent manner. After incubation with CON at 1 × MIC or 1 × MBC for 10 h, P. deceptionensis CM2 decreased significantly (p < 0.05) from approximately 8.23 log10 CFU/mL at the initial loading to 6.16 and 2.00 log10 CFU/mL, respectively (Fig. 1B). These data indicate that CON can effectively inactivate P. deceptionensis CM2 cells in a time and concentration-dependent fashion.
Fig. 1.
Effects of CON on the viability of P. deceptionensis CM2. (A) P. deceptionensis CM2 cells were treated with different concentrations of CON at 25 °C for 4 h. (B) Time-kill curves of CON at different concentrations against P. deceptionensis CM2 cells. Different lowercase letters represent significant differences between treatments at the same time according to the LSD test (p < 0.05).
3.2. Cell morphology of P. deceptionensis CM2 cells
The changes in the surface morphologies of CON-treated P. deceptionensis CM2 cells were characterized by SEM. As shown in Fig. 2A, the control cells demonstrated intact rod‐like structures and plump rod shapes with smooth surfaces. In contrast, cells suffered extensive structural damage on the surfaces after exposure to CON at 1 × MIC or 2 × MIC for 4 h. A few cells treated with CON at 1 × MIC exhibited some shrinkage and depression (Fig. 2B). Most severe structural damage was observed in bacterial cells treated with CON at 2 × MIC, such as shrinkage, formation of holes in the membrane, crushing, and loss of their normal shape, resulting in cell lysis and leakage of cellular components (Fig. 2C).
Fig. 2.
Representative FE-SEM micrographs of P. deceptionensis CM2 cells. (A) Control cells, (B) cells treated with CON at 1 × MIC for 4 h, (C) cells treated with CON at 2 × MIC for 4 h.
3.3. CON caused marked increased cell membrane permeabilization
The inner membrane is a fluid phospholipid bilayer that encloses the bacterial cytoplasm. In bacterial cells, the cytoplasmic membrane is enclosed by the cell wall and plays an essential role in maintaining proper cell functions, such as energy generation, diffusion of small molecules, cell division, and maintenance of electrochemical gradients [30]. In the present work, the effect of CON on the inner membrane permeability of P. deceptionensis CM2 cells was determined with PI. PI is a nucleic acid binding fluorescent probe that selectively enters cells with a compromised inner membrane. After entering the cells, PI binds to the DNA or RNA, causing a remarkable increase in the fluorescence intensity. As shown in Fig. 3A, PI fluorescence intensity of P. deceptionensis CM2 cells significantly increased after CON treatment. After incubation with CON at 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC for 4 h, the PI-fluorescence intensity of P. deceptionensis CM2 cells increased significantly (p < 0.05) by 20.1%, 32.0%, 140.1%, and 218.4%, respectively.
Fig. 3.
Effect of CON on the inner (A) and outer membrane (B) permeability of P. deceptionensis CM2 cells. Different lowercase letters above the bars show significant differences among the treatments (p < 0.05, LSD test).
In Gram-negative bacteria, the outer membrane is a complex and highly asymmetrical bilayer and is mainly comprised of phospholipids, lipopolysaccharides, and proteins, which usually act as a permeability barrier [31]. Additionally, the outer membrane of Gram-negative bacteria also plays a versatile role in cellular physiology and viability [32]. In the present work, the changes in the outer membrane permeabilization of P. deceptionensis CM2 cells were measured by NPN fluorescence assay. Hydrophobic NPN can only enter cells that have damaged outer membranes and therefore exhibits weak fluorescence in an aqueous. After entry into the cell, NPN exhibits increased fluorescence in the hydrophobic phospholipid layer of cell membranes [33]. As shown in Fig. 3B, remarkable increases in NPN fluorescence intensity were observed in CON-treated P. deceptionensis CM2 cells. For cells treated with CON at 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC, the NPN fluorescence intensity was increased by 161.4%, 351.4%, 395.8%, and 482.5%, respectively (p < 0.05). It is clear from these results that CON significantly disrupts the cell membranes of P. deceptionensis CM2, ultimately causing the leakage of intracellular components and leading to cell death.
3.4. Leakage of proteins and electrolytes
The bacterial cell membrane is a permeability barrier that limits the entry and exit of molecules from the cell, which are crucial for the normal function and survival of bacterial cells. The influence of CON on the integrity of the cell membrane was also assessed by measuring the release of intracellular substances. As shown in Fig. 4, the level of extracellular proteins increased significantly (p < 0.05) after exposure to CON for 4 h. The extracellular protein concentration of P. deceptionensis CM2 cells was 0.264 ± 0.01 mg/mL after exposure to CON at 4 × MIC for 4 h, which was significantly (p < 0.05) higher than that of control cells (0.206 ± 0.01 mg/mL).
