Abstract
This work aimed to evaluate the physicochemical changes during the roasting process of Robusta and Arabica coffee. The highest content of total phenolics was detected in roasted coffee at temperatures of 135 °C/20.20 min, 210 °C/9.02 min, 210 °C/11.01 min, and 220 °C/13.47 min for both species. Robusta coffee showed greater antioxidant activity compared to Arabica coffee, except for the profiles at 230 °C/17.43 min and 275 °C/7.46 min that did not differ between samples by the DPPH and FRAP methods. For Arabica coffee, the antioxidant activity was independent of the roasting profile used. Robusta coffee presented higher values of the indexes b* (intensity of yellow vs blue), c* (chroma) and hue, being characterized as lighter and with greater chroma and hue. The highest levels of caffeoylquinic acid (5-CQA) were observed in Robusta coffee. Arabica coffee had lower trigonelline values. Caffeic acid and hydroxymethylfurfural were identified only in Robusta coffee. However, the results provided solid knowledge for the design of general properties and chemical compounds generated from binomials of roasting time and temperature that are little used in the world market.
Keywords: Antioxidant activity, Phenolics totals, Coffee beans, Roasting, Food science
1. Introduction
Coffee is a drink of vast popularity and appreciated worldwide. The Brazilian coffee production, initially estimated for the 2023 harvest, was estimated at 54.94 million bags of 60 kg, of which 37.43 million bags are of the coffee especies Coffea arabica and 17.5 million bags of Coffea canephora (robusta and conilon). The Brazilian states with the largest coffee production are Minas Gerais, Espírito Santo, São Paulo and Bahia [1]. The most cultivated species are C. arabica and C. canephora (robusta), robust being considered of inferior quality when compared to arabica coffee because it has a more bitter and astringent taste [2].
Coffee is the second most important beverage after water, with an estimated annual consumption of approximately 500 billion cups [3,4]. Most coffee drinkers do not drink the beverage for health-related reasons. However, coffee has a variety of antioxidant compounds and numerous types of functional phenolic compounds that play a protective role against various diseases. The main phenolic compounds present in coffee are chlorogenic (CGA), caffeic and ferulic acids [[5], [6], [7]]. Antioxidants have been the subject of growing interest due to their ability to inhibit oxidative reactions and their role in food preservation. Total phenolics, a diverse class of bioactive compounds of plant origin, are widely recognized for their potent antioxidant potential and ability to eliminate free radicals [8]. In addition to providing functionality, the sensory profile (fragrance/aroma and flavor) is strongly enhanced and influenced by the coffee beans roasting process. In this process, the time-temperature binomial is considered a relevant parameter in obtaining a drink with different flavor and aroma profiles [9,10].
During the roasting process, modification and/or generation and release of various chemical compounds occur through Maillard reactions, Strecker degradation, caramelization and other chemical reactions. These reactions are responsible for the desired physicochemical and sensory attributes in the coffee beverage, such as flavor, aroma and color, but also the formation of undesirable compounds (Hidroximetilfurfural (HMF), acrylamide and others) [11,12].
During coffee roasting, it is essential to control the temperature and interrupt the process at the right time to obtain a product with good sensory and physicochemical properties. The degree of roasting of the coffee bean is determined by the habit and preference of the consumer. The roasting conditions usually employed are close to the temperature and time of 200–210 °C/8–12 min [13,14]. In this context, it is necessary to explore other time and temperature binomials to predict the impact on the chemical composition and antioxidant properties of coffee through the use of unusual temperatures for coffee processing. The study of different roasting profiles is important because it can generate data that will help in the implementation of new roasting indices that can benefit the chemical composition as well as the final quality of the product. Some studies have already evaluated the effect of different time/temperature binomials on the phenolic composition and antioxidant activities of coffee varieties [5,6,15]. However, in the present study, the binomials used were different from previous studies, using a wide temperature range. Therefore, this study aimed to evaluate colorimetric indices, chemical composition, antioxidant activity, and total phenolics in Robusta and Arabica coffees, alternating the binomial time and temperature.
2. Materials and methods
2.1. Materials
All reagents met the quality norms required for analytical grade reagents. Folin–Ciocalteu's phenol reagent (FCR), acetic acid, caffeic acid, chlorogenic acid, gallic acid (>98%), 6-hydroxy-2578-tet-ramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Triphenyltetrazolium chloride (TPTZ), caffeine, trigonelline and 5-hidroxymethylfurfural were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethyl acetate (≥99,7%), methanol (≥99.9%) were purchased from Chromasolv (Shanghai, China). Ethyl alcohol (≥99.5%), sodium carbonate, ferric chloride and sodium were purchased from Êxodo Científica (Sumaré, SP, Brasil).
2.2. Coffee samples
Coffee samples (Coffea arabica Catúai variety and Coffea canephora Pierre variety), dry processed, June–July/2020 crop, were supplied from coffee farms in the São Geraldo city, located in Zona da Mata Mineira, Minas Gerais, Brazil.
