Abstract
Background
Hearing loss has been reported as the most significant modifiable risk factor for dementia, but it is still unknown whether auditory rehabilitation can practically prevent cognitive decline. We aim to systematically analyze the longitudinal effects of auditory rehabilitation via cochlear implants (CIs).
Methods
In this systematic review and meta-analysis, we searched relevant literature published from January 1, 2000 to April 30, 2022, using electronic databases, and selected studies in which CIs were performed mainly on older adults and follow-up assessments were conducted in both domains: speech perception and cognitive function. A random-effects meta-analysis was conducted for each domain and for each timepoint comparison (pre-CI vs. six months post-CI; six months post-CI vs. 12 months post-CI; pre-CI vs. 12 months post-CI), and heterogeneity was assessed using Cochran's Q test.
Findings
Of the 1918 retrieved articles, 20 research papers (648 CI subjects) were included. The results demonstrated that speech perception was rapidly enhanced after CI, whereas cognitive function had different speeds of improvement for different subtypes: executive function steadily improved significantly up to 12 months post-CI (g = 0.281, p < 0.001; g = 0.115, p = 0.003; g = 0.260, p < 0.001 in the order of timepoint comparison); verbal memory was significantly enhanced at six months post-CI and was maintained until 12 months post-CI (g = 0.296, p = 0.002; g = 0.095, p = 0.427; g = 0.401, p < 0.001); non-verbal memory showed no considerable progress at six months post-CI, but significant improvement at 12 months post-CI (g = −0.053, p = 0.723; g = 0.112, p = 0.089; g = 0.214, p = 0.023).
Interpretation
The outcomes demonstrate that auditory rehabilitation via CIs could have a long-term positive impact on cognitive abilities. Given that older adults’ cognitive abilities are on the trajectory of progressive decline with age, these results highlight the need to increase the adoption of CIs among this population.
1. Research in context
1.1. Evidence before this study
As the global population ages, the prevalence of dementia is rapidly increasing. With recent reports that hearing loss in midlife is the highest modifiable risk factor for dementia, clinical outcomes regarding the association between auditory rehabilitation and cognitive function are also being reported. However, a systematic evaluation on this has not yet been conducted; in particular, few studies have systematically analyzed the long-term effects of cochlear implants (CIs) on individuals with severe-to-profound hearing loss (SPHL) for whom conventional amplification (such as hearing aids) is not beneficial. A search of electronic databases from Jan 1, 2000 to April 30, 2022 derived a few meta-analyses investigating the impact of CIs on speech perception abilities, whereas it yielded no systematic studies analyzing the effect on cognitive abilities, except for one study with a small sample size and limited outcome measures. Furthermore, no studies have comprehensively analyzed longitudinal changes in speech perception and cognitive function via multiple timepoints comparisons.
1.2. Added value of this study
We here performed a systematic review and meta-analysis encompassing two different domains (speech perception and cognitive function) to evaluate the efficacy of CIs over time in each domain and to reveal the trajectory of interactions between the two domains. The results demonstrated that among the subtypes of cognitive function, executive function or verbal memory, which is directly associated with auditory processing, begins improving along with the rapid enhancement of speech perception after CI, whereas other relatively non-relevant cognitive functions (such as non-verbal memory) start to improve after the recovery of speech perception is to some extent progressed. Based on the findings, we speculate that as “listening effort” is reduced through auditory rehabilitation via CIs, the functional reorganization of the brain is induced and cognitive resources are re-allocated, thereby improving overall cognitive function.
1.3. Implications of all the available evidence
The current results revealed that auditory rehabilitation via CIs would be beneficial for preventing cognitive decline among an aging population. Although more than 60 million people worldwide currently suffer from SPHL, less than 5% of them have received care services through interventions such as CIs and regular hearing ability monitoring. Providing early intervention would contribute to reducing the global societal cost of diseases associated with cognitive decline such as dementia. Furthermore, considering that the relevant issues are especially prominent in low- and middle-income nations, it is crucial to establish a global health care policy and provide guidelines that include hearing care as an early intervention for neurodegenerative disease, commencing in midlife.