Fig. 4.
Effect of CON on protein release in P. deceptionensis CM2 cells. Means with different letters denote significant differences between values using the LSD test at p < 0.05.
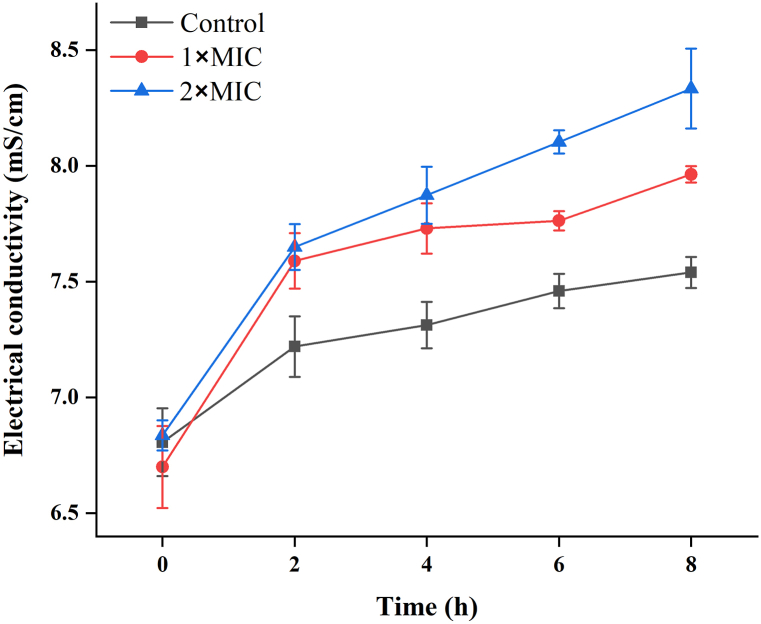
The electrical conductivity was measured in this work to assess the effect of CON on the electrolyte leakage of P. deceptionensis CM2 cells. As indicated in Fig. 5, the electrical conductivity of all bacterial suspensions increased with the extension of culture time. However, the increase in electric conductivity of cells treated with CON was much higher than that of the control cells. After being treated with CON at 1 × MIC and 2 × MIC for 8 h, the electrical conductivity of the bacterial suspension increased to approximately 7.93 and 8.33 mS/cm, respectively, both of which were higher than that of the control group (6.82 mS/cm).
Fig. 5.
Effect of CON on the electrical conductivity of P. deceptionensis CM2 suspensions. Different letters indicate significant differences between means based on the LSD test (p < 0.05).
3.5. Bacterial membrane depolarization
In this work, DiBAC4(3), a voltage-sensitive fluorescent probe, was used to investigate the effect of CON on the membrane potential of P. deceptionensis CM2 cells to further reveal the antibacterial mechanism of CON. Upon depolarization, DiBAC4(3) can enter the cells and exhibit increased fluorescence by binding to intracellular proteins or membranes [34]. As shown in Fig. 6, CON treatment caused significant increases in the DiBAC4(3) fluorescence intensity of P. deceptionensis CM2 cells. For cells treated with CON at 0.5 × MIC, 1 × MIC, 2 × MIC, and 4 × MIC for 4 h, the fluorescence intensity of DiBAC4(3) was markedly increased by 38.8%, 265.2%, 356.3%, and 417.3%, respectively. Collectively, the above data indicate that CON leads to increased depolarization of the cellular membrane.
Fig. 6.
DiBAC4(3) uptake of P. deceptionensis CM2 cells with the presence of CON for 4 h. Data are presented as means ± SD. Distinct lowercase letters indicate statistically significant differences (p < 0.05, LSD test).
3.6. CON induced oxidative stress in P. deceptionensis CM2 cells
In the present work, H2DCFDA was used to investigate the effect of CON on extracellular ROS levels in P. deceptionensis CM2 cells. Cell-permeable H2DCFDA can enter cells, where it is deacetylated by intracellular esterases to form the nonfluorescent 2′, 7′-dichlorodihydrofluorescein (H2DCF). Upon oxidation by ROS, H2DCF is oxidized to highly fluorescent 2′, 7′-dichlorodihydrofluorescein (DCF). As depicted in Fig. 7A, enhanced ROS generation was observed in P. deceptionensis CM2 cells after incubation with CON for 4 h. For cells treated with CON at 0.5 × MIC, 1 × MIC, and 2 × MIC for 4 h, the DCF fluorescence intensity was markedly increased (p < 0.05) by 16.8%, 54.7%, 75.2%, and 162.5%, respectively.
