Abstract
Background and aim
Numerous women of reproductive age experience physical or mental discomfort during their natural menstrual cycle due to paramenstrual symptoms, such as premenstrual syndrome (PMS). To date, there is no established biomarker for the diagnosis of PMS. This study investigated the relationship between skin gas composition and menstruation cycles, and evaluated the possibility of skin gas composition as a biomarker of paramenstrual symptoms.
Methods
We conducted an exploratory pilot study. Healthy Japanese women (aged 20–29 years) underwent blood and skin gas analyses on 1 day corresponding to menstruation, preovulatory, middle luteal, and late luteal phases. Skin gas was collected from the cubital fossa and armpit using a Passive Flux Sampler; samples were analyzed for 65 volatile organic compounds (VOCs) by gas chromatography-mass spectrometry (GC-MS). Non-parametric statistical analysis was performed to identify VOCs related to the menstrual cycle, levels of female hormones, and severity of PMS.
Results
Fourteen women participated; of those, 12 completed the study. Regarding the relationship with the menstrual cycles, seven and four VOCs were significantly and marginally changed, respectively, at the cubital fossa during menstruation. Of those 11 compounds, 10 were also correlated with the levels of serum female hormones. At the armpit, five and three compounds were significantly and marginally changed, respectively, during menstruation. Of those eight compounds, five were also correlated with the levels of serum female hormones. In the study of PMS severity, analysis of the changes in VOCs suggested that ketones and fatty acids are increased during menstruation in the severe PMS group versus the mild PMS group.
Conclusions
The results of this study suggest that certain VOCs emitted in skin gas related to the menstrual cycle, levels of female hormones, and severity of PMS. These findings may advance the metabolic understanding and development of diagnostic biomarkers for menstruation-related symptoms.
Keywords: Skin gas, Volatile organic compounds, Menstrual cycle, Estrogen, Progesterone, Premenstrual syndrome
1. Introduction
The natural menstrual cycle of women is a physiological phenomenon occurring from menarche to menopause. Each woman experiences approximately 400 menstruations during her reproductive age [1]. Menstruation is a typical physiological phenomenon controlled by endogenous hormones, and a normal menstruation cycle is essential for women's health and pregnancy. Menstruation could be an indicator of the physiological status and health of women, and can be used as a diagnostic tool [2]. Moreover, it is established that the rhythm of menstruation is easily disrupted by smoking, diet, obesity, depression, substance abuse, and physical and mental stress in daily life [3,4].
Numerous women experience physical or mental discomfort associated with menstruation, resulting in significant burden to daily life [[5], [6], [7]]. Such symptoms may occur before, during, or before and during menstruation. Those symptoms are termed premenstrual syndrome (PMS), dysmenorrhea or menstrual pain, and perimenstrual syndrome (PEMS), respectively. Problems associated with menstruation reduce the quality of life of individuals and impose burden on society and economy [8].
PMS is a psychological and somatic disorder that occurs during the late luteal phase; it is resolved by the end of menstruation. It is triggered by abnormal levels of neurotransmitters and hormones in the brain due to the reduction in the levels of estrogen and progesterone (PRG) at the late luteal phase [9]; however, its severity is diverse. Interestingly, >200 physical and mental indications have been reported, with typical symptoms including irritability, tension, and dysphoria. Approximately 80–90% of menstruating women experience at least one premenstrual symptom, while 2%–10% have incapacitating symptoms [10]. Those experiencing severe symptoms are diagnosed with premenstrual dysphoric disorder (PMDD). However, the diagnosis of PMS is difficult due to the diverse symptoms and mainly depends on a questionnaire administered by physicians. Hence, there is an urgent need to discover objective biomarkers for the diagnosis of PMS. For this purpose, several studies of inflammation markers [11], amino acid levels in plasma [12], and gut microbiota [13] have been performed.
In this study, we attempted to understand the natural menstrual cycle and severity of PMS from the perspective of skin gas composition. Skin gas is generally termed body odor. It has been reported that volatile organic compounds (VOCs) measured in skin gas, as well as in exhaled breath, may be markers of liver disease [14] and diabetes [15]. In addition, the content of skin gas may be related to biological phenotypes that cannot been detected using the exhaled breath, such as age [16], severe burn status [17], and attractiveness to mosquitoes [18]. This is because the composition of skin gas differs from that of exhaled breath [19].
In this exploratory pilot trial, we recruited young and healthy Japanese women with normal menstrual cycles and variations in PMS severity. We attempted to identify the VOCs that are closely related to the menstrual cycle or severity of PMS. For skin gas sampling, we used a recently developed technique termed Passive Flux Sampler (PFS) [20]. It has been reported that PFS reduces the time of sampling and physical burden on subjects undergoing skin gas collection [17,21,22] compared with the other methods of sample collection [15,[23], [24], [25]]. We sought to address the following questions:
Are any VOCs closely associated with the menstrual cycles?
Are any VOCs closely linked to the levels of female hormones?
Are any VOCs closely related to the severity of PMS?
2. Materials and Methods
2.1. Study design
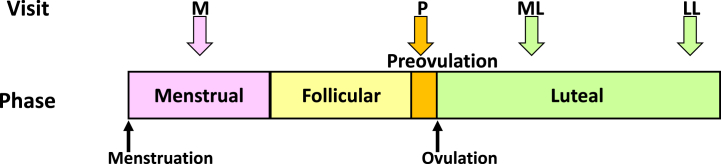
This was an open pilot trial, which involved four scheduled visits based on the menstrual cycle of each subject (Fig. 1). Each visit corresponded to 1 day of each menstrual (M) phase, 7 days after menstruation; preovulatory (P) phase, where ovulation day was determined by measuring the levels of luteinizing hormone (LH) in urine using Dotest LH II® (ROHTO Pharmaceutical Co., Ltd., Osaka, Japan); middle luteal (ML) phase, approximately 7 days after the P phase visit; and late luteal (LL) phase, 3 days before the predicted next menstruation date. At each visit, the subjects underwent blood sampling to assess plasma levels of sex hormones. Subsequently, they were given a set lunch, and skin gas samples were collected from the cubital fossa and armpit 2 h later according to the instructions provided by the manufacturer.
Fig. 1.
Four scheduled visits based on the menstrual cycle of the subjects.
The study involved four scheduled visits based on the menstrual cycle of the subjects. M: a visit during the menstruation phase; P: a visit during the preovulation phase; ML phase: a visit during the middle luteal phase; LL phase: a visit during the late luteal phase. Each visiting day was determined as described in the Materials and Methods section.
2.2. Subjects
The volunteers were recruited in December 2019 through the presentation of a poster in Nara Women's University. Each volunteer was asked to complete the background questionnaire to investigate their menstruation cycle and PMS.
The inclusion and exclusion criteria of the present study are shown in Table 1, Table 2, respectively. Healthy Japanese women aged 20–29 years with a standard body shape were selected to avoid the influence of possible confounding factors, including race, age, and body shape (i.e., skinny, obese).
Table 1.
Inclusion criteria in this study.
| (1) | Females aged 20–29 years |
| (2) | Females who experienced symptoms of premenstrual syndrome (PMS) with consistent severity in each menstrual cycle. |
| (3) | Females who provided written informed consent after receiving sufficient explanation regarding the purpose and details of the study, understanding the study well, and deciding to voluntarily participate in the study. |
Table 2.
