Abstract
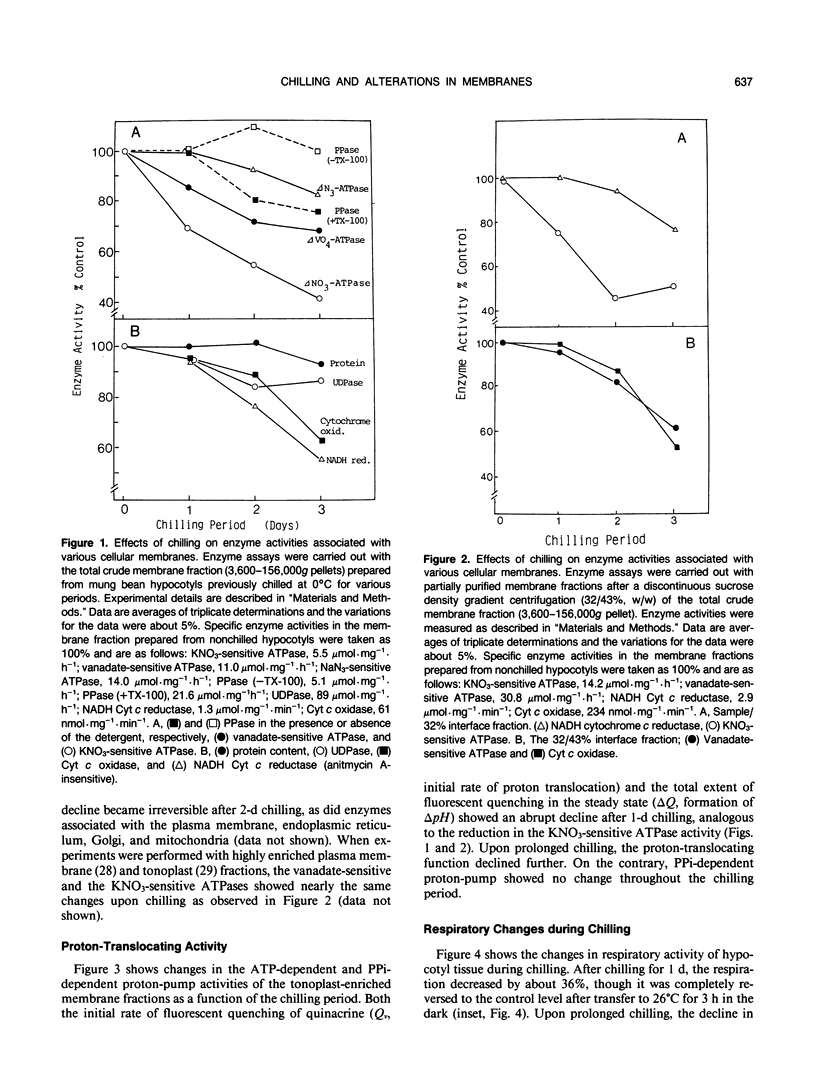
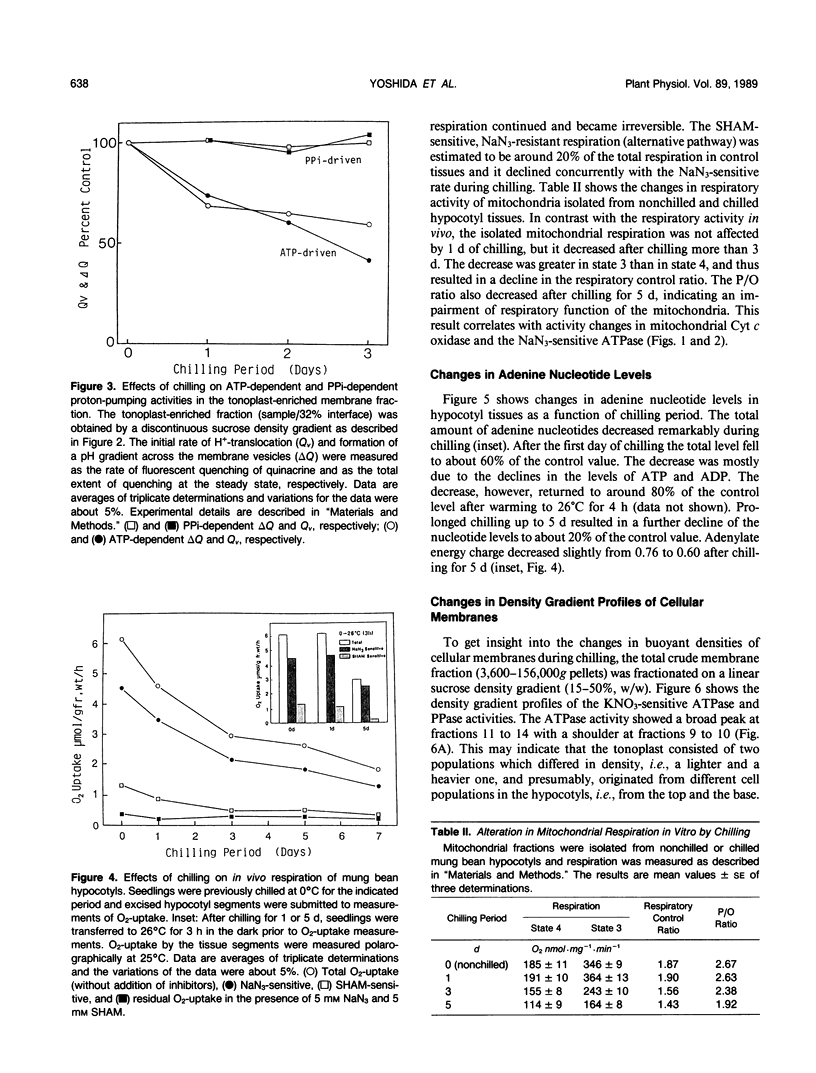
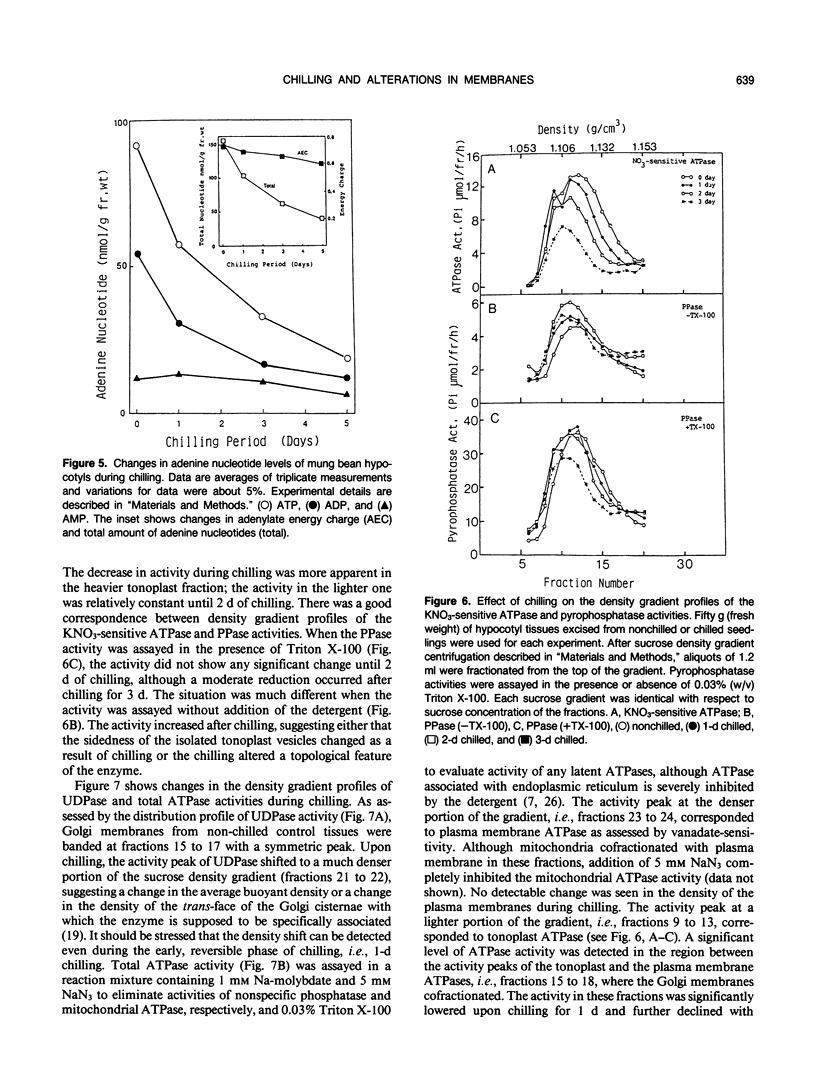
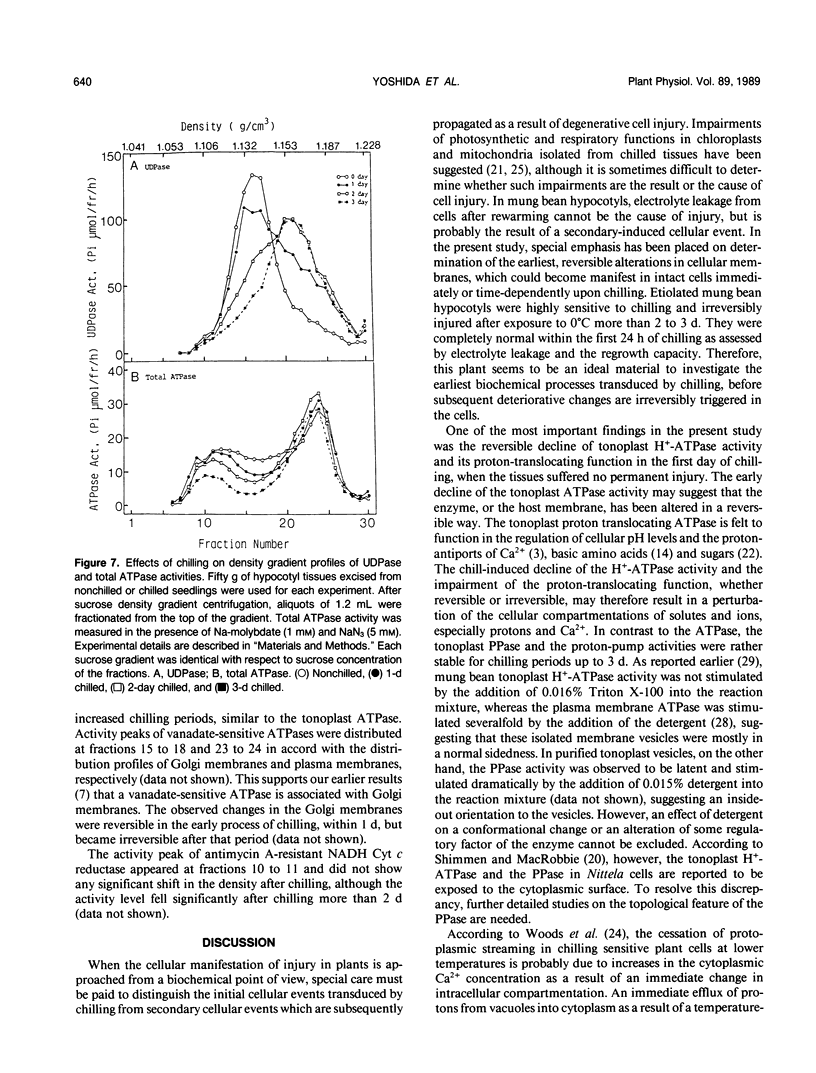
Biochemical alterations of cellular membranes in chilling-sensitive mung bean (Vigna radiata [L.] Wilczek) hypocotyls were investigated with reference to chilling injury. Reversible decreases in activities of tonoplast H+-ATPase and in vivo respiration became manifest within 24 hours of chilling when tissues suffered no permanent injury as assessed by electrolyte leakage and regrowth capacity. These changes were found to be the earliest cellular responses to chilling. A density-shift on a sucrose density gradient was observed in Golgi membranes early in the chilling treatment, suggesting that Golgi function and/or membrane biogenesis via the Golgi may have been altered upon chilling. After chilling more than 2 days, irreversible