Fig. 7.
Role of oxidative stress in the antimicrobial activity of CON against P. deceptionensis CM2 cells. (A) Effect of CON on ROS generation in P. deceptionensis CM2 cells. (B) Effect of GSH on the antimicrobial activity of CON (1 × MIC) against P. deceptionensis CM2. Different letters indicate statistical significance at p < 0.05 between groups with the LSD test.
Furthermore, GSH, one of the most studied antioxidants, was used to address the role of ROS in the antimicrobial activity of CON. As shown in Fig. 7B, the presence of GSH (2 mmol/L) in the growth medium markedly decreased the antimicrobial activity of CON against P. deceptionensis CM2, which might be due to its ROS scavenging capability.
4. Discussion
The antibacterial activity and mechanism of CON against P. deceptionensis CM2 was evaluated in this work. As shown in Fig. 1A, CON inhibited the growth of P. deceptionensis CM2 in a time- and dose-dependent manner. In our previous work, it was observed that CON could effectively decrease the total viable count and P. deceptionensis CM2 of chicken fillets during storage at 4 °C for up to 15 days [22]. Similarly, Alderees et al. [35] investigated the antifungal activities of nanoencapsulated essential oils extracted from Tasmanian pepper leaves, lemon myrtle, and anise myrtle. The results showed that the prepared EOs nanoemulsion exhibited dose- and time-dependent antimicrobial activity against Zygosaccharomyces bailii in clear apple juice [35]. The SEM results revealed that CON-treated cells showed a rough and deformed surface morphology. When compared to untreated cells. Similar findings were also observed that eugenol nanoemulsions ruptured the cell membrane of E. coli and S. aureus [36,37]. As revealed by transmission electron microscopy (TEM), linalool nanoemulsions disrupted the cell membranes of Aeromonas hydrophila and caused loss of cell contents [37].
The cell envelope of Gram-negative bacteria consists of three principal layers, the cytoplasmic or inner membrane, the peptidoglycan cell wall, and the outer membrane, which protects the cell from a changing environment [38]. In this work, the influences of CON on the integrity of the cytoplasmic and outer membranes were investigated with PI and NPN staining, respectively. After 4 h of incubation with CON at 1 × MIC, the relative fluorescence intensity of PI and NPN was increased by 32.0% and 351.4%, respectively, suggesting the disruption of the cytoplasmic and outer membranes after exposure to CON. These results are consistent with previous studies by Gao et al. [39], in which cinnamaldehyde nanoemulsions caused significant damage to the inner membranes of E. coli and S. aureus cells after SYTO 9/PI staining. In another study, Guo et al. [40] also observed a sharp increase in the proportion of PI-positive E. coli O157:H7 cells after the treatment with thyme essential oils nanoemulsions for 9 min. CON-induced cell membrane disruption may be due to the lipophilic nature of CO, causing the chemical constituents cross easily and destroy the phospholipid layer of cell membranes [30]. As reported in a previous work, linalool nanoemulsions caused severe damage to the outer membrane of A. hydrophila cells according to TEM results [37]. CON disrupted the cell membrane integrity of P. deceptionensis CM2, resulting in the release of intracellular substances such as proteins (Fig. 4) and electrolytes (Fig. 5) into the culture medium. Similar findings were also observed in previous studies [24,41]. For instance, Moghimi et al. [41] found that sage oil nanoemulsions caused leakage of proteins and nucleic acids from E. coli cells. These data suggest that CON may affect the various metabolic pathways of the bacterial cells, which contributes further to the resulting death of P. deceptionensis CM2 cells.
The electrical potential across the membrane plays pivotal roles in fundamental cellular functions such as ATP synthesis, motility, membrane transport, and environmental sensing [42]. As shown in Fig. 6, CON caused membrane depolarization of P. deceptionensis CM2 cells. In the study of Liu et al. [29], a significant increase in DiBAC4(3) fluorescence intensity was observed in methicillin-resistant S. aureus cells treated with garlic essential oil in water nanoemulsion, indicating membrane depolarization. Membrane depolarization is attributed to the abnormal movement of several different ion species across the cell membrane [29]. CON-induced depolarization of the membrane potential may further lead to a disruption of the lipid bilayers and permeabilization of plasma membranes, subsequently resulting in death of P. deceptionensis CM2.
