Abstract
Amoebae of the genus Acanthamoeba are etiological agents of amoebic keratitis, for which up to now there is no treatment of choice and one of its main risk factors is the use of contact lenses, including cosmetic contact lenses. Recently there has been an increase in amoebic keratitis cases due to the use of cosmetic contact lenses. Therefore, having a solution for the care of lenses with an efficient disinfectant effect that prevents the adhesion of trophozoites to lenses becomes essential. This study was carried out to determine the effect of 8 multipurpose contact lenses care solutions on Acanthamoeba castellanii trophozoites viability, and the efficiency of two of them to prevent the trophozoites adherence onto two cosmetic contact lenses (Acuvue 2, approved by the US Food and Drug Administration, and Magic Eye CCL, not approved). After 3 h of interaction, only AO Sept Plus, OPTI FREE Replenish, Renu Plus, Bio True and Multiplus significantly reduced the number of viable trophozoites with respect to the control; at 6 h Renu Plus, and at 12 h Conta Soft Plus and Multiplus, maintained the inhibitory effect. Only Opti Free Pure Moist did not significantly reduce the number of viable trophozoites. Multiplus and Opti Free Pure Moist (selected for their greater and lesser antiamibic effect) significantly reduced trophozoite adherence to both lenses; however, Opti Free Pure Moist was more efficient, despite the fact that A. castellanii adhered similarly to both lenses. Our results show that in all the multipurpose solutions evaluated, hundreds of viable A. castellanii trophozoites remain after several hours of incubation. Therefore, storage of the lenses in their case with MPS maintains the potential risk of amoebic keratitis in, cosmetic contact lenses wearers. Moreover, the use of CCL, not approved by the FDA, can increase the risk factor for AK since its poor manufacture can favor the permanence of amoebae, in addition to being a risk for corneal integrity.
Keywords: Acanthamoeba castellanii, Cosmetic contact lenses, Multipurpose solutions
1. Introduction
Several species of Acanthamoeba genus are etiological agents of humans and animal pathologies of the central nervous system, the skin, and more frequently corneal infections (AK); a sight-threatening corneal infection which can culminate in severe vision loss characterized by severe eye pain, photophobia, and a ring-shaped central or paracentral stromal inflammatory infiltrate [1]. To date, several therapeutic schemes have been implemented [1,2], as well, the amoebicidal effect of diverse drugs has been evaluated in vitro [[3], [4], [5]], without until now having a 100% efficient drug of choice. In addition, amoebae can encyst in tissue, being highly resistant to environmental conditions, biocides, and drugs [6].
Despite contact lenses (CL) correct vision problems, it is well known that their use is a leading risk factor for AK [1], including cosmetic contact lenses (CCL) [7], since they can be vectors of amoebae, may impact negatively the eye's natural defenses and favor microbial colonization and survival [[8], [9], [10]]. Prolonged CL use induces epithelial hypoxia, inhibition of the shedding of tears, and surface erosion compromising its integrity, which increases the risk of infection [11,12]. The relative risk of microbial keratitis as well as complications is 16.5 times higher in CCL than in conventional CL wearers [13], due to non-compliance with indications of use of the CL manufacturers is considered one of the key factors in AK [14]. In the particular case of CCL, other associated factors facilitate amoebae adhesion such as the quality of manufacturing, and coloring layer, which can be released with their use generating toxicity or can produce deposits of dye associated with the biofilms formation [15], as well as the presence of folds or roughness on the surface of the lens that can favor the anchoring of microorganisms such as amoebae.
Acanthamoeba trophozoites and cysts adhere to both soft and rigid contact lenses, an important step in the establishment of corneal infection [10,16]. Several studies have reported the importance of hydrophobicity and roughness surface for microbial adhesion [17].
In addition, it is necessary to consider the role played by solutions that preserve or clean the lenses, whose main function is to disinfect and preserve the characteristics of their manufacture [18] reducing the risk of eye infections. Particularly, multipurpose solutions (MPS) are formulated with an aqueous liquid medium, an antimicrobial, a surfactant, a buffer, a viscosity-inducing component, a tonicity component, and a chelating component. Although MPS are widely used for their simplicity and convenience, several studies on the disinfecting efficacy against Acanthamoeba trophozoites showed limited efficacies [19,20]. This study was carried out to determine the inhibitory effect of multipurpose solutions on Acanthamoeba castellanii trophozoites. In addition, not only the effect of these solutions during the recommended time for disinfection was evaluated, but it was also studied whether those solutions that showed the greatest and least effectiveness prevented adherence to two types of contact lenses in those trophozoites that remained viable after their interaction. Besides, was compared whether amoebae adherence differed according to the type of lenses studied.
2. Materials and methods
2.1. Amoebae
The study was carried out with an Acanthamoeba strain isolated of a contact lenses wearer keratitis case from Mexico City, attended in the “Hospital para evitar la ceguera en México; Luis Sánchez Bulnes” [21], and morphologically identified as A. castellanii, based on Page's taxonomic keys [22]. Belonging to the T4 group through molecular assays performed by genotyping through DNA sequencing of the DF3 region of 18S rRNA genes [4]. It is important to mention that with the strain in study some of the pathogenicity mechanisms carried out by these amoebae have been described through the murine model of GAE, the ex vivo model of AK, the experimental model of skin invasion, in vitro assays as well as contact lens adherence determination [23].
Trophozoites were cultured in borosilicate tubes (Pyrex, Mexico) with 2% Bacto Casitone medium (Difco) supplemented with 10% bovine serum at the optimal temperature of growth of the strain in study (30 °C). All assays were performed with trophozoites harvested by centrifugation at the end of the exponential growth phase (72 h) [23].
