Abstract
Ecological studies searching for drivers of biodiversity variation have frequently focused on taxonomic richness. However, more aspects of biodiversity, namely diversity facets can be considered to properly assess biotic-environment relationships. Here, we explore the environmental factors that could control the four biodiversity facets of aquatic Coleoptera from 93 regionally sampled Patagonian ponds. We also explore which are the ponds with high diversity values of all facets to prioritize them with a high conservation value. We fitted generalized additive models (GAM) to test relationships among environment (i.e., local and climatic variables) and aquatic beetles diversity facets (i.e., richness (SD), functional diversity (FD), phylogenetic diversity (PD), and local contribution to local beta diversity (LCBD). Climatic drivers were the most important predictors of beetle diversity facets, which exhibited linear and nonlinear responses. Thus, ponds from warmer Patagonia exhibited the highest values of SD and PD, whereas LCBD also peaked on colder sites suggesting that ponds under extreme temperatures sustain unique beetle assemblages. Moreover, ponds located in areas with higher precipitation variability exhibit the highest values of LCBD (i.e., unique assemblages). This result in addition to arid conditions in Patagonia prevailing since 16 m.y.a made us think that Patagonian beetle pond-dwellers are basally adapted to aridity. We calculated an index that summarizes the four facets patterns, to assign high conservation value to those ponds with higher index values. The relative importance of each facet varies from pond to pond. Hence, this multifaceteded approach not only allows us to identify priority areas for biodiversity conservation but also focuses on the importance of including multiple facets to understand biodiversity spatial patterns.
Keywords: Aquatic Coleoptera, Functional diversity, LCBD, Patagonia, Phylogenetic diversity, Traits
1. Introduction
Understanding the complex patterns of freshwater biodiversity variation could bring key clues to disentangle their potential drivers, having implications for their conservation [[1], [2], [3]]. Traditionally, studies focusing on the ecological factors that determine biodiversity variation across space have been based on taxonomic diversity, either considering species richness (i.e. presence-absence data) and/or their relative abundance. However, taxonomic diversity alone brings a limited notion of community structure, assuming that each taxon is equally distinct, and ignoring that their functional roles and evolutionary histories could determine the interactions among them and with the ecosystems [[4], [5], [6]].
Freshwater ecologists have recently drawn their attention to two additional facets of diversity: functional and phylogenetic diversity e.g. Refs. [[7], [8], [9]]. Functional diversity takes into consideration the value and range of organism traits that influence their performance and ecosystem functioning [3]. Phylogenetic diversity refers to the evolutionary relationships among species and therefore reflects their evolutionary histories, thus, sites with identical taxonomic diversity can be different in their phylogenetic diversities [4,10]. Another aspect of diversity that is growingly taken into consideration by researchers is the Local Contribution of Beta Diversity (LCBD), a metric developed from the partitioning of beta diversity by Legendre and De Cáceres[11], where the derived indices are comparative indicators for the ecological uniqueness of each site [12]. LCBD indices may allow the detection of high-priority conservation areas since higher values of LCBD may correspond to sites with an unusual combination of species and/or environmental variables or rare species [12,13]. Therefore, considering these four facets of biodiversity brings a more accurate picture of community assembly, revealing the underlying mechanisms of biodiversity maintenance along spatial and temporal gradients[14], and leading to the identification of sites with high conservation values.
Since macroinvertebrates play a pivotal ecological and functional role in every aquatic ecosystem, the study of their assemblages through multiple-facet diversity approaches could reflect the biodiversity status of the entire ecosystem [10]. There is strong evidence that macroinvertebrate taxonomic and functional alpha diversities may be more strongly influenced by local environmental variables than regional ones [2,3,15]. The environment could select those species featuring a set of traits that allow them to occur in a habitat with particular environmental conditions and “filter” those species that do not meet these requirements. Phylogenetic alpha diversity, on the other hand, seems more complex to understand since it would depend on the spatial scale analyzed. At small spatial scales, the phylogenetic diversity responses to the environment seem to be similar to functional diversity [2] but could be disproportionately affected by spatial factors at larger scales[2,3]. On the other hand, the association between macroinvertebrate LCBD and environmental predictors is not fully understood since studies aiming to answer that query are still scarce. However, there are a few contributions that found no relation [16] or some degree of linking between the environment and LCBD depending on the scale[13].
Among freshwater macroinvertebrates, water beetles are good surrogates of overall community patterns, hence excellent candidates to test ecological questions [17]. Aquatic Coleoptera is one of the most diverse orders of aquatic macroinvertebrates, exhibiting a wide morphological, functional, and ecological range; they are relatively easy to sample, and their taxonomy and biogeography are reasonably well-known [18]. Many studies have demonstrated that water beetles respond to variation across space and feature complex diversity patterns in freshwater ecosystems [1,[19], [20], [21], [22], [23], [24], [25]], but relationships within the four diversity attributes and their variation across wide spatial gradients have not been deeply studied.
Argentinian Patagonia is a very interesting region to test general ecological and biogeographical hypotheses, where freshwater studies at a regional scale are still incipient. The region is located between southern latitudes 35° and 55°, with complex geomorphology, an exponential west-east decreasing precipitation, strong and constant westerly winds, a wide variety of soils (i.e. entisols, aridisols, mollisols), and vegetation types (i.e. humid temperate forests, semideserts, shrub steppes, shrub-grass steppes, grass-shrub steppes) [[26], [27], [28], [29]]. The particular Patagonian topography and climate produce a redistribution of water that forms water meadows, called “mallines” by local inhabitants. These mallines are significant components of Patagonian ecosystems, sustaining a diverse fauna and flora, the regional economy based on livestock breeding, and other ecosystem services such as water supply, agricultural irrigation, and carbon sinks [[30], [31], [32], [33], [34]]. Across this heterogeneous region, the ponds generated by mallines (referred to as “ponds” hereafter) provide an excellent environment to examine the variation of multiple diversity facets and compare the outcoming patterns with other regions.
In this study, we used water beetles to explore the factors driving multiple facets of their diversity along a regional spatial scale. The objectives of this work were: 1) to compare the patterns within diversity facets, identifying those ponds with consistently high diversity values as priority sites for conservation; 2) to describe how different diversity facets of water beetle communities vary from north to south and across the regional aridity gradient; 3) to determine which are the main environmental controls of each diversity facet. We hypothesized that: (H1) all diversity facets are related to latitude, but their patterns also depend on aridity. To test this hypothesis, we will first test across facets relationships, and then their potential relationships with latitude and aridity (i.e., first and second objectives). We also hypothesized that: (H2) local environmental variables are the main drivers of the diversity variation of the water beetle communities from Patagonian ponds. We expect that local environmental conditions will more strongly influence taxonomic and functional diversity rather than phylogenetic diversity and LCBD.
Even though many contributions have studied macroinvertebrate community patterns of Patagonian ponds, a few have addressed the variation of biodiversity four facets. Furthermore, understanding the interactions between facets could bring valuable information to have a notion of the importance of ponds in terms of conservation priority. For this reason, the relevance of this study lies in the fact that we studied four facets of biodiversity and point to summarize this information in an index to detect ponds of high priority in terms of conservation.