ROS are crucial for the growth and survival of bacteria through many important signaling reactions. However, excessive ROS can induce the disruption of bacterial metabolic functions by causing oxidative damage to cellular components (e.g., proteins, nucleic acids, and lipids), subsequently leading to cell death. As depicted in Fig. 7A, a higher level of ROS was observed in P. deceptionensis CM2 cells incubated with CON for 4 h. Similar findings were reported by some researchers. According to Gao et al. [39], cinnamaldehyde nanoemulsions caused significant increases in intracellular ROS production and lipid peroxidation levels in E. coli and S. aureus cells. In another study, Das et al. [43] also found that Artemisia annua essential oil nanoemulsions directly caused increased ROS, peroxide, and superoxide generation in E. coli, S. aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Streptococcus pyogenes cells. Malondialdehyde (MDA) is one of the final secondary products of lipid peroxidation in the cells. As reported by Yang et al. [44], lavender essential oil treatment caused significant increases in the MDA amount of K. pneumoniae cells compared to the control sample. It is therefore speculated that CON may exhibit antibacterial activity against P. deceptionensis CM2 cells by inducing overproduction of ROS or by inhibiting the antioxidant systems. The main compounds in CO responsible for inducing oxidative stress should be determined in future research. Additionally, more attention should also be focused on the molecular mechanisms underlying CON-induced oxidative stress in P. deceptionensis CM2 cells by transcriptomic and proteomic approaches.
The results of the present study confirmed that CON caused irreversible damage to the cytoplasmic and outer membranes of P. deceptionensis CM2 cells, resulting in the loss of cell membrane integrity and even cell death (Fig. 8). CON also induced depolarization of the membrane potential, thereby affecting the cellular functions that are required for the survival of P. deceptionensis CM2 cells. Meanwhile, CON also increased oxidative stress in P. deceptionensis CM2 cells by enhancing intracellular ROS generation or inhibiting antioxidant systems. It is speculated that the antibacterial action of CON may involve the disruption of cell membrane integrity and oxidative stress. Multi-omics approaches should be used in future work to identify the molecular mechanism underlying the antibacterial action of CON.
Fig. 8.
Possible antibacterial mechanism of CON against P. deceptionensis CM2 cells.
5. Conclusions
In summary, CON was shown to effectively inactivate P. deceptionensis CM2 cells in a time- and concentration-dependent manner. CON treatment resulted in significant morphological damage and disruption of cell membranes, such as increased membrane permeability and depolarization of membrane potential. CON also caused a high level of ROS in P. deceptionensis CM2 cells, which might be one of the underlying mechanisms for the antibacterial action of CON. In summary, CON-induced membrane damage and oxidative stress may contribute to its antibacterial activity. The results of this study suggest that CON is a promising antimicrobial agent for potential use in food preservation and the control of foodborne pathogens in foods.
Author contribution statemen
Dianbo Zhao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper. Yanqing Ma, Wenwen Wang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Qisen Xiang: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Henan Province (No. 212300410090) and the Collaborative Innovation Special Project of Zhengzhou (No. 2021ZDPY0201).