Exclusion criteria in this study.
| (1) | Individuals who received medications or functional foods affecting female hormones. |
| (2) | Individuals with a menstrual cycle <18 days, >45 days, or extremely fluctuating. |
| (3) | Individuals who were pregnant, planning pregnancy, or breastfeeding during the test period. |
| (4) | Individuals who had or have a serious metabolic disease, including diabetes, thyroid disease, and adrenal disease. |
| (5) | Individuals with a body mass index (BMI) <18.5 or >32 kg/m2. |
| (6) | Individuals diagnosed as drug fiend or alcoholic. Individuals with a history of drug fiend or alcoholic. |
| (7) | Individuals who have smoking habitat. |
| (8) | Individuals who are going to be abroad for a long period and are difficult to participate to the study for at least one menstrual cycle. |
| (9) | Individuals who are participating or willing to participate in another clinical trial with intervention of foods, medications, and cosmetics. |
| (10) | Individuals who work for the company developing, producing, or selling functional foods. |
| (11) | Individuals who are judged unsuitable for this study by the investigator for other reasons. |
Pregnant women, those with an abnormal length of menstruation cycle, smoking habits, drug fiends, and alcoholics were excluded. All volunteers declared that their symptoms and severity of PMS is consistent in each menstrual cycle. Informed consent was obtained from all subjects after they were given the full details of the study in accordance with the Declaration of Helsinki (revised version of 2013).
During the test period, the use of medicines, herbs, and functional foods that could potentially affect the menstrual cycle or levels of female hormones was prohibited. On the day before each visit, subjects were not allowed to consume alcoholic beverages and foods containing strong herbs or spices that could affect their skin gas. Moreover, the subjects could not use strong-scented hair wash, body soap, or perfume for 24 h before undergoing sampling. Moreover, on the day of the test, the subjects did not use any product with a strong scent (e.g., antiperspirant, insect repellent, mosquito coil, deodorant spray, etc.). Subjects stayed in an air-conditioned room and avoided extreme stressful work or exercise while wearing the PFS samplers. The protocol of this study was approved by the Human Ethics Committee of Nara Women's University (approval number: 19–21). The study was registered through the University Hospital Medical Information Network (registration number: UMIN 000039171).
2.3. Skin gas sampling by PFS
PFS samplers were commercially provided by AIREX Inc. (Kanagawa, Japan). The human skin gas sampling method using PFS has been previously described [16,20]. Briefly, the device (MonoTrap®, SG DCC18; GL Science, Tokyo, Japan) consists of a glass vial with a polypropylene screw cap containing the trapping media and a polytetrafluorothylene O-ring as a stopper. The DCC18 trapping medium (surface area, >150 m2/g) is a three-dimensional silica monolith network with hydrophobic octadecylsilyl groups (C18H37Si) and activated carbon particles.
During use, the glass vial was removed, and the sampler was gently placed on the skin surface, while creating a headspace. The diffusion length between the skin surface and the trapping medium was fixed at 0.80 cm. The PFS was applied for 2 h to allow the skin gas to diffuse onto the trapping medium; the sampler was subsequently recapped with the glass vial. There was no special treatment of the skin performed before skin gas collection. After sampling, the samples were shipped to AIREX Inc. for gas chromatography-mass spectrometry (GC-MS) analysis with travel blank. The samples and travel blanks were maintained at −20 °C during storage and transportation.
2.4. GC-MS analysis
Gas emanating from the skin surface was analyzed by thermal desorption GC-MS [21], using a jms-Q1050GC mass spectrometer (JEOL, Tokyo, Japan) interfaced to a 7890B gas chromatography instrument (Agilent Technologies, Santa Clara, CA, USA). VOCs collected on the trapping media were extracted into 500 mL of carbon disulfide under ultrasound for 15 min. A sample (250 mL) was removed and N,O-Bis(trimethylsilyl)trifluoro acetamide with trimethylchlorosilane (25 mL) (Sigma Aldrich, Tokyo, Japan) was added for trimethylsilyl derivatization.
For all samples, blanks, and quantitation standards, aliquots (1 μL) were manually injected at a split ratio of 20:1 onto connected DB-1MS (30 m × 0.25 mm ID, film thickness: 0.25 μm; Agilent Technologies) and DB-35MS (30 m × 0.25 mm ID, film thickness: 0.25 μm; Agilent Technologies) columns. The carrier gas was helium at a flow rate of 1.0 mL min−1. The injector port was maintained at 300 °C. The following column temperature program was applied: 50 °C for 8 min; increased to 120 °C at 6 °C min−1; increased to 280 °C at 20 °C min−1; and 280 °C for 2 min. Data on the samples were acquired using the real-time simulation method. Quantitation ion and confirmation ion was decided on intensities of detection and selectivity (Table A1). Procedure blanks were not considered because we did not detect significant peaks of the compounds of interest.
To evaluate changes in skin gas over the course of the menstrual cycle, the emission flux of each VOC, E (ng cm−2 h−1) was determined using the following formula:
| (1) |
where W is the amount of substance (ng) collected by the PFS, S is the effective cross-section of the trapping media (0.777 cm2), and t is the sampling duration (2 h).
2.5. Measurement of female hormones
Blood sampling was conducted before lunch to avoid the stress of needle stabbing on the skin gas composition. Blood samples were collected using 8 mL vacutainer tubes and subsequently centrifuged at 1200 g for 10 min at room temperature. Serum levels of estrogen (estradiol [E2]) and PRG were determined using electrochemiluminescence immunoassay (ECLIA) by SRL Inc. (Tokyo, Japan).
2.6. Assessment of PMS severity
The severity of PMS was determined using the modified Menstrual Distress Questionnaire (m-MDQ) [26]. This is an improved version of the original MDQ [27] for Japanese women that has been used in a large population survey of PMS in Japan [8]. The questionnaire investigates a woman's physical and mental condition before, during, and after menstruation, and considers seven more common symptoms of PMS (i.e., abdominal distension, abdominal distension, appetite change, irritation, absence from school/at school, lack of self-confidence, suicidal ideation, and crying a lot), as well as 47 items from the original MDQ. Each item was scored for severity using a scale from 0 to 3, and the total score for the 54 items was calculated.
The subjects were asked to complete the m-MDQ on each visit, and the total score before and after menstruation was calculated for each patient. Subjects with a score higher than the median of all patients score were included in the heavy or middle severity PMS group (Severe group); those with a score below the median were included in the moderate or mild severity PMS group (Mild group).
2.7. Statistical analysis
Comparisons of continuous variables throughout the menstrual cycle were performed using the Kruskal–Wallis and Steel–Dwass tests. Multiplicity corrections were not performed in the Steel–Dwass test to obtain the maximum number of possible candidates. Correlations between each VOC and each female hormone were assessed using Spearman's rank correlation coefficient. According to a previous report [28], the strength of the association is classified as very weak (for absolute values of r 0–0.19), weak (0.2–0.39), moderate (0.40–0.59), strong (0.6–0.79), and very strong (0.8–1). Differences between the PMS groups were analyzed using the Mann–Whitney U test. Statistical significance was set at p < 0.05; marginal significance was set at p < 0.10.
Partial least square (PLS) regression models were used to calculate the predicted severity of PMS based on the change in skin gas emission flux from the LL phase to the P phase. VOCs that differed between the Severe group and the Mild group (p < 0.10 according to the Mann–Whitney U test) were used as explanatory variables and the change in total m-MDQ score from before to after menstruation was used as the response variable. All variables were mean-centered and unit variance-scaled before establishing the models. To avoid model over-fitting, k-fold cross-validation (k = 7) was performed, and the optimal number of latent variables was determined based on the maximum Q2 value. The quality of the model was evaluated by R2Y, Q2, and root mean square error of estimation (RMSEE), where R2Y indicates the percentage of variation explained by the model; Q2 shows the predictive ability of the model according to the cross-validation; and RMSEE describes the model fit. PLS regression was carried out with the statistical software SIMCA version 13.0 (Umetrics AB, Umeå, Sweden).
