Abstract
Wheat is an important food crop worldwide, providing substantial calories and nourishment. Genetic variability in wheat germplasm is crucial for the development of cultivars with desirable features. This two years study (2020–21 and 2021–22) was conducted to evaluate 13 diverse wheat genotypes factorially combined with foliar-applied zinc sulphate (0, 0.4, 0.6%) arranged in a triplicate randomized complete block design. Boxplot analysis revealed the significant (P < 0.01) phenotypic variation of wheat germplasm for all the studied traits, but maximum variation was observed for yield and Zn biofortification-related traits. Correlation and path analysis revealed a significant (P < 0.01) association among yield and biofortification-related traits. Zinc uptake showed maximum strength of association (r = 0.96, p < 0.01) with grain Zn concentration. The Biplot analysis showed the graphical representation of wheat accessions based on similar characteristics and then assort into distinct groups. Broadsense heritability (Hbs) was calculated to determine the proportion of variation transmitted to future generations. The high value of Hbs for yield and Zn biofortification-related traits indicates that these traits are governed by the additive type of gene action and can be fixed in early segregating generations. In crux, this study validated the genetic variability in existing wheat genotypes for yield and Zn biofortification-related traits and may be helpful to devise an efficient breeding program for wheat Zn biofortification.
Keywords: Heritability, PCA, Correlation, Path analyses, Zn biofortification
1. Introduction
Wheat is an important cereal crop globally, feeding over a third of the world's population [1]. Many countries rely on it as a staple food crop due to its richness in carbohydrates and dietary fiber [2,3]. In addition, it contains crucial nutrients such as vitamins B and E, as well as phosphorus, which are necessary for human health [4,5]. Global wheat production in 2021–22 reached 778 million metric tons, up by 4 million tons from 2020 to 21 [6]. In Pakistan, 9168 thousand hectares of wheat cultivation produced 27.63 million tons, contributing 8.2% to agricultural value addition and 1.9% to GDP in 2022–23 [7]. Despite its significance, wheat production faces several challenges, including low yields and deficiency in essential micronutrients. To meet the growing demands of wheat, it is important to improve the per hectare yield of wheat [8]. As the global population continues to increase, the need for sustainable measures to improve crop productivity becomes increasingly pressing. High-yielding wheat varieties have been developed over the years, but there is still room for improvement. Various factors affect wheat yield, including climate change, soil quality, and pest and disease outbreaks [9,10]. The wheat yield needs to be increased to meet the demands of the growing population. Additionally, wheat is deficient in several micronutrients [11]. Zinc (Zn) is an essential mineral for human health. However, over two billion people, mainly in developing countries, suffer from zinc deficiency due to a lack of Zn in their diet, leading to stunted growth, impaired immune function, and an increased risk of infections [12,13]. Zn biofortification is a sustainable way to improve the Zn contents in wheat and human diet.
Along-with being an important component of human being, Zn is also equally important for plant growth and development, where it is required only in minute quantity, but its deficiency leads to reduced crop yields and poor-grain quality [14,15]. The impact of zinc on crop yield and related attributes has been extensively researched, with multiple studies demonstrating significant improvements in productivity with zinc fertilization [14,16]. Inadequate Zn supply affects plant metabolism, leading to stunted growth, delayed maturity, and reduced grain yield [17,18]. Studies have shown that fertilizing with Zn can increase grain yield, as well as enhance plant height, grain weight, and tiller count [15,16,18]. In addition to improving crop yields, Zn fertilization also improves grain Zn contents and its uptake [19]. Several studies have shown that Zn fertilization increases the grain Zn content, improving nutritional quality [14,19,20]. Zinc biofortification by fertilization is an important strategy for addressing zinc deficiency in human diet, especially in developing countries where most of the soils are Zn deficient [9]. Therefore, Zn fertilization can significantly improve the crop productivity, nutritional quality of grains, and Zn availability in human diet.
Grain yield and nutritional quality traits are important traits for the plant breeders [21]. It is often challenging to select multiple traits together due to the variety of correlations. Therefore, it is essential to know the association among traits and their ultimate effect on yield and nutrient content in a breeding program [22]. To figure out the association of various characteristics, path analysis and principal component analyses (PCA) are most widely used because path analysis is used to determine the direct and indirect effects of traits on a dependent variable [23]. It provides a better understanding of the relationships between traits and the importance of each trait in determining the dependent variable. PCA is a multivariate technique that is used to reduce the dimensionality of data. It identifies the principal components explaining the maximum variation in the data set [23,24]. It is also useful in identifying the genotype group based on the association among different traits in a breeding program. Heritability of character is very important to be measured because the associations of various traits in genotypes are changed owing to the breakage of association among genes (i.e., crossing over) under changes in environmental conditions. Heritability of the multiple characteristics of plants related to yield and nutritional content is also determined to figure out better Zn biofortified wheat genotypes with improved yield [[24], [25], [26]].