2.2. Evaluation of multipurpose solutions on the viability of A. castellanii
The effect of 8 MPS on the viability of A. castellanii trophozoites was determined: Multiplus, Conta Soft, Conta Soft Plus, AO Sept Plus, Renu Plus, Opti Free Replenish, Optifree Pure Moist andBio True (Table 1). Briefly, 5 × 104 trophozoites in 40 μL of Bacto Casitone medium supplemented with bovine serum were placed in 96-well plates, then 260 μL of each MPS were added. Samples were placed in a wet chamber and incubated for 3, 6, and 12 h at 30 °C. A. castellanii trophozoites in Bacto Casitone medium were used as control. Assays were carried out sextuplicate. At the end of each time proposed, the samples were processed accordingly to the crystal violet viability technique, modified [24]. Briefly, the medium was removed by shaking the plate and gently washing it with saline. Trophozoites were fixed with 100 μL of methanol for 30 s, then they were removed by shaking the plaque. Next, 100 μL of violet crystal solution (0.1%) were added to each well, and incubated for 15 min at room temperature in darkness, then gently shacked and washed three times with saline solution. A total of 100 μL of sodium dodecyl sulfate (SDS) were added to 1% in 50% ethanol to solubilize the violet crystal contained in the amoebae. Finally, the plate was read on the microplate reader at 620 nm.
Table 1.
The chemical components of multipurpose solutions.
| Multipurpose solutions | Formula components | Manufacturer |
|---|---|---|
| 1. Multiplus (MP) | Boric acid, sodium borate, sodium chloride, EDTA, C.B.P. vehicle | Poyssa |
| 2. Conta Soft (CS) | Sodium hyaluronate, dyodic edetate, cq cosmocil, boric acid, sodium borate, el cremofor, poloxamer, hypromelosa, purified water | Laboratory Grin |
| 3. Conta Soft Plus (CSP) | Sodium borate, EDTA, sodium chloride, poloxamer 407, cosmocilCQ, creomophor EL, HPMC, purified water | Laboratory Grin |
| 4. AO Sept Plus (ASP) | Hydrogen peroxide (3%), stabilized with phosphonic acid, water, phosphate, sodium chloride, phosphoric acid, poloxamer | Ciba Vision |
| 5. Renu Plus (RP) | Dymed_polyaminopropylbiguanide (0.001%) | Bausch & Lomb |
| 6. OPTI-FREE RepleniSH (OPR) | Propylene glicol, PolyQuad (0.0001%), Aldoxn (0.0005%), sodium citrate, sodium chloride, sodium borate, TearGlyde, Tetronic 1304, nonanoyl ethylenediaminetriacetic acid | Alcon |
| 7. OPTI-FREE Pure Moist (OPM) |
Myristamidopropyl dimethylamine 0.0005%), Polyquaternium-1 (0.001%) | Alcon |
| 8. Bio True (BT) | Polyaminopropyl biguanide (0.00013%) Polyquaternium (0.0001%) | Bausch & Lomb |
To determine the number of viable trophozoites in the above assay, a standard curve was processed in parallel, for which 100 μL of Bacto Casitone were added to all columns of a 96-well plate. Subsequently, 2.5 × 105 amoebae were suspended in 100 μL of Bacto Casitone were added to the first column. Once there, were mixed with a micropipette, 10 times without bubbles. They were taken 100 μL and moved to the second well, where they were remixed 10 times, without bubbles. The same procedure was repeated until the twelfth well, thus obtaining a 1: 2 serial dilution; this was carried out six times. The plate was incubated at 30 °C for 30 min and was processed in the same way as the crystal violet viability test.
2.3. Adherence of trophozoites to Acuvue 2 and Magic Eye (FDA-approved and non-approved CCL)
Multiplus (MP) and Optifree Pure Moist (OPM) solutions were considered as those MPS with the greatest and lesser amoebicidal effects, respectively, which were used in subsequent assays to determine whether these solutions prevent A. castellanii trophozoites adherence to the CCL. Two types of CCL were chosen: one of them approved by FDA, Acuvue 2, and non-approved by FDA, Magic Eye. The lenses characteristics are shown in Table 2. New CCL were cut in segments of approximately 25 mm2. Twenty-four segments were used for each experimental group and lenses type. The CCL segments were placed in sterile 24-well boxes (Evergreen, Los Angeles, CA), with their concave side up, on which 200 μL of saline solution was dispensed to maintain the lens hydrated before the interaction [25].
Table 2.
Characteristics of lenses used in this study.
| Brand Name | Magic Eye | Acuvue 2 |
|---|---|---|
| Manufacture | Menicon | Johnson & Johnson Vision Care |
| Materials | 58% HEMA/GMA | Hydrogel ionic (negative charge) HEMA/MA |
| USAN | Hioxifilcon A | Etafilcon A |
| Water content | 42% | 58% |
| FDA Classification | Group II | Group IV |
| Diameter | 14.2 mm | 14.0 mm |
| Center thickness | 0.10 mm (−3.00 D) 0.08 mm | 0.085 mm (−3.00 D) 0.084 mm |
| Base curve | 8.6 mm | 8.3 mm, 8.7 mm |
| Package | Flat pack | Blister pack |
USAN (United States Adopted Name).
HEMA (poly-2-hydroxyethyl methacrylate).
MA (methacrylic acid).
GMA (glycerol methacrylate).
Hioxifilcon (A and D) is a copolymer of HEMA/GMA. It contains hydroxyl groups that tightly bind water and so it does not dehydrate as much as traditional HEMA materials do..
Etafilcon is a copolymer of HEMA/MA. It high water content ionic lens polymer..
The interactions were carried out accordingly in the following experimental conditions [25].
-
•
1: Segments of both lenses interacted with 250 trophozoites in 50 μL of Bacto Casitone medium for 15 min; then 250 μL of MP was added, the interaction was carried out for 4 h.
-
•
2: Segments of both lenses interacted with 250 trophozoites in 300 μL of MP during 4 h.
-
•
3: Segments of both lenses interacted with 250 trophozoites in 50 μL of Bacto Casitone medium for 15 min; after this time, 250 μL OPM solution was added, the interaction was carried out for 4 h.
-
•
4: Segments of both lenses interacted with 250 trophozoites in 300 μL of OPM solution for 4 h.
Bacto Casitone medium was used as a control. Samples were incubated at 30 °C in a wet chamber, fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 h, and washed with the same buffer. Trophozoites attached to the surface were counted in an inverted light microscope (Nikon Eclipse TS100, Tokyo, Japan).
