Abstract
Over time, mounting evidence has demonstrated extra-gastric manifestations of Helicobacter pylori infection. As such, a number of studies demonstrated the potential contribution of H. pylori infection to the incidence and progression of Alzheimer's disease (AD). Considering unanswered questions regarding the effect of H. pylori infection on brain activity, we sought to investigate the impact of H. pylori infection on the expression of AD-associated risk factors. We used two H. pylori clinical strains obtained from two patients with peptic ulcer and evaluated their influence on the expression level of AD-associated genes (APP, ApoE2, ApoE4, ABCA7, BIN1, Clu, CD33) and genes for inflammatory markers (TLR-4, IL-8, TNF-α) by RT-qPCR in human glioblastoma (U87MG) and astrocyte (1321N1) cell lines. The expression of inflammatory cytokines was further assessed by ELISA assay. The exposure of U97MG and 1321N1 cells to H. pylori strains resulted in a significant enhancement in the expression level of the risk allele ApoE4, while reducing the expression of the protective allele ApoE2. H. pylori infection remarkably increased the expression level of main AD-associated risk genes, and also pro-inflammatory cytokines. Furthermore, we noticed a substantial elevation in the mRNA expression level of transmembrane receptor TLR-4 following H. pylori infection. Our findings presented the potential for H. pylori to stimulate the expression of AD-associated risk genes and trigger neuroinflammation in the brain tissue. This, in principle, leads to the recommendation that AD patients should perhaps test for H. pylori infection and receive treatments upon positive detection.
Keywords: Helicobacter pylori, Alzheimer's disease, Neuroinflammation, Apolipoprotein, TLR-4
1. Introduction
Helicobacter pylori (H. pylori) is a Gram-negative bacterium classed as a human carcinogen that is strongly correlated with the development of gastric disorders. H. pylori specifically colonizes the gastric epithelium and usually persists for decades, developing a dynamic and persistent equilibrium with its human host [1]. H. pylori pathogenesis largely depends on the deleterious impact of virulence factors VacA (vacuolating cytotoxin A) and oncoprotein CagA (cytotoxin-associated gene A) [2]. The presence of H. pylori virulence factors in the gastric epithelium may stimulate various inflammatory and tumorigenic signaling pathways [3]. Most certainly, the presence of various gastrointestinal disorders is coupled with H. pylori infection, including chronic gastritis, peptic ulcer disease (PUD), chronic atrophic gastritis, and gastric adenocarcinoma. However, epidemiological studies further revealed the influence of H. pylori colonization on the pathogenicity of extra-gastrointestinal diseases including those in hematologic, cardiopulmonary, metabolic, neurologic, and dermatologic systems [[4], [5], [6], [7]]. Several mechanisms have been suggested through which H. pylori could affect the progression of disorders localized outside the stomach, such as low-grade systemic inflammation, molecular mimicry mechanisms, and alterations in the gut microbial composition [8,9]. In this regard, neurodegenerative diseases appeared as a field of particular interest, especially Alzheimer's disease (AD), due to its notable public health burden.
AD is a progressive age-related neurodegenerative disorder characterized by cognitive dysfunction and memory decline. Being the most common cause of dementia, AD develops due to the accumulation of amyloid and neurofibrillary tangles in the brain and within neurons, respectively [10]. Major risk factors for the development and progression of AD include the presence of risk loci, apolipoprotein E (ApoE) polymorphic alleles, and neuroinflammation [[11], [12], [13]]. Considering H. pylori-AD interrelationship, this bacterium and its metabolites can reach the central nervous system (CNS) through the oral-nasal olfactory pathway, retrograde gastrointestinal tract neural pathway, infection of circulating monocytes, and disrupted blood-brain barrier [14]. However, meticulous interconnections between H. pylori infection and the risk of AD development remain elusive. Thus, this study aimed to investigate the effect of H. pylori clinical strains on the expression levels of ApoE polymorphic alleles (ApoE2 and ApoE4), risk genes involved in neurodegenerative disorders, pro-inflammatory cytokines (IL-8/interleukin-8 and TNF-α/tumor necrosis factor-α), and inflammatory receptor (TLR-4/toll-like receptor-4), presenting the potential for H. pylori to induce neurodegenerative disorders.
2. Material and methods
2.1. H. pylori strains and growth condition
Two H. pylori clinical strains of PUD patients including H. pylori HC168 (CagA+/VacA s1m2/BabA2+/SabA+) and H. pylori OC824 (CagA+/VacA s1m2/BabA2-/SabA−) were obtained from the Helicobacter Research Laboratory collection of the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran [15]. Briefly, the strains were cultured on Brucella agar plates (Merck, Darmstadt, Germany) supplemented with 7% (v/v) horse blood, 10% fetal calf serum (FCS), Campylobacter-selective supplement (vancomycin 2.0 mg/L, polymyxin 0.05 mg/L, trimethoprim 1.0 mg/L), and amphotericin B (2.5 mg/L). The cultured plates were incubated at 37 °C under a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2) in a CO2 incubator for 3–7 days.