The mentioned research focuses on developing strategies for enhancing the zinc (Zn) content in wheat grains, which can contribute to the biofortification of wheat. However, several factors, such as climate fluctuations and soil conditions, can affect the performance of different wheat genotypes regarding biofortification [26,27]. Thus, the primary objective of the study is to investigate the heritability and phenotypic diversity of wheat genotypes for concentration and bioavailability of grain zinc content and yield. In addition, the study also examines the effect of Zn fertilization on the relationship among yield and biofortification related traits of wheat. The study aims to provide insights into the factors influencing Zn biofortification in wheat and identify potential breeding and agronomic strategies for improving he grain Zn content in wheat.
2. Materials and methods
2.1. Experimental site and treatments
The experiment was conducted in the field area of the College of Agriculture at the University of Layyah, Pakistan during two growing seasons (2020–2021 to 2021–2022). The field area experienced an average rainfall of 275 mm with a warm summers and cold winters. The soil was categorized as aridisol, containing 65% sand, 22% silt, and 20% clay. The soil pH was 8.1 and the concentration of primary nutrients (NPK) was recorded 438 mg/kg, 6.3 mg/kg and 120 mg/kg respectively and 675 μg/kg Zn (AB-DTPA-extractable).
The wheat's seed was drilled with a 45 cm row to row and a 30 cm plant to plant spacing at mid of November in both years and was harvested in last week of April. During crop life span, the recommended dose of NPK, irrigations and plant protection measures were applied for the uniform growth and development of the crop.
The experimental treatments were arranged in a two-factorial randomized complete block design with three replications. Treatment factors include 13 genotypes of wheat (Table S1) and three foliar applied Zn concentrations, i.e., 0, 0.4 and 0.6%, respectively. For all treatments, aquas solution was prepared and was applied at the grain filling stage (Z75 Zadoks growth scale). The experiment was repeated with the same experimental treatments in the next year.
2.2. Data collection
For all yield-related traits, data were collected for 10 randomly selected plants and average was calculated for further processing of the data. Plant height was measured from the soil surface to the top of the spike excluding awns. On each spike, the number of spikelets were counted. The harvested plants were manually threshed and weighed at maturity to obtain grain yield.
To conduct the grain mineral analyses, a unique sample of 01 g was taken and baked at 65 °C until the weight was constant. This was followed by the grinding of the samples to pass through a 1 mm sieve. To digest the ground materials, a solution of 2:1 perchloric and nitric acid was used according to the approach developed by Jones and Case [28]. The concentration of Zn in the digested samples was determined using an atomic absorption spectrophotometer (240FS AA, Agilent, Santa Clara, USA). Grain Zn uptake was calculated by multiplying the Zn concentration with the grain yield. To extract the phytate from the same samples used for estimating Zn concentrations, 0.2 M HCL was used following the procedure outlined by Haug and Lantzsch [29].
The bioavailability of Zn for human consumption was determined through trivariate model of Zn absorption as described by Miller et al. [30]. The model assumes that an adult consumes 300 g of wheat flour daily as their sole source of zinc and calculates the availability of zinc in milligrams per day (mg Zn d−1).
2.3. Statistical analyses
The statistical software STATISTIX 8.1 was used to analyze the data for the analyses of variance and to calculate the mean squares. Boxplot analyses were performed to estimate the presence of genetic variability of wheat genotypes under different levels of treatment. Correlation analyses were also performed to determine the degree of association among the traits under study by using equation (1) [31].
| (1) |
where n represents the number of observations, and ‘X’ and ‘Y’ are variables which were counted across the replications.
Path coefficient analysis was used to investigate the direct and indirect relationships among traits, as per the method (Equation (2)) described by Dewey and Lu [32].
| (2) |
In the above formula, outcome variable is represented by ‘Y’, the intercept is represented by ‘b0’ while ‘b1’, ‘b2’, …, ‘bn’ are the path coefficients and ‘X1’, ‘X2’, …, ‘Xn’ are the independent variable.
PCA biplot analysis was employed to group the genotypes based on multivariate relationship among the traits. Likewise, broad sense heritability was calculated separately for each Zn treatment, using equation (3) [33].
| (3) |
3. Results
3.1. Phenotypic variability for yield and zinc related traits
The studied genotypes have shown a range of phenotypic variability for plant height, number of spikelets per spike, grain yield, grain zinc content and bioavailability and zinc uptake and the interaction of ZnSO4 and genotypes was also significant (p < 0.01) for the all the traits except the number of spikelets per spike and grain zinc content (Table 1). Box plots were created to estimate the degree of genotypic variation among wheat genotypes under different Zn fertilization treatments (Fig. 1). There was a less amount of variation among genotypes for plant height, number of spikelets per spike and spike length under all Zn fertilization treatments (Fig. 1a, b, c). Grain yield showed a greater amount genetic variation among genotypes under 0.6% ZnSO4 fertilization relative to control and 0.4% (Fig. 1d). Zinc biofortification related traits demonstrated a maximum variation among genotypes across all three ZnSO4 levels. Variation among the genotypes for the grain zinc content and grain Zn bioavailability was maximum at 0.4% and 0.6% respectively (Fig. 1e and f). Phenotypic variation for the Zn uptake was nearly similar under all Zn fertilization treatments (Fig. 1g).