2.3. Coffee roasting process
The coffee beans were roasted using a Probat drum roaster (Probat-Werke). Each roast profile was performed only once. Then, the samples of roasted and ground coffee beans were individually packaged in an odor-free closed hermetic package and transported to the Laboratory of Natural Dyes and Bioactive Compounds of the Federal University of Viçosa Food Technology Department and kept in storage at 5 °C for further analysis. Six different roasting profiles were obtained by varying the roasting temperature and time. Five of the roasting profiles were created to obtain standard roasting [Underdeveloped (T1): 135 °C/20.20 min; Light (T2): 210 °C/9.02 min; Dark (T3): 220 °C/13.47 min; Baked (T4): 230 °C/17.43 min; and Scorched (T5): 275 °C/7.46 min)], while the control was the standard procedure commonly used in coffee roasting (T6: 210 °C/11.01 min) (Table 1) [16]. The choice of roasting defects was based reflecting a consensual configuration of pre-existing and applied roasting in the market, the most common being T6: 210 °C/11.01 min (standard).
Table 1.
Roasting profile with respective time/temperature binomial.
| Roasting profile | Roasting Temperature (°C) | Start time: 1° CRACK (min) | Total roasting time (min) |
|---|---|---|---|
| T1 | 135 | 2.16 | 20.20 |
| T2 | 210 | 0.13 | 9.02 |
| T3 | 220 | 4.55 | 13.47 |
| T4 | 230 | 6.20 | 17.43 |
| T5 | 275 | 1.56 | 7.46 |
| T6 | 210 | 2.38 | 11.01 |
2.4. Determination of color
The roasted coffee color was determined using a Colorquest XE Colorimeter (Hunter Lab, Reston, VA), with direct reading of the indexes values L* (brightness), a* (intensity of red vs green), and b* (intensity of yellow vs blue). The hue (h*) and chroma (c*) indexes were calculated from the values of a* and b*, according to equations (1), (2), respectively [17].
| (1) |
| (2) |
2.5. Total phenolic content
The quantification of total phenolic compounds was performed according to the method described by Ref. [18]. The results were expressed in gallic acid equivalent/g of coffee.
2.6. Determination of antioxidant activity
The scavenging activity on DPPH radicals was determined according to the method described by Ref. [19] and the Ferric Reducing Antioxidant Power (FRAP) according to the method of [20].
2.7. Analysis of caffeine, caffeoylquinic acid (5-CQA), trigonelline, caffeic acid and HMF contents in roasted and ground coffee
Caffeic acid, caffeoylquinic acid, caffeine, trigonelline, and hydroxymethylfurfural were determined in roasted coffee samples according to the methodology adapted by Ref. [21]. Analyzes were performed by high performance liquid chromatography (HPLC) on a Thermo Scientific Accela LC system (diode array detector (DAD), autoinjector, and Accela pump) (Thermo Fisher Scientific, Austin, TX). The column used for the separation was the reverse phase Lichrospher 100 RP-18 (250 × 4.6 mm, with a particle size of 5 μm and 10 nm pore) (Merck, Germany). The mobile phase consisted of water (A) and methanol (B), with elution in isocratic mode of 0–6 min (90% A and 10% B), gradient mode of 6–7 min (90 - 80% A and 10–20% B), isocratic mode of 7–23 min (80% A and 20%), gradient 23–24 min (80 - 0% A and 20–100% B), 24–25 min (0–90% A and 100–10% B) and ending with isocratic mode of 25–26 min (90% A and 10% B). The flow was 1 mL/min, and the injection volume was 1 μL (partial loop), with a temperature of 25 °C for the injector and 40 °C for the column. Peaks were detected at wavelengths of 272 nm. Caffeine, caffeoylquinic acid (5-CQA), trigonelline, caffeic acid, and HMF were identified by standards injection and calibration curve.
2.8. Statistical analysis
All analyses were performed in 3 repetitions. The data was expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) in a factorial design was used. A 2-factor factorial design was used (2x6), with the first factor being the coffee species (Robusta and Arabica) and the second factor the roasting profiles (Table 1), totaling 12 treatments. Differences in means were compared using Tukey's post hoc test. All analyses adopted a significance level of 5% and were performed using the R software (R Core Team, Vienna, Austria). Principal component analysis (PCA) based on the correlation matrix was conducted to differentiate the color indexes using the R program (R Core Team, Vienna, Austria).
3. Results and discussion
3.1. Analysis of color
The coffee bean color is related to the beverage quality and is an important factor in the product value. Changing the degree of temperature applied in the roasting process allows obtaining information on changes in coffee bean color, which is intrinsically related to the Maillard reaction. The color changes result from the formation of some Maillard reaction products that can impart functionality and appearance to roasted coffee beans [22,23]. The quantitative color evaluation of the roasted and ground coffee beans was reasoned on the indexes value L*, a *, b *, C*, and hue (Table 2).
Table 2.
