Abstract
This is the first study reporting the presence of airborne nano-sized plastic particles in the bronchoalveolar lavage fluid (BALF) samples of patients undergoing diagnostic bronchoscopy. The results represent the plastic pollution content in the lower airways of the residents of Northern Europe. Airborne micro- and nanoplastic particles (MP/NPs) are widely dispersed worldwide and intrude on human organisms to various extents, with the respiratory tract being the first line of exposure. The amounts of inhaled MP/NPs, their fate in the human respiratory tract, and the effects on the health of human airways and other exposed organs remain largely unknown.
In this clinical study, human BALF samples were assessed by means of optical and transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy (TEM-EDX). Results show that MP/NPs levels vary in the interval of 0.14–12.8 particles per 100 ml of BALF and are present in all samples tested, mainly in a fragmented form. External pollution by MP/NPs was excluded by carefully choosing methodology and equipment. This finding is a timely addition of valuable information and stimulates further research into the biological effects of inhaled MP/NPs.
Keywords: Microplastic, Nanoplastic, Bronchoalveolar lavage fluid, Air pollution
Graphical abstract
1. Introduction
For many years now, a considerable amount of plastic waste has been deposited into the environment, which subsequently degrades into microplastics (MPs) [1]. MPs in the environment undergo mechanical erosion and photooxidation via interaction with ultraviolet rays, resulting in fragmentation to even smaller, nano-sized plastic fractions and shapes, such as fibers and films [2,3]. The degradation and aging of plastic are major pathways for the increase of MPs levels in all ecosystems, affecting air, water, and soil [4,5].
While much research on MPs to date has focused on marine ecosystems and is well documented, airborne MP/NPs have been increasingly found in the air [5], especially in urban areas [6], where the sources include tire wear, synthetic clothing material, degradation of plastic surfaces, aerosolization from waters, and industrial emissions [5,6]. It is important to point out that there has been a rapid increase in the number of studies [[7], [8], [9]] reporting on particulate matter-associated MPs with a diameter of less than 2.5 μm or 10 μm (PM2.5/PM10), responsible for transferring MPs to humans' respiratory tract. Moreover, 99% of the world's population lives in places where the World Health Organization air quality guidelines for PM2.5 and PM10 are not met. Rapid urbanization further increases human exposure to PM and subsequently MP/NPs. The toxic effects of inhaled MPs on human health increase as their size decreases. This is attributed to the fact that smaller aerosol particles that contain MPs penetrate human tissues more efficiently. Airborne MPs are not only a source of toxicity but also a highly adsorbent transportation vector for heavy metals, polycyclic aromatic hydrocarbons, dioxins, furans, and other air pollutants [10].
Research on microplastics in human samples is currently limited to a few regions of the world (48% in Asia and 44% in Europe). However, it is developing rapidly, and more studies are expected in the near future. The assessment of microplastics in a single human biological sample was the focus of most of the selected studies (85%), however, mostly in feces (30%) [11]. MPs were also found to accumulate in human lungs [12]. It was demonstrated in a simulation study that MPs, depending on their size, may reach all levels of the human respiratory tract [13]. It is also hypothesized that MPs can cause damage to the tissues of the human respiratory system depending on individual host responses and the nature of the particles [14]. MPs may accumulate in tissues and potentially induce local cytotoxicity, enhancing hosts’ immune responses and tumorigenesis [[15], [16], [17]]. Such notions require further clinical data-based proof. Although a post-mortem study on human lung tissue-associated MPs has shown the chemistry and geometry of the particles, there was no linkage between clinical data and MPs load [12]. To date, only three studies have reported evidence of the presence of microplastics, but not nanoplastics, in human BALF samples [[18], [19], [20]]. Research conducted by Lu et al. [18] and Qiu et al. (2023) in China has revealed the presence of MPs in all examined BALF samples from the population of Zhuihai city, by employing laser direct infrared spectroscopy (LDIR). Studies have reported that MPs were commonly found as fibers. Lu et al. [18] have shown that BALF samples from smokers contained a higher concentration of MPs compared to non-smokers, 25.86 and 13.37 particles/g, respectively. Qiu et al. [19] have reported the composition of MPs in BALF samples from never-smokers mostly diagnosed with lung cancer, where most concentrations of MPs ranged from 0.2 to 140.9 particles/g of BALF and were composed of polyethylene (86.1%) and poly(ethylene terephthalate) (7.5%) within the size range of 20–80 μm. The only testing of MPs from BALF samples conducted in Europe was carried out by Baeza-Martínez et al. [20] and involved the analysis of samples from the Spanish population. In contrast to Chinese studies, MPs were not detected in all samples analyzed. The mean concentration of MPs was 9.18 ± 2.45 particles/100 ml of BALF for microfiber shaped MPs and only 0.57 ± 0.27 particles/100 ml of BALF for particulate-shaped MPs. The average size of MPs was found to be 1730 ± 150 μm and it did not correlate with environmental, physiological, or clinical factors.