2.2. Cell culture
Human glioblastoma U-87MG (ATCC HTB-14) and astrocyte 1321-N1 (ATCC HTB-16) cell lines were obtained from the Tarbiat Modares University, Tehran, Iran. The U87MG and 1321N1 cells were routinely grown in high-glucose Dulbecco's Modified Eagle Medium (Gibco-Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Gibco-Invitrogen, Carlsbad, CA), 2 mM of l-glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin and were incubated in a 5% CO2 humidified atmosphere at 37 °C.
2.3. Cell viability assay
U87MG and 1321N1 cells were seeded in 96-well plates at a density of 1 × 105 cells/well, and treated with H. pylori strains at multiplicity of infection (MOI) 50 and 100, when reached ∼80% confluence for 24 h. The treated groups and untreated controls were then incubated with MTT solution (Sigma Aldrich, St. Louis, MO, USA) at a concentration of 5 mg/L for 4 h and consequently dissolved in 100 μl of DMSO. The cell viability was assessed by a microplate reader (Eon, BioTek Instruments, USA) with an absorbance of 570 nm and a reference wavelength of 630 nm.
2.4. Cell culture treatment
The U87MG and 1321N1 cells were counted and seeded in 24-well tissue culture plates at a density of 1 × 105 cells/well and grown in a CO2 incubator for 24 h. Prior to treatment, the 80–90% confluent monolayers were washed three times with PBS (pH 7.2) (Gibco-Invitrogen, Carlsbad, CA), and the media were replaced with antibiotic- and serum-free complete DMEM overnight. Then, the cells were infected for 3 h, 6 h, 12 h, and 24 h at 37 °C with an MOI of 100 for each H. pylori strain. The untreated cells were harvested as the control group. The experiments were performed in duplicate and repeated at least three times.
2.5. ELISA measurement for pro-inflammatory cytokines
Cell culture supernatants were collected from H. pylori-treated and untreated control cells and subsequently centrifuged at 4 °C and 1000×g for 10 min. The concentration of pro-inflammatory cytokines IL-8 and TNF-α were assessed using enzyme-linked immunosorbent assay (ELISA) kits (Thermo Scientific, MA, USA) according to the manufacturer's instructions. A nonlinear regression model was used to determine the concentration of each cytokine with GraphPad Prism software version 8 (Inc., San Diego, CA, USA).
2.6. RNA extraction and cDNA synthesis
Total RNA from 1321N1 and U87MG cells was extracted using RNeasy Plus Mini Kit (Qiagen, GmbH, Germany) following the manufacturer's instructions. RNA concentration and purity were assessed using a NanoDrop spectrophotometer (ND-1000, Thermo Scientific, MA, USA) by the A260/280 ratio, and ribosomal RNA (rRNA) was assessed by electrophoresis. The RNA samples were frozen at −80 °C until used for cDNA synthesis. The RNA was reverse-transcribed to cDNA using the PrimeScript™ RT Reagent Kit (Takara, Kyoto, Japan) according to the manufacturer's protocol. All cDNA preparations were frozen at −20 °C until further use.
2.7. Quantitative real-time PCR (RT-qPCR)
The RT-qPCR was performed by the Rotor-Gene® Q real-time PCR system (Qiagen, GmbH, Germany) using BioFACT™ 2X Real-Time PCR Master Mix (BIOFACT CO., Ltd. Daejeon, South Korea). The oligonucleotide sequences used for gene expression analysis are listed in Table 1. To confirm amplification specificity, a melting analysis and subsequent agarose gel electrophoresis were performed after each run. All reactions were run in triplicate and the results of fold change in mRNA expression were given relative to the control samples using the comparative Ct formula “2-ΔΔCT”, and the RNA input was normalized against the housekeeping gene β-actin [16].
Table 1.
Specific primers used for RT-qPCR.