Average attributes color of canephora (Robusta) and arabica coffee variety Catúai samples.
| Samples | Roasting Profiles |
|||||
|---|---|---|---|---|---|---|
| 135 °C/20.20 min | 210 °C/9.02 min | 220 °C/13.47 min | 230 °C/17.43 min | 275 °C/7.46 min | 210 °C/11.01 min | |
| L* (brightness) | ||||||
| C. canephora | 37.26 ± 0.78Aab | 36.76 ± 0.38Aab | 38.32 ± 1.64Aa | 37.96 ± 2.27Aab | 34.15 ± 2.92Ab | 37.36 ± 2.14Aab |
| C. arabica | 24.25 ± 1.24Bb | 24.97 ± 0.37Bb | 26.12 ± 0.60Bb | 35.28 ± 0.80Ba | 31.60 ± 1.75Aa | 25.52 ± 1.38Bb |
| a*(intensity of red vs green) | ||||||
| C. canephora | 26.10 ± 0.42Aa | 25.66 ± 0.46Aa | 26.12 ± 0.83Aa | 25.29 ± 0.92Aa | 22.50 ± 0.18Ab | 25.56 ± 0.82Aa |
| C. arabica | 4.96 ± 0.63Bd | 7.99 ± 0.87Bc | 11.93 ± 1.71Bb | 24.55 ± 0.50Aa | 11.38 ± 0.40Bb | 12.38 ± 1.60Bb |
| b* (intensity of yellow vs blue) | ||||||
| C. canephora | 23.08 ± 1.05Ab | 22.38 ± 0.65Ab | 26.56 ± 0.68Aa | 26.53 ± 0.65Aa | 15.36 ± 0.44Ac | 21.56 ± 1.22Ab |
| C. arabica | 0.81 ± 0.27Be | 2.18 ± 0.41Bde | 4.18 ± 1.02Bcd | 19.57 ± 1.36Ba | 10.14 ± 0.84Bb | 4.49 ± 0.92Bc |
| h* (hue) | ||||||
| C. canephora | 41.48 ± 0.87Ab | 41.09 ± 0.32Ab | 45.47 ± 1.17Aa | 46.38 ± 1.37Aa | 41.65 ± 0.63Ac | 40.12 ± 1.52Ab |
| C. arabica | 9.11 ± 1.93Bb | 15.14 ± 1.21Bc | 19.16 ± 1.71Bb | 38.52 ± 1.41Ba | 34.31 ± 1.34Ba | 19.81 ± 1.43Bb |
| c* (chroma) | ||||||
| C. canephora | 34.85 ± 1.81Aabc | 34.04 ± 1.00Abc | 37.25 ± 0.77Aa | 36.66 ± 0.77Aab | 27.25 ± 0.72Ad | 33.45 ± 0.37Ac |
| C. arabica | 5.02 ± 0.66Bd | 8.29 ± 0.94Bc | 12.65 ± 1.96Bb | 31.41 ± 1.24Ba | 15.24 ± 0.86Bb | 13.17 ± 1.81Bb |
Means followed by the same lowercase letters in the row and uppercase letters in the column do not differ by Tukey's test (p > 0,05).
Regarding the L* indexes values, significant differences (p < 0.05) were observed between the two species and corresponding roasting conditions, except for roasting performed at 275 °C/7.46 min, which presented values equal for Robusta and Arabica coffees. Robusta coffee samples were significantly lighter. The Arabica coffee samples had a darker color, and the samples roasted at lower temperatures had lower values of the L* index. Robusta coffee samples had higher a* values than Arabica coffee, except for roasting at 230 °C/17.43 min, in which the values did not differ from each other. As for the b* index, the Robusta coffee samples showed significantly higher values (p < 0.05) for all the roasts applied. The values of the indexes h* and C* of Arabica coffee in the different roasting conditions used were significantly lower than those of Robusta, characterizing the samples of Robusta coffee with higher values of chroma and hue, respectively.
According to Ref. [24] depending on the roasting intensity used, roasted and ground coffee may have a brownish, yellow, lighter, or stronger color. At average temperature (210 °C) can present a reddish brown color and high temperature (>220 °C) darker color brown. In the present study, Robusta coffee showed greater luminosity and higher chroma and hue values than Arabica coffee [25]. reported lower luminosity values for Arabica coffee mixed with Robusta when compared to pure Robusta.
The data obtained in the color evaluation of the roasted coffees were also analyzed by PCA (Fig. 1), commonly used to interpret the correlation between the color indexes of roasted Robusta and Arabica coffee beans. Data matrices for PCA study were set up configuring that every line was equivalent to a sample (coffee species) and each column to a color principle. The first two principal components (PCs) explained 52.4% and 46% of the data variance, respectively, with both components accumulating 98.37% of the variation. From the sample's spatial dispersion, it is possible to identify four distinct groups, separated by quadrant: 230 °C/17.43 min (PC1 positive, PC2 positive); 220 °C/13.47 min (PC1 negative, PC2 positive); 135 °C/20.20 min, 210 °C/9.02 min and 210 °C/11.01 min (PC1 negative, PC2 negative); 275 °C/7.46 min (PC1 positive, PC2 negative). The first component allowed the division between darker and lighter samples, being mainly affected by luminosity values.
Fig. 1.
Scatter plot of Principal Component Analysis (PCA) scores of L ∗ a ∗ b ∗ color indexes for Robusta and Arabica coffee samples after different roasting treatments (PC1 vs. PC2).
The color of coffee beans varies between light brown and dark brown due to the pyrolysis of organic compounds and the formation of melanoidins [26]. The decreases in L* values and increases in a* and b* values after greater exposure of the coffee beans to the roasting process (increased time and temperature) may be due to the development of dark pigments through non-enzymatic browning and degradation of phospholipids, as well as thermal total phenolics oxidation [27,28].