2.4. Statistical analysis
Statistical analysis was performed using Statistica version 10 program. For statistical comparisons, differences between groups were analyzed by the Kruskal-Wallis nonparametric test since the trophozoite number data did not behave normally. To determine differences between the groups, Dunn non-parametric multiple comparison test was applied. A p < 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of multipurpose solutions on the viability of A. castellanii
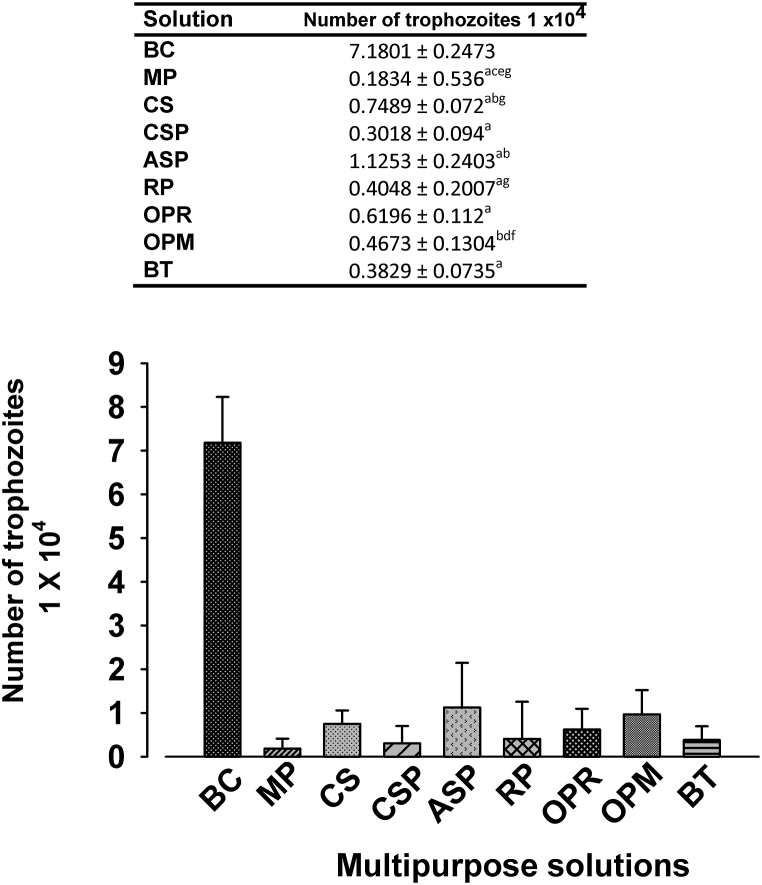
The analysis of the interaction of A. castellanii trophozoites with MPS through Kruskal-Wallis test showed significant differences between treatments (H = 115.22, p<0.01). At 3 h of incubation, trophozoites number in control condition (BC) was higher than that in ASP, OPR, RP, BT, and MP solutions. At 6 h, trophozoites number in BC was significantly higher than that in RP. At 12 h, BC trophozoites number was significantly higher than that in CSP, and MP solutions. Regarding the inhibitory effect of each MPS through time, it was determined that all the solutions did not show significant differences in the number of amoebae that remained viable at 3, 6, and 12 h. Nevertheless, it is important to mention the effect presented in the ASP solution, in which the trophozoites showed an initial decrease of amoebic viability similar to the other solutions, and after 6 h there was a tendency to increase in the number of amoebae, to decrease again to the 12 h of incubation (Fig. 1).
Fig. 1.
Effect of MPS on the number of viable trophozoites of A. castellanii through the time. BC: Bactocasitone medium, MP: Multiplus, CS: Conta Soft, CSP: Conta Soft Plus, ASP: AO Sept Plus, RP: Renu Plus, OPR: OPTI-FREE RepleniSH, OPM: OPTI-FREE Pure moist and BT: Bio True. Denote in the table that MP; ASP, RP, OPR and BT, significantly diminished trophozoites respect BC at 3 h, while only RP (6 h), and MP and CSP (12 h) shown that effect. There were not significant differences within MPS through the time. Superscript letters indicate significant differences: a3 hour BC vs solutions, b6 hour BC vs solutions, and c12 h BC vs solutions. In all cases p < 0.05.
Since there were no statistical differences through the time in any evaluated condition, we consider the mean value for each condition, independently of time, to make a global analysis (Fig. 2). According to Kruskal-Wallis test, there were significant differences between groups (H = 83.07, p<0.01). All MPS significantly decreased the trophic forms with respect to BC medium, except OPM. The lowest number of trophozoites was observed in MP, which was significantly smaller than that in CS, ASP, and OPM solutions. In contrast, the OPM solution inhibited trophozoites proliferation lesser than CSP, RP, and MP. As general synthesis, the interaction of A. castellanii trophozoites with 7 of the 8 evaluated MPS significantly decreased the trophic forms since the first 3 h post interaction, with a percentage greater than 80%, but MP, CSP, RP, and BT were the most effective solutions, reducing approximately 90% of the trophozoites, while the interaction with ASP induced a lesser effect on the trophozoites. It is important to emphasize that despite the antiamibic effect of all MPS, thousands of trophozoites remained viable in all of them after 12 h of incubation.
Fig. 2.
General analysis of the effect of MPS on the viability of A. castellanii. For each MPS, the mean number of viable trophozoites is presented, independently of interaction time. BC: Bactocasitone medium, MP: Multiplus, CS: Conta Soft, CSP: Conta Soft Plus, ASP: AO Sept Plus, RP: Renu Plus, OPR: OPTI-FREE RepleniSH, OPM: OPTI-FREE Pure moist and BT: Bio True. Superscript letters indicate significant differences: aBC vs MPS; bMP vs CS, ASP and OPM; cCS vs MP; dOPM vs CSP; eASP vs MP; fRP vs OPM, and gOPM vs MP, CSP, RP. In all cases p < 0.05.
3.2. Adherence of trophozoites to Acuvue 2 and Magic Eye (FDA approved and non-approved CCL)
As mentioned previously, the interaction of the trophozoites with the MP solution induced the greatest reduction of these protozoa, while OPM was one of the MPS that induced the lesser effect. Based on these results, we decided to use MP and OPM solutions to determine their effect on the trophozoites adherence to LCC: Acuvue 2 (FDA approved) and Magic Eye (not FDA approved). Adherence results are shown in Fig. 3. In the different treatments were observed 1–15 viable trophozoites adhered to the lenses surface (Fig. 4A–D). According to the Kruskall-Wallis test, significant differences between groups (H = 140, p < 0.01) were observed. The Dunn multiple comparison tests revealed the following significant differences. In Acuvue 2 lenses, the trophozoites number, incubated with MP at 4 h was lesser than with BC. In both Acuvue 2 and Magic eye lenses, trophozoites numbers in OPM at both 15 min, and 4 h, were lesser than in those incubated with BC, and respect those incubated during 15 min with BC, and after added MP. In fact, the mentioned trophozoites numbers in Magic eye lenses incubated with OPM at both 15 min, and 4 h were the lowest values we founded.