| Primer | Primer sequence (5′–3′) | Size of amplicon (bp) | Reference |
|---|---|---|---|
| APP | F: GCCATCATCGGACTCATGGT R: ATCCGTTCTGCTGCATCTTG |
169 | [29] |
| ApoE2 | F: CGGACATGGAGGACGTGT R: CTGGTACACTGCCAGGCA |
173 | [49] |
| ApoE4 | F: CGGACATGGAGGACGTGC R: CTGGTACACTGCCAGGCG |
173 | [49] |
| Bin1 | F: CCTGCTGTGGATGGATTACC R: GCTTTCTCAAGCAGCGAGAC |
219 | [50] |
| ABCA7 | F: TCCTTTGGAACAGCCTTTTG R: CTGCCCTTGAGATGTTGC |
157 | [51] |
| CD33 | F: GATCTTCTCCTGGTTGTCAG R: CTGTGGAACATAGGTGACGTTG |
184 | This study |
| Clu | F: ACATTTGGTGCCCAGAAGTC R: CTGTGGTCCAGGGAAAGGTA |
190 | [52] |
| TLR4 | F: CGAGGAAGAGAAGACACCAGT R: CATCATCCTCACTGCTTCTGT |
106 | [53] |
| TNF-α | F: CCCAGGGACCTCTCTCTAATC R: ATGGGCTACAGGCTTGTCACT |
84 | [54] |
| IL-8 | F: CTCTTGGCAGCCTTCCTGATT R: ACTCTCAATCACTCTCAGTTCT |
147 | [54] |
| β-actin | F: CTGGAACGGTGAAGGTGACA R: AAGGGACTTCCTGTAACAATGCA |
140 | [55] |
2.8. Statistical analysis
Statistical analysis was carried out with GraphPad Prism software version 8 (Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) and Tukey's post hoc test were used to determine the statistical significance between the groups. The data were presented as the averages of at least three independent experiments; error bars represent the standard deviations (SD). Differences were considered statistically significant when P < 0.05; *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
3. Results
3.1. Cell viability
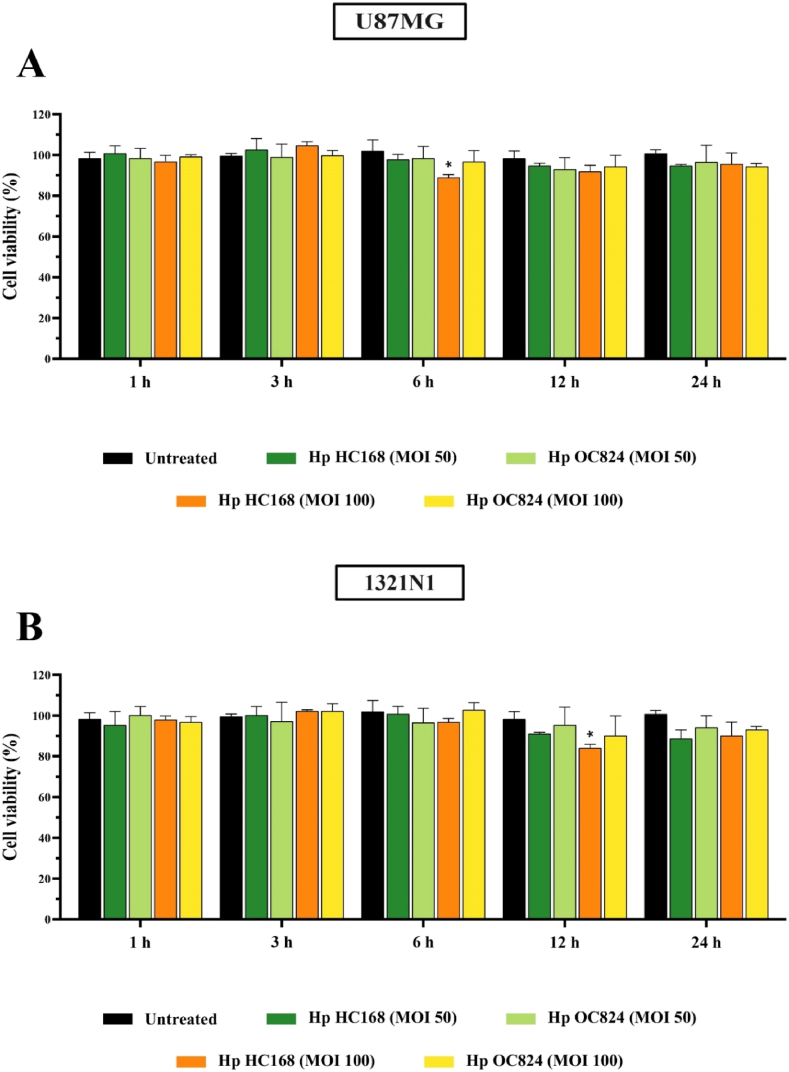
For evaluating the toxicity of H. pylori strains on U87MG and 1321N1 cells, the MTT assay was performed to measure the viability of U87MG and 1321N1 cells treated with H. pylori (MOI 50 and 100) bacteria. MTT results presented a non-significant reduction in the viability of both cell lines particularly after 24 h of infection, compared to the untreated control (Fig. 1A and B). Considering the cell viability results, H. pylori at MOI 100 was selected for further cell culture experiments.
Fig. 1.
Cell viability was determined using MTT assay for (A) U87MG and (B) 1321N1 cell lines treated with H. pylori (MOI 50 and 100) for 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
3.2. Effects of H. pylori on the gene expression level of ApoE and APP in brain cells
To evaluate the effects of H. pylori on the gene expression level of the apolipoprotein isoforms ApoE2 and ApoE4, U87MG and 1321N1 cells were infected with H. pylori bacteria for different time points. In U87MG cells, ApoE2 expression was reduced by H. pylori HC168 after 24 h (P < 0.01) and by H. pylori OC824 after 12 h (P < 0.05) and 24 h (P < 0.01). The exposure of 1321N1 cells to H. pylori HC168, however, demonstrated a significant reduction in the expression level of ApoE2 isoform after 1 h (P < 0.05) and 12 h (P < 0.05) of infection while H. pylori OC824 substantially reduced ApoE2 expression after 3 h (P < 0.05), 6 h (P < 0.05), and 24 h (P < 0.01) (Fig. 2A). On the contrary, H. pylori infection significantly increased ApoE4 gene expression in both the U87MG cells and 1321N1 cells (Fig. 2B). Furthermore, our results presented a remarkable time-dependent overexpression of APP gene in both cell lines after H. pylori infection (Fig. 2C).