3.2. Phenolic content and antioxidant activity
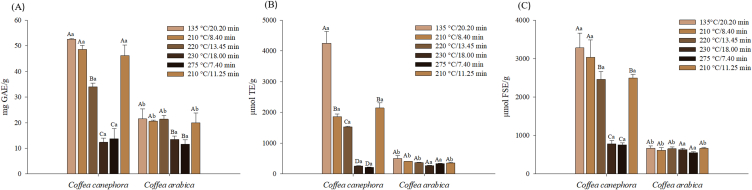
Different roasting conditions result in different final product quality, that, different colors, flavors, aroma, and acidity [29]. Futhermore, the chemical coffee bean properties depend on other factors such as species and geographic location [[29], [30], [31], [32], [33]]. Different roasting profiles in two coffee species were evaluated in this study. The results of the impact of these conditions on the TPC and antioxidant activities by the DPPH and FRAP methods are introduced in Fig. 2.
Fig. 2.
Total phenolic content (TPC) (A); DPPH radical scavenging activity (B); Ferric Reducing Antioxidant Power (FRAP) (C) of two coffee species after different roasting treatments. Lowercase letters represent Tukey's test at 5% probability between species and uppercase letters between treatments for the same species.
The TPC ranged from 12.31 to 52.47 mg GAE/g for Robusta and 11.52–21.47 mg GAE/g for Arabica. The highest values of TPC were detected in roasted coffee at temperatures of 135 °C/20.20 min, 210 °C/9.02 min, 210 °C/11.01 min and 220 °C/13.47 min for both species. The TPC contents of Robusta coffee were significantly higher than those of Arabica coffee (p < 0.05), except for roasts at 230 °C/17.43 min and 275 °C/7.46 min. The results also indicated a significant decrease in TPC when roasting profiles at higher temperatures of 230 °C/17.43 min and 275 °C/7.46 min were used. Long roasting times at low temperatures result in little exposure to heat and oxygen and less total phenolic content decrease [34].
The results of the DPPH method (Fig. 2) revealed that the Robusta coffee exhibited greater antioxidant activity when low or standard temperatures in the roasting process were used. Comparing the antioxidant properties determined in the DPPH assay within each coffee species, it was observed that the highest Robusta coffee samples antioxidant activity was provided by treatments at 135 °C/20.20 min, 210 °C/9.02 min, and 210 °C/11.01 min, followed by 220 °C/13.47 min. The methods at 230 °C/17.43 min and 275 °C/7.46 min were the ones that provided samples with lower antioxidant activity, which complies with the TPC. Arabica coffee did not show significant differences (p > 0.05) in antioxidant activity by the DPPH method among the pre-established roasts. A higher antioxidant activity was also observed in robusta coffee samples compared to Arabica coffee in different roasting profiles, except for treatments at 230 °C/17.43 min and 275 °C/7.46 min that showed the same antioxidant activity for both species. Similar results were found by Ref. [35] (roasting at 203–205 °C/11–13 min), indicating that the coffee antioxidant properties are affected by the species and roasting, with Robusta coffee being the one with higher antioxidant activity.
The ferric reducing antioxidant power (FRAP) of each roasted coffee sample was also evaluated and the results are pictured in Fig. 2. The ferric reducing power oscillated from 547.28 to 666.83 μmol FSE/g and 838,02–3150.62 μmol FSE/g for Arabica and Robusta samples, respectively. Robusta coffee showed greater antioxidant activity in coffees roasted at 135 °C/20.20 min, 210 °C/9.02 min, 220 °C/13.47 min and 220 °C/11.01 min. For Arabica coffee no significant differences (p > 0.05) were observed in the samples antioxidant activity. Therefore, after roasting, Robusta samples showed significantly higher antioxidant activity values (FRAP) compared to Arabica samples, except for samples roasted at 230 °C/17.43 min and at 275 °C/7.46 min. The higher antioxidant activity in Robusta coffee can be attributed to its caffeine content, which is an alkaloid and has antioxidant properties, whose content can be changed along the roasting standard [36].
The causes of changes in antioxidant activity in coffee beans subjected to different roasting temperatures are associated with chlorogenic acid degradation and the development of products from the advanced glycation [15]. Following the Maillard reaction during the coffee bean roasting process, non-covalent interactions between the phenolic compounds and the reaction products (melanoidins) occur and cause the complexes production that have varying degrees of antioxidant activity [37].
The roasting process impact on the antioxidant properties was verified in several studies, which revealed an increase in the antioxidant activity when using roasts at low temperatures and a subtraction in the antioxidant activity when using roasts at high temperatures [15,38]. The present study, Robusta coffee showed significantly higher antioxidant activity values (DPPH and FRAP) in treatments using roasts at lower temperatures (<210 °C) and significantly lower activities when roasting at high temperatures.
It is noteworthy that several methodologies are used in the characterization of antioxidant activity in foods, with no single, standardized and universal method in the process of performing the analysis. To determine the antioxidant activity, the ideal is to use at least two evaluation methods, with the DPPH, FRAP and total phenolic methods widely applied in the determination, as seen in the related work [39,40].