2. Materials and methods
2.1. Study area
Argentinian Patagonia is an extensive area located in southern South America that occupies approximately 780,000 km2. From east to west, this region extends from the Atlantic Ocean to the Andes mountains. The Andes ultimately determine the heterogeneous landscape of Argentinian Patagonia since act as a barrier for humid air masses coming from the Pacific Ocean that discharges the humidity in the western flank establishing a strong west-east precipitation gradient and modeling two main phytogeographical provinces: a thin Sub-Antarctic Forest strip along the Andes Mountains; and the dominant Patagonian Steppe. Most of the region could be considered dryland (86.7%) based on the aridity index. These drylands are classified as arid, semiarid, and dry subhumid [29].
We sampled 93 ponds distributed from the northern 37°50′S to the southern 54°52′ Patagonia (Fig. 1). Each pond was sampled once during summer (late December to early January), between 2006 and 2018, distributed as follows: 10 in 2006, 16 in 2007, 6 in 2011, 55 in 2014, and 6 in 2018. We used a handheld GPS (Garmin Etrex 10) to obtain geographic coordinates and the altitude of each pond and checked later using the elevation model of Google Earth Pro software.
Fig. 1.
Map of the study area in Argentine Patagonia showing the location of the 93 sampled ponds.
2.2. Water beetle collection and identification
We used a D-frame net to sample the aquatic beetles. We swept the net horizontally (1.5 m) from 12 to 32 times (depending on the pond's area) from the margins to the middle part of the pond see Ref. [1]. We then pooled the content of each sweep into one composite sample.
Beetles were fixed in the field with 5% formalin, sorted at the laboratory, and stored in 70% ethyl alcohol. Specimens were sorted and identified at the possible lowest taxonomic level following regional taxonomic keys and original descriptions; we use this source of information either to reach to family or genus level [[35], [36], [37], [38], [39]] or species level [[40], [41], [42], [43], [44], [45], [46], [47], [48]]. By considering the area of the D-frame net and the distance covered in the water, we express pond invertebrate abundance as the number of individuals per unit volume (ind m−m) [1].
2.3. Predictor variables
To test our hypotheses and accomplish our objectives we consider local environmental and climatic variables. Within the first ones, we measured wetland aerea (Garmin Etrex 10 or Google Earth Pro software), and mean depth (calibrated stick). For each pond, we also registered water temperature, conductivity, total dissolved solids, salinity, pH, dissolved oxygen, and percentage of saturation using multiparameter probes (Hach Sension 156). We collected water samples and then frozen, for later analysis of total phosphorus and total nitrogen in unfiltered water samples, and soluble reactive phosphorus, nitrate + nitrite, and ammonium in field-filtered water samples (Sartorius, cellulose acetate filter). We visually estimated the percentage cover of the plants, including both emergent and submerged species (referred to as aquatic plant cover). We then focused on climatic variables. We obtained mean annual precipitation (mm), maximum temperature of the warmest month (°C) (maxT), minimum temperature of the coldest month (°C), precipitation seasonality (i.e., coefficient of variation that provides a percentage of precipitation variability where larger percentages represent greater variability of precipitation, PSE) and mean annual wind speed (m/s), using models available at WorldClim v.2 databases for the 1970–2000 period (1 km2 resolution; [49]. We got information on the aridity of each pond location from the Global Aridity Index Climate Database v2 (1 km2 resolution [50]).
2.4. Response variables
We calculated four facets of diversity: taxonomic richness (SD), functional diversity (FD), phylogenetic diversity (PD), and local contribution to beta diversity (LCBD). Taxonomic richness was calculated as the number of different taxa found in each pond, thus using presence-absence data. We calculated FD, PD, and LCBD using beetle abundance data. All facets were calculated and then analyzed using the R software v.4.1.0 [51] and RStudio software [52]. To calculate functional diversity we first selected adequate functional traits related to distributional patterns and environmental filtering to test our hypotheses. Following Schmera et al. [53], we set 47 traits in 15 trait groups (Table S1). Using the trait coding methodology of Schmera et al. [ [54]] as a guide, we used a fuzzy code to codify each trait state. This is, we coded each trait with an integer that ranges from 0 (low affinity) to 3 (high affinity) reflecting the relative affinity of a taxon to a particular trait (see [54]). The affinity values were set by three of the authors (MA, LBE, and NMR) who are familiar with the Patagonian aquatic beetle fauna; we also consulted literature to be sure of the importance of some traits [[35], [36], [37],46,55,56]. We then calculated Rao's quadratic entropy (RaoQ) (hereafter referred to as FD) as a measure of functional diversity using the dbFD function of the ‘FD’ package[57].
We used delta taxonomic distinctness based on the Linnaean hierarchy (see Table S2) as a surrogate for true phylogenetic information [58,59] because of the lack of available phylogenetic reconstructions that cover all the species of aquatic Coleoptera considered in this study. To calculate this index, we first estimated mean pairwise distances among species and then calculated the delta taxonomic distinctness (hereafter referred to as Phylogenetic Diversity, PD), using the taxa2dist and taxondive functions of the ‘vegan’ package [60]. Although relevant information may be lost using taxonomy hierarchy instead of true phylogenies, some studies have demonstrated that taxonomic distinctness could be a reliable proxy of phylogenetic diversity when there is no data of truly phylogenetic relationships among taxa [61,62]. We calculated the LCBD index using the R package ‘adespatial’ [63]. The LCBD index assesses the degree of uniqueness in assemblage composition from each pond, with higher values indicating a higher singularity value for each site.
2.5. Multifaceted index
To assess which are the higher-priority conservation ponds we calculate a named index “Multifaceted index” (MF), that takes into account all four facets of biodiversity as follows: we first scale them from 0 to 1 dividing all values of a particular diversity facet to each pond by the highest value of that attribute. Then we proceeded to sum the four values and scaled the outcome value from 0 to 1. Based on index results, we considered four pond conservation priorities as follows: high-priority (>0.75); moderate-priority (0.75–0.5); low-priority (0.5–0.25); and non-critical-priority (<0.25).
2.6. Statistical analyses
In order to explore potential relationships among response variables (i.e., diversity facets), we performed single non-parametric correlations tests (Spearman rank) using ‘corrplot’ package [ [64]]. We used a significance level of 0.05. We also performed a one-factor analysis (Table S3) of the variance (ANOVA) to test if the contribution of each facet to the MF index was similar.