3. Results
3.1. Subject characteristics
Fourteen eligible subjects were enrolled in the study. During the test period, two subjects were lost to follow-up owing to an irregular menstrual cycle; notably, those two subjects did not have an ovulation day according to Do-test. As a result, data from 12 subjects were included in the analysis. Baseline characteristics of the participants are summarized in Table 3.
Table 3.
Baseline characteristics (mean ± standard deviation) of the study subjects.
| Total | Mild | Severe | p-value*1 | |
|---|---|---|---|---|
| Number | 12 | 6 | 6 | |
| Age (years) | 21.8 ± 0.9 | 22.3 ± 0.5 | 21.2 ± 0.8 | 0.012 |
| Height (cm) | 160.4 ± 5.9 | 160.4 ± 2.3 | 160.5 ± 8.4 | 0.975 |
| Weight (kg) | 52.5 ± 3.7 | 52.0 ± 3.9 | 52.9 ± 3.9 | 0.693 |
| BMI (kg/m2)*2 | 20.4 ± 1.3 | 20.2 ± 1.3 | 20.6 ± 1.4 | 0.649 |
| m-MDQ*3 | 24.6 ± 14.4 | 13.4 ± 6.2 | 35.8 ± 10.8 | 0.002 |
BMI: body mass index; m-MDQ: modified Menstrual Distress Questionnaire.
*1: p-values calculated using Student's t-test. *2: BMI. *3: total score of m-MDQ.
3.2. Identification of VOCs that depend on the menstruation phase
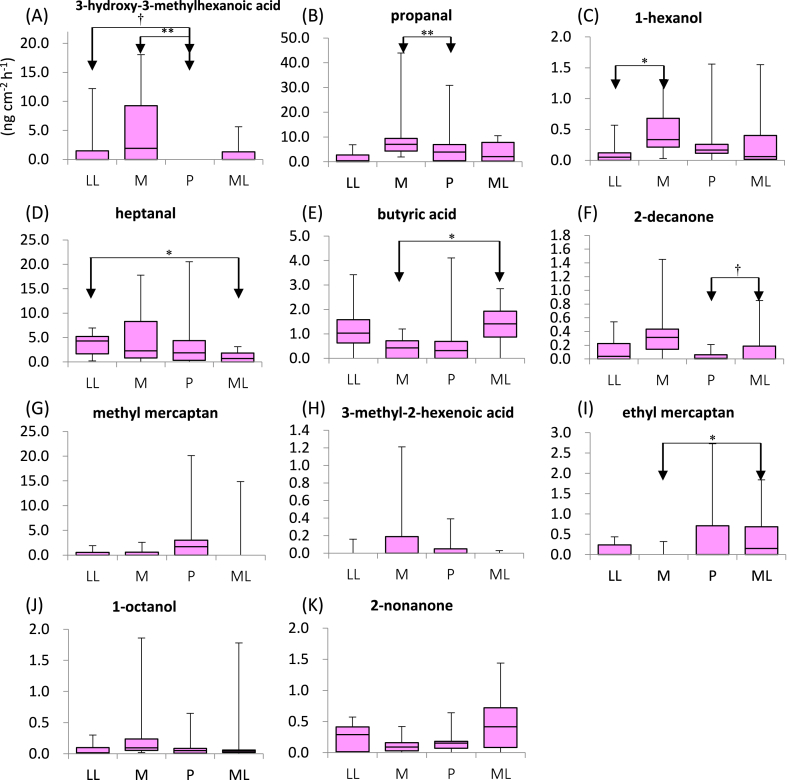
We analyzed skin gas samples for 65 VOCs (Table A1) to determine the components of skin gas that exhibited changes in emission flux between the four phases of menstruation. The emission flux values were analyzed using the Kruskal–Wallis test (Table 4; Fig. 2, Fig. 3). At the cubital fossa, 3-hydroxy-3-methylhexanoic acid, a VOC that has been reported in axial sweat [29], showed the most marked change, while 3-methyl-2-hexenoic acid, which has also been reported as an axial odor [30], showed a marginal difference. The emission flux values of six other compounds (i.e., propanal, 1-hexanol, butyric acid, heptanal, 2-decanone, and methyl mercaptan) showed significant differences, while those of three other compounds (i.e., ethyl mercaptan, 1-octanol, and 2-nonanone) showed marginal differences. Steel–Dwass analysis of changes in these VOCs was also performed. The results showed that several compounds, including 1-hexanol and propanal, were emitted more at the M phase than other phases. In contrast, ethyl mercaptan and butyric acid were emitted less at the M phase versus other phases.
Table 4.
VOCs affected by menstrual cycles.
| VOC | Functional group | Kruskal-Wallis |
Steel-Dwass |
||
|---|---|---|---|---|---|
| p-value | p-value | Phase | |||
| Cubital fossa | |||||
| 3-hydroxy-3-methylhexanoic acid | Fatty acid | 0.007 | 0.005 | M-P | |
| 0.007 | 0.070 | LL-P | |||
| Propanal | Aldehyde | 0.008 | 0.003 | LL-M | |
| 1-hexanol | Alcohol | 0.018 | 0.021 | LL-M | |
| Butytic acid | Fatty acid | 0.025 | 0.020 | M-ML | |
| Heptanal | Aldehyde | 0.037 | 0.020 | LL-ML | |
| 2-decanone | Ketone | 0.037 | 0.069 | M-P | |
| Methyl mercaptan | Sulfur compound | 0.038 | ND | ||
| 3-methyl-2-hexenoic acid | Fatty acid | 0.063 | ND | ||
| Ethyl mercaptan | Sulfur compound | 0.065 | 0.049 | M-ML | |
| 1-octanol | Alcohol | 0.068 | ND | ||
| 2-nonanone | Ketone | 0.098 | ND | ||
| Armpit | |||||
| Heptanoic acid | Fatty acid | 0.011 | 0.046 | P-ML | |
| 0.072 | LL-ML | ||||
| Propanal | Aldehyde | 0.031 | 0.033 | LL-M | |
| 0.031 | 0.057 | M-P | |||
| 2-dodecanone | Ketone | 0.044 | 0.067 | LL-ML | |
| Hexanoic acid | Fatty acid | 0.044 | ND | ||
| Propionic acid | Fatty acid | 0.047 | 0.046 | M-ML | |
| 2-nonanone | Ketone | 0.088 | 0.071 | M-ML | |
| 1-heptanol | Alcohol | 0.089 | ND | ||
| Allyl methyl disulfide | Sulfur compound | 0.098 | ND | ||
LL: late luteal; M: menstrual; ML: middle luteal; ND: no statistical difference; P: preovulatory; VOC: volatile organic compound.
Fig. 2.
Comparison of the emission flux of VOCs at the cubital fossa during the menstrual cycle.
Vertical axes show the emission rates of skin gas (ng cm-2 h-1). Boxes show the upper quartiles, median, and lower quartiles. Whiskers show the upper and lower limits. Arrows indicate differences between menstrual phases identified by the Steel–Dwass test. *: p < 0.05; †: p < 0.10. M: a visit during the menstruation phase; P: a visit during the preovulation phase; ML phase: a visit during the middle luteal phase; LL phase: a visit during the late luteal phase; VOCs: volatile organic compounds.