Fig. 3.
Adherence of A. castellanii trophozoites to Acuvue 2 and Magic eye cosmetic contact lenses. BC: Bactocasitone medium, MP: Multiplus, and OPM: OPTI-FREE Pure moist. Superscript letters indicate significant differences: aAcuvue 2 lenses BC vs MP at 4 h, and vs OPM at both 15 min and 4 h bAcuvue 2 lenses MP 15 min vs OPM at both 15 min and 4 h cMagic eye lenses BC vs OPM at both 15 min and 4 h dMagic eye lenses MP 15 min vs OPM at both 15 min and 4 h eAcuvue 2 with MP at 15 min vs Magic eye MP 4 h, and vs Magic eye OPM at 15 min or 4 h fAcuvue 2 with MP at 4 h vs Magic eye OPM at 15 min or 4 h. In all cases p < 0.01.
Fig. 4.
Photomicrographs showing the interaction of A. castellanii to cosmetic contact lenses in presence of MPS. A-B) Arrows indicate several trophozoites adhered on Acuvue 2 and Magic eye lenses, respectively. C-D) Some amoebae (arrows), were observed adhering near or inside fissures (arrow head) in Magic eye lenses. E) Magic eye lenses shown rough and discontinuous surface (arrow head). F) Irregular edges in Magic eye lenses (arrow head). Magnification A) 40×; B–F) 20×.
No significant differences were observed between both CCL types for the same experimental condition.
Finally, it is very important to highlight that by light microscopy, CCL Magic Eye (not approved by FDA) shows irregular edges, and rough and discontinuous surfaces, which can cause abrasions to the corneal epithelium (Fig. 4C–F). Moreover, one amoeba was observed adhered inside fissures of the lenses (Fig. 4C).
4. Discussion
Amoebic keratitis is a corneal infection that has gained importance in recent years, being a multifactorial infection in which the use of CLs is one of the most relevant since it implies disinfection processes, handling, adequate storage, use of adequate times to avoid hypoxia and keep the corneal surface in optimal conditions. Furthermore, the use of CCL increases the risk of infection or multi-infection [13].
A. castellanii is one of the species most frequently isolated from clinical cases of Acanthamoebiasis, and it has been reported in both new and used lenses, as in contact lenses desinfecting solutions [26]. In particular, the species evaluated in this study was isolated from a clinical case of AK, demonstrating its ability to invade different target tissues, as well as to adhere to different types of CL [25]. Therefore, we considered necessary to determine the susceptibility of its trophozoites to contact lens MPS and their ability to adhere to CCL post solutions interaction. With this strategy, we tried to evaluate the MPS effectiveness on trophozoites number decrease, and to highlight and describe that those amoebae that survive disinfection processes, may continue to adhere to CL, and for the same reason, to the cornea as well, with the potential risk of inducing corneal infection. We also highlight that we work with trophic forms, that is, with invasive forms, which are more susceptible to the effect of solutions and drugs, with respect to cystic forms. Through this study, we demonstrate that the risk factors of AK in people who wear corrective CL and CCL increase with the lack of effective solutions for their elimination.
As mentioned before, the number of CCL wearers has been increasing over time, since some people use them for aesthetic purposes, without knowing the risks that their inappropriate use implicates. Consequently, the number of cases of microbial keratitis associated with its use has increased [7]. Particularly in United States CCL are considered medical devices, and their safety and efficacy are monitored [27]. In contrast, in Mexico there is no regulation for the CCL acquisition and disposal which implies that persons can acquire them without receiving indications of use and handling, despite that exist several reports of infectious keratitis associated with them [4,8,21,28]. A system of care is necessary as lenses are exposed to microbial contamination, which makes them vectors of microorganisms able to invade corneal surface and conjunctiva, risks that can be aggravated by improper handling.
Our results show that only the solutions MP, ASP, RP, OPR, and BT, exert a significant effect on the viability of the trophozoites under study since the earliest time 3 h post interaction, which corresponds to a lesser time than the minimum suggested of 4 h for the disinfection of the lenses, after 6 h only RP maintained a significant proliferation inhibitory effect with respect to the control in BC medium and, after 12 h, only MP and CSP showed a significant difference. It is very important to highlight that none of evaluated solutions totally eliminated the A. castellanii trophozoites, even after 12 h of interaction. In addition, only MPS MP and CSP reduced 1 log of the initial inoculum as FDA recommended [29], however maintaining more than 1 × 103 viable trophozoites after almost all times evaluated. By contrast, with ASP more than 2 × 104 viable trophozoites were determined after 6 h incubation.
Hiti et al. [30] suggested that only a surviving trophozoite and cyst can give rise to a new “amoeba population”. Lenses and cases contaminated by these amoebae may endanger the visual health of individuals.
Furthermore, despite amoebae interaction with solutions such as ASP and OPR, which initially reduce the number of protozoa, favored an important increase in amoeba number after 6 and 12 h of interaction. It is possible that its effect decreases or that the trophozoites that remain viable maintain their ability to adhere and invade the target tissue; suggesting that viable trophozoites are able to reproduce after being interacted with solution for several hours, either by adaptation to experimental conditions or perhaps as a consequence of decreased effectiveness of the MPS active compounds, as has been documented with peroxide hydrogen.
The discrepancy in the effectiveness of the MPS is attributed to the different formulations of each commercial solution including buffering agents (such as sodium chloride), cleaning agents (such as sodium citrate), conditioning agents (such as Tetronic), surfactants (such as propylene glycol). The specific formulation of each product significantly affects its disinfectant efficacy [[30], [31], [32]]. In this study, a reduction of up to 90% of the trophozoites occurred with the MP, CSP, RP, and BT solutions. MP and CSP containing boric acid were the MPS that had the greatest amoebicidal effect; RP, and BT contain polyaminopropyl biguanide. Polyhexamethylene biguanide is an effective drug for the treatment of Acanthamoeba-induced keratitis and eliminate both cysts and trophozoites [33,34], inducing programmed cell death (PCD) (98.3%) in trophozoites [35], but is toxic to human corneal epithelial cells [36].