We performed generalized linear models (GLMs) for modeling the effect of latitude and aridity on diversity facets (Hypothesis H1). The response variable taxonomic richness (SD) was modelled assuming a Poisson distribution and log link function. Whereas, functional diversity (FD), with a Binomial distribution and logit link. Gaussian distribution with an identity link and a Gamma with a log link were used for phylogenetic diversity (PD) and local contribution to beta diversity (LCBD), respectively. To test the second hypothesis (H2), we fitted another set of generalized additive models (GAMs). We analyzed the effects of environmental predictors on the diversity facets mentioned above. Collinearity between environmental variables dissolved oxygen, total phosphorus, total nitrogen, aridity, the maximum temperature of the warmest month, minimum temperature of the coldest month, precipitation seasonality, and mean annual wind speed, was checked using a nonparametric rank correlation and a threshold of r = ±0.7. An automatic backward step-wise approach was applied for model selection using the command “drop1”. Residual plots were examined for model validation following the protocol described by Zuur et al. [65]. Modeling was performed using ‘lme4’ [66], ‘mgcv’ [67] and ‘ggplot2’ [68] R packages.
3. Results
3.1. General patterns of aquatic beetles
The aquatic Coleoptera assemblages of the 93 studied ponds included 28 species in 9 families and 18 genera (Table S2). The most frequent families were Dytiscidae and Hydrophilidae, followed by Haliplidae (74, 67, and 14 ponds, respectively). Dytiscids and hydrophilids were also dominant in most sites, showing higher mean densities (70.86 ind/m3 and 21, respectively); the other aquatic Coleoptera mean densities were below 3 ind/m³. Local richness per pond ranged between 0 and 10, with 6 ponds lacking water beetles and 24 ponds having 5 or more taxa. Lancetes sp., Tropisternus setiger, and Liodessus patagonicus were the most frequent (74, 54, and 50 ponds, respectively) and abundant taxa (mean relative abundance of 37.87%, 18.75%, and 16.33%, respectively).
3.2. Diversity facets patterns and Multifaceted index
SD, FD, and PD were positively and moderately to strongly correlated with each other (Fig. S1), and LCBD was weakly and negatively correlated with SD (r = −0.28; p < 0.05). SD was significantly and positively correlated with both FD and PD (r = 0.68 and r = 0.58, respectively; p < 0.05), and the two latter metrics were correlated among them (FD and PD: r = 0.8; p < 0.05).
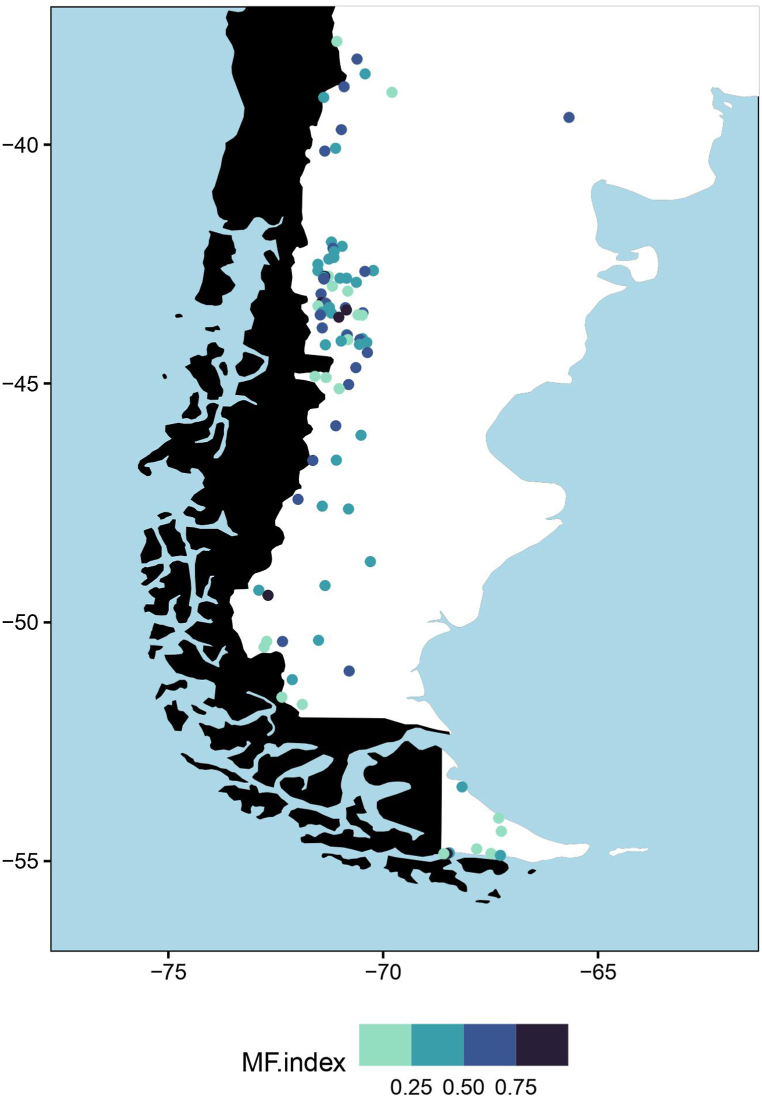
From the multifaceted index, we identified 13 high-priority ponds (index value > 0.75), 39 moderated-priority ponds (index value 0.50–0.75), 33 low-priority ponds (index value 0.25–0.50) and 8 non-critical ponds (index value < 0.25) (Fig. 2). All diversity facets contributed differently to each high-priority site: SD contribution varied from 9 to 35%, FD from 23 to 42%, PD from 18 to 32%, and LCBD from 13 to 40% (Fig. S2). Nevertheless, the mean proportional contribution of each facet to the overall Multifaceted index was similar (F = 2.58, p = 0.06, Table S3).
Fig. 2.
Map of the study area showing the values of Multifaceted index (MF index) corresponding to each pond. Colors show the index values range and pond conservation priority category: dark blue = 1.00–0.75, high-priority; blue = 0.75–0.50, moderated-priority; light-blue = 0.50–0.25, low-priority; light green = 0.25–0.00, non-critical priority.
The GLM models did not retain any predictor, and no significant relationship between neither latitude nor aridity and diversity facets (i.e., SD, PD, FD, LCBD) was detected (Table S4).
From the GAM models, only three predictors out of eight were retained (Fig. 3, Table S5). There was a non-linear relationship between SD, PD, and the climatic predictor maxT (p < 0.05; p < 0.001). According to these models, SD increased linearly with maxT and remains constant above 22 °C (Fig. 3a), whereas the PD decreased until 11 °C approximately, and then displays two maximum values (19 °C and 24 °C; Fig. 3b). For both diversity measures, model uncertainty raised to warmer temperatures (Fig. 3). LCBD was explained by three main predictors (Fig. 3c), being linearly related with the climatic predictor precipitation seasonality and the local one dissolved oxygen (positively and negatively, respectively), and having a “U” shaped distribution with maxT, featuring two peaks at extreme temperatures: one near 0 °C and one near 30 °C, with lower values towards around 20 °C temperatures (i.e, assemblages are unique in colder and warmer ponds). Again, an increasing uncertainty trend towards higher temperatures was observed.
Fig. 3.