We believe the finding of the current study is the first evidence of nanoplastic particles in human BALF samples. Up until now, the presence of NPs has solely been identified in human peripheral blood samples through nanocytometry analysis [21]. A study by Sun et al. [22] has also shown blood uptake and urine excretion of ingested nanoplastics in animal models.
Many recent studies have identified the physical and chemical characteristics of MPs in BALF, but there have been no confirmatory results on the concentration levels and no synergy analysis with elements. These are crucial and necessary for assessing the dose-response relationship between the exposure to MPs and health effects and would be of extreme importance to clinicians, scientists, and policymakers. We hereby report that MP/NPs might be detected in BALF of the inhabitants of Northern Europe by means of a relatively new and powerful TEM-EDX methodology. The further impact of this and similar studies leads to the understanding that the airborne pathway of the impact of microplastics needs to be considered on a full scale and that wide environment and human biomonitoring measures are needed.
2. Materials and methods
2.1. Bronchoalveolar fluid (BALF) sample collection
2.1.1. Ethics declaration
The study has been approved by the Lithuanian Bioethics Committee (approval #2021/2-1308-786) in accordance with current guidelines and regulations. Informed consent was obtained from all participants.
De-identified human BALF samples were obtained from patients undergoing bronchoscopy at Vilnius University Hospital Santaros Clinics. The bronchoalveolar lavage was collected via standard fibrobronchoscopy procedure in an outpatient setting on 10 randomly selected patients. The site of BALF was selected depending on high-resolution chest computed tomography findings (targeting pathological findings). Segmental or subsegmental bronchi were obturated with a fibrobronchoscope distal ending, then 100 ml of saline was introduced into the affected area and then aspirated. BALF was then immediately transported to the laboratory for the characterization of MPs by optical and TEM microscopes. Participants were given a questionnaire to assess their possible exposure routes and other relevant circumstances. The questionnaire included inquiries about professional activities, residence area, age, smoking habits, use of public transportation, etc.
2.2. Analysis of human BALF samples by optical and TEM-EDX microscopy
After the samples were collected, they were taken to the laboratory for further examination. All samples were stored in glass tubes in a dark cabinet at room temperature. Prior to microscopic measurements, organic and inorganic contaminants were removed from the BALF samples through chemical digestion, density separation, and filtration methods, that is commonly known and used to isolate microplastics from various other samples [17]. To remove organic and inorganic matter from the BALF samples, acids, alkalis, and salts were used in various concentrations. The amount and concentration used varied depending on the amount of the sample, its purity, and the presence of foreign matter. In order not to damage the MPs contained in the BALF samples, minimal concentrations of acidic and alkaline substances and temperatures below 60 °C degrees were used. During the study, care was taken to avoid environmental pollution and to ensure the reliability of the experimental results. No foreign materials containing plastic (plastic utensils, dishwashing detergents, sponges, etc.) were used in the microplastic research laboratory. Only glassware was used in the experiments, which was washed three times with ultrapure water (Milli-Q water purification system) and dried in a closed drying cabinet before each use. All reagents used in the study were filtered through glass fiber filters (“Branchia”, diameter size of 47 mm, porosity of 1.6 μm). Laboratory surfaces (fuge, work surfaces) were thoroughly cleaned with 70% ethanol and Milli-Q water prior to each test. Personnel involved in sampling and laboratory work wore cotton gowns, masks, and plastic-free gloves.
The morphology, surface structure, shape, and elemental analysis of the isolated MPs in the BALF samples were measured using optical and TEM-EDX microscopes. Optical microscopy was used to quantify MPs (from 40 μm to 1 mm) and evaluate their shape and size. Significantly smaller particles, i.e., down to nanometers in size, were identified and their chemical composition was determined using a TEM-EDX microscope. Images and EDX spectra were acquired using a Tecnai G2 F20 X-TWIN (FEI, The Netherlands, 2011) microscope with a Schottky-type field emission electron source, a high-angle annular dark field (HAADF) detector, a single- and double-tilted sample holder, and an 11 MPix ORIUS SC1000B (Gatan) CCD camera. BALF samples with microplastic particles were mounted directly on a holey carbon-coated copper grid (Agar Scientific Ltd., Stansted, UK).