3.3. Caffeine, caffeoylquinic acid (5-CQA), trigonelline, caffeic acid, and HMF in roast and ground coffee
Aiming to evaluate whether the applied roasting process affected the caffeine, 5-CQA, trigonelline, caffeic acid, and HMF concentration, the analysis was carried out in HPLC.
Caffeine contents in roasted and ground coffee samples varied in a wide range from 9.27 mg/g to 33.29 mg/g in Robusta coffee and from 5.55 mg/g to 10.15 mg/g in coffee Arabica, with the highest content found in roasting at 135 °C/20.20 min for Robusta and at 220 °C/13.47 min for Arabica. (Table 3). The presence of caffeine in the roasts may be due to the compound thermostability and the loss of mass of thermolabile compounds due to the roasting temperature applied [41]. The presence of caffeine in the samples may be due to the compound thermostability and the loss of mass of thermolabile compounds due to the roasting temperature applied [41]. Notably, this compound's thermal resistance was predominantly higher in almost all treatments in relation to caffeic acids and 5-CQA, as well as trigonelline and HMF, under the pre-established roasting conditions (Table 3). As reported, the coffee samples caffeine content in both species was in agreement with the ranges reported in the literature [21,42], with higher values in Robusta coffee.
Table 3.
Caffeine, caffeoylquinic acid (5-CQA), trigonelline, caffeic acid and hydroxymethylfurfural (HMF) (mg/g) content in canephora (Robusta) and arabica coffee variety Catúai affected by different degrees of roasting.
| Samples | |||||||
|---|---|---|---|---|---|---|---|
| 135 °C/20.20 min | 210 °C/9.02 min | 220 °C/13.47 min | 230 °C/17.43 min | 275 °C/7.46 min | 210 °C/11.01 min | ||
| Caffeine | |||||||
| C. canephora | 33.29 ± 0.50Aa | 31.16 ± 2.29Aa | 19.27 ± 2.24Ab | 12.40 ± 1.63Abc | 9.27 ± 0.10Ac | 30.19 ± 2.28Aa | |
| C. arabica | 5.55 ± 2.09Ba | 6.71 ± 1.95Ba | 10.15 ± 2.18Ba | 6.19 ± 1.18Ba | 9.33 ± 0.12Aa | 7.19 ± 0.37Ba | |
| 5-CQA | |||||||
| C. canephora | 17.27 ± 0.19Aa | 17.67 ± 1.5Aa | 10.26 ± 2.15Ab | 3.50 ± 0.08Ac | 0.93 ± 0.05Ac | 15.03 ± 1.15Aab | |
| C. arabica | 6.03 ± 2.05Bab | 7.08 ± 1.92Bab | 8.85 ± 1.13Aa | 2.13 ± 0.98Ab | 3.07 ± 1.58Aab | 6.11 ± 1.06Bab | |
| Trigonelline | |||||||
| C. canephora | 9.11 ± 0.12Aa | 9.72 ± 0.75Aa | 6.47 ± 0.41Ab | 4.36 ± 0.07Ab | 1.27 ± 0.03Ac | 5.34 ± 0.06Ab | |
| C. arabica | 5.02 ± 1.39Bab | 3.95 ± 0.00Bab | 5.73 ± 0.00Aa | 2.59 ± 0.82Bbc | 0.94 ± 0.02Ac | 5.58 ± 0.39Aa | |
| Caffeic acid | |||||||
| C. canephora | 0.08 ± 0.02Ab | 0.55 ± 0.11Aa | 0.00 ± 0.00Ab | 0.00 ± 0.00Ab | 0.00 ± 0.00Ab | 0.42 ± 0.02Aa | |
| C. arabica | 0.00 ± 0.00Aa | 0.00 ± 0.00Ba | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Ba | |
| HMF | |||||||
| C. canephora | 0.27 ± 0.22 | 0.06 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.01 | |
| C. arabica | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
Means followed by the same lowercase letters in the row and uppercase letters in the column do not differ by Tukey's test (p > 0,05).
The highest levels of 5-CQA were observed in Robusta coffee at roasting profiles of 135 °C/20.20 min, 210 °C/11.01 min, and 210 °C/9.02 min, with average values of 17, 27, 15.03, and 17.67 mg/g, respectively. The results also indicated that there was a significant decrease (p < 0.05) of 5-CQA at higher temperatures of 220 °C/13.47 min, 230 °C/17.43 min, and 275 °C/7.46 min (Table 3). Concentrations of 5-CQA decrease drastically when more severe roast conditions (between 180 and 200 °C) are used in the process of obtaining the roasted coffee powder, with Robusta coffee being the species with a slightly higher amount of chlorogenic acids (CQAs) [43].