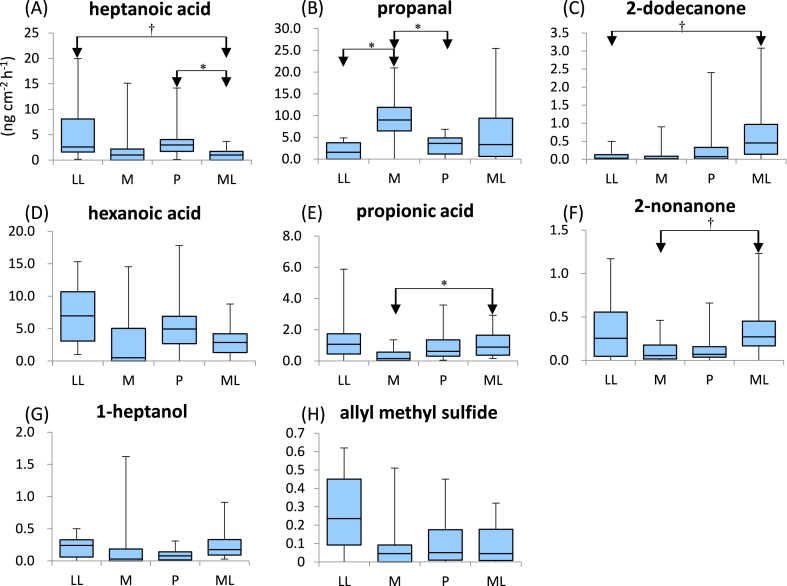
Fig. 3.
Comparison of the emission flux of VOCs at the armpit during the menstrual cycle.
Vertical axes show the emission rates of skin gas (ng cm-2 h-1). Boxes show the upper quartiles, median, and lower quartiles. Whiskers show the upper and lower limits. Arrows indicate differences between menstrual phases identified by the Steel–Dwass test. *: p < 0.05, †: p < 0.10, M: a visit during the menstruation phase, P: a visit during the preovulation phase, ML: a visit during the middle luteal phase, LL: a visit during the late luteal phase, VOCs: volatile organic compounds.
At the armpit, the emission flux of five compounds (i.e., heptanoic acid, propanal, 2-dodecanone, hexanoic acid, and propionic acid) was significantly different, while that of three other compounds (i.e., 2-nonanone, 1-heptanol, and allyl methyl disulfide) was marginally different. Steel–Dwass analysis suggested that propanal was emitted more, whereas 2-nonanone and propionic acid were emitted less in the M phase versus other phases. Collectively, these results suggested that the emission flux of specific skin gas components varies during the menstrual cycle.
3.3. Identification of VOCs that correlate with serum E2
We sought to identify skin gas components related to the serum levels of female hormones. For this purpose, we used Spearman's rank correlation analysis to assess the relationship between the skin gas emission flux and serum E2 concentration. VOCs that showed correlation to E2 with significance or marginal significance are shown in Table 5. At the cubital fossa, the emission flux of ethyl mercaptan showed a significant and moderate positive correlation (r = 0.4–0.7) with serum E2. Nine compounds (i.e., propanal, 1-hexanol, 2-decanone, 3-hydroxy-3-methylhexanoic acid, 7-octenoic acid, 1-octanol, γ-decanolactone, 3-methyl 2-hexenoic acid, and butyric acid) showed a significant and weak correlation (r = 0.2–0.4) with serum E2. Eight of those ten VOCs that exhibited a significant correlation with E2, except for 7-octenoic acid and γ-decanolactone, were also identified as VOCs related to the menstruation cycle by the Kruskal–Wallis test (Table 4). The other seven compounds showed marginal significance and weak correlation.
Table 5.
Correlation of emission flux of VOCs with the levels of estradiol (E2) in the blood.
| VOC | Functional group | Correlation coefficient (r) | p-value*1 | |
|---|---|---|---|---|
| Cubital fossa | ||||
| Ethyl mercaptan | Sulfur compounds | 0.439 | 0.002 | |
| Propanal | Aldehyde | −0.347 | 0.016 | |
| 1-hexanol | Alcohol | −0.330 | 0.022 | |
| 2-decanone | Alcohol | −0.322 | 0.026 | |
| 3-hydroxy-3-methylhexanoic acid | Fatty acid | −0.316 | 0.029 | |
| 7-octenoic acid | Fatty acid | 0.310 | 0.032 | |
| 1-octanol | Alcohol | −0.307 | 0.034 | |
| γ-decanolactone | Lactone | 0.301 | 0.038 | |
| 3-methyl 2-hexenoic acid | Fatty acid | −0.296 | 0.041 | |
| Butyric acid | Fatty acid | 0.285 | 0.049 | |
| Octanoic acid | Fatty acid | −0.283 | 0.051 | |
| Isovaleraldehyde | Aldehyde | −0.272 | 0.062 | |
| 2-nonanone | Ketone | 0.247 | 0.090 | |
| Valeric acid | Fatty acid | −0.246 | 0.091 | |
| 2-ethyl-1-hexanol | Alcohol | −0.246 | 0.092 | |
| Butanal | Aldehyde | 0.245 | 0.093 | |
| 2-tetradecanone | Ketone | −0.241 | 0.099 | |
| Armpit | ||||
| 7-octenoic acid | Fatty acid | 0.510 | 0.000 | |
| Ethyl mercaptan | Sulfur compounds | 0.340 | 0.018 | |
| Propionic acid | Fatty acid | 0.333 | 0.021 | |
| 2-dodecanone | Ketone | 0.321 | 0.026 | |
| Octanal | Aldehyde | −0.300 | 0.038 | |
| Heptanol | Alcohol | 0.297 | 0.041 | |
| Isovaleraldehyde | Aldehyde | −0.284 | 0.050 | |
| Propanal | Aldehyde | −0.275 | 0.058 | |
| 2-decanone | Ketone | −0.266 | 0.068 | |
| Valeric acid | Fatty acid | 0.265 | 0.069 | |
| Pentanol | Alcohol | −0.254 | 0.082 | |
| Hexanal | Aldehyde | −0.244 | 0.094 | |
VOC: volatile organic compound.
*1: p-values were calculated using Spearman's rank correlation test.
At the armpit, the emission flux of 7-octenoic acid showed a significant and moderate positive correlation with serum E2. Five compounds (i.e., ethyl mercaptan, propionic acid, 2-dodecanone, octanal, and heptanol) showed a significant and weak correlation with the serum levels of E2. However, only two of those six VOCs that exhibited a significant correlation with E2 (i.e., propionic acid and 2-dodecanone) were also identified as VOCs related to the menstruation cycle by the Kruskal–Wallis test (Table 4). The other six compounds showed marginal significance and weak correlation.
3.4. Identification of VOCs that correlate with PRG
We also used Spearman's rank correlation analysis to assess the relationship between the skin gas emission flux and serum PRG concentration. The VOCs that showed significant correlation with PRG are shown in Table 6. At the cubital fossa, propanal and 3-methyl-2-hexenoic acid showed a significant and middle correlation. Eight compounds (i.e., octanoic acid, 1-pentanol, 2-nonanone, butyric acid, 1-octanol, hexanal, 2-hexenal, and 1-hexanol) showed a significant and weak correlation with serum PRG. Six of those ten VOCs that showed significant correlation with PRG (i.e., propanal, 3-methyl-2-hexenoic acid, 2-nonanone, butyric acid, 1-octanol, and 1-hexanol) were also identified as VOCs related to the menstruation cycle by the Kruskal–Wallis test (Table 4). The other seven compounds showed marginal significance and weak correlation.
Table 6.