Table 1.
Mean square of different yield and zinc related traits of wheat as affected by ZnSO4 levels.
| Source of Variance | Plant Height | Number of Spikelets/Spike | Spike Length | Grain Yield/Plant | Grain Zinc Content | Grain Zinc Bioavailability | Zinc Uptake |
|---|---|---|---|---|---|---|---|
| Genotype (G) | 235.75** | 5.33** | 2.78** | 1.57** | 116.47** | 1.74** | 0.01** |
| ZnSO4 | 266.84** | 11.36** | 0.12ns | 5.31** | 716.68** | 21.11** | 0.09** |
| ZnSO4 *G | 64.31** | 1.80ns | 1.50** | 0.60* | 7.61ns | 0.20** | 0.00ns |
| Error | 8.73 | 1.23 | 0.53 | 0.31 | 4.97 | 0.01 | 0.00 |
*Significant at 5%, **Significant at 1%.
Fig. 1.
Box plot analyses for genetic variability of thirteen wheat genotypes for (a) plant height, (b) number of spikelets per spike, (c) spike length, (d) grain yield per plant, (e) grain Zn contents, (f) grain Zn bioavailability and (g) Zn uptake under various foliar applied treatments of zinc sulphate.
3.2. Heritability
Heritability is the proportion of variance that is passed on to future generations. The highest broad sense heritability (98% and 94%) was recorded for grain zinc bioavailability and plant height, respectively, under 0.6% ZnSO4 foliar application, whereas the coefficient of broad sense heritability changed as the concentration of zinc sulphate application is changed. Under 0.4% ZnSO4 foliar application, maximum broad sense heritability was recorded 97% for grain zinc bioavailability, followed by plant height (96%) and grain zinc content (95%), while in the control condition, maximum broad sense heritability (92% and 81%) was recorded for grain zinc bioavailability and plant height, respectively (Table 2).
Table 2.
Heritability coefficient of different yield and zinc related traits of wheat as affected by ZnSO4 levels.
| Plant Height | Number of Spikelets/Spike | Spike Length | Grain Yield/Plant | Grain Zinc Content | Grain Zinc Bioavailability | Zinc Uptake | |
|---|---|---|---|---|---|---|---|
| Control | 0.81 | 0.46 | 0.60 | 0.29 | 0.61 | 0.92 | 0.50 |
| 0.4% ZnSO4 | 0.95 | 0.67 | 0.83 | 0.71 | 0.95 | 0.96 | 0.93 |
| 0.6% ZnSO4 | 0.93 | 0.74 | 0.74 | 0.79 | 0.84 | 0.97 | 0.76 |
3.3. Correlation and path analyses
The knowledge about the association among the traits is crucial for the selection of multiple traits simultaneously. The correlation between the studied attributes changed under different levels of zinc sulphate application. Under control conditions, zinc uptake and grain zinc content (r = 0.96) had a highly significant connection, followed by grain yield and zinc uptake (r = 0.88) (Table 3). In the case of 0.4% ZnSO4 fertilization, correlation values were relatively lower. A highly positive correlation was observed between grain zinc content and zinc uptake (r = 0.91) followed by grain zinc bioavailability and zinc uptake (r = 0.70) (Table 3). In the case of 0.6% ZnSO4 application, the correlation between grain zinc content and Zinc uptake (r = 0.85) decreased as compared to 0.4% ZnSO4 application and the control (Table 4).
Table 3.
Correlation among different yield and zinc related traits of wheat under control and 0.4% ZnSO4 foliar application. In table values over diagonal are from control condition and lower are 0.4% ZnSO4 application.
| Traits | Plant Height | Number of Spikelets/Spike | Spike Length | Grain Yield/Plant | Grain Zinc Content | Grain Zinc Bioavailability | Zinc Uptake |
|---|---|---|---|---|---|---|---|
| Plant Height | 1 | 0.26ns | 0.29ns | 0.01ns | −0.03ns | 0.32* | −0.01ns |
| Number of Spikelets/Spike | −0.07ns | 1 | 0.82** | 0.45** | 0.48** | 0.36* | 0.50** |
| Spike Length | −0.10ns | 0.63** | 1 | 0.45** | 0.33* | 0.24ns | 0.41** |
| Grain Yield/Plant | 0.03ns | 0.29ns | 0.30ns | 1 | 0.70** | 0.54** | 0.88** |
| Grain Zinc Content | 0.42** | 0.24ns | 0.23ns | 0.11ns | 1 | 0.58** | 0.95** |
| Grain Zinc Bioavailability | 0.44** | 0.32* | 0.28ns | 0.30ns | 0.66** | 1 | 0.61** |
| Zinc Uptake | 0.36* | 0.34* | 0.33* | 0.51** | 0.91** | 0.69** | 1 |
*Significant at 5%, **Significant at 1%.
Table 4.