Other solutions evaluated, OPR, and OPM contain a dual disinfection system, POLYQUAD (polyquaternium-1) 0.001% and ALDOX (myristamidopropyl dimethylamine) 0.0006%. POLYQUAD has been shown to have predominantly antibacterial activity, while ALDOX has antifungal and antiamoebic activity [37,38]. Mowrey-McKee and George (2007) [39] demonstrated that this disinfectant solution exhibited a restricted amoebicidal effect after the suggested disinfection duration of 6 h upon being examined against a strain of A. castellanii, leading to 0.5 and 2.5 log reductions for cysts and trophozoites, Ustunturk and Zeybek (2014) [40] found that OPTI-FREE Express achieved total destruction of trophozoites but it had limited cysticidal activity.
The ASP solution, containing 3% hydrogen peroxide, exhibited the highest number of viable trophozoites. The disinfecting ability of hydrogen peroxide is directly relation to the time of exposure of the organisms to the active ingredients of the solution [18]. For neutralization, the one-step 3% hydrogen peroxide uses a neutralizer catalyst (AO Sept 1 Step and NOVASEPT) or catalase tablet (Oxysept 1 Step), thus the decomposition of hydrogen peroxide into water and oxygen occurs very early before disinfection occurs. Perhaps, for this reason, a 3% hydrogen peroxide solution is ineffective for Acanthamoeba. The amoebicidal activity of one-step hydrogen peroxide could perhaps be improved by slowing the rate of neutralization. Currently, there are no drugs that eliminate 100% trophozoites or Acanthamoeba cysts, so the treatment of patients with AK is complicated and in many cases with a poor prognosis, which emphasizes the need to have a more efficient MPS against these amoebae to prevent infections. In this regard, Hendiger et al. (2020) [41] reported a good antiamibic activity of silver nanoparticles on a strain of A. castellanii, and verified that when these nanoparticles interact with contact lens solutions, trophozoite adherence to CL was reduced [42]. Through the analysis of these results, it is very important to evaluate and propose solutions that eliminate both trophic and cysts forms to guarantee their elimination. Studies have been reported describing CL characteristics associated with Acanthamoeba trophozoites adherence, such as physical and chemical characteristics: chemical composition, manufacturing materials, ionic properties, water content, among others [12,25,43]. Omaña-Molina et al., 2014 [25], carried out using this same strain in which adherence to hydrogel CL of different generations was evaluated, concluding that a rough surface contributes to trophozoites adhering more efficiently to silicone hydrogel lenses and to a lesser extent they adhered to smooth surface lenses. Likewise, they reported that homemade saline solutions can contribute to the persistence of trophozoites, especially when an adequate hygiene regimen is not used with CL cases. This agrees with our results, in which the irregular surface of the lenses can be a factor that favors the adhesion of the trophozoites and we also saw how different formulations favor the survival of these amoebae, increasing the risk of infection with poor handling and with lack of instructions for use in the case of CCL. Respect previous reports with Acuvue 2 CCL, our results were similar. For example, it has been reported that Opti-Free Express (similar in composition with our OPM), significantly reduced the number of adherent Acanthamoeba lugdunensis L3a trophozoites in CCL after 6 h of incubation [44].
Magic Eye is manufactured with Hioxifilcon A hydrogel and Acuvue 2 with Etafilcon A silicone hydrogel. The water content of the hydrogel lenses ranges between 54% and 66%, and between 46% and 69% in silicone hydrogels [44]. In this study, a tendency higher adherence of A. castellanii to Acuvue 2 CCL (58% water content) than to Magic Eye CCL was observed, perhaps because of lower water content in Magic Eye CCL (42% water content), in a similar way that previous reports [45]. However, the difference was not significant.
The adhesion of Acanthamoeba trophozoites to HEMA-based soft contact lenses is material dependent, and Etafilcon A composite lenses, the material from which Acuvue 2 lenses are made, showed the highest adhesion [12,45]. The interaction with MPS induced an adverse effect on the trophozoites, either reducing the number of amoebae that adhered to the lenses surface or favoring their detachment when they were pre-incubated for 15 min comparing it with the control with BC medium.
Despite the fact that MP solutions exerted the greatest effect on the trophozoites under study, those that survived adhered to the lenses very efficiently. Even though OPM induced a lesser effect on the amoebae viability, it was more effective than MP to inhibit trophozoites adherence to both lenses. Exposed results suggest that the contamination of CCL by A. castellanii depends on the interaction of factors such as the lenses material, its water content, the MPS used for its care, the exposure time of the lenses to the MPS, as well as the conditions in which the amoebae adhered to the lenses [12,46].
Acanthamoeba remains trophic and viable to adhere to both CCL in the presence of MPS. We emphasize that the interaction was carried out with only 250 trophozoites, an inoculum closer to what can occur in reality, which reaffirms the idea that a reduced number of amoebae is capable of adhering and initiating invasive processes as has been shown through ex vivo studies of the invasion of A. castellanii in the cornea of hamsters and human corneas, demonstrating that trophozoites, alone or in groups, are capable of invading and causing damage to damaged or intact tissues, initiating the process with adherence to the corneal surface, followed by migration and invasion into deeper layers of the corneal epithelium. In agreement with our results, an Opti-Free Express solution significantly, but not totally, reduced the adherence of A. lugdunensis to CL [20].
Silver nanoparticles (AgNPs) are used as modern antimicrobial agents. Their effectiveness against Acanthamoeba spp., with the addition of plant metabolite tannic acid, has been confirmed. The obtained results showed an increased anti-adhesion activity of CL solutions in conjunction with tannic acid-modified silver nanoparticles (AgTANPs) with a limited cytotoxicity effect compared to contact lenses solutions acting alone [47].