Correlation of emission flux of VOCs with the levels of progesterone (PRG) in the blood.
| VOC | Functional group | Correlation coefficient (r) | p-value*1 | |
|---|---|---|---|---|
| Cubital fossa | ||||
| Propanal | Aldehyde | −0.452 | 0.001 | |
| 3-methyl-2-hexenoic acid | Fatty acid | −0.422 | 0.003 | |
| Octanoic acid | Fatty acid | −0.397 | 0.005 | |
| 1-pentanol | Alcohol | −0.371 | 0.009 | |
| 2-nonanone | Ketone | 0.339 | 0.018 | |
| Butyric acid | Fatty acid | 0.332 | 0.021 | |
| 1-octanol | Alcohol | −0.330 | 0.022 | |
| Hexanal | Aldehyde | −0.311 | 0.031 | |
| 2-hexenal | Aldehyde | −0.300 | 0.038 | |
| 1-hexanol | Alcohol | −0.289 | 0.047 | |
| 1-butanol | Alcohol | −0.277 | 0.057 | |
| 2-decanone | Ketone | −0.276 | 0.057 | |
| Diallyl disulfide | Sulfur compounds | 0.276 | 0.058 | |
| Heptanal | Aldehyde | −0.270 | 0.063 | |
| Ethyl mercaptan | Sulfur compounds | 0.265 | 0.069 | |
| 2-ethyl-1-hexanol | Alcohol | −0.258 | 0.076 | |
| γ-octanolactone | Lactone | 0.251 | 0.085 | |
| Armpit | ||||
| 1-heptanol | Alcohol | 0.506 | 0.000 | |
| 7-octenoic acid | Fatty acid | 0.382 | 0.007 | |
| 2-octanone | Ketone | 0.354 | 0.014 | |
| Propionic acid | Fatty acid | 0.348 | 0.016 | |
| 3-methyl-2-hexenoic acid | Fatty acid | −0.344 | 0.017 | |
| Propanal | Aldehyde | −0.308 | 0.033 | |
| Diallyl disulfide | Sulfur compounds | 0.302 | 0.037 | |
| Ethyl mercaptan | Sulfur compounds | 0.275 | 0.059 | |
| Valeric acid | Fatty acid | 0.270 | 0.063 | |
| Hexanoic acid | Fatty acid | 0.257 | 0.077 | |
| Hexanal | Aldehyde | −0.255 | 0.081 | |
| 2-decanone | Ketone | −0.246 | 0.091 | |
VOC: volatile organic compound.
*1: p-values were calculated using Spearman's rank correlation test.
At the armpit, the emission flux of 1-heptanol showed a significant and middle correlation, while six compounds (i.e., 7-octenoic acid, 2-octanone, propionic acid, 3-methyl 2-hexenoic acid, propanal, and diallyl disulfide) showed a significant and weak correlation with serum PRG. Three of those seven VOCs that were significantly correlated with PRG (i.e., 1-heptanol, propionic acid and propanal) were also identified as VOCs related to the menstruation cycle by the Kruskal–Wallis test (Table 4). The other five compounds showed marginal significance and weak correlation.
3.5. Identification of VOCs related to the severity of PMS
Next, we evaluated whether the change in emission flux of skin gas components during menstruation is linked to PMS severity. PMS is a phenomenon observed during the LL phase and resolves by the end of menstruation. Thus, the emission flux of each skin gas compound at the P phase was subtracted from that recorded at the LL phase. The resulting values were compared between the mild and severe PMS groups using the Mann–Whitney U test (Table 7).
Table 7.
Change in the emission flux of VOCs from the LL phase to the P phase.
| VOC | Functional group | Mild group*1 | Severe group*1 | p-value*2 | |
|---|---|---|---|---|---|
| Cubital Fossa | |||||
| 2-decanone | Ketone | −0.04 ± 0.15 | 0.19 ± 0.20 | 0.036 | |
| 2-ethyl-1-hexanol | Alcohol | −3.77 ± 8.07 | 6.55 ± 12.75 | 0.037 | |
| Acetone | Ketone | −8.65 ± 14.05 | 6.29 ± 13.70 | 0.037 | |
| 2-hexanone | Ketone | −0.52 ± 1.43 | −0.22 ± 3.29 | 0.076 | |
| Decanoic acid | Fatty acid | −5.41 ± 9.06 | 2.59 ± 6.61 | 0.078 | |
| Nonanoic acid | Fatty acid | −3.93 ± 8.25 | 3.80 ± 5.74 | 0.078 | |
| Acetic acid | Fatty acid | 4.48 ± 8.34 | −6.70 ± 11.54 | 0.078 | |
| Armpit | |||||
| Acetone | Ketone | −10.01 ± 7.16 | 10.30 ± 11.11 | 0.010 | |
| Nonanoic acid | Fatty acid | −7.94 ± 8.90 | 2.32 ± 7.64 | 0.055 | |
| 2-nonanone | Ketone | −0.03 ± 0.18 | 0.41 ± 0.44 | 0.055 | |
| γ-hexanolactone | lactone | −0.14 ± 1.91 | 1.60 ± 2.47 | 0.055 | |
| 2-hexenal | Aldehyde | −1.13 ± 2.49 | 2.54 ± 3.37 | 0.076 | |
| 2-octanone | Ketone | −0.61 ± 0.96 | 2.55 ± 5.70 | 0.078 | |
LL: late luteal; P: preovulatory; VOC: volatile organic compound.
*1: mean ± standard devition (ng cm-2 h-1) *2: p-values were calculated using the Mann–Whitney U test.
At the cubital fossa, three compounds (i.e., 2-decanone, 2-ethyl-1-hexanol, and acetone) showed a significant difference between the Mild and Severe groups. Notably, four compounds (i.e., 2-hexanone, decanoic acid, nonanoic acid, and acetic acid) showed a marginal difference between the two groups. Except for acetic acid, the difference from the P phase to the LL phase was higher in the Severe group versus the Mild group.
At the armpit, acetone showed a significant difference between the Mild and Severe groups. Five compounds (i.e., nonanoic acid, 2-nonanone, 2-hexanal, γ-hexanolactone and 2-octanone) showed a marginal difference between the two groups. For all compounds, the difference from the P phase to the LL phase was higher in the Severe group versus the Mild group.
3.6. PLS analysis of skin gas in PMS
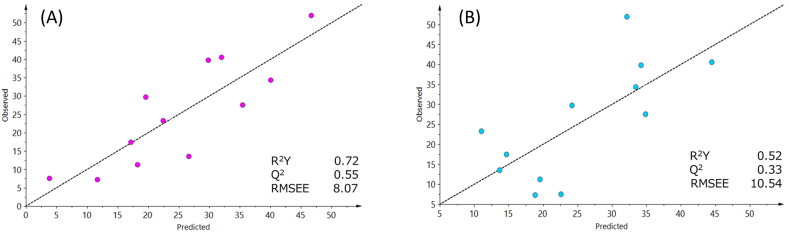
We examined the potential of the emission flux of skin gas as a marker of the severity of PMS. PLS regression models were established based on the change in skin gas from the LL phase to the P phase for the components listed in Table 7. The model based on the data of seven VOCs identified at the cubital fossa (i.e., 2-decanone, 2-ethyl-1-hexanole, acetone, 2-hexanone, decanoic acid, nonanoic acid, and acetic acid) yielded values of 0.72, 0.55, and 8.07 for R2Y, Q2, and RMSEE, respectively (Fig. 4A). The model based on the data of six VOCs identified at the armpit (i.e., acetone, nonanoic acid, 2-nonanone, γ-hexanolactone, 2-hexanal, and 2-octanone) yielded values of 0.52, 0.33, and 10.54 for R2Y, Q2, and RMSEE, respectively (Fig. 4B). These results suggest that the model based on skin gas obtained from the cubital fossa was better than that based on skin gas obtained from the armpit.