2. Introduction
Dementia, which causes a decline in cognitive function and is typically chronic or progressive, is becoming more prevalent as the global population ages. Approximately 55 million individuals worldwide have dementia, more than 60% of whom reside in low- and middle-income nations. This figure is anticipated to increase to 78 million by 2030 and 139 million by 2050 due to the increasing percentage of older people in almost every country [1]. Dementia causes not only a decline in intellectual abilities but also behavioral changes and a loss of personality, and results in emotional disorders such as anxiety and depression, which negatively affects the daily activities and social lives of individuals and significantly reduces their quality of life [2]. It also has enormous social and economic repercussions, both in terms of the direct costs of medical and social care and the indirect costs of informal care. In 2019, the estimated global societal cost of dementia was US$ 1·3 trillion, and as the number of individuals with dementia and the associated cost of care increase, these expenses are projected to reach US$ 2·8 trillion by 2030 [1].
Although dementia was formerly thought to be neither curable nor avoidable, considerable progress has been achieved in this regard. The Lancet International Commission on Dementia Prevention and Care has convened to integrate a new understanding about what can be done and what people should do to prevent and treat dementia [3,4]. The 2017 Commission provided a life-course model for possibly modifiable dementia risk factors. According to their report, hearing loss presented the highest value of the weighted population attributable fraction (9.1%), followed by hypertension (2%) and obesity (0.8%) as potentially modifiable risk factors for dementia in midlife (age 45–65) [3]. Their latest report shows that the attributable fraction changed as new risk factors were added, including traumatic brain injury and excessive alcohol consumption; however, hearing loss remained the greatest modifiable risk factor for dementia [4].
Midlife hearing impairment is associated with brain atrophy [5]. Several studies have reported that the degree of hearing loss is correlated with gray matter volume loss, particularly in the temporal lobe [[5], [6], [7]], and Lin et al. demonstrated that such volume loss accelerated over time in older adults with significant hearing loss compared to those with normal hearing [7]. This brain atrophy was observed not only in the superior and transverse temporal gyri, which are directly involved in auditory perception, but also in the regions surrounding them, such as the hippocampus, entorhinal cortex, and parahippocampal gyrus, which are involved in cognitive processing. These findings indicate that the deterioration of sensory input can cause long-term deprivation effects on auditory pathways, leading to structural decline and functional changes in the brain, which may explain the link between hearing impairment and cognitive decline [5].
Recent long-term follow-up studies have reported that the use of hearing aids (HAs) was beneficial and protective against cognitive decline [4,8]. In particular, Bucholc et al. demonstrated that in hearing-impaired individuals with mild cognitive impairment (MCI), the use of HAs delayed the transition to dementia [8]. These outcomes suggest that hearing loss treatment may reduce the risk of developing dementia. However, in terms of hearing rehabilitation, conventional amplification with HAs may bring little benefit to individuals with a high degree of hearing impairment, such as severe-to-profound hearing loss (SPHL). For these people, cochlear implants (CIs) are often offered as a useful option.
The CI is a surgically implanted device that electrically stimulates the auditory nerve and facilitates speech processing. In general, with increasing age, speech comprehension deteriorates as degeneration occurs in the peripheral and central auditory system [9]. This is a complex phenomenon caused by multiple reasons, including not only a shift in the audiometric threshold, but also weakened ability to process and integrate inputs from both ears [[9], [10], [11]]. In particular, the degradation of binaural processing that detects and processes interaural time and intensity differences for auditory signals arriving at both ears reduces speech segregation ability; this phenomenon is associated with the fact that speech perception in a noisy environment becomes more difficult with aging. Several research groups have indicated that CIs may be useful for aging populations, by demonstrating that CIs resulted in significant improvement in speech perception in adults with moderate-to-profound sensorineural hearing loss [[11], [12], [13], [14], [15]]. Such improvements in speech perception remained stable even after 10 years of follow-up [11].