Relationships between water beetles and local environmental and climatic variables from 93 Patagonian ponds (each location is represented with a black tick mark over the x-axis). Graphical results for the effects of maximum temperature of the warmest month modelled with Generalized Additive Models (GAMs) on a) species richness and b) phylogenetic diversity. c) Graphical results for the effects of precipitation seasonality, maximum temperature of warmest month and dissolved oxygen modelled with GAMs on local contribution to beta diversity. The y-axes represent the estimated smoothing curve and the 95% confidence bands obtained from fitted GAMs. DO: dissolved oxygen. maxT: maximum temperature of the warmest month. PSE: Precipitation seasonality.
4. Discussion
4.1. Relationships between latitude, aridity, and biodiversity facets
We did not find any clear relation between latitude nor aridity and pond Coleoptera biodiversity, rejecting our first hypothesis (H1). As was stated by Heino [68], freshwater insects feature many latitudinal diversity patterns including positive, negative, and no relationship with increasing latitude and this variation seems to depend on the latitudinal extent and region-specific conditions [68,69], especially regarding local species biodiversity or alpha diversity. According to this author, a weak or no relationship between diversity facets and latitude could be explained by the high within-region variation of environmental variables across latitude. Ribera et al. [70] explored some of these issues. They studied richness patterns of aquatic beetles in ten European countries and found that species richness from lotic environments decreases with latitude whereas no clear pattern was observed in lentic habitats. Mainly, the authors attributed this outcome to the low geographical turnover and larger range size of lentic beetles which may indicate dispersal as being fundamental in explaining their respective species richness patterns. In line with previous explanations, the lack of a significant relationship between beetle diversity facets and latitude may be explained by Patagonian beetles good dispersal capacity and adaptability to harsh environments (i.e., drier or colder locations).
Aridity, a measure of west-east Patagonian precipitation decrease, did not explain the diversity patterns of pond aquatic beetles. This outcome contrast with other studies conducted at shorter spatial scales in Patagonian ponds, in which either beetles [71] or other macroinvertebrates[1,72] responded to water conductivity as an indirect measure of site aridity [ [31,33]]. Water conductivity in Patagonia is related to the exponential west-east precipitation gradient (i.e., increasing aridity towards the east)[27]. One possible explanation for the lack of a link between aridity and water beetle diversity is that the pond-dwellers Coleoptera lineages of Patagonia are basally adapted to arid conditions by displaying physiological mechanisms to face desiccation and salinity [73,74,75]. For example, Pallarés et al.[76], conducted an experimental study finding that regardless of the ability to cope with aridity, eight Lumetus species (Hydrophilidae) occurred in arid areas. Hence, the authors hypothesized that the studied Lumetus species have a high ancestral desiccation resistance that allows them to face aridity. Argentinian Patagonia has been highly dominated by arid conditions since the late Miocene (∼16 Mya)[75,77] therefore beetle ability to deal with salinity might have evolved as an adaptation to freshwater desiccation.
4.2. Ecological drivers of biodiversity facets
Our second hypothesis (H2) was only partially supported since dissolved oxygen was the unique local environmental predictor explaining a diversity facet (LCBD), and two climatic variables (i.e., the maximum temperature of the warmer month and precipitation seasonality) played important roles in predicting SD, PD, and LCBD (Fig. 3, Table S5).
We found a negative relationship between LCBD and dissolved oxygen (DO), meaning that sites with lower values of DO sustained unique assemblages of aquatic Coleoptera (Fig. 3c, right panel). As well-adapted pond-dwellers, the aquatic beetles of the sampled ponds have developed a wide variety of respiration strategies that may allow them to live in environments with low oxygen levels [71]. In most cases, the adult breathes via the retention of an air bubble stored under the elytra [78]. Among beetle larvae, scirtid (Coleoptera, Scirtidae), Tropisternus, and Enochrus larvae (Coleoptera, Hydrophilidae), have to reach water surface to restore air supply, but larvae of other species have thoracic and abdominal lateral cuticular projections with tracheae reaching in order to increase surface breathing area (i.e., Berosus spp. or Haliplus spp.) [79,80]. Hence, those ponds with high LCBD may have unique combinations of anoxic adapted taxa (like Liodessus patagonicus, Rhantus signatus (Dytiscidae), Haliplus subseriatus (Haliplidae), Enochrus sp., Tropisternus setiger (Hydrophilidae), Pseudomicrocara (Scirtidae), that are able to exploit these harsh environments.
Climatic variables maximum temperature of the warmest month (maxT) and precipitation seasonality (PSE) seem to play critical roles in most beetle diversity facets (FD excepted). Interestingly, maxT relationships with SD and PD were similar, tending to increase positively. Temperature affects not only various biological and physicochemical processes related to metabolism, growth, development, reproduction, and food availability for the aquatic fauna [81,82], but also determines many physical factors such as evapotranspiration and the availability of dissolved oxygen. Moreover, in general, water temperature enhances the biological activity and the rate of chemical reactions, favoring the coexistence of more species [3]. For instance, our results suggest that aquatic Coleoptera SD and PD are constrained by lower temperatures so in a scenario where temperatures are getting globally higher, these relatively good aerial dispersers might expand their distributions taking advantage of current colder locations (i.e., higher mountains and higher latitudes).
According to our model, maxT and LCBD relationship exhibited a “U” shaped form, where ponds from colder (near 0 °C) and warmer (near 30 °C) sites had higher values of the LCBD index (Fig. 3c, middle panel). This outcome suggests that ponds under extreme temperatures sustain unique assemblage compositions. Colder ponds (located in southern or montane Patagonia) would sustain low numbers of cold-adapted species that would be vulnerable to global warming due to the potential range shifts of other generalist species [83]. Moreover, assemblages from warmer ponds would be unique due to a distinctive combination of several species (i.e., higher taxonomic richness). In this line of thought, co-existing species of mild-temperature ponds are more similar to each other than ponds from higher and lower temperatures.
In line with previous studies [83,84], we found that precipitation seasonality was a secondary control of LCBD (Fig. 3c, left panel). Since, this climatic coefficient peaks in those areas where precipitation events are concentrated in time, our results indicate that ponds located in areas with high precipitation seasonality harbor species that would be adapted to desiccation. In particular, those beetles with high flying strength like dytiscids (i.e., Desmopachria, Laccophilus, Lancetes, Liodessus, Meridiorhantus, Rhantus), haliplids (i.e., Haliplus subseriatus) and the hydrophilids (i.e., Tropisternus) and to a lesser extent, the hydrophilids Hemiosus dejeanii and Enochrus tend to fly fleeing across ponds located in less predictable weathers. Even more, the strong adult sclerotization that minimizes cuticular transpiration, plus produced water as a product of the metabolization of fat reserves during aerial dispersal [76,85], suggest that these beetles would be resilient to the Patagonian aridization.