It is very important to highlight that the microscopic observation of CCL Magic Eye evidenced irregular edges, a rough and discontinuous surface in which there were amoebae inside that can cause abrasions to the corneal epithelium, coinciding with previous reports [25,43]. The smoothness of and electrical charge on the CL surface have previously been shown to be related to bacterial adhesion. In particular surface pigments increase the surface roughness of CCL, and this CCL characteristic favors to Staphylococcus aureus and Pseudomonas aeruginosa to be adhered mainly on the colored surface. The adherence of A. castellanii trophozoites on CCL was proved in the present study and it might be related to the irregular surface of the CCL, particularly in Magic eye CCL. This result its in accord with those obtained with Acanthamoeba lugdunensis L3a [16].
In summary, OPM and MP did not totally inhibit the adherence of A. castellanii trophozoites to Acuvue 2 and Magic Eye CCL, nor did they remove all trophozoites previously attached to the lenses.
Finally, it is important to mention that storage of the CL in their case with MPS may not totally inhibit the adherence of the A. castellanii trophozoites to the surface of both CCL; likewise, it does not favor the detachment of trophozoites previously adhered to the lenses, which is very important since it maintains the potential risk of amoebic keratitis in both CL and CCL users. In addition, it is important to also evaluate the efficacy of these MPS on the cysts of this strain under study, considering the importance of this stage of the Acanthamoeba life cycle and its high capacity for resistance to disinfectants and biocides, which could contaminate the cases of cosmetic contact lenses and promote AK.
5. Conclusions
Of the 8 MPS evaluated only MPS and CSP reduced 1 log of the initial inoculum as FDA recommended [29], however maintaining more than 1 × 103 viable trophozoites after almost all times evaluated, and none of these eliminate A. castellanii trophozoites, even after 12 h of incubation. The MP and OPM solutions allowed viable amoebae to adhere to the Acuvue 2 and Magic Eye CCL, and only slightly reduced the number of previously attached trophozoites, despite using low inoculum during lenses interaction. This confirms the risk of AK in lenses wearers if CL is not handled respecting the hygienic care procedures established for them. In addition, Magic Eye lenses showed poor surface quality which can promote debris deposition and even damage the corneal epithelium. Therefore, it is important to note that the use of non-FDA-approved cosmetic lenses such as Magic Eye may not meet established quality standards, which could significantly increase the risk factor for infectious diseases such as AK.
Finally, although the solutions eliminate a high percentage of trophozoites, it is evident that those that survive are able to adhere to contact lenses and therefore invading the cornea.
Author contribution statement
Dolores Hernández-Martínez; Maritza Omaña-Molina: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Edson Castro-Pot; Perla Hernández-Olmos; Sandra Villa Ramírez: Performed the experiments.
Elizabeth Alejandrina Guzmán Hernández; David Segura Cobos; Tomás Ernesto Villamar Duque: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ángel Durán Díaz: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lorenzo-Morales, Khan N.A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:1–20. doi: 10.1051/parasite/2015010. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha-Cabrera P., Reyes-Batlle M., Martín-Navarro C.M., Dorta-Gorrín A., López-Arencibia A., Sifaoui I., Martínez-Carretero E., et al. Detection of Acanthamoeba on the ocular surface in a Spanish population using the Schirmer strip test: pathogenic potential, molecular classification and evaluation of the sensitivity to chlorhexidine and voriconazole of the isolated Acanthamoeba strains. J. Med. Microbiol. 2015;64(8):849–853. doi: 10.1099/jmm.0.000103. [DOI] [PubMed] [Google Scholar]
- 3.Taravaud A., Loiseau P.M., Pomel S. In vitro evaluation of antimicrobial agents on Acanthamoeba sp. and evidence of natural resilience to amphotericin B. Int J Parasitol Drugs Drug Resist. 2017;7(3):328–336. doi: 10.1016/j.ijpddr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernández-Martínez D., Reyes-Batlle M., Castelan-Ramírez I., Hernández-Olmos P., Vanzzini-Zago V., Ramírez-Flores E., Sifaoui I., et al. Evaluation of the sensitivity to chlorhexidine, voriconazole and itraconazole of T4 genotype Acanthamoeba isolated from Mexico. Exp. Parasitol. 2019;197:29–35. doi: 10.1016/j.exppara.2019.01.006. https://DOI: 10.1016/j.exppara.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Martín-Navarro C.M., López-Arencibia A., Arnalich-Montiel F., Valladares B., Piñero J.E., Lorenzo-Morales J. Evaluation of the in vitro activity of commercially available moxifloxacin and voriconazole eye-drops against clinical strains of Acanthamoeba. Graefes Arch. Clin. Exp. Ophthalmol. 2013;251(9):2111–2117. doi: 10.1007/s00417-013-2371-y. https://DOI: 10.1007/s00417-013-2371-y [DOI] [PubMed] [Google Scholar]
- 6.Marciano F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003;16(2):273–307. doi: 10.1128/CMR.16.2.273-307.2003. https://DOI: 10.1128/CMR.16.2.273-307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyomarch J., van Nuoï D.N., Beral L., Donnio A., Desbois N., Olive C., Theodose R., Merle H. Kératites infectieuses et lentilles cosmétiques: étude rétrospective de cinq cas [Infectious keratitis and cosmetic lenses: a five-case retrospective study] J. Fr. Ophtalmol. 2010;33(4):258–262. doi: 10.1016/j.jfo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Sauer A., Bourcier T., French Study Group for Contact Lenses Related Microbial Keratitis. Microbial keratitis as a foreseeable complication of cosmetic contact lenses: a prospective study. Acta Ophthalmol. 2011;89(5):e439–e442. doi: 10.1111/j.1755-3768.2011.02120.x. https://DOI: 10.1111/j.1755-3768.2011.02120.x [DOI] [PubMed] [Google Scholar]