More recently, several studies have examined the effects of hearing rehabilitation with CIs on cognitive function as well as on speech perception. Most of these studies have demonstrated that overall cognitive abilities could be improved with CIs through follow-up assessments [[16], [17], [18], [19], [20], [21]]. In particular, Mosnier et al. have shown that 32% of older adults who had MCI returned to normal cognition in a follow-up evaluation seven years post-CI, strongly suggesting that hearing rehabilitation in older adults can have a positive effect on neurocognitive functioning [17]. Although several studies have demonstrated the enhancement effect of cognitive abilities due to CIs, studies showing insignificant effects have also been reported [22]. Furthermore, recent studies that performed various cognitive tests capable of evaluating different subtypes of cognitive function have indicated that different outcomes might be derived depending on each subtype or assessment tool [17,19,[23], [24], [25], [26]]. As such, the effect of CIs on cognitive function is still controversial since the results have been found to vary with each study, subtype of cognitive function, or follow-up period after CI intervention. Therefore, a systematic analysis regarding this is required.
Considering that the association between auditory rehabilitation and cognitive function is a recent area of research interest, there are no standardized criteria for applying demographic factors of subjects, such as age, education level, baseline hearing and cognitive levels, or etiology. Thus, the recent studies, mainly conducted according to in-house criteria, demonstrated some variance. The sample size also varied from study to study. Concerning the neurocognitive test battery, various tests were employed in each study: some studies used multiple tests for in-depth assessment of different subtypes of cognitive function [17,[23], [24], [25], [26]], while others used only screening instruments for global function, which might have overlooked slight cognitive changes [16,18,21,27]. Consequently, the studies reported to date have quite different characteristics in terms of study group, study design, and the statistical evaluation of the results, and have some limitations regarding the difficulty including a control group (subjects with hearing loss who were followed-up without hearing rehabilitation) due to the nature of the research topic.
To systematically analyze the efficacy of CIs by considering differences in the characteristics of the studies, a meta-analysis including multiple studies could offer an alternative approach, although few such studies have been reported to date. However, even previously reported meta-analyses have primarily focused on comparing hearing-related abilities, such as speech perception, before and after CIs [14,28]. Very recently, a study investigating changes in cognitive function was published [29]; however, the number of studies included in the analysis was quite small (seven), and the analysis was based on limited cognitive tests. To date, no meta-analysis has comprehensively analyzed changes in speech perception and cognitive function. A systematic meta-analysis encompassing two different domains (speech perception and cognitive function) is crucial, as it could not only evaluate the efficacy of CIs over time in each domain but also reveal the trajectory of interactions between the two domains.
The current study investigated the impact of CIs over time on older adults by classifying the subtypes of cognitive abilities to reveal the tradeoff trajectory between speech perception and cognitive domains. The aims of this study were: 1) to assess the long-term effects of CIs on speech perception and cognitive domains, including global function, executive function, verbal memory, and non-verbal memory and 2) to examine time-related changes in speech perception and cognitive domains by analyzing the following three timepoints: i) pre-CI vs. six months post-CI, ii) six months post-CI vs. 12 months post-CI, and iii) pre-CI vs. 12 months post-CI.
3. Materials and Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [30], and the protocol was not registered.
3.1. Search strategy and study selection criteria
Studies published between January 1, 2000 and April 30, 2022 were retrieved from the following three electronic databases: PubMed, EMBASE, and Web of Science. Keywords included “cochlear implant,” “cognition,” “dementia,” “Alzheimer disease,” and “old adults”, and the final search statement was extended to “cochlear implant OR cochlear implants OR cochlear implantation” AND “cognition OR cognitions OR cognitive OR cognitively OR cognitives” AND “dementia OR dementias” AND “Alzheimer disease OR Alzheimer OR Alzheimers” AND “adult OR adults OR old OR (aging OR ageing) OR aged” through systematic searches of the three databases.
The inclusion criteria for the study were as follows.
-
(1)
Participants: consist of, or include, older adults aged 60 years or older (mean age of participants ≥55 years) with post-lingual SPHL
-
(2)
Intervention: CI with a multi-electrode
-
(3)
Outcomes: change in performance on speech perception test and cognitive function test after CIs compared to baseline (pre-CI)
-
(4)
Study design: longitudinal studies with a baseline measurement and at least one post-CI measurement (minimum six months after).