Fig. 2.
The mRNA expression level of (A) ApoE2 and (B) ApoE4 in U87MG and 1321N1 cell lines upon exposure to H. pylori (Hp) HC168 and Hp OC824 strains at 1 h, 3 h, 6 h, 12 h, and 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
3.3. Effects of H. pylori on the expression level of genes related to AD in brain cells
In this study, the effects of H. pylori HC168 and OC824 on gene expression levels of ATP binding cassette subfamily A member 7 (ABCA7), bridging integrator 1 (BIN1), clusterin (Clu), and CD33 were investigated in U87MG and 1321N1 brain cells during different time points of infection. As shown in Fig. 3A, a significant increase was found for ABCA7 gene expression level in both U87MG (P < 0.0001) and 1321N1 (P < 0.0001) cells infected with H. pylori HC168 after 12 h (P < 0.0001). However, a notable reduction in the expression level of the ABCA7 gene was observed for H. pylori HC168 and OC824 in both U87MG (P < 0.0001) and 1321N1 (P < 0.0001) cells after 24 h of infection, in comparison with untreated cells.
Fig. 3.
The mRNA expression level of (A) ABCA7, (B) Bin1, (C) Clu, and (D) Cd33 in U87MG and 1321N1 cell lines upon exposure to H. pylori (Hp) HC168 and Hp OC824 strains at 1 h, 3 h, 6 h, 12 h, and 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
We found a notable time-dependent enhancement in the gene expression level of BIN1 in U87MG cells treated with H. pylori HC168. Except for the remarkable reduction at 1 h (P < 0.01), this strain could significantly increase BIN1 expression in U87MG cells and only after 6 h (P < 0.01) of infection in 1321N1 cell, compared with the control group. Whilst, H. pylori OC824 could induce a significant upregulation in the expression level of the BIN1 gene in 1321N1 cells after 3 h (P < 0.05), 6 h (P < 0.0001), and 12 h (P < 0.0001) of infection (Fig. 3B).
As depicted in Fig. 3C, H. pylori HC168 could markedly increase the expression level of the Clu gene in U87MG cells, whereas significantly reduced its expression in 1321N1 cells. Moreover, the Clu gene expression was substantially induced by H. pylori OC824 infection in U87MG cells only after 3 h (P < 0.0001) and 24 h (P < 0.0001) of infection, whilst H. pylori OC824 significantly reduced Clu gene expression after 3 h (P < 0.0001), 12 h (P < 0.0001), and 24 h (P < 0.0001) of infection in 1321N1 cells.
As shown in Fig. 3D, the gene expression level of CD33 was markedly upregulated in U87MG cells after 1 h (P < 0.0001), 3 h (P < 0.0001), 6 h (P < 0.0001), and 12 h (P < 0.0001) and also in 1321N1 cells after 3 h (P < 0.0001), 6 h (P < 0.0001), and 12 h (P < 0.0001) of infection with H. pylori HC168, in comparison with control cells. H. pylori OC824 could induce the gene expression level of CD33 in U87MG cells only at 12 h (P < 0.0001) and in 1321N1 cells at 3 h (P < 0.01), 12 h (P < 0.001), and 24 h (P < 0.001), in comparison with untreated control cells.
3.4. H. pylori induced TLR-4 gene expression in brain cells
We sought to examine whether H. pylori strains could upregulate the TLR-4 gene expression in U87MG and 1321N1 cells. As shown in Fig. 4, both H. pylori strains increased the expression levels of TLR-4 in infected cells when compared to the untreated cells. H. pylori HC168 significantly increased TLR-4 gene expression in U87MG cells at 3 h (P < 0.0001), 6 h (P < 0.05), 12 h (P < 0.05), and 24 h (P < 0.0001) and in 1321N1 cells at 3 h (P < 0.0001), 12 h (P < 0.01), and 24 h (P < 0.01), compared to the untreated control. Considering H. pylori OC824, however, TLR-4 expression level was substantially upregulated after 24 h (P < 0.0001) of infection in U87MG cells and following 12 h (P < 0.01) and 24 h (P < 0.001) of infection in 1321N1 cells.
Fig. 4.