2.3. Quality assurance and quality control (QA/QC)
There are no standardized sampling and analysis protocols that have been developed for studies of this type. To ensure the quality of the results, a plastic-free approach was applied during the experiments, ensuring the reliability of the data collected. To assess possible plastic contamination during the bronchoscopy the blank measurements were done. The results were then normalized to the MPs’ values of 1.8–1.9 MP/100 ml of BALF found in the samples and were used as background data. Also, blank samples of laboratory air were measured to determine the potential effect of airborne MPs. The filters were kept open for 9 h and then examined under an optical microscope to determine whether there were any trapped unwanted particles or fibers. A few (up to 5) MP particles were detected, but all of them were of the fiber type and larger than 5 mm, which is outside the range selected for our study.
3. Results
The presence of MP/NPs in BALF samples was confirmed in all samples tested. Characteristics of 10 randomized patients who underwent bronchoscopy are presented in Table 1. Gender, age, living area, employment status, smoking habits, and a clinical indication for bronchoscopy were recorded. The study was composed of almost an equal number of males and females (40% female/60% male). The age range of the participants varied from 39 to 64 years for males and from 55 to 70 years for females. The place of residence of the study participants was noted (40% lived in rural areas, 60% - in urban areas) and their smoking habits were recorded (60% never smokers, 40% smokers). Rural area living style includes single-family detached houses, while city inhabitants live in multi-flat buildings. In this pilot study, occupational activities varied a lot. MPs were detected in all BALF samples and ranged from 0.11 ± 0.02 MPs/100 ml of BALF to 12.80 ± 0.64 MPs/100 ml of BALF with a mean value of 2.61 MPs/100 ml of BALF. The highest amount of MPs (12.80 ± 0.64 MPs/100 ml of BALF) was found in a 39-year-old non-smoking male donor, working in an administrative office and living in the countryside. In contrast, the lowest amount of MPs (0.11 ± 0.02 MPs/100 ml of BALF) was found in a 56-year-old male who smoked, worked in the construction industry, and lived in the city. Since the number of patients is not large, we cannot claim any correlations between the level of MP/NPs and diagnosis. However, the largest amount of microplastic particles, i.e., 12.80 ± 0.64 MP/ml, 5.74 ± 0.45 MP/ml, and 2.91 ± 0.13 MP/ml were found in samples from people who either had or were suspected of having tuberculosis or similar lung disease.
Table 1.
The main characteristics of study participants and the amount of MP/NPs detected.
| Participant Number | Sexa | Age in years | Employment | Living area | Smoking habits | Indication for bronchoscopy | MP/NPs/100 ml of BALF |
|---|---|---|---|---|---|---|---|
| P1 | M | 64 | Loader in warehouse | Rural area | No | Hemoptysis | 1.13 ± 0.05 |
| P2 | F | 55 | Cook | City | No | Lymphadenopathy | 0.52 ± 0.03 |
| P3 | M | 56 | Driver, Metalworks | Rural area | Yes | Focal infiltration | 5.74 ± 0.45 |
| P4 | M | 39 | Leader, office | Rural area | No | Tuberculosis suspected | 12.80 ± 0.64 |
| P5 | F | 56 | Sales, garden goods store | City | No | Bronchiectasis | 0.72 ± 0.04 |
| P6 | M | 63 | Hotel manager | City | Yes | Focal infiltration | 0.14 ± 0.01 |
| P7 | F | 70 | Packer at the factory | City | No | Bronchiectasis | 1.03 ± 0.06 |
| P8 | F | 62 | Nurse, hospital | City | No | Bronchiectasis | 1.22 ± 0.08 |
| P9 | M | 56 | Concrete mixer, Construction | City | Yes | Tuberculosis suspected | 0.11 ± 0.02 |
| P10 | M | 60 | Welder, Construction | Rural area | Yes | Tuberculosis suspected | 2.91 ± 0.13 |
Sex: M − Male, F - Female.