The present study imposed no restrictions on the participants’ language and only included original research articles written in English. Three researchers (SA, JES, and SBJ) independently performed the search and deduplication processes, and screened article titles and abstracts to obtain provisional articles for inclusion. The three researchers then conducted eligibility assessment procedures through a cross-checking procedure and discussions to determine the final studies in line with the inclusion criteria. If overlapping data were identified, only the most comprehensive data were included in the meta-analyses. The literature search was performed on April 30, 2022, and the screening and eligibility assessment processes were completed on May 15, 2022.
3.2. Data extraction
Two researchers (SA and EJ) independently extracted data from each study, including basic characteristics, assessment tools, time points of measurements, and outcome measures. If outcome measures could not be extracted because they were presented only in graphs, the values were estimated using PlotDigitizer software [31]. When only median and quartile values were available, the mean and standard deviation (SD) values were estimated using the quantile method by Wan et al. (2014) [32].
3.3. Quality assessment
The quality of each study was evaluated using the validated Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) [33], which consists of the following six domains: participant selection, confounding variables, measurement of exposure, blinding of outcome assessments, incomplete outcome data, and selective outcome reporting. Two researchers (SA and EJ) independently rated each study as having either a low, high, or unclear risk of bias, and disagreements were resolved through discussion until a consensus was reached.
3.4. Statistical analyses
All analyses were conducted using Comprehensive Meta-Analysis Version 3. Meta-analyses were performed separately for each domain (speech perception and cognitive function) and timepoint comparison (pre-CI vs. six months post-CI, six months post-CI vs. 12 months post-CI, and pre-CI vs. 12 months post-CI). For the cognitive domain, a meta-analysis was performed for each of the four subtypes: global function, executive function, verbal memory, and non-verbal memory. In the current paper, the global function represents the performance on general cognitive screening tests such as Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). A random-effects model was employed because several characteristics (e.g., the sample size, measurement points, and outcome measures) varied between studies [34]. The effect sizes for repeated measures designs were calculated as Hedges' g, an adjusted mean difference [35], using means, SDs, and other statistics (e.g., p values, sample sizes, and pre–post correlation values). When no pre-post correlation values were reported, a medium correlation was assumed, and a fixed value of 0.5 was used for the analyses [36]. If an individual study included more than one outcome measure in the analysis of a particular domain, the effect size was calculated for each outcome measure, and then the combined effect size was used for analysis. Effect sizes of 0.30, 0.50, and 0.80 were considered to have small, medium, and large effects, respectively. The significance of the effect size was verified based on the significance level of 0.05, i.e., a 95% confidence interval. Heterogeneity between studies was assessed by Cochran's Q test using statistics [37]. The statistics of 25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively. Publication bias was evaluated through a visual inspection of funnel plots and Egger's regression analysis [38]. Suspected publication bias was addressed using the trim-and-fill method to estimate the unbiased effect size [39].
4. Results
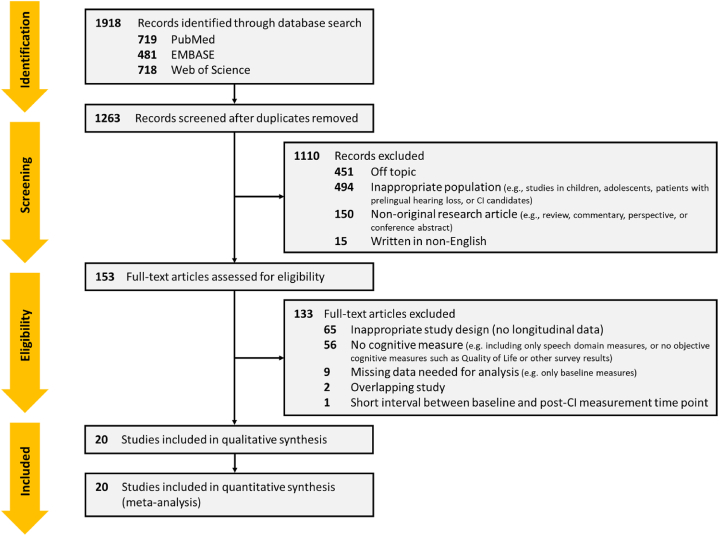
A total of 1918 articles were identified following the search of the three electronic databases mentioned in the previous section. After removing duplicates, 1263 articles remained, and a further 1110 articles that were off-topic, inappropriate for the target population (e.g., studies in children, adolescents, or patients with prelingual hearing loss), not original research articles (e.g., reviews, commentaries, perspectives, or conference abstracts), or not written in English were excluded via title and abstract screening. The full texts of the 153 remaining articles were then further evaluated for eligibility. According to the inclusion criteria in Materials and Methods section, 65 articles that did not contain longitudinal data due to inappropriate study design (e.g., a cross-sectional study) were excluded, 56 articles that did not present objective cognitive measures were also excluded. Nine articles that did not include all the necessary information for the analysis were excluded, and one article with too short of an interval between the baseline and the post-CI measurement time point was further excluded. In cases of overlapping data, only the articles with the most comprehensive data were included. Consequently, 20 articles that met the inclusion criteria were finally selected (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram [30].