changes were generally produced in cellular membranes including the plasma membrane, endoplasmic reticulum, and mitochondria. Respiratory functions remained intact in mitochondria isolated from tissues prechilled for 24 hours, but were impaired after prechilling for 3 days. Given the important role of the tonoplast H+-ATPase in the active transport of ions and metabolites, the early decline in the tonoplast H+-ATPase activity may give rise to an alteration of the cytoplasmic environment and, consequently, trigger a series of degenerative reactions in the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball W. J., Jr, Atkinson D. E. Adenylate energy charge in Saccharomyces cerevisiae during starvation. J Bacteriol. 1975 Mar;121(3):975–982. doi: 10.1128/jb.121.3.975-982.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chanson A., Pilet P. E. Localization in sucrose gradients of the pyrophosphate-dependent proton transport of maize root membranes. Plant Physiol. 1987 Aug;84(4):1431–1436. doi: 10.1104/pp.84.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Lew R. R., Spanswick R. M. Characterization of Anion Effects on the Nitrate-Sensitive ATP-Dependent Proton Pumping Activity of Soybean (Glycine max L.) Seedling Root Microsomes. Plant Physiol. 1985 Feb;77(2):352–357. doi: 10.1104/pp.77.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Slack C. R., McPherson H. G. Plants under Climatic Stress: VI. Chilling and Light Effects on Photosynthetic Enzymes of Sorghum and Maize. Plant Physiol. 1974 Nov;54(5):696–701. doi: 10.1104/pp.54.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Properties of Plasma Membrane Isolated from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):152–160. doi: 10.1104/pp.80.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