Fig. 4.
Scatter plots of observed (total m-MDQ score) and predicted severity of PMS.
(A) Predicted scores were calculated using skin gas components identified at the cubital fossa. (B) Predicted scores were calculated using skin gas components identified at the armpit. m-MDQ: modified Menstrual Distress Questionnaire, PMS: premenstrual syndrome, RMSEE: root mean square error of estimation.
4. Discussion
In this study, we attempted to reveal whether the emission ratio of any of the VOCs in skin gas is related to the menstruation cycle or levels of two female hormones. We collected skin gas at two body sites, namely the cubital fossa and armpit. This approach was employed because several previous reports suggested that the emission flux of VOCs may differ between body sites [21,31].
At the cubital fossa, seven and four compounds exhibited significant and marginal changes during menstruation, respectively, as assessed by the Kruskal–Wallis test (Table 4). Of note, all these compounds, except for methyl mercaptan, were also correlated with the levels of E2 or PRG. Eight and nine compounds were correlated with the serum levels of E2 and PRG, respectively (Table 5, Table 6, respectively). These results suggest that most of the skin gas components at the cubital fossa reflect physical changes due to hormonal regulation during the menstrual cycle.
At the armpit, five and three compounds demonstrated significant and marginal changes during menstruation, respectively, as assessed by the Kruskal–Wallis analysis. Five of those compounds were also correlated with the serum levels of E2 or PRG. Four of those compounds were also correlated with the serum levels of E2 (Table 5), while three compounds were correlated with the serum levels of PRG (Table 6). These results suggest that the cubital fossa is a more suitable body site for identifying compounds related to the menstrual cycle compared with the armpit. The armpit is a well-established site of body odor and is rich in apocrine glands. The cubital fossa is one of the most commonly used sites for venipuncture and is rich in superficial veins. It is possible that VOCs at the armpit are mainly affected by sweat or fluid secreted from the apocrine glands, while those at the cubital fossa may reflect VOCs presence in the blood. Another possible difference between the cubital fossa and armpit is the skin microbiota. The microbiome at the armpit has been studied extensively and is related to skin gas formation [32,33]. In contrast, few studies have focused on the microbiota present at the cubital fossa. Thus, further analysis comparing VOCs detected in skin gas with those found in blood, as well as with skin microbial compositions, is warranted.
The emission flux of propanal and 2-nonanone was affected by the phase of menstruation cycle both at the cubital fossa and armpit. The levels of propanal differed significantly between the cubital fossa and armpit, while those of 2-nonanone differed marginally at both sites. In addition, propanal showed significant correlation with E2 and PRG at the cubital fossa, and demonstrated marginal and significant correlation with E2 and PRG at the armpit, respectively. It has been reported that propanal is produced by OH-initiated degradation of isoprene [34], which originates from the mevalonate pathway that produces cholesterol and is a precursor of steroid hormones [35]. In addition, the level of isoprene in exhaled breath has been reported to be affected by menstrual phase [36]. Collectively, these observations suggest that the metabolic pathways of steroid hormones, such as E2 and PRG, may affect the emission flux of isoprene and propanal in biogas.
Other VOCs at the cubital fossa were found to be affected by the menstrual cycle. 3-hydroxy-3-methylhexanoic acid, which showed the most marked change, and 3-methyl-2-hexenoic acid, which showed a marginal difference during the menstruation cycle (Table 4), have been previously reported as components of body odor [29,37]. Ethyl mercaptan and methyl mercaptan were also significantly changed during the menstrual cycle, as assessed by the Kruskal–Wallis test. Mercaptans are metabolized in the liver, and elevated levels of mercaptans are related to halitosis and brain disease [38,39]. Butyric acids are one of the well-known metabolites of the gut microbiota as a result of the fermentation of non-digestible polysaccharides, and have been detected in exhaled breath [40]. Collectively, our results suggest that the menstrual cycle affects the metabolism in women, as well as the emission ratio of several compounds in skin gas. In addition, 1-hexanol and 2-decanone were also identified as menstruation cycle-related compounds. However, limited information was obtained regarding the metabolism of these compounds.
We also conducted screening of VOCs related to the severity of PMS. Our data suggest that the emission ratios of several VOCs are related to the severity of PMS. Seven VOCs at the cubital fossa and six VOCs at the armpit were significantly or marginally different between the Severe and Mild groups. Three of the seven VOCs at the cubital fossa were fatty acids, while three others were ketones. At the armpit, three of the six VOCs were ketones, while another was a fatty acid. All these ketones and fatty acids, except for acetic acid, showed a larger emission flux in the Severe group versus the Mild group. This suggests that oxidative stress is enhanced and the levels of oxidants (e.g., lipid hydroperoxide) are increased in the Severe group versus the Mild group. Oxidative stress has been implicated in many diseases, including psychiatric disorders. It should be noted that antioxidant levels are correlated with PMS severity [30]. Moreover, it has been suggested that oxidative stress and the serum levels of antioxidants fluctuate during the menstrual cycle [41,42]. Our findings suggest that these changes in the degree of oxidation might be detected by monitoring skin gas.
The levels of 2-ethyl-1-hexanol also differed significantly between the Mild group and the Severe group at the cubital fossa. This compound is widely used as a raw material for the production of di(2‐ethylhexyl) phthalate (DEHP), a plasticizer for polyvinyl chloride (PVC), and is an air pollutant [43]. Sola-Martínez et al. reported that 2-ethyl-1-hexanol and acetone in the exhaled breath of women of childbearing age were indicators of asthma and other coexisting atopic diseases [44]. Acetone was also detected as a discriminant at the cubital fossa in this study. We speculate that the accumulation and metabolism of 2-ethyl-1-hexanol in a woman's body is affected by PMS and other disorders.
Lastly, using the VOCs that exhibited significant differences between the Severe and the Mild group, we performed PLS analysis to evaluate the potential of VOCs emitted in skin gas as diagnostic markers for the severity of PMS. According to previous reports, R2Y > 0.65 indicates a quantitative possible model, and Q2 > 0.50 denotes a good predictive model [45]. Our results imply that the severity of PMS may be predicted by the skin gas profile at the cubital fossa. Our results showed that the predictive model using VOCs identified at the cubital fossa was better than that using VOCs identified at the armpit. This also supports our hypothesis that the cubital fossa is an appropriate body site for the collection of skin gas in the diagnosis of menstruation-related symptoms. Currently, the diagnosis of PMS mainly depends on a questionnaire administered by gynecologists. It is possible that the profile of skin gas obtained from the cubital fossa may be a diagnostic marker of PMS. Nevertheless, further studies are required to construct a more practical predictive model.
As far as we know, this is the first report showing that the composition of skin gas changes during the menstrual cycle. Regarding exhaled breath, several reports investigated the relationship between skin gas profile and menstruation cycle. Dragonieri et al. showed that the menstruation cycle may alter the exalted VOC patterns of an electronic nose [46]. Sukul et al. reported that the levels of ammonia, acetone, isoprene, and dimethyl sulfide were significantly changed between the follicular and luteal phases [36]. However, most of the VOCs identified in the present study were not detected in their analysis. The generation mechanisms of VOCs in skin gas and exhaled breath may differ and should be clarified in the future to accelerate the development of gas as non-invasive biomarkers for diagnosis.