The 20 studies included in the final analysis investigated changes in speech perception and cognitive function induced by auditory rehabilitation through CIs and contained follow-up data for at least six months including pre- and post-CI. Most of the studies (14 of 20) were single-group-based, longitudinal studies involving patients with post-lingual SPHL. The other six studies were multi-group-based studies analyzing differences in longitudinal changes in two or more groups classified according to either the method or execution of intervention (e.g., CI group vs. control group, CI group vs. HA group). In this meta-analysis, only data corresponding to the CI group were extracted and used, and as a result, data from a total of 648 CI subjects from 20 studies were included.
Most of the participants included in the analyses were older adults aged 60 years or more. In 10 studies, all participants were older adults (>60 years) [13,17,20,22,24,25,27,[40], [41], [42], [43]],and in the other studies, young adults (≥18 years, <35 years) and middle-aged adults (≥36 years, <60 years) were included, but the average age of the participants in each study was 60 years or older, with the exception of 55 years in one study [44]. Regarding the cognitive level of the participants, most studies were conducted in individuals with SPHL excluding those with severe cognitive impairments based on cognitive screenings such as the MMSE and MoCA or on medical history (i.e., in a cognitively normal group [24,26,42,43,45,46], or a group including participants with MCI [13,[16], [17], [18],[20], [21], [22],25,27,40]. The gender ratio of participants varied by study from 14:86 to 67:33 (female-to-male ratio as a percentage). The spoken language was English in six studies [18,21,25,44,45,47], German in five studies [20,26,42,43,46], French in four studies [13,16,17,22], Italian in two studies [24,40], Dutch in one study [23], Japanese in one study [27], and mixed (Dutch, Spanish, Polish, and English) in one study [19]. More details of the participants’ demographics are presented in Table 1.
Table 1.
Characteristics of studies included in the meta-analyses – participant demographic data.