The size and shape distribution of the microplastic particles in BALF samples were determined by optical microscopy, considering length as the distance and longest part of the microplastic particle (Fig. 1). The width of the particles varied from 20 μm to 283 μm, with an average of 49 μm. Similarly, the length of the particles exhibited variability, ranging from 35 μm to 1020 μm with an average of 203 μm. About 4.47% of microplastic particles were larger than 500 μm and about 3.12% were larger than 300 μm. Via the optical analysis most of the microplastic particles, i.e., 92.41%, were found in the size range of 10–300 μm. These results are consistent with the study that determined the size distribution of microplastic particles in indoor and outdoor air samples. The results showed that the vast majority of microplastic particles in the air were smaller than 100 μm [23]. The size distribution of microplastic particles in Fig. 1A is also presented according to the place of residence of the participants, i.e., urban vs. rural area. Yet, no significant difference was found between the two groups.
Fig. 1.
Size (A) and shape (B) distribution of microplastic particles in BALF samples.
Based on the determined dimensions, all measured microplastic particles were classified according to their shape: fragment or fiber. Only particles with a length ten times the width were considered to be fibers. Other possible forms of microplastics, such as granules, film, or foam, when added together, accounted for less than one percent and are therefore not presented in the work. Fragments accounted for 84.42% of the total microplastic content, while fibers constituted the remaining 15.65% of the particles (Fig. 1B). The analysis also included the 25th to 75th percentile range for both fiber and fragment percentages. The range for fragments spanned from 76.39% to 94.87%, indicating the variability in fragment distribution within the sample. Similarly, the fiber percentage ranged from 5.13% to 23.71%.
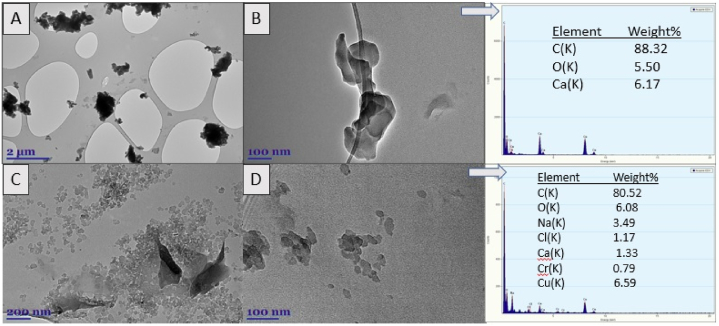
The presence and chemical composition of nanoplastic particles extracted from BALF samples and smaller than 1 μm were confirmed using the TEM-EDX microscopy (Fig. 2). Analysis of images from TEM showed that despite the relatively small amount of microplastic particles detected by optical microscopy, the amount of nano-plastic particles in BALF samples can be very high (Fig. 2A–D). Depending on the patient's information, occupation, and place of residency, various metals were identified in their BALF in addition to MPs. For example, patient #5, who worked in a garden supply store, had a Calcium (Ca) content of about 6% in his BALF sample. Calcium is commonly used in agriculture to reduce soil and water acidity. According to the EDX data, many different metals were found in minimal concentrations in the lungs of patient #9, who works in the construction industry, e.g., Na, Ca, Cr, Cu (3–6%).
Fig. 2.
TEM images (A–D) and EDX spectra (right panel) of NPs in human BALF samples (n = 10). Arrows indicate in which TEM image the EDX spectrum was measured.
4. Discussion
The presence of MPs in the human respiratory tract was demonstrated, i.e., fragments and fibers of MPs were detected [12,[18], [19], [20],24]. The findings of our study not only corroborate and extend upon existing knowledge but also contribute novel insights, as we are the first to report the evidence of both microplastics and nanoplastics in human BALF samples by employing optical and TEM-EDX microscopy. Our study confirmed the presence of MP/NPs in the lower respiratory tract of the residents of a Northern European country. We demonstrated that MP/NPs were present in the airways of all tested individuals. Similar results have been reported by Lu et al. [18] and Qiu et al. [19] in China, where all analyzed samples contained microplastics. Meanwhile, a study in South Europe by Baeza-Martínez et al. [20] has reported that 31.82% of participants had no MPs in their BALF samples.
Our results showed that MP/NPs levels vary in the interval of 0.11–12.8 particles per 100 ml of BALF. These values are close to the first findings reported by Baeza-Martínez et al. [20], with a mean of 9.18 ± 2.45 MPs/100 ml of BALF and, similar to our findings, no significant relationship with environmental, physiological, or clinical factors was identified. It should be noted that in the study by Baeza-Martínez et al., 97.06% of MPs were found in the form of microfibers, while only 5.88% with a mean of 0.57 ± 0.27 MPs/100 ml of BALF were particulate MPs, meanwhile, in our study particulate fragments accounted for 84.42% of the total microplastic content, while fibers constituted the remaining 15.65%.