This study has some limitations. Firstly, the sample size was small and not determined based on the statistical power analysis. Instead, it was determined based on several previously unpublished pilot studies performed by the manufacturer of PFS. To verify our results, studies involving larger numbers of subjects should be performed in the future. Secondly, the subjects in this study were heathy young women; the analysis did not include patients diagnosed with PMS or PMDD who experienced more severe symptoms than PMS. More studies involving older healthy women and patients diagnosed with PMS or PMDD should be carried out. Thirdly, other VOCs not investigated in this study might be useful as markers of the menstrual cycle or PMS severity.
In the future, more studies are necessary to develop skin gas as a biomarker of health conditions. We are interested in studying the relationship between skin gas and other menstrual cycle-related symptoms, such as dysmenorrhea or menstrual pain, and perimenstrual syndrome. We are also interested in developing skin gas as a marker of menopause. Menopause is an event occurring at the end of the reproductive age in women caused by changes in the levels of female hormones [47]. Multiple symptoms develop during the transition to menopause, and the severity of menopause differs between individuals [48]. Skin gas could be a non-invasive biomarker of menopause that may assist in maintaining the quality of life of women. In addition, studying the skin gas generation metabolism is important for understanding the causality of skin gas. Particularly, a comprehensive analysis investigating the effect of female hormones on the metabolism and emission ratio of VOCs is essential.
Finally, more efficient and time-saving methods for skin gas analysis, such as electronic nose [49], should be developed for the study of large number of subjects and practical use of skin gas as a biomarker.
In conclusion, the results of this study suggest that certain VOCs emitted in skin gas are associated with the menstrual cycle, serum female hormones, and severity of PMS in young and healthy women. Our findings may provide a new scope for the basic and clinical understanding of the effects of menstrual cycle and related symptoms on metabolism.
Funding
This study was funded by Kirin Holdings Company, Limited.
Ethics and approval and consent to participate.
This study was performed in accordance with the guidelines laid out in the Declaration of Helsinki and was conducted with the approval of the Institutional Review Board, Nara women's University (No. 19–21). Written informed consent was obtained from all participants.
Author contribution statement
Toshio Fujii: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nozomi Matsuura: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yuji Morita: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Keiko Morimoto: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr, Daisuke Oikawa, AIREX Inc. for useful discussions. We also thank Ms. Yuho Yamauchi for her technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19627.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Estanislau do Amaral M.C., Hardy E., Hebling E.M., Faúndes A. Menstruation and amenorrhea: opinion of Brazilian women. Contraception. 2005;72(2):157–161. doi: 10.1016/j.contraception.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt K.M., Dimmock P.W., Hayes-Gill B., Crowe J., O'Brien P.M.S. Menstrual symptometrics: a simple computer-aided method to quantify menstrual cycle disorders. Fertil. Steril. 2002;78(1):96–101. doi: 10.1016/s0015-0282(02)03161-8. [DOI] [PubMed] [Google Scholar]
- 3.Wallach E.E., Baker E.R. Menstrual dysfunction and hormonal status in athletic women: a review. Fertil. Steril. 1981;36(6):691–696. doi: 10.1016/s0015-0282(16)45908-x. [DOI] [PubMed] [Google Scholar]
- 4.Ju H., Jones M., Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014;36:104–113. doi: 10.1093/epirev/mxt009. [DOI] [PubMed] [Google Scholar]
- 5.Wasiak R., Filonenko A., Vanness D.J., Wittrup-Jensen K.U., Stull D.E., Siak S., Fraser I. Impact of estradiol-valerate dienogest on work productivity and activities of daily living in European and Australian women with heavy menstrual bleeding. Int J Womens Health. 2012;4:271–278. doi: 10.2147/IJWH.S31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser I.S., Langham S., Uhl-Hochgraeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Rev Obstet Gynecol. 2009;4(2):179–189. [Google Scholar]
- 7.Liu Z., Doan Q.V., Blumenthal P., Dubois R.W. A systematic review evaluating haealth-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka E., Momoeda M., Osuga Y., Rossi B., Nomoto K., Hayakawa M., Kokubo K., Wang E.C.Y. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J. Med. Econ. 2013;16(11):1255–1266. doi: 10.3111/13696998.2013.830974. [DOI] [PubMed] [Google Scholar]
- 9.Rapkin A.J., Akopians A.L. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. 2012;18(2):52–59. doi: 10.1258/mi.2012.012014. [DOI] [PubMed] [Google Scholar]
- 10.Angst J., Sellaro R., Stolar M., Merikangas K.R., Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr. Scand. 2001;104(2):110–116. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertone-Johnson E.R., Ronnenberg A.G., Houghton S.C., Nobles C., Zagarins S.E., Takashima-Uebelhoer B.B., Faraj J.L., Whitcomb B.W. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum. Reprod. 2014;29(9):1987–1994. doi: 10.1093/humrep/deu170. [DOI] [PubMed] [Google Scholar]
- 12.Di Florio A., Alexander D., Schmidt P.J., Rubinow D.R. Progesterone and plasma metabolites in women with and in those without premenstrual dysphoric disorder. Depress. Anxiety. 2018;35(12):1168–1177. doi: 10.1002/da.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda T., Yoshimi K., Kai S., Ozawa G., Yamada K., Hiramatsu K. Characteristics of the gut microbiota in women with premenstrual symptoms: a cross-sectional study. PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nose K., Mizuno T., Yamane N., Kondo T., Ohtani H., Araki S., Tsuda T. Identification of ammonia in gas emanated from human skin and its correlation with that in blood. Anal. Sci. 2005;21(12):1471–1474. doi: 10.2116/analsci.21.1471. [DOI] [PubMed] [Google Scholar]
- 15.Yamane N., Tsuda T., Nose K., Yamamoto A., Ishiguro H., Kondo T. Relationship between skin acetone and blood β-hydroxybutyrate concentrations in diabetes. Clin. Chim. Acta. 2006;365(1–2):325–329. doi: 10.1016/j.cca.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Kimura K., Sekine Y., Furukawa S., Takahashi M., Oikawa D. Measurement of 2-nonenal and diacetyl emanating from human skin surface employing passive flux sampler—GCMS system. J. Chromatogr. B. 2016;1028:181–185. doi: 10.1016/j.jchromb.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Kimura K., Sekine Y., Umezawa K., Furukawa S., Takahashi M., Asai S., MiyachiI H., Tukamoto T., Ozano T. Clinical application of ammonia emanating from severe burn patients during critical care. J. Jpn. Assoc. Odor Environ. 2016;47(6):421–429. [Google Scholar]
- 18.Ghaninia M., Larsson M., Hansson B.S., Ignell R. Natural odor ligands for olfactory receptor neurons of the female mosquito Aedes aegypti : use of gas chromatography-linked single sensillum recordings. J. Exp. Biol. 2008;211(18):3020–3027. doi: 10.1242/jeb.016360. [DOI] [PubMed] [Google Scholar]
- 19.Mochalski P., Unterkofler K., Teschl G., Amann A. Potential of volatile organic compounds as markers of entrapped humans for use in urban search-and-rescue operations. Trends Anal. Chem. 2015;68:88–106. [Google Scholar]