- 9.Lee G.H., Yu H.S., Lee J.E. Effects of multipurpose solutions on the adhesion of Acanthamoeba to rigid gas permeable contact lenses. Ophthalmic Physiol. Opt. 2016;36(2):93–99. doi: 10.1111/opo.12277. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton F. The epidemiology of infectious keratitis. Ocul. Surf. 2021;19 doi: 10.1016/j.jtos.2021.08.007. S1542-0124(21)00089-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.S., Hahn T.W., Choi S.H., Yu H.S., Lee J.E. Acanthamoeba keratitis related to cosmetic contact lenses. Clin. Exp. Ophthalmol. 2007;35(8):775–777. doi: 10.1111/j.1442-9071.2007.01622.x. https://DOI: 10.1111/j.1442-9071.2007.01622.x [DOI] [PubMed] [Google Scholar]
- 12.Bakay B.B., Polat Z.A. In vitro evaluation of adhesion of two Acanthamoeba strains to cosmetic contact lenses. Eye Contact Lens. 2018;44(Suppl 2):S241–S246. doi: 10.1097/ICL.0000000000000457. https://DOI: 10.1097/ICL.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 13.Bourcier T., Sauer A., the French Study Group of Contact Lenses-Related Microbial Keratitis. Cosmetic contact lenses related microbial keratitis as a foreseeable disaster: a prospective study. Invest. Ophthalmol. Vis. Sci. 2010 51;(13):2884. [Google Scholar]
- 14.Padzik M, Starościak B, Szaflik JP, Pietruczuk-Padzik A, Siczek P, Chomicz L. Assessment of in Vitro Dynamics of Pathogenic Acanthamoeba Strains Originating from Contact Lens Wearers with Infectious Keratitis.10.17420/ap6204.69. [DOI] [PubMed]
- 15.De Lacerda A.G., Lira M. Acanthamoeba keratitis: a review of biology, pathophysiology and epidemiology. Ophthalmic Physiol. Opt. 2021 Jan;41(1):116–135. doi: 10.1111/opo.12752. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.M., Lee J.E., Lee D.I., Yu H.S. Adhesion of Acanthamoeba on cosmetic contact lenses. J. Kor. Med. Sci. 2018;33(4):e26. doi: 10.3346/jkms.2018.33.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stapleton F., Naduvilath T., Keay L., Radford C., Dart J., Edwards K., Carnt N., Minassian D., Holden B. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One. 2017 Aug 16;12(8) doi: 10.1371/journal.pone.0181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willcox M., Keir N., Maseedupally V., Masoudi S., McDermott A., Mobeen R., Purslow C., Santodomingo-Rubido J., Tavazzi S., Zeri F., Jones L. Clear - contact lens wettability, cleaning, disinfection and interactions with tears. Contact Lens Anterior Eye. 2021 Apr;44(2):157–191. doi: 10.1016/j.clae.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Dutot M., Reveneau E., Pauloin T. Multipurpose solutions and contact lens: modulation of cytotoxicity and apoptosis on the ocular surface. Cornea. 2010;29:541–549. doi: 10.1097/ICO.0b013e3181bd4bc1. https://DOI: 10.1097/ICO.0b013e3181bd4bc1 [DOI] [PubMed] [Google Scholar]
- 20.Lee G.H., Lee J.E., Park M.K., Yu H.S. Adhesion of Acanthamoeba on silicone hydrogel contact lenses. Cornea. 2016;35(5) doi: 10.1097/ICO.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 21.Omaña-Molina M.A., González-Robles A., Salazar-Villatoro L., Bernal-Escobar A., Durán-Díaz A., Méndez-Cruz A.R., et al. Silicone hydrogel contact lenses surface promote Acanthamoeba castellanii trophozoites adherence: qualitative and quantitative analysis. Eye Contact Lens. 2014;40(3):132–139. doi: 10.1097/ICL.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 22.Page F.C. The Freshwater Biological Association; Cumbria: 1988. A New Key to Freshwater and Soil Gymnamoebae. [Google Scholar]
- 23.Hernández-Jasso M., Hernández-Martínez D., Avila-Acevedo J.G., Benítez-Flores J.D.C., Gallegos-Hernández I.A., García-Bores A.M., Espinosa-González A.M., Villamar-Duque T.E., Castelan-Ramírez I., González-Valle M.D.R., Omaña-Molina M. Morphological description of the early events during the invasion of Acanthamoeba castellanii trophozoites in a murine model of skin irradiated under UV-B light. Pathogens. 2020 Sep 27;9(10):794. doi: 10.3390/pathogens9100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feoktistova M., Geserick P., Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016 Apr 1;2016(4) doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 25.Omaña-Molina M.A., González-Robles A., Salazar-Villatoro L., Bernal-Escobar A., Durán-Díaz A., Méndez-Cruz A.R., et al. Silicone hydrogel contact lenses surface promote Acanthamoeba castellanii trophozoites adherence: qualitative and quantitative analysis. Eye Contact Lens. 2014;40(3):132–139. doi: 10.1097/ICL.0000000000000024. https://DOI: 10.1097/ICL.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 26.Hassan F.A.M., Tolba M.E.M., Abed G.H., Omar H.M., Abdel-Hakeem S.S. Contact lenses contamination by Acanthamoeba spp. in upper Egypt. PLoS One. 2021 Nov 15;16(11) doi: 10.1371/journal.pone.0259847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration . 2022. Medical Device Database.https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm [Google Scholar]
- 28.Omaña-Molina M., Vanzzini-Zago V., Hernandez-Martinez D., Gonzalez-Robles A., Salazar-Villatoro L., Ramirez-Flores E., Oregon-Miranda E., Lorenzo-Morales J., Martinez-Palomo A. Acanthamoeba genotypes T3 and T4 as causative agents of amoebic keratitis in Mexico. Parasitol. Res. 2016;115(2):873–878. doi: 10.1007/s00436-015-4821-4. https://DOI: 10.1007/s00436-015-4821-4 [DOI] [PubMed] [Google Scholar]