The conventional methods used to detect MPs include Raman spectroscopy, μFTIR spectroscopy, and optical and fluorescent microscopy [12,18,24]. Among these techniques, Raman is more extensively employed than μFTIR, primarily due to its ability to identify particles smaller than 1 μm. Additionally, only a limited number of studies have employed advanced approaches like LDIR, a significant drawback of this method is its constrained capability to detect smaller sized (>20 μm) particles [11]. We have used a different detection method, i.e., TEM-EDX, which enabled nanoplastics detection and acquisition of elemental information. Other authors used μFTIR spectroscopy for the qualitative analysis of the organic and inorganic matter. However, despite all attention given to the field, the data remains scarce and additional methods are needed to confirm, facilitate and enhance the diagnostic capabilities of μFTIR.
The detection method employed in our study includes a minimally invasive sampling of human lower airways via bronchoscopy and the TEM-EDX investigation of BALF samples which is increasingly used by scientists and clinicians as a tool to study incoming airborne pollutants, including nonfibrous mineral particles, asbestos fibers, or to identify pathogenic infectious agents, cellular abnormalities, disbalance of immune components, molecular markers of microorganisms or biochemical processes. Compared to surgical or autopsy samples, BALF samples provide access to a significantly larger pool of patients, thereby enabling the detection of potential associations between pathologies or exposures.
Once in the lower airways, MP/NPs may be deposited in the alveoli, accumulate, and enter the bloodstream rather than degrade [25]. It is, therefore, important to timely detect and characterize intrabronchial MP/NPs. As we show in this study, MPs and NPs are present in the human lower airways and the majority of them are in fragmented, rectangular form. The amount of MP/NPs varied a lot among study participants. Further studies are needed to quantify airway MPs and to investigate possible pathogenetic consequences induced in the host tissues. So far, oxidative stress induction, microbiome shifts, and inflammatory responses were reported as MP-associated cellular events [26].
This study has several strengths. Due to the heterogeneous distribution of MPs in lung tissue, previous studies on lung tissue may have produced biased results. The BALF provides a more complete picture of the exposure to the MP/NPs in the lower respiratory tract. Patients with underlying respiratory diseases from the same region (Northern Europe, along the southeastern shore of the Baltic Sea), but different environments (urban and rural) were included in this study. As shown in Fig. 2, synergy analysis of MP/NPs and elements in BALF can reveal more insightful results.
The results of our study expand the current knowledge on microplastic presence in the human respiratory tract. It will also provide an incentive for scientists and clinicians to look for possible preventive strategies and advocate for cleaner air policies. Study participants include patients undergoing diagnostic bronchoscopy, i.e., coming with a spectrum of diagnoses, thus, few clinical implications can be preliminary inferred. The results of our study also underscore the importance of interdisciplinary collaboration in order to deliver concise and reliable results, where clinicians are equipped with reliable sampling tools, proper controls are taken, and clinical data is closely correlated with physical findings. Yet another implication of this study is additional information on how future in vitro studies should be designed to assess the effects of MP/NPs, i.e., particle size, form, concentration, and co-factors for in vitro exposure modeling.
Limitations of the study include a small number of participants and missing younger population data. The use of TEM-EDX microscopy for small particles is a powerful technique, but it has its own limitations. Quantification of small nanoparticles is not possible. Also, it requires careful sample preparation, and the results may be influenced by factors such as particle orientation and the presence of other materials in the sample. Despite that, the findings of our study significantly contribute to the existing understanding of microplastic presence within the human respiratory tract.
Furthermore, it is essential to continue researching the potential health impacts of MP/NPs to better understand their effects on respiratory and overall human health and to develop effective strategies to prevent the associated negative effects.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Authors contributions
IU performed the experiments; analyzed and interpreted the data; wrote the paper.
AV conceived and designed the experiments; performed the experiments; wrote the paper.
MS performed the experiments.
DB analyzed and interpreted the data; wrote the paper.
EB and VG conceived and designed the experiments; contributed reagents, materials, analysis tools or data.
RA and SB analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
All authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Grant # 01.2.2-LMT-K-718-03-0079 by Lithuanian Research Council.