In the present study, Robusta coffee had a higher trigonelline content in almost all roasting profiles, with the highest levels found in coffees roasted at mild temperatures, especially in roasting profiles 135 °C/20.20 min and 210 °C/9.02 min, with values of 9.11 and 9.72 mg/g, respectively. Arabica coffee had lower trigonelline content (Table 3). The local climate, species, temperature, and roasting time can perform an important role in trigonelline content [44], which possibly explains the higher trigonelline content in Robusta coffee. Caffeic acid is a hydroxycinnamic compound that partially originates from the hydrolysis of caffeoylquinic and dicaffeoylquinic acids of roasting technique [36] and is found in low levels. Minimum contents of this compound were found in the analyzed Robusta coffee samples. In Arabica coffee samples, the compound was not found (Table 3). It was verified the absence of HMF in the Arabica coffee used in this study in all roasting profiles and a minimum amount of this compound in Robusta coffee, showing the ability of this compound to decompose quickly. These results indicated that the coffee species and temperatures employed did not interfere with the concentration of HMF, thus resulting in a non-significant interaction. The HMF degradation could have occurred from the reaction between the furan compound and the amino acid decomposition products or compact with sugar alcohols and nitrogen-free polymer to trigger flavor compounds and melanoidins [45].
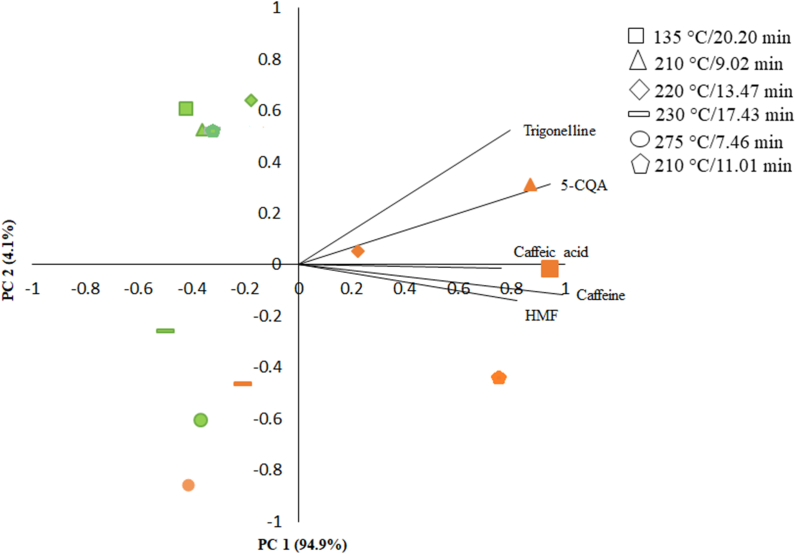
For the analysis of composition and caffeine, trigonelline, 5-CQA, caffeic acid, and HMF levels, in order to investigate the correlation between the studied parameters, the statistical method known as Principal Component Analysis (PCA) was employed. This procedure allowed for a graphical evaluation of the variable values' dispersions with respect to component 1 and component 2 through a visual representation on the PCA score plot (Fig. 3).
Fig. 3.
Scatter plot of Principal Component Analysis (PCA) scores of chemical compounds for Robusta and Arabica coffee samples after different roasting treatments (PC1 vs. PC2).
Fig. 3 illustrates the PCA score plot, where the samples are represented in a two-dimensional space formed by two axes or coordinate components. In this context, samples that cluster closely to these components possibly share similar chemical concentrations. It was observed that the first principal component (PC1) was able to explain 94.9% of the variation present in the data, while the second principal component (PC2) explained only 4.1% of the variation. This finding reinforces the representativeness of the analysis performed.
Consequently, it was observed that the analyzed chemical compounds presented higher concentrations in Robusta coffee. Additionally, it is noteworthy that temperature played a relevant role in altering the concentrations of the chemical compounds, with roasting at 275 °C/7.46 min being the most drastic condition for the reduction of these compounds.
These results underscore the importance of PCA as a valuable tool in investigating the chemical composition and variations of components in different coffee samples, providing a more comprehensive and precise understanding of the factors influencing the quality and chemical profile of this globally cherished beverage.
4. Conclusion
The present study investigated the physicochemical changes during the roasting process of Robusta and Arabica coffees. In general, TPC gradually reduced with the addition of temperature and roasting time for both species. Robusta coffee showed higher antioxidant capacity than arabica coffee samples, except for profiles 230 °C/17.43 and 275 °C/7.46 min, in which both samples showed equivalent antioxidant capacity in all methods applied. Robusta coffee samples were characterized as lighter and with higher chroma and hue values. The thermal resistance of caffeine was higher than that of chlorogenic (5-CQA) and caffeic acids. Robusta coffee samples in all roasting profiles showed higher values of trigonelline content. In Arabica coffee, the absence of HMF was verified in all roasting profiles. The results showed that different roasting profiles directly influence the physicochemical characteristics and antioxidant properties of coffee.
Author contribution statement
Valdeir Viana Freitas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Larissa Lorrane Rodrigues Borges: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gabriel Abranches Dias Castro: Performed the experiments; Analyzed and interpreted the data.
Marcelo Henrique dos Santos: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Márcia Cristina Teixeira Ribeiro Vidigal: Analyzed and interpreted the data; Wrote the paper.
Sergio Antonio Fernandes: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Paulo Cesar Stringheta: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are grateful for the financial support provided by the Fundação de Amparo á Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Cientifíco e Tecnoloǵico (CNPq), Embrapa-Café, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES, Finance Code 001). M.H.S., P.C.S. and S.A.F. were supported by Research Fellowships from CNPq. To coffee producers in the city of São Geraldo, Minas Gerais, Brazil, we thank you for making samples available.