Correlation among different yield and zinc related traits of wheat under control and 0.6% ZnSO4 foliar application. In table values over diagonal are from control condition and lower are 0.6% ZnSO4 application.
| Traits | Plant Height | Number of Spikelets/Spike | Spike Length | Grain Yield/Plant | Grain Zinc Content | Grain Zinc Bioavailability | Zinc Uptake |
|---|---|---|---|---|---|---|---|
| Plant Height | 1 | 0.267ns | 0.29ns | 0.01ns | −0.03ns | 0.32* | −0.01ns |
| Number of Spikelets/Spike | 0.07ns | 1 | 0.82** | 0.45** | 0.48** | 0.36* | 0.50** |
| Spike Length | 0.32* | 0.50** | 1 | 0.45** | 0.33* | 0.24ns | 0.41** |
| Grain Yield/Plant | 0.05ns | −0.06ns | 0.00ns | 1 | 0.70** | 0.54** | 0.88** |
| Grain Zinc Content | −0.13ns | 0.50** | 0.30ns | −0.22ns | 1 | 0.58** | 0.95** |
| Grain Zinc Bioavailability | −0.13ns | 0.64** | 0.44** | 0.14ns | 0.63** | 1 | 0.61** |
| Zinc Uptake | −0.09ns | 0.46** | 0.29ns | 0.32* | 0.84** | 0.67** | 1 |
*Significant at 5%, **Significant at 1%.
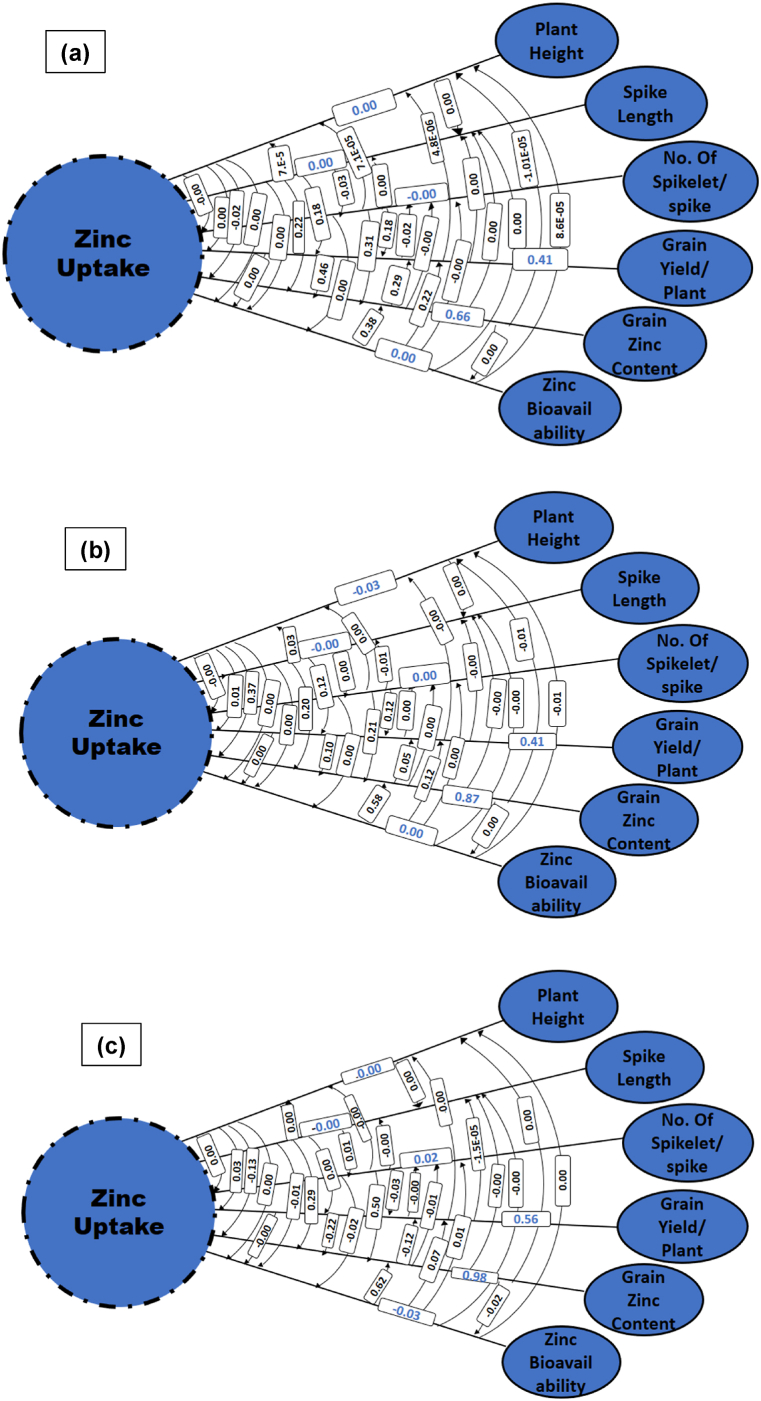
For Grain Zn biofortification, Zn uptake efficiency of the genotypes is crucial. Path coefficient analysis was conducted to dissect the association of different traits with Zn uptake into direct and indirect effects (Fig. 2a–c). Although strength of association (Between Zn uptake and other trats) was different under different Zn fertilization treatments, but trend of strength of direct and indirect effects was nearly similar under all three treatments. Direct effect of plant height, spike length and number of spikelets per spike was very small compared to the correlation values, which showed that these attributes contribute indirectly via other traits mainly via grain Zn contents (Fig. 2a–c). Whereas strong direct effects were observed grain yield, grain Zn contents and grain bioavailability which means these attributes play a direct role in uptake of Zn to the grains.