| Study | Participants | Gender | Language | Age | Duration of deafness | CI | Post-CI condition | Institution |
|---|---|---|---|---|---|---|---|---|
| Mosnier et al., 2015 [13] | N = 94 | Female = 49, Male = 45 | French | Mean = 72, Median = 71, Range = 65–85 |
Mean ± SD = 11 ± 15.1, Range = 1–61 | Unilateral = 93 (Right = 50, Left = 43), Bilateral = 1 |
Unknown | Multi-center (10) |
| Castiglione et al., 2016 [40] (for speech measure, Castiglione et al., 2015 [41]) | N = 125 CI group: N = 15 (N = 30) |
CI group: Female = 7, Male = 8 |
Italian | CI group: Median = 71, Range = 65–75 (Median = 70.5, Range = 65–79) |
Unknown | Unilateral | Unknown | Single center |
| Ambert-Dahan et al., 2017 [16] | N = 18 | Female = 7, Male = 11 | French | Mean ± SD = 64 ± 3.5, Range = 23–83 |
Mean ± SD = 6.5 ± 2.1, Range = 0.3–35 | Unilateral = 17, Bilateral = 1 |
CI + contralateral HA = 12, Only CI = 6 |
Single center |
| Jayakody et al., 2017 [47] | N = 39 CI group: N = 16 |
CI group: Female = 9, Male = 7 |
English | CI group: Mean ± SD = 61.8 ± 15.6 |
Mean ± SD = 34.4 ± 18.6 | Unknown | Unknown | Single center |
| Sonnet et al., 2017 [22] | N = 16 | Female = 10, Male = 6 | French | Mean age = 72.5, Range = 65–80 |
Mean ± SD = 15.7 ± 11.8, Range = 1–41 | Unilateral (Right = 10, Left = 6) | Unknown | Single center |
| Claes et al., 2018 [23] | N = 20 | Female = 8, Male = 12 | Dutch | Mean = 71.5, Range = 54.8–84.8 |
Mean ± SD = 26.9 ± 16.7, Range = 0.3–55 | Unilateral (Right = 10, Left = 10) | CI + contralateral HA = 4, Only CI = 5, N/A = 11 |
Single center |
| Mosnier et al., 2018 [17] | N = 94 (same population with Mosnier et al., 2015) | |||||||
| Anzivino et al., 2019 [24] | N = 44 CI group: N = 25 |
CI group: Female = 14, Male = 11 |
Italian | CI group: Mean ± SD = 66.4 ± 5.8 |
Unknown | Unilateral | CI + contralateral HA = 16, Only CI = 9 |
Multi-center (2) |
| Macpherson et al., 2019 [44] | N = 15 | Female = 10, Male = 5 | English | Mean = 55, Range = 34–83 |
Mean ± SD = 29.1 ± 12.9, Range = 2–50 | Sequential (unilateral → bilateral) | CI + contralateral HA = 15 (at post-CI 6, 12 months) | Single center |
| Buchman et al., 2020 [18] | N = 96 | Female = 34, Male = 62 | English | Median = 71, Range = 23–91 |
Mean ± SD = 27 ± 14, Range = 1–66 | Unilateral (Right = 56, Left = 40) | CI + contralateral HA = 94, Only CI = 2 |
Multi-center (13) |
| Zhan et al., 2020 [45] | N = 19 | Female = 8, Male = 11 | English | CI group: Mean ± SD = 72 ± 7 |
Unknown | Unilateral = 18, Bilateral = 1 |
Unknown | Single center |
| Huber et al., 2021 [42] | N = 58 CI group: N = 29 |