References
- 1.CONAB . 2019. Companhia Nacional de Abastecimento.https://www.conab.gov.br Avalilable: Acess: 29 mar. 2019. [Google Scholar]
- 2.Wang X., Lim L.-T., Fu Y. Review of analytical methods to detect adulteration in coffee. J. AOAC Int. 2020;103:295–305. doi: 10.1093/jaocint/qsz019. [DOI] [PubMed] [Google Scholar]
- 3.Agunbiade H.O., Fagbemi T.N., Aderinola T.A. Antioxidant properties of beverages from graded mixture of green/roasted coffee and hibiscus sabdariffa calyx flours. Appl. Food Res. 2022;2 doi: 10.1016/j.afres.2022.100163. [DOI] [Google Scholar]
- 4.Endeshaw H., Belay A. Optimization of the roasting conditions to lower acrylamide content and improve the nutrient composition and antioxidant properties of Coffea arabica. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz A.E., Hernández S.S., Tolosa A.R., Burillo S.P., Olalla Herrera M. Evaluation of differences in the antioxidant capacity and phenolic compounds of green and roasted coffee and their relationship with sensory properties. LWT. 2020;128 doi: 10.1016/j.lwt.2020.109457. [DOI] [Google Scholar]
- 6.Liao Y.-C., Kim T., Silva J.L., Hu W.-Y., Chen B.-Y. Effects of roasting degrees on phenolic compounds and antioxidant activity in coffee beans from different geographic origins. LWT. 2022;168 doi: 10.1016/j.lwt.2022.113965. [DOI] [Google Scholar]
- 7.Pastoriza S., Rufián-Henares J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014;164:438–445. doi: 10.1016/j.foodchem.2014.04.118. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A., Mahmood N., Hussain M., Aiman U., Al-Mijalli S.H., Raza M.A., Al Jbawi E. Improvement in oxidative stability and quality characteristics of functional chicken meat product supplemented with aqueous coriander extract. Int. J. Food Prop. 2023;26:855–865. doi: 10.1080/10942912.2023.2189086. [DOI] [Google Scholar]
- 9.Liu C., Yang N., Yang Q., Ayed C., Linforth R., Fisk I.D. Enhancing Robusta coffee aroma by modifying flavour precursors in the green coffee bean. Food Chem. 2019;281:8–17. doi: 10.1016/j.foodchem.2018.12.080. [DOI] [PubMed] [Google Scholar]
- 10.Liu C., Yang Q., Linforth R., Fisk I.D., Yang N. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019;272:251–257. doi: 10.1016/j.foodchem.2018.07.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguiar J., Estevinho B.N., Santos L. Microencapsulation of natural antioxidants for food application – the specific case of coffee antioxidants – a review. Trends Food Sci. Technol. 2016;58:21–39. doi: 10.1016/j.tifs.2016.10.012. [DOI] [Google Scholar]
- 12.Seninde D.R., Chambers E. Coffee flavor: a review. Beverages. 2020;6:44. doi: 10.3390/beverages6030044. [DOI] [Google Scholar]
- 13.Malaquias J.V., Celestino S.M.C., Xavier M.F.F. Optimization of the roasting conditions of arabica coffee cultivated in the cerrado area of Brazil. Brazilian J. Food Technol. 2018;21 doi: 10.1590/1981-6723.16216. [DOI] [Google Scholar]
- 14.Nakilcioglu-tas E., Otles S. Physical characterization of Arabica ground coffee with different roasting degrees. An. Acad. Bras. Cienc. 2019;91 doi: 10.1590/0001-3765201920180191. [DOI] [PubMed] [Google Scholar]
- 15.Mehaya F.M., Mohammad A.A. Thermostability of bioactive compounds during roasting process of coffee beans. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacalone D., Degn T.K., Yang N., Liu C., Fisk I., Münchow M. Common roasting defects in coffee: aroma composition, sensory characterization and consumer perception. Food Qual. Prefer. 2019;71:463–474. doi: 10.1016/j.foodqual.2018.03.009. [DOI] [Google Scholar]
- 17.Rocha J. de C.G., de Barros F.A.R., Perrone Í.T., Viana K.W.C., Tavares G.M., Stephani R., Stringheta P.C. Microencapsulation by atomization of the mixture of phenolic extracts. Powder Technol. 2019;343:317–325. doi: 10.1016/j.powtec.2018.11.040. [DOI] [Google Scholar]
- 18.V L., Singleton R.J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 19.Kim D.-O., Lee K.W., Lee H.J., Lee C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 20.M.. V.J.. C.S.. M.D. Boroski, Antioxidantes: Princípios e Métodos Analíticos, first ed., appris, n.d.