References
- 1.Liu J., et al. Microplastic pollution in China, an invisible threat exacerbated by food delivery services. Bull. Environ. Contam. Toxicol. 2021;107:778–785. doi: 10.1007/s00128-020-03018-1. [DOI] [PubMed] [Google Scholar]
- 2.Sarinah Basri K., et al. Detection of exposure to microplastics in humans: a systematic review. Open Access Macedonian Journal of Medical Sciences. 2021;9:278–280. doi: 10.3889/oamjms.2021.6494. [DOI] [Google Scholar]
- 3.Enyoh C.E., et al. Microplastics exposure routes and toxicity studies to ecosystems: an overview. Environ Anal Health Toxicol. 2020;35:1. doi: 10.5620/eaht.e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul M.B., et al. Micro and nanoplastics: current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020;2:4350–4367. doi: 10.1039/D0NA00539H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 6.Kooi M., Koelmans A.A. Simplifying microplastic via continuous probability distributions for size, shape, and density. Environ. Sci. Technol. Lett. 2019;6(9):551–557. doi: 10.1021/acs.estlett.9b00379. [DOI] [Google Scholar]
- 7.Abbasi S., et al. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019;244:153–164. doi: 10.1016/j.envpol.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Wright S.L. Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ. Sci. Technol. 2019;53(15):8947–8956. doi: 10.1021/acs.est.8b06663. [DOI] [PubMed] [Google Scholar]
- 9.Rahman L., et al. Microplastics and nanoplastics science: collecting and characterizing airborne microplastics in fine particulate matter. Nanotoxicology. 2021;15(9):1253–1278. doi: 10.1080/17435390.2021. [DOI] [PubMed] [Google Scholar]
- 10.Rai P.K., et al. Adsorption of environmental contaminants on micro- and nano-scale plastic polymers and the influence of weathering processes on their adsorptive attributes. J. Hazard Mater. 2022;427 doi: 10.1016/j.jhazmat.2021.127903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutralam-Muniasamy G. Microplastic diagnostics in humans: "The 3Ps" Progress, problems, and prospects. Sci. Total Environ. 2023;856(Pt 2) doi: 10.1016/j.scitotenv.2022.159164. [DOI] [PubMed] [Google Scholar]
- 12.Amato-Lourenço L.F., et al. Presence of airborne microplastics in human lung tissue. J. Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126124. [DOI] [PubMed] [Google Scholar]
- 13.Vianello A., et al. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019;9:8670. doi: 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prata J.C. Airborne microplastics: consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Agullo A., et al. Airborne microplastic particle concentrations and characterization in indoor urban microenvironments. Environ. Pollut. 2022;308 doi: 10.1016/j.envpol.2022.119707. [DOI] [PubMed] [Google Scholar]
- 16.Campanale C., et al. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Publ. Health. 2020;17:1212. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amato-Lourenço L., et al. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu W., et al. New evidence of microplastics in the lower respiratory tract: inhalation through smoking. Environ Sci Technol. 13. 2023;57(23):8496–8505. doi: 10.1021/acs.est.3c00716. [DOI] [PubMed] [Google Scholar]
- 19.Qiu L., et al. Evidence of microplastics in bronchoalveolar lavage fluid among never-smokers: a prospective case series. Environ Sci Technol. 14. 2023;57(6):2435–2444. doi: 10.1021/acs.est.2c06880. [DOI] [PubMed] [Google Scholar]
- 20.Baeza-Martínez C., et al. First evidence of microplastics isolated in European citizens' lower airway. J. Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129439. [DOI] [PubMed] [Google Scholar]
- 21.Salvia R., et al. Fast-screening flow cytometry method for detecting nanoplastics in human peripheral blood. MethodsX. 2023;10 doi: 10.1016/j.mex.2023.102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun W., et al. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci Total Environ. 20. 2022;848 doi: 10.1016/j.scitotenv.2022.157639. [DOI] [PubMed] [Google Scholar]
- 23.Yichun X., et al. Inhalable microplastics prevails in air: exploring the size detection limit. Environ. Int. 2022;162 doi: 10.1016/j.envint.2022.107151. [DOI] [PubMed] [Google Scholar]
- 24.Jenner L.C., et al. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- 25.Law B.D., et al. Solubility of polymeric organic fibers and manmade vitreous fibers in gambles solution. Inhal. Toxicol. 1990;2:321–339. doi: 10.3109/08958379009145261. [DOI] [Google Scholar]
- 26.Zha H., et al. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice. Chemosphere. 2023;310 doi: 10.1016/j.chemosphere.2022.136764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.