- 29.Kackar S., Suman E., Kotian M.S. Bacterial and fungal biofilm formation on contact lenses and their susceptibility to lens care solutions. Indian J. Med. Microbiol. 2017;35(1):80–84. doi: 10.4103/ijmm.IJMM_16_273. https://DOI: 10.4103/ijmm.IJMM_16_273 [DOI] [PubMed] [Google Scholar]
- 30.Hiti K., Walochnik J., Maria Haller-Schober E., Faschinger C., Aspöck H. Efficacy of contact lens storage solutions against different Acanthamoeba strains. Cornea. 2006;25(4):423–427. doi: 10.1097/01.ico.0000214204.22200.7f. https://DOI: 10.1097/01.ico.0000214204.22200.7f [DOI] [PubMed] [Google Scholar]
- 31.Beattie T.K., Tomlinson A., McFadyen A.K., Seal D.V., Grimason A.M. Enhanced attachment of Acanthamoeba to extended-wear silicone hydrogel contact lenses: a new risk factor for infection? Ophthalmology. 2003;110(4):765–771. doi: 10.1016/S0161-6420(02)01971-1. https://DOI: 10.1016/S0161-6420(02)01971-1 [DOI] [PubMed] [Google Scholar]
- 32.Borazjani R.N., Kilvington S. Efficacy of multipurpose solutions against Acanthamoeba species. Contact Lens Anterior Eye. 2005;28(4):169–175. doi: 10.1016/j.clae.2005.10.001. https://DOI: 10.1016/j.clae.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 33.Lorenzo-Morales J., Martín-Navarro C.M., López-Arencibia A., Arnalich-Montiel F., Piñero J.E., Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013;29(4):181–187. doi: 10.1016/j.pt.2013.01.006. https://DOI: 10.1016/j.pt.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 34.Dart J.K., Saw V.P., Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am. J. Ophthalmol. 2009;148(4):487–499. doi: 10.1016/j.ajo.2009.06.009. https://DOI: 10.1016/j.ajo.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 35.Moon E.K., Choi H.S., Kong H.H., Quan F.S. Polyhexamethylene biguanide and chloroquine induce programmed cell death in Acanthamoeba castellanii. Exp. Parasitol. 2018;191:31–35. doi: 10.1016/j.exppara.2018.06.002. https://DOI: 10.1016/j.exppara.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 36.Mafra C.S., Carrijo-Carvalho L.C., Chudzinski-Tavassi A.M., Taguchi F.M., Foronda A.S., Carvalho F.R., de Freitas D. Antimicrobial action of biguanides on the viability of Acanthamoeba cysts and assessment of cell toxicity. Invest. Ophthalmol. Vis. Sci. 2013;54(9):6363–6372. doi: 10.1167/iovs.13-11990. https://DOI: 10.1167/iovs.13-11990 [DOI] [PubMed] [Google Scholar]
- 37.Codling C.E., Maillard J.Y., Russell A.D. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J. Antimicrob. Chemother. 2003;51(5):1153–1158. doi: 10.1093/jac/dkg228. https://DOI: 10.1093/jac/dkg228 [DOI] [PubMed] [Google Scholar]
- 38.Schuster F.L., Buck S., Rosenthal R.A., Schlech B.A. Efficacy of myristamidopropyl dimethylamine (Aldox) against corneal isolates of Acanthamoeba spp. J. Eukaryot. Microbiol. 2003;50(Suppl):520–521. doi: 10.1111/j.1550-7408.2003.tb00616.x. https://DOI: 10.1111/j.1550-7408.2003.tb00616.x [DOI] [PubMed] [Google Scholar]
- 39.Mowrey-McKee M., George M. Contact lens solution efficacy against Acanthamoeba castellani. Eye Contact Lens. 2007 Sep;33(5):211–215. doi: 10.1097/ICL.0b013e31805d8662. [DOI] [PubMed] [Google Scholar]
- 40.Ustüntürk M., Zeybek Z. Amoebicidal efficacy of a novel multi-purpose disinfecting solution: first findings. Exp. Parasitol. 2014 Nov;145(Suppl):S93–S97. doi: 10.1016/j.exppara.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Hendiger E.B., Padzik M., Sifaoui I., Reyes-Batlle M., López-Arencibia A., Rizo-Liendo A., Bethencourt-Estrella C.J., Nicolás-Hernández D.S., Chiboub O., Rodríguez-Expósito R.L., Grodzik M., Pietruczuk-Padzik A., Stępień K., Olędzka G., Chomicz L., Piñero J.E., Lorenzo-Morales J. Silver nanoparticles as a novel potential preventive agent against Acanthamoeba keratitis. Pathogens. 2020;9(5):350. doi: 10.3390/pathogens9050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendiger E.B., Padzik M., Sifaoui I., Reyes-Batlle M., López-Arencibia A., Zyskowska D., Grodzik M., Pietruczuk-Padzik A., Hendiger J., Olędzka G., Chomicz L., Piñero J.E., Lorenzo-Morales J. Silver nanoparticles conjugated with contact lens solutions may reduce the risk of Acanthamoeba keratitis. Pathogens. 2021;10(5):583. doi: 10.3390/pathogens10050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraldez M.J., Resua C.G., Lira M., Oliveira M.E., Magariños B., Toranzo A.E., Yebra-Pimentel E. Contact lens hydrophobicity and roughness effects on bacterial adhesion. Optom. Vis. Sci. 2010;87(6):E426–E431. doi: 10.1097/OPX.0b013e3181da8656. https://DOI: 10.1097/OPX.0b013e3181da8656 [DOI] [PubMed] [Google Scholar]
- 44.Lee Seung-Mok, Lee Ji-Eun, Da-In Lee, Hak-Sun Yu. Adhesion of Acanthamoeba on cosmetic contact lenses. J. Kor. Med. Sci. 2018;33(4):e26. doi: 10.3346/jkms.2018.33.e26. 2018 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reverey J.F., Fromme R., Leippe M., Selhuber-Unkel C. In vitro adhesion of Acanthamoeba castellanii to soft contact lenses depends on water content and disinfection procedure. Contact Lens Anterior Eye. 2014;37(4):262–266. doi: 10.1016/j.clae.2013.11.010. https://DOI: 10.1016/j.clae.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 46.Kilvington S. Acanthamoeba trophozoite and cyst adherence to four types of soft contact lens and removal by cleaning agents. Eye(Lond). 1993;7(Pt 4):535–538. doi: 10.1038/eye.1993.116. https://DOI: 10.1038/eye.1993.116 [DOI] [PubMed] [Google Scholar]
- 47.Padzik M., Chomicz L., Bluszcz J., Maleszewska K., Grobelny J., Conn D.B., Hendiger E.B. Tannic acid-modified silver nanoparticles in conjunction with contact lens solutions are useful for progress against the adhesion of Acanthamoeba spp. to contact lenses. Microorganisms. 2022;10(6):1076. doi: 10.3390/microorganisms10061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.