4.3. Relationships between facets and priority conservation ponds
We found that the four facets of biodiversity bring complementary information from Patagonian ponds. Recently published research [2,86] remark on the importance of understanding how diversity metrics interact with each other for planning conservation policies because of the difficulty of preserving all diversity facets simultaneously under limited resources (e.g., low budgets, limited equipment, few human resources, etc.). Our results show that SD, FD, and PD are positively correlated indicating, at first sight, that planning efforts could cover these aspects of diversity; the strong and positive correlation between FD and PD suggests some degree of evolutionary conservatism due to closely related species sharing the same functional traits, and it seems that the functional traits we selected have a phylogenetic signal [87]. LCBD, however, showed a statistically weak negative correlation with SD (i.e. the species richest ponds were the most similar in species structuring). Therefore, by conserving the most speciose ponds, we would preserve sites with the highest number of functional groups and evolutionary lineages, but we would be forgetting about ponds with unique combinations of species. To address this issue, we constructed the “multifaceted index” in an attempt to provide all the information from the four diversity facets. As the four biodiversity aspects have different contributions to each pond, a multifaceted approach would cover all angles.
We calculated the multifaceted index, assuming that the output would give a conservation value to each pond. Using this index, we identified 13 sites of high conservation priority in which it seems that all facets contribute to the index in a similar proportion. All of these sites are located in northwestern Patagonia, in Chubut Province, except for two sites which are located far south, one in southern Santa Cruz Province and one in Tierra del Fuego Province, near the australmost latitude in the South-American continent. These sites support between 10 and 3 species each and bring all together 24 species out of 30. Interestingly, only using beetle assemblages and four diversity metrics, our findings are in line with those from Epele et al. [34]. In that research, authors detected 10 high-priority conservation ponds including terrestrial plants and vertebrates, and aquatic macroinvertebrates. For this reason, the proposed multifaceted index approach could also be useful to identify priority areas for the conservation of other organisms.
Author contribution statement
Nicolás Martínez-Román: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Luis Epele: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Luz Manzo: Miguel Archangelsky: Contributed reagents, materials, analysis tools or data.
Marta Grech: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
NMR would like to express his gratitude to the National Research Council for a postdoctoral fellowship. This contribution was possible due to the partial financial support of grant R/8, No. 108/2013 UNPSJB. All authors were funded by CONICET. We also are grateful to INTA personnel for access and field assistance to some of the study sites, particularly V. Nakamatsu, G. Ciari, and W. Opazo. We also thank C. Brand, C.Y. Di Prinzio, Y. Assef, M. Grech, R. Suarez, A.M. Kutschker, and N. Nakamura for their assistance in the field and lab work. We thank the private landowners who gave us gate and road access. This work was partially supported by the project. We thank the National Parks and Provinces administrations for sampling permits. We deeply thank two anonymous reviewers for their valuable suggestions that considerably helped to improve this manuscript. https://www.freepikcompany.com/ This is contribution 173 to LIESA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19666.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Epele L.B., Brand C., Miserendino M.L. Ecological drivers of alpha and beta diversity of freshwater invertebrates in arid and semiarid Patagonia (Argentina) Sci. Total Environ. 2019;678:62–73. doi: 10.1016/j.scitotenv.2019.04.392. [DOI] [PubMed] [Google Scholar]
- 2.Hill M.J., Heino J., White J.C., Ryves D.B., Wood P.J. Environmental factors are primary determinants of different facets of pond macroinvertebrate alpha and beta diversity in a human-modified landscape. Biol. Conserv. 2019;237:348–357. doi: 10.1016/J.BIOCON.2019.07.015. [DOI] [Google Scholar]
- 3.Li Z., Jiang X., Wang J., Meng X., Heino J., Xie Z. Multiple facets of stream macroinvertebrate alpha diversity are driven by different ecological factors across an extensive altitudinal gradient. Ecol. Evol. 2019;9:1306–1322. doi: 10.1002/ECE3.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb C.O., Ackerly D.D., McPeek M.A., Donoghue M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Systemat. 2002;33:475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448. [DOI] [Google Scholar]
- 5.Cardoso P., Rigal F., Carvalho J.C., Fortelius M., Borges P.A.V., Podani J., Schmera D. Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components. J. Biogeogr. 2014;41:749–761. doi: 10.1111/JBI.12239. [DOI] [Google Scholar]
- 6.Saito V.S., Siqueira T., Fonseca-Gessner A.A. Should phylogenetic and functional diversity metrics compose macroinvertebrate multimetric indices for stream biomonitoring? Hydrobiol. (Sofia) 2014;7451 745:167–179. doi: 10.1007/S10750-014-2102-3. 2014. [DOI] [Google Scholar]
- 7.Heino J., Tolonen K.T. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017;62:2431–2444. doi: 10.1002/LNO.10577. [DOI] [Google Scholar]
- 8.Schmera D., Heino J., Podani J., Erös T., Dolédec S. Functional diversity: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia. 2017;787:27–44. doi: 10.1007/S10750-016-2974-5. [DOI] [Google Scholar]