Fig. 2.
Path diagram showing direct and indirect effect (association) of different traits on zinc uptake of wheat grain under control (a), 0.4% (b) and 0.6% (c) foliar applied zinc sulphate.
3.4. Biplot analyses
Principal Component Analyses (PCA) was carried out to draw the biplot and for each fertilization treatment, a separate PCA was conducted to check whether change in the grouping of the genotypes occur under different Zn fertilization treatments or not. Under control conditions, the first two principal components (PCs) of the biplot manifested 61.26% and 17.42% variability, respectively (Fig. 3a). Whereas, under 0.4% ZnSO4 foliar application, the first two PCs' respective contributions were 50.56% and 25.37% and under 0.6% ZnSO4 foliar application, the first two PCs' contributions were 51.50% and 18.06%, respectively (Fig. 3b). Under the control conditions, the biplot classified all genotypes into three distinct groups, each of which reflected a unique indication of different attributes. However, the genotypes were classified into four groups under 0.4% and 0.6% ZnSO4 foliar application (Fig. 3c).
Fig. 3.
Biplot diagrams showing the genotypic diversity and grouping of genotypes under control (a), 0.4% (b) and 0.6% (c) foliar applied zinc sulphate.
Under control condition, 1st group under consisted of ‘Fakher-e-Bhakar’, ‘Anaj-17′, ‘Galaxy-13′, and ‘Borlaug’ genotypes which had higher plant height, spike length, number of spikelets per spike and grain zinc bioavailability. The 2nd group comprised of genotypes' Johar’, ‘Markez’, ‘Punjab-11′, and ‘Faisalabad-08′, which had lower grain yield and zinc content and number of spikelets per spike relative to the 1st group genotypes. The 3rd group consisted of genotypes' Akbar-19′, ‘Zincol’ and ‘AARI-11′ which had higher grain yield, grain zinc content and zinc uptake compared to both 1st and 2nd group. The genotypes' SH-2002′, and ‘Ujala’ were outliers representing extreme cases that displayed different patterns compared to all three groups under control conditions (Fig. 3a). Under 0.4% ZnSO4 fertilization, four distinct groups of the genotypes were created. The 1st group consisted of ‘Fakher-e-Bhakar’, ‘Akbar-19′, ‘Galaxy-13′, and ‘Zincol’ genotypes which had higher plant height, grain zinc content, grain zinc bioavailability and zinc uptake. The 2nd group comprised of only two genotypes i.e., ‘Markez’ and ‘Borlaug’, which had low grain yield, grain zinc content, number of spikelets per spike, grain zinc bioavailability and zinc uptake as compared to rest of genotypes'. The 3rd group consisted of genotypes' Faisalabad-08′, ‘Punjab-11′, ‘Ujala’ and ‘SH-2002′, which had higher grain yield, grain zinc content and zinc uptake than the genotypes in other groups. The 4th group consisted of genotypes' Anaj-17′, ‘Johar’, and ‘AARI-11′, which had higher grain yield, number of spikelets per spike and spike length than other groups but had low plant height, grain zinc content, grain zinc bioavailability and zinc uptake as compared to the 1st group (Fig. 3b). Under 0.6% ZnSO4 foliar application, four distinct groups of the genotypes had been created. The 1st group contained only a single genotype, ‘Fakher-e-Bhakar’ which manifested more spikelets per spike, grain zinc bioavailability and zinc uptake than other genotypes. The 2nd group comprised Galaxy-13′, Johar’ and ‘Punjab-11′, which had higher plant height and grain yield than the rest of the genotypes. The 3rd group consisted of genotypes' Markez’, ‘Anaj-17′, ‘Ujala’, ‘Borlaug’ and ‘SH-2002′, which had lower grain yield, grain zinc content and zinc uptake than the genotypes in other groups. The 4th group consisted of genotypes' Akbar-19′, ‘Faisalabad-08′, ‘Zincol’, and ‘AARI-11′, which had comparatively higher grain zinc content and spike length than the genotypes present in other groups (Fig. 3c).
4. Discussion
Genetic diversity is crucial for any breeding program in order to improve yield and quality. In current study genetic diversity among wheat genotypes was estimated separately for different Zn fertilization treatments to estimate that how the Zn fertilization affects genetic variation in the genotypes. Variation on the rage of genotypic variability for different studied traits under different Zn might be attributed to the differential response of wheat genotypes to the foliar applied zinc sulphate [34,35]. A broad range of variation among genotypes for grain yield and zinc-related traits such as Zn concentration, bioavailability and Zn uptake under variable Zn application (Fig. 1), depicts the potential of the studied genotypes to be used in a breeding program aimed at Zn biofortification. A smallest amount of variation for plant height, number of spikelets per plant, and spike length indicates the selective breeding for these attributes which has narrowed the genetic diversity [35,36].