- 20.Sekine Y., Toyooka S., Watts S.F. Determination of acetaldehyde and acetone emanating from human skin using a passive flux sampler—HPLC system. J. Chromatogr. B. 2007;859(2):201–207. doi: 10.1016/j.jchromb.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Sato S., Sekine Y., Kakumu Y., Hiramoto T. Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic. Sci. Rep. 2020;10(1):465. doi: 10.1038/s41598-019-57258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine Y., Sato S., Kimura K., Sato H., Nakai S., Yanagisawa Y. Detection of tobacco smoke emanating from human skin surface of smokers employing passive flux sampler – GCMS system. J. Chromatogr. B. 2018;1092:394–401. doi: 10.1016/j.jchromb.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Nose K., Nunome Y., Kondo T., Araki S., Tsuda T. Identification of gas emanated from human skin: methane, ethylene, and ethane. Anal. Sci. 2005;21(6):625–628. doi: 10.2116/analsci.21.625. [DOI] [PubMed] [Google Scholar]
- 24.Mochalski P., Unterkofler K., Hinterhuber H., Amann A. Monitoring of selected skin-borne volatile markers of entrapped humans by selective reagent ionization time of flight mass spectrometry in NO + mode. Anal. Chem. 2014;86(8):3915–3923. doi: 10.1021/ac404242q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarumi W., Shinohara K. Women's body odour during the ovulatory phase modulates testosterone and cortisol levels in men. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odagawa H., Shirato N., Nagatsuka M., Chiba H., Kimura T., Okai T. An investigation of menstruation symptoms in young women by measuring modified MDQ scores. Showa-ikaishi. 2008;68(3):155–161. . Japanese. [Google Scholar]
- 27.Moos R.H. The development of a menstrual distress questionnaire. Psychosom. Med. 1968;30(6):853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Campbell M.J. twelfth ed. 2021. Statistics at Square One. [Google Scholar]
- 29.Hasegawa Y., Yabuki M., Matsukane M. Identification of new odoriferous compounds in human axillary sweat. Chem. Biodivers. 2004;1(12):2042–2050. doi: 10.1002/cbdv.200490157. [DOI] [PubMed] [Google Scholar]
- 30.Heidari H., Amani R., Feizi A., Askari G., Kohan S., Tavasoli P. Vitamin D Supplementation for Premenstrual Syndrome-Related inflammation and antioxidant markers in students with vitamin D deficient: a randomized clinical trial. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-51498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher M., Wysocki C.J., Leyden J.J., Spielman A.I., Sun X., Preti G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008;159(4):780–791. doi: 10.1111/j.1365-2133.2008.08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D., Daulby A., Grimshaw S., James G., Mercer J., Vaziri S. Characterization of the microflora of the human axilla. Int. J. Cosmet. Sci. 2003;25(3):137–145. doi: 10.1046/j.1467-2494.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 33.James A.G., Austin C.J., Cox D.S., Taylor D., Calvert R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol. Ecol. 2013;83(3):527–540. doi: 10.1111/1574-6941.12054. [DOI] [PubMed] [Google Scholar]
- 34.Dibble T.S. A quantum chemical study of the C−C bond fission pathways of alkoxy radicals formed following OH addition to isoprene. J. Phys. Chem. A. 1999;103(42):8559–8565. [Google Scholar]
- 35.Stone B.G., Besse T.J., Duane W.C., Dean Evans C., DeMaster E.G. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men. Lipids. 1993;28(8):705–708. doi: 10.1007/BF02535990. [DOI] [PubMed] [Google Scholar]
- 36.Sukul P., Schubert J.K., Trefz P., Miekisch W. Natural menstrual rhythm and oral contraception diversely affect exhaled breath compositions. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-29221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng X., Leyden J.J., Lawley H.J., Sawano K., Nohara I., Preti G. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 1991;17(7):1469–1492. doi: 10.1007/BF00983777. [DOI] [PubMed] [Google Scholar]
- 38.Newman A. Breath-analysis tests in gastroenterology. Gut. 1974;15(4):308–323. doi: 10.1136/gut.15.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al Mardini H., Bartlett K., Record C.O. Blood and brain concentrations of mercaptans in hepatic and methanethiol induced coma. Gut. 1984;25(3):284–290. doi: 10.1136/gut.25.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meurs J., Sakkoula E., Cristescu S.M. Real-time non-invasive monitoring of short-chain fatty acids in exhaled breath. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.853541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duvan C.I., Cumaoglu A., Turhan N.O., Karasu C., Kafali H. Oxidant/antioxidant status in premenstrual syndrome. Arch. Gynecol. Obstet. 2011;283(2):299–304. doi: 10.1007/s00404-009-1347-y. [DOI] [PubMed] [Google Scholar]
- 42.Yama K., Minami E., Machida M., Hayase N., Miura J. Relation between premenstrual syndrome, oxidative stress and depression. Pharmacometrics. 2018;95(1/2):19–24. [Google Scholar]
- 43.Wakayama T., Ito Y., Sakai K., Miyake M., Shibata E., Ohno H., Kamijima M. Comprehensive review of 2‐ethyl‐1‐hexanol as an indoor air pollutant. J. Occup. Health. 2019;61(1):19–35. doi: 10.1002/1348-9585.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sola-Martínez R.A., Lozano-Terol G., Gallego-Jara J., Morales E., Cantero-Cano E., Sanchez-Solis M., García-Marcos L., Jiménez-Guerrero P., Noguera-Velasco J.A., Cánovas Díaz M., de Diego Puente T., Candel-Torralba M.E., Garcia-Marcos L., Gimenez-Banon M.J., Martinez-Torres A., Morales E., Perez-Fernandez V., Sanchez-Solis M., Nieto A., Prieto-Sanchez M.T., Sanchez-Ferrer M., Fernandez-Palacios L., Gomez-Gomez V.P., Martinez-Gracia C., Peso-Echarri P., Ros-Berruezo G., Santaella-Pascual M., Gazquez A., Larque E., Pastor-Fajardo M.T., Sanchez-Campillo M., Serrano-Munuera A., Zornoza-Moreno M., Jimenez-Guerrero P., Adomnei E., Arense-Gonzalo J.J., Mendiola J., Navarro-Lafuente F., Torres-Cantero A.M., Salvador-Garcia C., Segovia-Hernández M., Yagüe-Guirao G., Valero-Guillén P.L., Aviles-Plaza F.V., Cabezas-Herrera J., Martinez-Lopez A., Martinez-Villanueva M., Noguera-Velasco J.A., Cantero-Cano E., Franco-Garcia A., Garcia-Serna A.M., Hernandez-Caselles T., Martin-Orozco E., Norte-Muñoz M., Cánovas Díaz M., de Diego Puente T., Pastor J.M., Sola-Martínez R.A., Esteban-Gil A., Fernández-Breis J.T., Alcántara M.V., Hernández S., López-Soler C. Exhaled volatilome analysis as a useful tool to discriminate asthma with other coexisting atopic diseases in women of childbearing age. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eriksson L., Byrne T., Johansson E., Trygg J., Vikstrom C. third ed. 2013. Multi- and Megavariate Data Analysis. [Google Scholar]
- 46.Dragonieri S., Quaranta V.N., Carratu P., Ranieri T., Resta O. The ovarian cycle may influence the exhaled volatile organic compound profile analyzed by an electronic nose. J. Breath Res. 2018;12(2) doi: 10.1088/1752-7163/aa9eed. [DOI] [PubMed] [Google Scholar]
- 47.Hall J.E. Endocrinology of the menopause. Endocrinol Metab Clin North Am. 2015;44(3):485–496. doi: 10.1016/j.ecl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santoro N., Epperson C.N., Mathews S.B. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44(3):497–515. doi: 10.1016/j.ecl.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzgerald J., Fenniri H. Cutting edge methods for non-invasive disease diagnosis using E-tongue and E-nose devices. Biosensors. 2017;7(4):59. doi: 10.3390/bios7040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.