- 9.Martini S., Larras F., Boyé A., Faure E., Aberle N., Archambault P., Bacouillard L., Beisner B.E., Bittner L., Castella E., Danger M., Gauthier O., Karp-Boss L., Lombard F., Maps F., Stemmann L., Thiébaut E., Usseglio-Polatera P., Vogt M., Laviale M., Ayata S.D. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021;66:965–994. doi: 10.1002/LNO.11655. [DOI] [Google Scholar]
- 10.Tolonen K.T., Vilmi A., Karjalainen S.M., Hellsten S., Heino J. Do different facets of littoral macroinvertebrate diversity show congruent patterns in a large lake system? Community Ecol. 2017;18:109–116. doi: 10.1556/168.2017.18.1.12. [DOI] [Google Scholar]
- 11.Legendre P., De Cáceres M. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol. Lett. 2013;16:951–963. doi: 10.1111/ELE.12141. [DOI] [PubMed] [Google Scholar]
- 12.Legendre P. Interpreting the replacement and richness difference components of beta diversity. Global Ecol. Biogeogr. 2014;23:1324–1334. doi: 10.1111/GEB.12207. [DOI] [Google Scholar]
- 13.Hill M.J., White J.C., Biggs J., Briers R.A., Gledhill D., Ledger M.E., Thornhill I., Wood P.J., Hassall C. Local contributions to beta diversity in urban pond networks: implications for biodiversity conservation and management. Divers. Distrib. 2021;27:887–900. doi: 10.1111/DDI.13239. [DOI] [Google Scholar]
- 14.Alahuhta J., Erös T., Kärnä O.-M., Soininen J., Wang J., Heino J. Understanding environmental change through the lens of trait-based, functional, and phylogenetic biodiversity in freshwater ecosystems. Environ. Rev. 2018;27:263–273. doi: 10.1139/ER-2018-0071. [DOI] [Google Scholar]
- 15.Heino J., Tolonen K.T. Untangling the assembly of littoral macroinvertebrate communities through measures of functional and phylogenetic alpha diversity. Freshw. Biol. 2017;62:1168–1179. doi: 10.1111/FWB.12934. [DOI] [Google Scholar]
- 16.Heino J., Grönros M. Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia. 2017;183:151–160. doi: 10.1007/s00442-016-3754-7. [DOI] [PubMed] [Google Scholar]
- 17.Ortega J.C.G., Geijer J., Bergsten J., Heino J., Herrmann J., Johansson F., Bini L.M. Spatio-temporal variation in water beetle assemblages across temperate freshwater ecosystems. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148071. [DOI] [PubMed] [Google Scholar]
- 18.Bilton D.T., Ribera I., Short A.E.Z. Water beetles as models in ecology and evolution. Annu. Rev. Entomol. 2019;64:359–377. doi: 10.1146/annurev-ento-011118-111829. [DOI] [PubMed] [Google Scholar]
- 19.Miserendino M.L., Archangelsky M. Aquatic Coleoptera distribution and environmental relationships in a large Patagonian river. Int. Rev. Hydrobiol. 2006;91:423–437. doi: 10.1002/iroh.200510854. [DOI] [Google Scholar]
- 20.Ribera I. In: Aquatic Insects: Challenges to Populations. Lancaster J., Briers R.A., editors. CAB International; Wallingford, UK: 2008. Habitat constraints and the generation of diversity in freshwater macroinvertebrates; pp. 289–311. [DOI] [Google Scholar]
- 21.Miserendino M.L., Archangelsky M., Brand C., Epele L.B. Environmental changes and macroinvertebrate responses in Patagonian streams (Argentina) to ashfall from the Chaitén Volcano (May 2008) Sci. Total Environ. 2012;424:202–212. doi: 10.1016/j.scitotenv.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 22.Valente-Neto F., Saito V.S., Siqueira T., Fonseca-Gessner A.A. Evidence of species sorting driving aquatic beetles associated with woody debris in a transitional region between Cerrado and Atlantic Forest biomes. Aquat. Ecol. 2016;50:209–220. doi: 10.1007/s10452-016-9569-0. [DOI] [Google Scholar]
- 23.Heino J., Alahuhta J., Ala-Hulkko T., Antikainen H., Bini L.M., Bonada N., Datry T., Erös T., Hjort J., Kotavaara O., Melo A.S., Soininen J. Integrating dispersal proxies in ecological and environmental research in the freshwater realm. Environ. Rev. 2017 doi: 10.1139/er-2016-0110. [DOI] [Google Scholar]
- 24.Heino J., Alahuhta J., Fattorini S., Schmera D. Predicting beta diversity of terrestrial and aquatic beetles using ecogeographical variables: insights from the replacement and richness difference components. J. Biogeogr. 2018;46:304–315. doi: 10.1111/jbi.13485. [DOI] [Google Scholar]
- 25.González-Córdoba M., Chará J., Zúñiga M., del C., Giraldo L.P., Ramírez Y.P. Sensibilidad de Elmidae (Insecta: Coleoptera) a la perturbación del hábitat y la calidad fisicoquímica del agua en ambientes lóticos de los Andes colombianos. Rev. Biol. Trop. 2020;68:601–622. [Google Scholar]
- 26.León R.J.C., Bran D., Collantes M., Paruelo J.M., Soriano A. Grandes unidades de vegetación de la Patagonia extra andina. Ecol. Austral. 1998;8:125–144. [Google Scholar]
- 27.Paruelo J.M., Jobbágy E.G., Sala O.E. Biozones of Patagonia. Ecol. Austral. 1998;8:145–153. [Google Scholar]
- 28.Premoli A., Aizen M., Kitzberger T., Raffaele E. In: La Situación Ambiental Argentina 2005. Brown A.D., Martínez Ortiz U., Acerbi M., Corcuera J., editors. Fundación Vida Silvestre Argentina; Buenos Aires, Argentina: 2006. Situación ambiental de los bosques patagónicos; pp. 279–301. [Google Scholar]
- 29.Gaitán J.J., Bran D.E., Oliva G.E. Encyclopedia of the World's Biomes. 2019. Patagonian Desert; pp. 163–180. [DOI] [Google Scholar]
- 30.Raffaele E. Susceptibility of a Patagonian mallín flooded meadow to invasion by exotic species. Biol. Invasions. 2004;6:473–481. doi: 10.1023/B:BINV.0000041560.33770.97. [DOI] [Google Scholar]
- 31.Chimner R.A., Bonvissuto G.L., Cremona M.V., Gaitan J.J., López C.R. Ecohydrological conditions of wetlands along a precipitation gradient in Patagonia, Argentina. Ecol. Austral. 2011;21:329–337. [Google Scholar]
- 32.Gaitán J.J., López C.R., Bran D.E. Vegetation composition and its relationship with the environment in mallines of north Patagonia, Argentina. Wetl. Ecol. Manag. 2011;19:121–130. doi: 10.1007/S11273-010-9205-Z. 2010 192. [DOI] [Google Scholar]