A plant breeder often selects a number of traits in any breeding program, therefore information about relationship of these traits plays a crucial role in a successful selection [37]. In current study, strong association of Zn biofortification traits with grain yield depicts potential of simultaneous improvement in both traits. Simultaneous improvement in grain Zn traits and yield has also been reported by the other researchers [37,38]. Thapa et al. [37] demonstrated that improvement in grain Zn contents does not compromise the grain yield in wheat. The change in correlation values under different fertilization conditions may be attributed to differential response of the genotypes for specific traits under different fertilization conditions [14,20]. The PCA analysis partitioned the studied wheat germplasm into 3–4 groups across all three ZnSO4 levels, indicating the performance of different genotypes for a variety of yield and zinc-related traits. The performance of genotypes changed under different environmental conditions due to the change in the association of functional genes and some specific transcription factors [[39], [40], [41]] which has caused a change in the grouping of the genotypes under different Zn fertilization treatments. Change in the grouping pattern of the genotypes has also been reported by Sharma et al. [41] who reported change in the grouping pattern under different irrigation conditions. Better performing genotypes from different groups like Zincole, Fakhr-e-Bhakkr, Akbar-19 and Punjab-11 can be used in the breeding programs aimed at grain yield and Zn biofortification. Maximum genotypic variability among genotypes for zinc uptake was shown under 0.6% ZnSO4 foliar application which represents high variability in the tested genotypes for their performance under high Zn fertilization [42]. This variability may be attributed to presence or absence of transporter genes in different genotypes which might led a variable uptake in different genotypes [43,44].
Heritability is essential to study for the degree of transmission of traits to future generations in any breeding program [45,46]. Although high broad-sense heritability for almost all the traits were observed under all fertilization conditions, there were some differences which might be due to variation in the variability under different fertilization conditions. Velu et al. [46] demonstrated that high heritability across environments might be due to non-cross over type of interaction. Strong correlation along-with higher heritability among the yield and Zn biofortification related traits manifested that these traits are beneficial and valuable for breeding and can prove helpful in substantial genetic advance for wheat germplasm.
5. Conclusion
The study depicted the high genotypic diversity among the genotypes for grain yield and Zn biofortification related traits. The Studied traits manifested strong correlation and high heritabilites which depicts that the selection for these traits can be done in early segregating generations. Due to high genotypic variation in Znicol, Fakhar-e-Bhakkar, and Akbar-19 (as depicted by PCA biplot), these genotypes can be utilized in a breeding program aimed at high yield and Zn biofortification. Higher Broadsense heritability for yield and Zn biofortification related traits suggests selection in early segregating generations.
Funding
The was no external funding for this study.
Declarations
Author contribution statement
Asad Azeem: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Sami Ul-Allah: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Farukh Azeem: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data. Muhammad Naeem: Contributed reagents, materials, analysis tools or data; Wrote the paper. Abdul Sattar: Performed the experiments. Muhammad Ijaz: Analyzed and interpreted the data. Ahmad Sher: Conceived and designed the experiments; Analyzed and interpreted the data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19643.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Fao F. Food and Agriculture Orgainzation of the United Nation; 2019. Cereal Supply and Demand Brief. [Google Scholar]
- 2.Saini P., Islam M., Das R., Shekhar S., Sinha A.S.K. Wheat bran as potential source of dietary fiber: prospects and challenges. J. Foodserv. 2022;116 https://www.sciencedirect.com/science/article/pii/S0889157522006482 [Google Scholar]
- 3.Iqbal M.J., Shams N., Fatima K. 2022. Nutritional Quality of Wheat, Wheat.https://www.intechopen.com/chapters/81564 [Google Scholar]
- 4.Siddiqui S.A., Mahmud M.M.C., Abdi G., Wanich U., Farooqi M.Q.U., Settapramote N., Khan S., Wani S.A. New alternatives from sustainable sources to wheat in bakery foods: science, technology, and challenges. J. Food Biochem. 2022;46 doi: 10.1111/jfbc.14185. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar H. The importance of cereals (Poaceae: Gramineae) nutrition in human health: a review. J. Cereals Oilseeds. 2013;4:32–35. [Google Scholar]