The mRNA expression level of TLR-4 in U87MG and 1321N1 cell lines upon exposure to H. pylori (Hp) HC168 and Hp OC824 strains at 1 h, 3 h, 6 h, 12 h, and 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
3.5. H. pylori upregulated the expression and production of IL-8 and TNF-α in brain cells
We examined the effect of H. pylori on the gene expression level of pro-inflammatory cytokines in U87MG and 1321N1 cells. As expected, both H. pylori strains enhanced the expression of pro-inflammatory cytokines IL-8 and TNF-α (Fig. 5). H. pylori HC168 significantly induced IL-8 expression at 6 h (P < 0.0001) and 12 h (P < 0.0001) in U87MG cells and also at 3 h (P < 0.0001), 6 h (P < 0.0001), and 12 h (P < 0.05) in 1321N1 cells. Whilst, H. pylori OC824 notably increased the mRNA expression level of IL-8 at 3 h (P < 0.0001), 12 h (P < 0.05), and 24 h (P < 0.01) in U87MG cells and also at 1 h (P < 0.0001), 3 h (P < 0.001), and 6 h (P < 0.001) in 1321N1 cells (Fig. 5A). The gene expression of TNF-α was increased following the treatment of U87MG cells with H. pylori HC168 at 6 h (P < 0.01) and 12 h (P < 0.0001) and also in 1321N1 cells at 12 h (P < 0.001) and 24 h (P < 0.0001), in comparison to control cells. Moreover, H. pylori OC824 significantly induced the expression of TNF-α at 6 h (P < 0.0001) in U87MG cells and at 6 h (P < 0.0001) and 24 h (P < 0.0001) in 1321N1 cells (Fig. 5B). Consistent with the RT-qPCR results, ELISA assay presented noticeable increase in the production of IL-8 and TNF-α pro-inflammatory cytokines after H. pylori treatment in both the U87MG and 1321N1 cells (Fig. 6A and B).
Fig. 5.
The mRNA expression level of (A) IL-8 and (B) TNF-α in U87MG and 1321N1 cell lines upon exposure to H. pylori (Hp) HC168 and Hp OC824 strains at 1 h, 3 h, 6 h, 12 h, and 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Fig. 6.
The production of (A) IL-8 and (B) TNF-α in U87MG and 1321N1 cell lines upon exposure to H. pylori (Hp) HC168 and Hp OC824 strains at 1 h, 3 h, 6 h, 12 h, and 24 h (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
4. Discussion
The gut microbiota-brain axis is the biological network of bidirectional interactions between the gut microbiota and the brain, preserving homeostasis of the gastrointestinal, nervous, and microbial systems in humans. Considering the involvement of multiple biological systems, these communication networks are orchestrated through various mechanisms and pathways that signal by chemical transmitters, multi-neuronal networks, and the immune system [17]. Several cross-sectional studies have demonstrated the alteration of the gut microbiota composition in patients with neurological disorders, indicating the contribution of specific microbiota to brain pathology [[18], [19], [20]]. Accumulating evidence supports the potential influence of H. pylori on brain function and AD development. Clinical studies have reported significantly higher serum anti-H. pylori IgG and IgA titer in patients with AD or mild cognitive impairment [[21], [22], [23]]. Furthermore, H. pylori eradication improved the cognitive and functional status of AD patients and was associated with a significantly higher survival rate [24,25]. Studying activated genes and dysregulated pathways of human gastric MNK-45 cell line by a RNA-seq approach following treatment with H. pylori peptide Hp(2–20), Contaldi et al. [26] demonstrated alterations in the expression level of 77 genes, some of which (APP, ApoE, PSEN1, and PSEN2) contributing to AD development. Furthermore, similar dysregulated pathways during AD were also presented in the gastric cell line, emphasizing the biological possibility of the connection between H. pylori infection and predisposition to AD incidence. There are, however, inconsistencies about the potential influence of H. pylori on brain function, and different hypotheses have been presented so far (Fig. 7). It has been proposed that H. pylori might translocate to the brain through the oral-nasal-olfactory pathway or by circulating monocytes through the disrupted blood-brain barrier. H. pylori can also reach the brain tissue via the gastrointestinal retrograde neural pathways [27]. Moreover, H. pylori metabolites and by-products, such as outer membrane vesicles (OMVs), could cross biological barriers and ultimately access the brain [28]. To investigate the impact of H. pylori infection on the brain tissue, we treated U87MG and 1321N1 cell lines with two H. pylori strains (HC168, OC824) obtained from patients with PUD and evaluated the expression of pro-inflammatory cytokines and AD-associated risk genes.
Fig. 7.
Potential mechanisms through which H. pylori might access and affect brain function. H. pylori can penetrate the gastric epithelial barrier and reach the lamina propria. Thereafter, a disrupted blood-brain barrier (BBB) might allow H. pylori to reach the brain through (1) the gastrointestinal retrograde neural pathway, (2) the oral-nasal-olfactory pathway, or (3) circulating monocyte pathway. Furthermore, (4) H. pylori-derived OMVs could also cross biological barriers and eventually reach the brain (H. pylori OMV-mediated pathway). Subsequently, H. pylori induces neuroinflammation (e.g. IL-8 and TNF-α production) and increases the accumulation of amyloid plaque, ultimately leading to behavior and brain disorders.