- 33.Epele L.B., Manzo L.M., Grech M.G., Macchi P., Claverie A., Lagomarsino L., Miserendino M.L. Disentangling natural and anthropogenic influences on Patagonian pond water quality. Sci. Total Environ. 2018;613–614:866–876. doi: 10.1016/j.scitotenv.2017.09.147. [DOI] [PubMed] [Google Scholar]
- 34.Epele L.B., Grech M.G., Manzo L.M., Macchi P.A., Hermoso V., Miserendino M.L., Bonada N., Cañedo-Argüelles M. Identifying high-priority conservation areas for Patagonian wetlands biodiversity. Biodivers. Conserv. 2021;30:1359–1374. doi: 10.1007/s10531-021-02146-2. [DOI] [Google Scholar]
- 35.Archangelsky M., Manzo V., Torres P.L.M., Michat M.C. In: Macroinvertebrados Bentónicos Sudamericanos. Sistemática Y Biología. Domínguez E., Fernández H.R., editors. Fundación Miguel Lillo; Tucumán, Argentina: 2009. Coleoptera; pp. 411–468. [Google Scholar]
- 36.Manzo V., Archangelsky M. In: Roig-Juñent S., Claps L.E., Morrone J.J., editors. vol. 3. Editorial INSUE-UNT, San Miguel de Tucumán; Argentina: 2014. Elmidae; pp. 487–499. (Biodiversidad de Artrópodos Argentinos). [Google Scholar]
- 37.Torres P.L.M., Archangelsky M. In: Roig-Juñent S.A., Claps L.E., Morrone J.J., editors. vol. 3. Editorial INSUE-UNT, San Miguel de Tucumán; Argentina: 2014. Hydraenidae; pp. 475–486. (Biodiversidad de Artrópodos Argentinos). [Google Scholar]
- 38.Benetti C.J., Michat M.C., Ferreira-Jr N. In: Thorp and Covich's Freshwater Invertebrates. Volume III. Keys to Neotropical Hexapoda. Hamada N., Thorp J.H., Rogers D.C., editors. Academic Press; United States/United Kingdom: 2018. Family Dytiscidae; pp. 539–560. [Google Scholar]
- 39.Clarkson B., Archangelsky M., Torres P.L.M., Short A.E.Z. In: Thorp and Covich's Freshwater Invertebrates. Volume III. Keys to Neotropical Hexapoda. Hamada N., Thorp J.H., Rogers D.C., editors. Academic Press; United States/United Kingdom: 2018. Family Hydrophilidae; pp. 561–576. [Google Scholar]
- 40.Bachmann A.O., Trémouilles E.R. El género Lancetes en la Argentina continental (Coleoptera, Dytiscidae) Physis Secc B. 1981;39:103–118. [Google Scholar]
- 41.Trémouilles E.R. El género Rhantus Dejean en la Argentina (Coleoptera, Dytiscidae) Physis Secc B. 1984;42:9–24. [Google Scholar]
- 42.Oliva A., Fernández L.A., Bachmann A.O. Sinopsis de los Hydrophiloidea Acuáticos de la Argentina (lnsecta, Coleoptera) Monogr del Mus Argentino Ciencias Nat. 2002;2:1–67. [Google Scholar]
- 43.Delgado J.A., Archangelsky M. Description of the larval stages of Gymnochthebius jensenhaarupi and phylogenetic analysis of the relationships with other species of the subfamily Ochthebiinae (Coleoptera: hydraenidae) Eur. J. Entomol. 2005;102:231–240. doi: 10.14411/eje.2005.036. [DOI] [Google Scholar]
- 44.Alarie Y., Michat M.C., Archangelsky M., Barber-James H.M. Larval morphology of Liodessus Guignot, 1939: generic characteristics, descriptions of five species and comparisons with other members of the tribe Bidessini (Coleoptera: Dytiscidae: Hydroporinae) Zootaxa. 2007:1–21. doi: 10.11646/zootaxa.1516.1.1. [DOI] [Google Scholar]
- 45.Michat M.C., Archangelsky M. Descriptions of larvae of Desmopachria babington (Coleoptera: Dytiscidae: hydroporinae): the D. Vicina sharp species group. Coleopt. Bull. 2007;61:264–276. doi: 10.1649/0010-065X. 2007)61[264:DOLODB]2.0.CO;2. [DOI] [Google Scholar]
- 46.Archangelsky M., Michat M.C. In: Roig-Juñent S.A., Claps L.E., Morrone J.J., editors. vol. 3. Editorial INSUE-UNT, San Miguel de Tucumán; Argentina: 2014. Haliplidae; pp. 467–473. (Biodiversidad de Artrópodos Argentinos). [Google Scholar]
- 47.Libonatti M.L. Unpublished Ph.D. thesis, UBA, Department of Biodiversity and Experimental Biology, Faculty of Sciences, Buenos Aires University; 2016. Morfología, sistemática, filogenia y bionomía de Scirtidae (Insecta: Coleoptera: Polyphaga) de la Argentina. [Google Scholar]
- 48.Balke M., Hájek J., Hendrich L. Generic reclassification of species formerly included in Rhantus dejean (Coleoptera, Dytiscidae, colymbetinae) Zootaxa. 2017;4258 doi: 10.11646/ZOOTAXA.4258.1.7. 91–100–91–100. [DOI] [PubMed] [Google Scholar]
- 49.Fick S.E., Hijmans R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/JOC.5086. [DOI] [Google Scholar]
- 50.Trabucco A., Zomer R.J. 2019. ‘Global Aridity Index and Potential Evapotranspiration (ET0) Climate Database V2’, CGIAR Consortium for Spatial Information (CGIAR-CSI), (January) p. 10.https://cgiarcsi.community/2019/01/24/global-aridity-index-and-potential-evapotranspiration-climate-database-v2/ Available at: [DOI] [Google Scholar]
- 51.R Core Team . R Foundation for Statistical Computing; Vienna: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ Available at: [Google Scholar]
- 52.RStudio Team . RStudio, Inc; Boston: 2021. RStudio: Integrated Development for R. [Google Scholar]
- 53.Schmera D., Podani J., Heino J., Erös T., Poff N.L.R. 2015. A Proposed Unified Terminology of Species Traits in Stream Ecology; pp. 823–830. [DOI] [Google Scholar]
- 54.Schmera D., Heino J., Podani J. Characterising functional strategies and trait space of freshwater macroinvertebrates. Sci. Rep. 2022;121 12:1–9. doi: 10.1038/s41598-022-16472-0. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archangelsky M., Rodriguez G., Torrers, Coleoptera (in press), in: Claps L. E., Roig-Juñent S., Morrone) J. J. (Eds.), hydrophiloidea. In: Biodiversidad de Artrópodos Argentinos, 5, Editorial INSUE - Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina.
- 56.Michat, M. C., Archangelsky, M. and Benetti, C. J. 2023.Coleoptera, Dytiscidae. In: Biodiversidad de Artrópodos Argentinos, Vol. vol. 5. (Eds. L. E. Claps, S. Roig-Juñent, and J. J. Morrone); Editorial INSUE - Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina..