References
- 1.Machado-Moreira B., Richards K., Brennan F., Abram F., Burgess C.M. Microbial contamination of fresh produce: what, where, and how? Compr. Rev. Food Sci. Food Saf. 2019;18(6):1727–1750. doi: 10.1111/1541-4337.12487. [DOI] [PubMed] [Google Scholar]
- 2.Wu D., Forghani F., Daliri E.B.M., Li J., Liao X.Y., Liu D.H., Ye X.Q., Chen S.G., Ding T. Microbial response to some nonthermal physical technologies. Trends Food Sci. Technol. 2020;95:107–117. doi: 10.1016/j.tifs.2019.11.012. [DOI] [Google Scholar]
- 3.Carocho M., Barreiro M.F., Morales P., Ferreira I.C. Adding molecules to food, pros and cons: a review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014;13(4):377–399. doi: 10.1111/1541-4337.12065. [DOI] [PubMed] [Google Scholar]
- 4.Javanmardi F., Rahmani J., Ghiasi F., Gahruie H.H., Khaneghah A.M. The association between the preservative agents in foods and the risk of breast cancer. Nutr. Cancer. 2019;71(8):1229–1240. doi: 10.1080/01635581.2019.1608266. [DOI] [PubMed] [Google Scholar]
- 5.Baptista R.C., Horita C.N., Sant'Ana A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: a review. Food Res. Int. 2020;127 doi: 10.1016/j.foodres.2019.108762. [DOI] [PubMed] [Google Scholar]
- 6.Pisoschi A.M., Pop A., Georgescu C., Turcus V., Olah N.K., Mathe E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 7.Webb L., Ma L.Y., Lu X.N. Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual. Saf. 2022;6 doi: 10.1093/fqsafe/fyac045. fyac045. [DOI] [Google Scholar]
- 8.Calo J.R., Crandall P.G., O'Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems-A review. Food Control. 2015;54:111–119. doi: 10.1016/j.foodcont.2014.12.040. [DOI] [Google Scholar]
- 9.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils' chemical characterization and investigation of some biological activities: a critical review. Via Medici. 2016;3(4):25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barradas T.N., de Holanda e Silva K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: a review. Environ. Chem. Lett. 2021;19(2):1153–1171. doi: 10.1007/s10311-020-01142-2. [DOI] [Google Scholar]
- 11.Maurya A., Prasad J., Das S., Dwivedy A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021;5 doi: 10.3389/fsufs.2021.653420. [DOI] [Google Scholar]
- 12.Perricone M., Arace E., Corbo M.R., Sinigaglia M., Bevilacqua A. Bioactivity of essential oils: a review on their interaction with food components. Front. Microbiol. 2015;6:76. doi: 10.3389/fmicb.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh B.K., Tiwari S., Dubey N.K. Essential oils and their nanoformulations as green preservatives in boosting food safety against mycotoxin contamination of food commodities: a review. J. Sci. Food Agric. 2021;101(12):4879–4890. doi: 10.1002/jsfa.11255. [DOI] [PubMed] [Google Scholar]
- 14.Donsì F., Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- 16.Maurya A., Singh V.K., Das S., Prasad J., Kedia A., Upadhyay N., Dubey N.K., Dwivedy A.K. Essential oil nanoemulsion as eco-friendly and safe preservative: bioefficacy against microbial food deterioration and toxin secretion, mode of action, and future opportunities. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.751062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidotti-Takeuchi M., Ribeiro L.N.D., dos Santos F.A.L., Rossi D.A., Della Lucia F., de Melo R.T. Essential oil-based nanoparticles as antimicrobial agents in the food industry. Microorganisms. 2022;10(8):1504. doi: 10.3390/microorganisms10081504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W., Chen H.L., He Z.Z., Han C., Liu S.L., Li Y. Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT--Food Sci. Technol. 2015;62(1):39–47. doi: 10.1016/j.lwt.2015.01.012. [DOI] [Google Scholar]
- 19.Liang R., Xu S.Q., Shoemaker C.F., Li Y., Zhong F., Huang Q.R. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012;60(30):7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- 20.Jayaprakasha G.K., Rao L.J.M. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011;51(6):547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 21.Vasconcelos N.G., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: a review. Microb. Pathog. 2018;120:198–203. doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Wang W.W., Zhao D.B., Xiang Q.S., Li K., Wang B.H., Bai Y.H. Effect of cinnamon essential oil nanoemulsions on microbiological safety and quality properties of chicken breast fillets during refrigerated storage. LWT--Food Sci. Technol. 2021;152 doi: 10.1016/j.lwt.2021.112376. [DOI] [Google Scholar]