Amyloid accumulation is a major characteristic of AD development, which is the consequence of proteolytic processing of APP transmembrane protein. As the first identified protein with a major contribution to AD, APP physiological activity has been vastly studied [29]. High expression levels of APP have been reported in AD and Down syndrome patients, which cause amyloid generation and neuritic plaque formation [30]. Therefore, we evaluated the impact of H. pylori infection on APP gene expression and demonstrated a marked time-dependent increase in its mRNA expression level in both cell lines. It could be interpreted from our results that H. pylori infection might contribute to the development of AD through the overexpression of APP and subsequent accumulation of amyloid in the brain.
A major cholesterol transporter in the brain, ApoE consists of three main allelic variants ε2, ε3, ε4, of which ApoE4 contributes to a higher risk while ApoE2 contributes to a lower risk of AD, compared to the common ApoE3 phenotype [31]. We noticed a significant elevation in the expression level of ApoE4 in both cell lines upon infection with either H. pylori strain. On the contrary, the exposure of U87MG and 1321N1 cells to H. pylori notably reduced ApoE2 expression level. Although H. pylori strains demonstrated these substantial changes at different time points in the cell lines, H. pylori infection presented potential modification in the expression of AD-associated risk factor ApoE. By changing the ApoE expression level, H. pylori could affect neurodegeneration [32], microglial homeostasis [33], synaptic integrity [34], lipid transport [35], and glucose metabolism [36]. Furthermore, a recent study presented the influence of the gut microbiota and metabolome alteration on neurodegeneration in an ApoE-dependent manner in a mouse model [37]. Therefore, H. pylori-mediated changes in the structure of the gut microbiome and metabolome might contribute to the pathogenicity of AD.
Following ApoE, BIN1 is considered the second most important genetic susceptibility locus for AD [38]. Furthermore, genome-wide association studies (GWASs) have identified several other risk genes for AD development such as Clu, ABCA7, and CD33 [[39], [40], [41]]. Except for a significant reduction in the expression level of ABCA7 at 24 h and BIN1 at 1 h in the U87MG cell line, as well as reduced expression of Clu at different time points in the 1321N1 cell line, both H. pylori strains presented a remarkable enhancement in the expression levels of AD-associated risk genes. Overall, we noticed a more substantial elevation in the expression of risk genes following H. pylori HC168 infection, compared to the H. pylori OC824 infection. This might be probably due to the absence of H. pylori adhesion proteins BabA and SabA in the H. pylori OC824 strain, hindering its ability to properly attach to glioblastoma and astrocyte cells [42]. The attachment of H. pylori to the host cell critically contributes to its pathogenicity and intracellular translocation of H. pylori virulence factors CagA and VacA [43]. The presence of different variants of H. pylori virulence factors such as variants of CagA EPIYA motifs could be another ration for distinctive pathogenicity of these strains [44]. Also, different cellular characteristics of glioblastoma and astrocyte cells including their morphological differences could be the underlying reason for their similar yet unique responses to the same H. pylori strain. Nevertheless, our findings demonstrated the capacity of H. pylori to simulate the expression of AD-associated risk genes.
Solid evidence suggests the involvement of TLR-4-mediated signaling in the pathogenesis of AD [45]. The detection of danger/damage-associated molecular patterns (DAMPs), such as high mobility group box 1 (HMGB1), or pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), by TLR-4 can cause neuroinflammation and neuronal damage contributing to AD incidence [46]. H. pylori LPS, which is considered as an essential pathogenic virulence factor, is well known to induce inflammatory reactions through TLR-4 activation. Furthermore, neuroinflammation, which is an inflammatory response within the CNS featured by the excessive production of pro-inflammatory cytokines, has been demonstrated as a key risk factor for AD [13]. Therefore, we evaluated the impact of H. pylori infection on the expression level of TLR-4 and exhibited a substantial elevation in the expression of this receptor by both H. pylori strains in both cell lines. To further evaluate the potential capacity for H. pylori to induce neuroinflammation, we assessed the expression level of pro-inflammatory cytokines IL-8 and TNF-α. Our findings showed significant enhancement in the expression of these cytokines by both H. pylori strains, particularly IL-8 in the 1321N1 cell line. In this regard, H. pylori LPS and H. pylori-induced expression of HMGB1 can stimulate TLR-4 and subsequent NFKB/NF-κB signaling [47,48], eventually inducing pro-inflammatory cytokine production and neuroinflammation.
5. Conclusion
In a nutshell, we demonstrated the increased expression of AD-associated risk genes in U87MG and 1321N1 cell lines following H. pylori infection at different time points. Our findings further exhibited the potential capacity of H. pylori to stimulate the expression of pro-inflammatory cytokines in neural cells and induce neuroinflammation. Therefore, it is plausible that H. pylori infection predisposes AD incidence and progression, which is yet to be further validated by preclinical and clinical studies. Limitations to our study include the absence of in vivo models or human subjects to validate the results of this study. The results of the present study should also be evaluated by protein expression assays. Further mechanism-oriented studies and clinical trials are required to elucidate H. pylori mechanistic action of inducing AD development and progression.