- 6.FAO . Statista; 2023. Global Whet Production.www.statista.com available on. Last accessed on. [Google Scholar]
- 7.Govt. Of Pakistan. Pakistan Economic Survey, Finance Division, Economic Advisory Wing; Islamabad: 2022-2023. [Google Scholar]
- 8.Kheir A.M.S., Ammar K.A., Attia A., Elnashar A., Ahmad S., El-Gioushy S.F., Ahmed M. In: Global Agricultural Production: Resilience to Climate Change. Ahmed M., editor. Springer International Publishing; Cham: 2022. Cereal crop modeling for food and nutrition security; pp. 183–195. [Google Scholar]
- 9.Chapman S.C., Chakraborty S., Dreccer M.F. Plant adaptation to climate change—opportunities and priorities in breeding. Crop Pasture Sci. 2012 https://www.publish.csiro.au/cp/cp11303 [Google Scholar]
- 10.Prasad P., Bhardwaj S.C., Thakur R.K., Adhikari S., Gangwar O.P., Lata C., Kumar S. Prospects of climate change effects on crop diseases with particular reference to wheat. Journal of Cereal. 2021 [Google Scholar]
- 11.Verma V., Kaur M., Shivay Y.S., Nisar S., Gaber A. 2022. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security, Molecules.https://www.mdpi.com/1420-3049/27/4/1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiran A., Wakeel A., Mahmood K., Mubaraka R. Agronomy; 2022. Biofortification of Staple Crops to Alleviate Human Malnutrition: Contributions and Potential in Developing Countries.https://www.mdpi.com/1494058 [Google Scholar]
- 13.Chasapis C.T., Ntoupa P.-S.A., Spiliopoulou C.A., Stefanidou M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 14.Mazhar M.W., Ali Q., Ishtiaq M., Ghani A., Maqbool M., Hussain T., Mushtaq W. Zinc-aspartate-mediated drought amelioration in maize promises better growth and agronomic parameters than zinc sulfate and L-aspartate. SABRAO Journal Breeding and Genetics. 2021;53:290–310. [Google Scholar]
- 15.Saquee F.S., Diakite S., Kavhiza N.J., Pakina E., Zargar M. The efficacy of micronutrient fertilizers on the yield formulation and quality of wheat grains. Agronomy. 2023;13:566. [Google Scholar]
- 16.Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302:1–17. [Google Scholar]
- 17.Pandey M., Shrestha J., Subedi S., Shah K.K. Role of nutrients in wheat: a review. Tropical Agrobiodiversity. 2020;1:18–23. [Google Scholar]
- 18.Verma R., Verma O., Chandra S., Shankhdhar S.C., Gautam P. Yield performance of normal and late sown wheat in response to seed priming and foliar applied nutrients. J. Plant Nutr. 2023;46:852–866. [Google Scholar]
- 19.Liu D.-Y., Liu Y.-M., Zhang W., Chen X.-P., Zou C.-Q. Zinc uptake, translocation, and remobilization in winter wheat as affected by soil application of Zn fertilizer. Front. Plant Sci. 2019;10:426. doi: 10.3389/fpls.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanitha J., Amudha K., Mahendran R., Srinivasan J., Robin S., Kumari R. Genetic variability studies for zinc efficiency in aerobic rice. SABRAO Journal Breeding and Genetics. 2022;48:425–433. [Google Scholar]
- 21.Karadi A., Kajjidoni S.T. Genetic variability and diversity for productivity traits and grain quality including nutritional quality traits in selected mini core and promising released varieties of. J. Pharmacogn. 2019;8:2091–2097. https://www.phytojournal.com/archives?year=2019&vol=8&issue=4&ArticleId=9271 [Google Scholar]
- 22.Amegbor I.K., van Biljon A., Shargie N., Tarekegne A., Labuschagne M.T. Heritability and associations among grain yield and quality traits in quality protein maize (QPM) and non-QPM hybrids. Plants. 2022;11 doi: 10.3390/plants11060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naeem M., Sattar A., Sher A., Ijaz M., Azeem A. Phenotypic characterization of wheat germplasm for heritability and dissection of association among post anthesis traits under variable sowing dates. Journal of King Saud. 2023;35 https://www.sciencedirect.com/science/article/pii/S101836472300040X [Google Scholar]
- 24.Khan S.H., Aslam M., Bibi A., Khan H.Z. GGE biplot analysis for zinc quality and yield stability of exotic maize hybrids. SABRAO Journal Breeding and Genetics. 2023;(55):268–278. [Google Scholar]
- 25.Rabieyan E., Bihamta M.R., Mostashari M.M., Moghaddam M.E., Mohammadi V., Alipour H. Applying genetic biofortification for screening of Iranian bread wheat genotypes with high grain yield and nutritional quality. J. Soil Sci. Plant Nutr. 2023;23:1235–1253. [Google Scholar]
- 26.Kumar J., Saripalli G., Gahlaut V., Goel N., Meher P.K., Mishra K.K., Mishra P.C., Sehgal D., Vikram P., Sansaloni C., Singh S., Sharma P.K., Gupta P.K. Genetics of Fe, Zn, β-carotene, GPC and yield traits in bread wheat (Triticum aestivum L.) using multi-locus and multi-traits GWAS. Euphytica. 2018;214:219. [Google Scholar]