- 23.Kang C.D., Xiang Q.S., Zhao D.B., Wang W.J., Niu L.Y., Bai Y.H. Inactivation of Pseudomonas deceptionensis CM2 on chicken breasts using plasma-activated water. Food Sci. Technol. 2019;56(11):4938–4945. doi: 10.1007/s13197-019-03964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foerster S., Unemo M., Hathaway L.J., Low N., Althaus C.L. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol. 2016;16:216. doi: 10.1186/s12866-016-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao J.X., Y J., Zhang, Zheng X.Q., Zhao D.D. Bactericidal efficacy of slightly acidic electrolyzed water (SAEW) against Listeria monocytogenes planktonic cells and biofilm on food-contact surfaces. Food Qual. Saf. 2022;6 doi: 10.1093/fqsafe/fyab038. fyab038. [DOI] [Google Scholar]
- 26.Xiang Q.S., Kang C.D., Niu L.Y., Zhao D.B., Li K., Bai Y.H. Antibacterial activity and a membrane damage mechanism of plasma activated water against Pseudomonas deceptionensis CM2. LWT--Food Sci. Technol. 2018;96:395–401. doi: 10.1016/j.lwt.2018.05.059. [DOI] [Google Scholar]
- 27.Zhang R., Ma Y.F., Wu D., Fan L.M., Bai Y.H., Xiang Q.S. Synergistic inactivation mechanism of combined plasma-activated water and mild heat against Saccharomyces cerevisiae. J. Food Protect. 2020;83(8):1307–1314. doi: 10.4315/JFP-20-065. [DOI] [PubMed] [Google Scholar]
- 28.Liu X., Li Y.F., Zhang R., Huangfu L.L., Du G.H., Xiang Q.S. Synergistic antimicrobial activity of plasma-activated water and propylparaben: mechanism and applications for fresh produce sanitation. LWT--Food Sci. Technol. 2021;146 doi: 10.1016/j.lwt.2021.111447. [DOI] [Google Scholar]
- 29.Liu M.M., Pan Y., Feng M.X., Guo W., Fan X., Feng L., Huang J.R., Cao Y.G. Garlic essential oil in water nanoemulsion prepared by high-power ultrasound: properties, stability and its antibacterial mechanism against MRSA isolated from pork. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mykytczuk N.C.S., Trevors J.T., Leduc L.G., Ferroni G.D. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog. Biophys. Mol. Biol. 2007;95(1–3):60–82. doi: 10.1016/j.pbiomolbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Delcour A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta-Protei. 2009;1794(5):808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J.W., Rutherford S.T., Silhavy T.J., Huang K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2021;20(4):236–248. doi: 10.1038/s41579-021-00638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helander I.M., Mattila-Sandholm T. Fluorometric assessment of Gram-negative bacterial permeabilization. J. Appl. Microbiol. 2000;88(2):213–219. doi: 10.1046/j.1365-2672.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 34.Adams D.S., Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012;2012:459–464. doi: 10.1101/pdb.prot067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderees F., Akter S., Mereddy R., Sultanbawa Y. Antimicrobial activity of nanoencapsulated essential oils of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum against weak-acid resistant Zygosaccharomyces bailii in clear apple juice. Beverages. 2021;7(3):67. doi: 10.3390/beverages7030067. [DOI] [Google Scholar]
- 36.Fu X., Gao Y., Yan W.Y., Zhang Z.L., Sarker S., Yin Y.Y., Liu Q., Feng J.G., Chen J. Preparation of eugenol nanoemulsions for antibacterial activities. RSC Adv. 2022;12(6):3180–3190. doi: 10.1039/d1ra08184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong W.M., Tang P.Y., Liu T., Zhao T.Y., Guo J.J., Gao Z.P. Linalool nanoemulsion preparation, characterization and antimicrobial activity against Aeromonas hydrophila. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey A.B., Ashenden A., Köper I. Model architectures for bacterial membranes. Biophys Rev. 2022;14(1):111–143. doi: 10.1007/s12551-021-00913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y., Liu Q., Wang Z.X., Zhuansun X.X., Chen J., Zhang Z.L., Feng J.G., Jafari S.M. Cinnamaldehyde nanoemulsions; physical stability, antibacterial properties/mechanisms, and biosafety. J. Food Meas. Char. 2021;15(6):5326–5336. doi: 10.1007/s11694-021-01110-6. [DOI] [Google Scholar]
- 40.Guo M.M., Zhang L.J., He Q., Arabi S.A., Zhao H.H., Chen W.J., Ye X.Q., Liu D.H. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- 41.Moghimi R., Aliahmadi A., McClements D.J., Rafati H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT--Food Sci. Technol. 2016;71:69–76. doi: 10.1016/j.lwt.2016.03.018. [DOI] [Google Scholar]
- 42.Benarroch J.M., Asally M. The microbiologist's guide to membrane potential dynamics. Trends Microbiol. 2020;28(4):304–314. doi: 10.1016/j.tim.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Das S., Vörös-Horváth B., Bencsik T., Micalizzi G., Mondello L., Horváth G., Kőszegi T., Széchenyi A. Antimicrobial activity of different artemisia essential oil formulations. Molecules. 2020;25(10):2390. doi: 10.3390/molecules25102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S.K., Yusoff K., Thomas W., Akseer R., Alhosani M.S., Abushelaibi A., Lim S.H.E., Lai K.S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci. Rep. 2020;10(1):819. doi: 10.1038/s41598-019-55601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.