Ethics statement
The study protocol conformed to the ethical guidelines of the Institutional Ethical Review Committee of Research Institute for Gastroenterology and Liver Diseases at Shahid Beheshti University of Medical Sciences, Tehran, Iran (RIGLD 1050, Project No. IR. SBMU.RIGLD.REC.1398.023).
Author contribution statement
Maryam Noori: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Ramina Mahboobi; Shaghayegh Jamshidizadeh; Farzaneh Fakharian: Performed the experiments. Ali Nabavi-Rad: Analyzed and interpreted the data; Wrote the paper. Abbas Yadegar: Conceived and designed the experiments; Analyzed and interpreted the data. Mohammad Reza Zali: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We sincerely thank all the laboratory staff of the Foodborne and Waterborne Diseases Research Center at the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Polk D.B., Peek R.M., Jr. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari S., Yamaoka Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins. 2019;11(11) doi: 10.3390/toxins11110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavros Y., Merchant J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022;19(7):451–467. doi: 10.1038/s41575-022-00591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsay F.-W., Hsu P.-I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018;25(1):65. doi: 10.1186/s12929-018-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim T.J., et al. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J. Gastroenterol. 2017;52(11):1201–1210. doi: 10.1007/s00535-017-1337-y. [DOI] [PubMed] [Google Scholar]
- 6.Huang B., et al. CagA-positive Helicobacter pylori strains enhanced coronary atherosclerosis by increasing serum OxLDL and HsCRP in patients with coronary heart disease. Dig. Dis. Sci. 2011;56(1):109–114. doi: 10.1007/s10620-010-1274-6. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y., et al. Helicobacter pylori infection and inflammatory bowel disease: a crosstalk between upper and lower digestive tract. Cell Death Dis. 2018;9(10):961. doi: 10.1038/s41419-018-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi F., et al. Clinical effects of Helicobacter pylori outside the stomach. Nat. Rev. Gastroenterol. Hepatol. 2014;11(4):234–242. doi: 10.1038/nrgastro.2013.243. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Nuñez G.M., et al. Gut microbiota: the missing link between Helicobacter pylori infection and metabolic disorders? Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.639856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aisen P.S., et al. Early-stage Alzheimer disease: getting trial-ready. Nat. Rev. Neurol. 2022;18(7):389–399. doi: 10.1038/s41582-022-00645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellenguez C., et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat. Genet. 2022;54(4):412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C.-C., et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 2021;17(3):157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 14.Doulberis M., et al. Review: impact of Helicobacter pylori on Alzheimer's disease: what do we know so far? Helicobacter. 2018;23(1) doi: 10.1111/hel.12454. [DOI] [PubMed] [Google Scholar]
- 15.Yadegar A., Mohabati Mobarez A., Zali M.R. Genetic diversity and amino acid sequence polymorphism in Helicobacter pylori CagL hypervariable motif and its association with virulence markers and gastroduodenal diseases. Cancer Med. 2019;8(4):1619–1632. doi: 10.1002/cam4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nabavi-Rad A., et al. The synergistic effect of Levilactobacillus brevis IBRC-M10790 and vitamin D3 on Helicobacter pylori-induced inflammation. Front. Cell. Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1171469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morais L.H., Schreiber H.L.t., Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 18.Sampson T.R., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469–1480 e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S., et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly J.R., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Kountouras J., et al. P1–166: relationship between Helicobacter pylori infection and Alzheimer's disease. Alzheimer's Dementia. 2006;2:S144. doi: 10.1016/j.jalz.2006.05.542. 3S_Part_5. S144. [DOI] [PubMed] [Google Scholar]
- 22.Kountouras J., et al. Association between Helicobacter pylori infection and mild cognitive impairment. Eur. J. Neurol. 2007;14(9):976–982. doi: 10.1111/j.1468-1331.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 23.Malaguarnera M., et al. Helicobacter pylori and Alzheimer's disease: a possible link. Eur. J. Intern. Med. 2004;15(6):381–386. doi: 10.1016/j.ejim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Kountouras J., et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer's disease. J. Neurol. 2009;256(5):758–767. doi: 10.1007/s00415-009-5011-z. [DOI] [PubMed] [Google Scholar]
- 25.Kountouras J., et al. Five-year survival after Helicobacter pylori eradication in alzheimer disease patients. Cognit. Behav. Neurol. 2010;23(3) doi: 10.1097/WNN.0b013e3181df3034. [DOI] [PubMed] [Google Scholar]