CI group: Female = 12, Male = 17 |
German | Mean ± SD = 67.8 ± 9.4, Range = 49–82 |
Unknown | Unknown | Unknown | Multi-center (2) |
| Issing et al., 2021 [20] | N = 33 | Female = 20, Male = 13 | German | Mean ± SD = 79.4 ± 7.4 | Unknown | Unilateral | Unknown | Single center |
| Knopke et al., 2021 [43] | N = 21 | Female = 10, Male = 11 | German | Mean ± SD = 70.6 ± 4.7 | Unknown | Unilateral | Unknown | Single center |
| Mertens et al., 2021 [19] | N = 48 CI group: N = 24 |
CI group: Female = 10, Male = 14 |
Dutch, Spanish, Polish, English | Mean ± SD = 73.3 ± 4.8, Range = 65–86 |
Unknown | Unilateral | Unknown | Multi-center (5) |
| Vasil et al., 2021 [21] | N = 77 | Female = 24, Male = 53 | English | Mean ± SD = 77.1 ± 5.5 | Mean ± SD = 28.5 ± 13.1, Range = 8–66 | Unilateral | Unknown | Multi-center (13) |
| Völter et al., 2021 [46] | N = 176 CI group: N = 71 |
CI group: Female = 47, Male = 24 |
German | Mean ± SD = 72.4 ± 6.4, Range = 55–85 |
Mean ± SD = 22.9 ± 14.7 | Unilateral = 60 Bilateral = 11 |
Unknown | Single center |
| Gurgel et al., 2022 [25] | N = 37 | Female = 5, Male = 32 | English | Mean ± SD = 66.3 ± 9.2 Range = 50–84 |
Unknown | Unilateral | Unknown | Single center |
| Ohta et al., 2022 [27] | N = 21 | Female = 14, Male = 7 | Japanese | Median = 69, Range = 65–85 |
Mean ± SD = 10.7 ± 10.6, Range = 0.8–35 | Unilateral (Right = 10, Left = 11) | CI + contralateral HA = 6, Only CI = 15 |
Single center |
| Völter et al., 2022 [26] | N = 71 (same population with Völter et al., 2021) | |||||||
To examine longitudinal changes associated with CI intervention, all 20 studies performed speech perception tests and cognitive function tests, which can derive objective scores. For speech perception evaluation, 18 studies performed at least one word recognition test (using a monosyllabic, disyllabic, or trisyllabic word list), nine of which also conducted a sentence recognition test [16,18,[23], [24], [25],40,42,45,47]. One study performed a sentence recognition test alone [44], and the other study was a multi-center study (in different countries) based on local clinical test batteries and did not include outcomes due to language differences (appendix pp 1–2) [19]. For the cognitive function assessment, all studies employed various neuropsychological tests capable of evaluating multiple cognitive domains; these are summarized in the appendix (pp 3–6). In this meta-analysis, the different tests employed in the studies were categorized into the following four cognitive domains—global function, executive function, verbal memory, and non-verbal memory—and the results were analyzed.