- 27.Koç E., Karayiğit B. Assessment of biofortification approaches used to improve micronutrient-dense plants that are a sustainable solution to combat hidden hunger. J. Soil Sci. Plant Nutr. 2022;22:475–500. doi: 10.1007/s42729-021-00663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones J.B., Jr., Case V.W. SSSA Book Series. Soil Science Society of America; Madison, WI, USA: 2018. Sampling, handling, and analyzing plant tissue samples; pp. 389–427. [Google Scholar]
- 29.Haug W., Lantzsch H.-J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983;34:1423–1426. [Google Scholar]
- 30.Miller L.V., Krebs N.F., Hambidge K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007;137:135–141. doi: 10.1093/jn/137.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher R.A. In: Breakthroughs in Statistics: Methodology and Distribution. Kotz S., Johnson N.L., editors. Springer New York; New York, NY: 1992. Statistical methods for research workers; pp. 66–70. [Google Scholar]
- 32.Dewey D.R., Lu K.H. Agron. J.; 1959. A Correlation and Path‐Coefficient Analysis of Components of Crested Wheatgrass Seed Production1.https://acsess.onlinelibrary.wiley.com/doi/abs/10.2134/agronj1959.00021962005100090002x [Google Scholar]
- 33.Schmidt P., Hartung J., Rath J., Piepho H.P. Crop Sci.; 2019. Estimating Broad‐sense Heritability with Unbalanced Data from Agricultural Cultivar Trials.https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2018.06.0376 [Google Scholar]
- 34.Ambati D., Phuke R.M., Vani V., Sai Prasad S.V., Singh J.B., Patidar C.P., Malviya P., Gautam A., Dubey V.G. Assessment of genetic diversity and development of core germplasm in durum wheat using agronomic and grain quality traits. Cereal Res. Commun. 2020;48:375–382. [Google Scholar]
- 35.Vanitha J., Amudha K., Mahendran R., Srinivasan J., Robin S., Kumari R.U. Genetic variability studies for zinc efficiency in aerobic rice. SABRAO Journal of Breeding and Genetics. 2016;48:425–433. [Google Scholar]
- 36.Dervishi A., Rumano M., Ruzi P. Annales UMCS Sectio E.; 2022. The Genetic Diversity and Variation in Crude Protein Content of Wheat (Triticum aestivum L.) Promising Cultivars for Breeding in Albania.https://czasopisma.up.lublin.pl/index.php/as/article/download/4816/3254/ scholar.archive.org/work/wlicihfsg5ff7ihm2vwv6mwwgi/access/wayback. [Google Scholar]
- 37.Thapa D.B., Subedi M., Yadav R.P., Shrestha K.P., Magar P.B., Pant K.R., Gautam N.R. Variation in grain zinc and iron concentrations, grain yield and associated traits of biofortified bread wheat genotypes in Nepal. Front. Plant Sci. 2022;(13) doi: 10.3389/fpls.2022.881965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia H., Wang L., Qiao Y., Kong W., Xue Y., Wang Z., Sizmur T. Elucidating the source–sink relationships of zinc biofortification in wheat grains: a review. Food Energy Secur. 2020;9:e243. [Google Scholar]
- 39.Saeidnia F., Taherian M., Nazeri S.M. Graphical analysis of multi-environmental trials for wheat grain yield based on GGE-biplot analysis under diverse sowing dates. BMC Plant Biol. 2023;23:198. doi: 10.1186/s12870-023-04197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosová K., Prášil I.T., Klíma M., Nesvadba Z., Vítámvás P., Ovesná J. Proteomics of wheat and barley cereals in response to environmental stresses: current state and future challenges. J. Proteonomics. 2023;282 doi: 10.1016/j.jprot.2023.104923. [DOI] [PubMed] [Google Scholar]
- 41.Sharma H., Singh S., Shamshad M., Padhy A.K., Kaur R., Kaur S., Sharma A., Sohu V.S. Variability in iron, zinc, phytic acid and protein content in pre-breeding wheat germplasm under different water regimes. Plant Growth Regul. 2023;(100):531–543. [Google Scholar]
- 42.Jalal A., Galindo F.S., Freitas L.A., da Silva Oliveira C.E., de Lima B.H., Pereira Í.T., Ferraz G.F., de Souza J.S., da Costa K.N., Nogueira T.A.R., Filho M.C.M. Yield, zinc efficiencies and biofortification of wheat with zinc sulfate application in soil and foliar nanozinc fertilisation. Crop Pasture Sci. 2022 [Google Scholar]
- 43.Rengel Z. Genotypic differences in micronutrient use efficiency in crops, Commun. Soil Sci. Plant Anal. 2001;32:1163–1186. [Google Scholar]
- 44.Kamble U., Mishra C.N., Govindan V., Sharma A.K., Pawar S., Kumar S., Krishnappa G., Gupta O.P., Singh G.P., Singh G. Ensuring nutritional security in India through wheat biofortification: a review. Genes. 2022;13 doi: 10.3390/genes13122298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ul-Allah S., Azeem A., Sher A., Ijaz M., Sattar A. Assessment of genetic variability and direct-indirect contribution of post-anthesis traits to the grain yield in bread wheat (Triticum aestivum) at different sowing dates. Int. J. Agric. Biol. 2021;26:193–200. [Google Scholar]
- 46.Velu G., Singh R.P., Huerta-Espino J., Peña R.J., Mujahid M.Y., Sohu V.S., Mavi G.S., Crossa J., Alvarado G. Performance of biofortified spring wheat genotypes in target environments for grain zinc and iron concentrations. Field Crops Res. 2012;137:261–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.