- 26.Contaldi F., et al. The hypothesis that Helicobacter pylori predisposes to Alzheimer's disease is biologically plausible. Sci. Rep. 2017;7(1):7817. doi: 10.1038/s41598-017-07532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravina A.G., et al. Helicobacter pylori and extragastric diseases: a review. World J. Gastroenterol. 2018;24(29):3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J., et al. Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling. J. Extracell. Vesicles. 2023;12(2) doi: 10.1002/jev2.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cha H.J., Shen J., Kang J. Regulation of gene expression by the APP family in the adult cerebral cortex. Sci. Rep. 2022;12(1):66. doi: 10.1038/s41598-021-04027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y., et al. Regulation of global gene expression and cell proliferation by APP. Sci. Rep. 2016;6 doi: 10.1038/srep22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki Y., et al. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 2019;15(9):501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao N., et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat. Commun. 2018;9(1):4388. doi: 10.1038/s41467-018-06783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasemann S., et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566–581 e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweet R.A., et al. Apolipoprotein E*4 (APOE*4) genotype is associated with altered levels of glutamate signaling proteins and synaptic coexpression networks in the prefrontal cortex in mild to moderate alzheimer disease. Mol. Cell. Proteomics. 2016;15(7):2252–2262. doi: 10.1074/mcp.M115.056580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J., et al. APOE epsilon4/epsilon4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum. Mol. Genet. 2017;26(14):2690–2700. doi: 10.1093/hmg/ddx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekblad L.L., et al. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurol. Now. 2018;90(13):e1150–e1157. doi: 10.1212/WNL.0000000000005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo D.O., et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science. 2023;379(6628) doi: 10.1126/science.add1236. eadd1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu H., et al. Association between methylation of BIN1 promoter in peripheral blood and preclinical Alzheimer's disease. Transl. Psychiatry. 2021;11(1):89. doi: 10.1038/s41398-021-01218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dib S., Pahnke J., Gosselet F. Role of ABCA7 in human health and in alzheimer's disease. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster E.M., et al. Clusterin in Alzheimer's disease: mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu X., et al. Peripheral level of CD33 and Alzheimer's disease: a bidirectional two-sample Mendelian randomization study. Transl. Psychiatry. 2022;12(1):427. doi: 10.1038/s41398-022-02205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarmohammadi M., et al. Effects of a potential probiotic strain lactobacillus gasseri ATCC 33323 on Helicobacter pylori-induced inflammatory response and gene expression in coinfected gastric epithelial cells. Probiotics Antimicrob Proteins. 2021;13(3):751–764. doi: 10.1007/s12602-020-09721-z. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y., et al. Adhesion and invasion of gastric mucosa epithelial cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadegar A., Alebouyeh M., Zali M.R. Analysis of the intactness of Helicobacter pylori cag pathogenicity island in Iranian strains by a new PCR-based strategy and its relationship with virulence genotypes and EPIYA motifs. Infect. Genet. Evol. 2015;35:19–26. doi: 10.1016/j.meegid.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Calvo-Rodriguez M., et al. Role of toll like receptor 4 in alzheimer's disease. Front. Immunol. 2020;11:1588. doi: 10.3389/fimmu.2020.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momtazmanesh S., Perry G., Rezaei N. Toll-like receptors in Alzheimer's disease. J. Neuroimmunol. 2020;348 doi: 10.1016/j.jneuroim.2020.577362. [DOI] [PubMed] [Google Scholar]
- 47.Lin H.J., et al. Helicobacter pylori activates HMGB1 expression and recruits RAGE into lipid rafts to promote inflammation in gastric epithelial cells. Front. Immunol. 2016;7:341. doi: 10.3389/fimmu.2016.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su B., et al. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 2003;71(6):3496–3502. doi: 10.1128/IAI.71.6.3496-3502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raska J., et al. Generation of six human iPSC lines from patients with a familial Alzheimer's disease (n = 3) and sex- and age-matched healthy controls (n = 3) Stem Cell Res. 2021;53 doi: 10.1016/j.scr.2021.102379. [DOI] [PubMed] [Google Scholar]
- 50.De Rossi P., et al. Predominant expression of Alzheimer's disease-associated BIN1 in mature oligodendrocytes and localization to white matter tracts. Mol. Neurodegener. 2016;11(1):59. doi: 10.1186/s13024-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasquez J.B., Fardo D.W., Estus S. ABCA7 expression is associated with Alzheimer's disease polymorphism and disease status. Neurosci. Lett. 2013;556:58–62. doi: 10.1016/j.neulet.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoang B., et al. Docetaxel-carboxymethylcellulose nanoparticles display enhanced anti-tumor activity in murine models of castration-resistant prostate cancer. Int. J. Pharm. 2014;471(1–2):224–233. doi: 10.1016/j.ijpharm.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y., et al. Gene silencing of Toll-like receptor 2 inhibits proliferation of human liver cancer cells and secretion of inflammatory cytokines. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0038890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan X., et al. Inhibition of epidermal growth factor receptor attenuates LPS-induced inflammation and acute lung injury in rats. Oncotarget. 2017;8(16):26648–26661. doi: 10.18632/oncotarget.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.JanssenDuijghuijsen L.M., et al. Mitochondrial ATP depletion disrupts caco-2 monolayer integrity and internalizes claudin 7. Front. Physiol. 2017;8:794. doi: 10.3389/fphys.2017.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.