- 21.Vignoli J.A., Viegas M.C., Bassoli D.G., Benassi M. de T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014;61:279–285. doi: 10.1016/j.foodres.2013.06.006. [DOI] [Google Scholar]
- 22.Dong W., Hu R., Chu Z., Zhao J., Tan L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017;234:121–130. doi: 10.1016/j.foodchem.2017.04.156. [DOI] [PubMed] [Google Scholar]
- 23.Toci A.T., Farah A. Volatile fingerprint of Brazilian defective coffee seeds: corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014;153:298–314. doi: 10.1016/j.foodchem.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Bicho N.C., Leitão A.E., Ramalho J.C., Lidon F.C. Use of colour parameters for roasted coffee assessment. Food Sci. Technol. 2012;32:436–442. doi: 10.1590/S0101-20612012005000068. [DOI] [Google Scholar]
- 25.Wongsa P., Khampa N., Horadee S., Chaiwarith J., Rattanapanone N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019;283:579–587. doi: 10.1016/j.foodchem.2019.01.088. [DOI] [PubMed] [Google Scholar]
- 26.Cid M.C.P.M.P. Coffee: analysis and composition. Encycl. Food Heal. 2016:225. 23. [Google Scholar]
- 27.Budryn G., Nebesny E., Podsędek A., Żyżelewicz D., Materska M., Jankowski S., Janda B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009;228:913–922. doi: 10.1007/s00217-008-1004-x. [DOI] [Google Scholar]
- 28.Patras A., Brunton N.P., O'Donnell C., Tiwari B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- 29.Zhu M., Long Y., Ma Y., Chen Y., Yu Q., Xie J., Li B., Tian J. Comparison of chemical and fatty acid composition of green coffee bean (Coffea arabica L.) from different geographical origins. LWT. 2021;140 doi: 10.1016/j.lwt.2020.110802. [DOI] [Google Scholar]
- 30.Bertone E., Venturello A., Giraudo A., Pellegrino G., Geobaldo F. Simultaneous determination by NIR spectroscopy of the roasting degree and Arabica/Robusta ratio in roasted and ground coffee. Food Control. 2016;59:683–689. doi: 10.1016/j.foodcont.2015.06.055. [DOI] [Google Scholar]
- 31.Sandoval Z., Prieto F., Betancur J. 2010 IEEE Electron. Robot. Automot. Mech. Conf. IEEE; 2010. Digital image processing for classification of coffee cherries; pp. 417–421. [DOI] [Google Scholar]
- 32.Zheng C., Sun D.-W., Zheng L. Recent developments and applications of image features for food quality evaluation and inspection – a review. Trends Food Sci. Technol. 2006;17:642–655. doi: 10.1016/j.tifs.2006.06.005. [DOI] [Google Scholar]
- 33.Wu D., Sun D.-W. Colour measurements by computer vision for food quality control – a review. Trends Food Sci. Technol. 2013;29:5–20. doi: 10.1016/j.tifs.2012.08.004. [DOI] [Google Scholar]
- 34.Kieu Tran T.M., Kirkman T., Nguyen M., Van Vuong Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora) Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andueza S., Cid C., Cristina Nicoli M. Comparison of antioxidant and pro-oxidant activity in coffee beverages prepared with conventional and “Torrefacto” coffee. LWT - Food Sci. Technol. 2004;37:893–897. doi: 10.1016/j.lwt.2004.04.004. [DOI] [Google Scholar]
- 36.Vignoli J.A., Bassoli D.G., Benassi M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the influence of processing conditions and raw material. Food Chem. 2011;124:863–868. doi: 10.1016/j.foodchem.2010.07.008. [DOI] [Google Scholar]
- 37.Wolfe K.L., Liu R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 38.Pokorná J., Venskutonis P.R., Kraujalyte V., Kraujalis P., Dvorák P., Tremlová B., Kopriva V., Oštádalová M. Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Aliment. 2015;44:454–460. doi: 10.1556/066.2015.44.0017. [DOI] [Google Scholar]
- 39.Batista N.N., de Andrade D.P., Ramos C.L., Dias D.R., Schwan R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016;90:313–319. doi: 10.1016/j.foodres.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Bressani A.P.P., Batista N.N., Ferreira G., Martinez S.J., Simão J.B.P., Dias D.R., Schwan R.F. Characterization of bioactive, chemical, and sensory compounds from fermented coffees with different yeasts species. Food Res. Int. 2021;150 doi: 10.1016/j.foodres.2021.110755. [DOI] [PubMed] [Google Scholar]
- 41.Crozier T.W.M., Stalmach A., Lean M.E.J., Crozier A. Espresso coffees, caffeine and chlorogenic acid intake: potential health implications. Food Funct. 2012;3:30–33. doi: 10.1039/C1FO10240K. [DOI] [PubMed] [Google Scholar]
- 42.Farah A. Wiley-Blackwell; Oxford: 2012. Coffee Constituents, Coffee: Emerging Health Effects and Disease Prevention. [Google Scholar]
- 43.Farah A., de Paulis T., Trugo L.C., Martin P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005;53:1505–1513. doi: 10.1021/jf048701t. [DOI] [PubMed] [Google Scholar]
- 44.Murkovic M., Bornik M.-A. Formation of 5-hydroxymethyl-2-furfural (HMF) and 5-hydroxymethyl-2-furoic acid during roasting of coffee. Mol. Nutr. Food Res. 2007;51:390–394. doi: 10.1002/mnfr.200600251. [DOI] [PubMed] [Google Scholar]
- 45.Rigo D., Polidoro D., Perosa A., Selva M. Diversified upgrading of HMF via acetylation, aldol condensation, carboxymethylation, vinylation and reductive amination reactions. Mol. Catal. 2021;514 doi: 10.1016/j.mcat.2021.111838. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.