- 57.Laliberté E., Legendre P., Shipley B. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1. 2014 doi: 10.1890/08-2244.1. 0-12. [DOI] [PubMed] [Google Scholar]
- 58.Warwick R.M., Clarke K.R. Taxonomic distinctness and environmental assessment. J. Appl. Ecol. 1998;35:532–543. doi: 10.1046/J.1365-2664.1998.3540532.X. [DOI] [Google Scholar]
- 59.Clarke K.R., Warwick R.M. A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001;216:265–278. doi: 10.3354/meps216265. [DOI] [Google Scholar]
- 60.Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., O'Hara R., Solymos P., Dray S., Bauman D., Guillaume B., Borcard D., Clappe S., Guenard G., 2020, Package, adespatial, Stevens M., Szoecs E., Wagner H., Barbour M., Bedward M., Bolker B., Borcard D., Carvalho G., Chirico M., De Caceres M., Durand S., Evangelista H., FitzJohn R., Friendly M., Furneaux B., Hannigan G., Hill M., Lahti L., McGlinn D., Ouellette M., Ribeiro Cunha E., Smith T., Stier A., Ter Braak C., Weedon J. 2022. Vegan: Community Ecology Package. R Package Version 2.https://CRAN.R-project.org/package=vegan 6-2. [Google Scholar]
- 61.Ricotta C., Bacaro G., Marignani M., Godefroid S., Mazzoleni S. Computing diversity from dated phylogenies and taxonomic hierarchies: does it make a difference to the conclusions? Oecologia. 2012;170:501–506. doi: 10.1007/S00442-012-2318-8/METRICS. [DOI] [PubMed] [Google Scholar]
- 62.Heino J., Alahuhta J., Fattorini S. Phylogenetic diversity of regional beetle faunas at high latitudes: patterns, drivers and chance along ecological gradients. Biodivers. Conserv. 2015;24:2751–2767. doi: 10.1007/s10531-015-0963-z. [DOI] [Google Scholar]
- 63.Dray S., Bauman D., Guillaume B., Borcard D., Clappe S., Guenard G. 2020. Package ‘adespatial. [Google Scholar]
- 64.Wei T., Simko V. 2021. R Package 'corrplot': Visualization of a Correlation Matrix. [Google Scholar]
- 65.Zuur A.F., Ieno E.N., Walker N.J., Saveliev A.A., Smith G.M. Springer; New York: 2009. Mixed Effects Models and Extensions in Ecology with R. [Google Scholar]
- 66.Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]; [66] Wood S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. Roy. Stat. Soc. 2011;73(1):3–36. [Google Scholar]
- 67.Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 68.Heino J. Biodiversity of aquatic insects: spatial gradients and environmental correlates of assemblage-level measures at large scales. Freshw Rev. 2009;2:1–29. doi: 10.1608/frj-2.1.1. [DOI] [Google Scholar]
- 69.Heino J. A macroecological perspective of diversity patterns in the freshwater realm. Freshw. Biol. 2011 doi: 10.1111/j.1365-2427.2011.02610.x. [DOI] [Google Scholar]
- 70.Ribera I., et al. Does habitat use explain large scale species richness patterns of aquatic beetles in Europe? Wiley Online Library. 2003;26(2):145–152. doi: 10.1034/j.1600-0587.2003.03271.x. [DOI] [Google Scholar]
- 71.Epele L.B., Archangelsky M. Spatial variation of water beetle communities in arid and semi-arid Patagonian wetlands and their value as environmental indicators. Zool. Stud. 2012;51:1418–1431. [Google Scholar]
- 72.Epele L.B., Miserendino M.L. Temporal dynamics of invertebrate and aquatic plant communities at three intermittent ponds in livestock grazed Patagonian wetlands. J. Nat. Hist. 2015;1–20 doi: 10.1080/00222933.2015.1062930. [DOI] [Google Scholar]
- 73.Greenwood M.T., Wood P.J. Effects of seasonal variation in salinity on a population of Enochrus bicolor Fabricius 1792 (Coleoptera: Hydrophilidae) and implications for other beetles of conservation interest. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003;13:21–34. doi: 10.1002/aqc.517. [DOI] [Google Scholar]
- 74.Pallarés S., Arribas P., Bilton D.T., Millán A., Velasco J., Ribera I. The chicken or the egg? Adaptation to desiccation and salinity tolerance in a lineage of water beetles. Mol. Ecol. 2017;26:5614–5628. doi: 10.1111/mec.14334. [DOI] [PubMed] [Google Scholar]
- 75.Hernández F., Ríos C., Perotto-Baldivieso H.L. Evolutionary history of herbivory in the Patagonian steppe: the role of climate, ancient megafauna, and guanaco. Quat. Sci. Rev. 2019;220:279–290. [Google Scholar]
- 76.Pallarés S., Millán A., Lobo J.M. Abraham Pérez |, david Sánchez-Fernández. Lack of congruence between fundamental and realised aridity niche in a lineage of water beetles. Freshw Biol. 2022:1–14. doi: 10.1111/fwb.13912. [DOI] [Google Scholar]
- 77.Trayler R.B., Kohn M.J., Bargo M.S., Cuitiño J.I., Kay R.F., Strömberg C.A.E., Vizcaíno S.F. Patagonian Aridification at the onset of the mid-miocene climatic optimum. Paleoceanogr Paleoclimatology. 2020 doi: 10.1029/2020PA003956. [DOI] [Google Scholar]
- 78.Batzer D.P., Boix D. Invertebrates in Freshwater Wetlands: an International Perspective on Their Ecology. Springer International Publishing; 2016. An introduction to freshwater wetlands and their invertebrates; pp. 1–23. [DOI] [Google Scholar]
- 79.Rodriguez G., Archangelsky M., Torres P.L.M. Description of immatures of Berosus decolor Knisch, 1924 (Coleoptera: Hydrophilidae: Berosini), with emphasis on chaetotaxy and morphometry. Zootaxa. 2015;3981:577–591. doi: 10.11646/zootaxa.3981.4.8. [DOI] [PubMed] [Google Scholar]
- 80.Michat M.C., Archangelsky M., Alarie Y. Morphology and chaetotaxy of neotropical Haliplus larvae (Coleoptera: Haliplidae) Rev. Mex. Biodivers. 2020;91 doi: 10.22201/IB.20078706E.2020.91.3541. [DOI] [Google Scholar]
- 81.Huryn A.D., Wallace B.J., Anderson N.H. In: An Introduction to the Aquatic Insects of North America. Merritt R.W., Cummins K.W., Berg M.B., editors. Kendall/Hunt Publishing Company; Dubuque: 2008. Habitat, life history, secondary production, and behavioral adaptations of aquatic insects; pp. 55–103. [Google Scholar]
- 82.Fernández-Cirelli A., Volpedo A.V. In: La bioindicación en el monitoreo y evaluación de los sistemas fluviales de la Argentina. Domínguez E., Giorgi A., Gómez N., editors. Eudeba; Buenos Aires, Argentina: 2020. Indicadores físico-químicos: ¿qué, cómo y cuánto reflejan la calidad del agua? pp. 27–39. [Google Scholar]
- 83.Epele L.B., Grech M.G., Williams-Subiza E.A., Stenert C., McLean K., Greig H.S., Maltchik L., Pires M.M., Bird M.S., Boissezon A., Boix D., Demierre E., García P.E., Gascón S., Jeffries M., Kneitel J.M., Loskutova O., Manzo L.M., Mataloni G., Mlambo M.C., Oertli B., Sala J., Scheibler E.E., Wu H., Wissinger S.A., Batzer D.P. Perils of life on the edge: climatic threats to global diversity patterns of wetland macroinvertebrates. Sci. Total Environ. 2022;820 doi: 10.1016/j.scitotenv.2022.153052. [DOI] [PubMed] [Google Scholar]
- 84.Mouton T.L., et al. Increasing climate-driven taxonomic homogenization but functional differentiation among river macroinvertebrate assemblages. Global Change Biol. 2020;26(12):6904–6915. doi: 10.1111/gcb.15389. [DOI] [PubMed] [Google Scholar]
- 85.Chapman R.F. Cambridge University Press; New York: 2013. The Insects: Structure and Function. [Google Scholar]
- 86.Cai Y., Zhang M., Xu J., Heino J. Geographical gradients in the biodiversity of Chinese freshwater molluscs: implications for conservation. Divers. Distrib. 2018;24:485–496. doi: 10.1111/DDI.12695. [DOI] [Google Scholar]
- 87.De Pauw K., Meeussen C., Govaert S., Sanczuk P., Vanneste T., Bernhardt-Römermann M., Bollmann K., Brunet J., Calders K., Cousins S.A.O., Diekmann M., Hedwall P.O., Iacopetti G., Lenoir J., Lindmo S., Orczewska A., Ponette Q., Plue J., Selvi F., Spicher F., Verbeeck H., Vermeir P., Zellweger F., Verheyen K., Vangansbeke P., De Frenne P. Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 2021 doi: 10.1111/1365-2745.13671. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.