The results of the study quality assessment based on RoBANS are provided in the appendix (pp 7–9). Briefly, all 20 studies were conducted by prospectively recruiting patients who were clinically determined to require CIs, used validated assessment tools to evaluate speech perception and cognitive function, and clearly reported the results of the tests performed at each measurement timepoint. Accordingly, the studies were evaluated as having a low risk of bias overall. However, for the “confounding variables” domain, the risk of bias was rated as low in five studies [16, 20, 24, 27 40],high in nine studies [13,19,22,23,25,26,[45], [46], [47]], and unclear in six studies [17,18,21,[42], [43], [44]], depending on whether individual factors that could affect the outcome measures were controlled. For the “incomplete outcome data” domain, risk of bias was assessed as high in three studies [22,24,47], depending on the proportion of participants who dropped out during the follow-up evaluations.
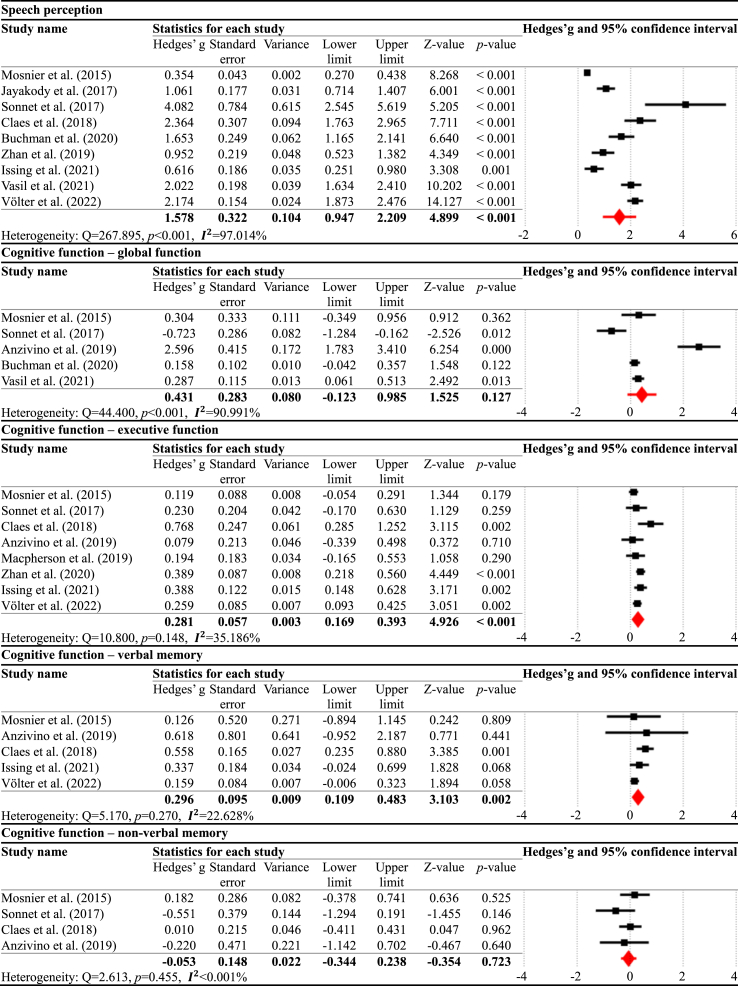
Meta-analyses were performed separately for the speech perception and cognitive domains for each timepoint comparison. In the pre-CI vs. six months post-CI comparison (Table 2), the results demonstrated that speech perception significantly improved at six months post-CI compared to pre-CI, with a large effect size (g = 1.578, p < 0.001, = 97.014%). For the cognitive domains, there were significant improvements in executive function (g = 0.281, p < 0.001, = 35.186%) and verbal memory (g = 0.296, p = 0.002, = 22.628%). However, there were no significant changes in global function (g = 0.431, p = 0.127, = 90.991%) and non-verbal memory (g = −0.053, p = 0.723, <0.001%).
Table 2.
Meta-analysis results for pre-CI vs. six months post-CI.
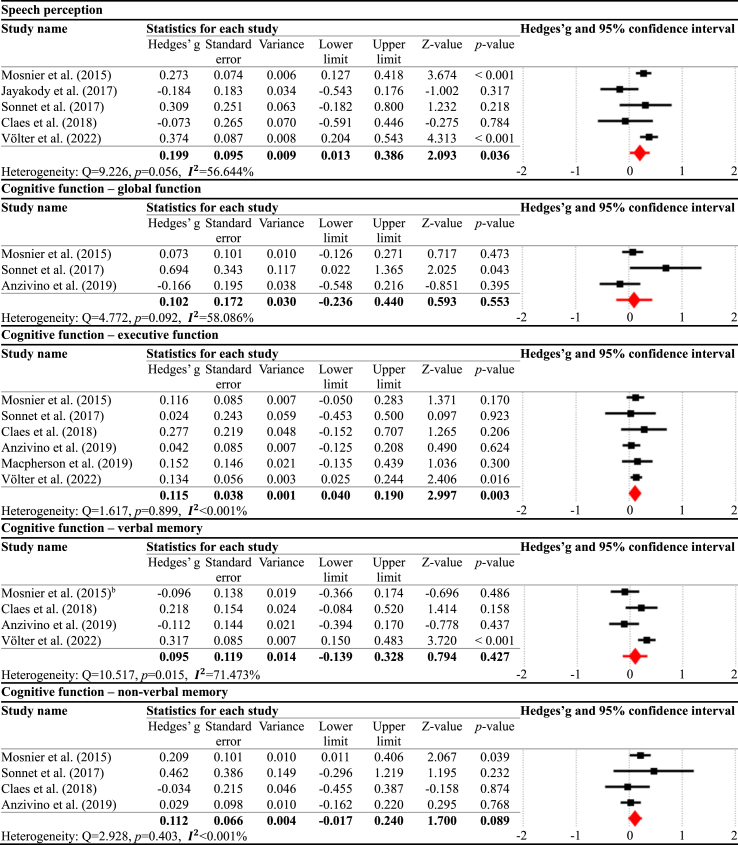
In the six months post-CI vs. 12 months post-CI comparison (Table 3), the results showed that speech perception was significantly enhanced at 12 months post-CI compared to six months post-CI, presenting the small effect size (g = 0.199, p = 0.036, = 56.644%). For the cognitive domains, executive function (g = 0.115, p = 0.003, <0.001%) significantly improved, whereas the other cognitive domains did not show significant changes: global function (g = 0.102, p = 0.553, = 58.086%), verbal memory (g = 0.095, p = 0.427, = 71.473%), and non-verbal memory (g = 0.112, p = 0.089, <0.001%).
Table 3.
Meta-analysis results for six months post-CI vs. 12 months post-CI.
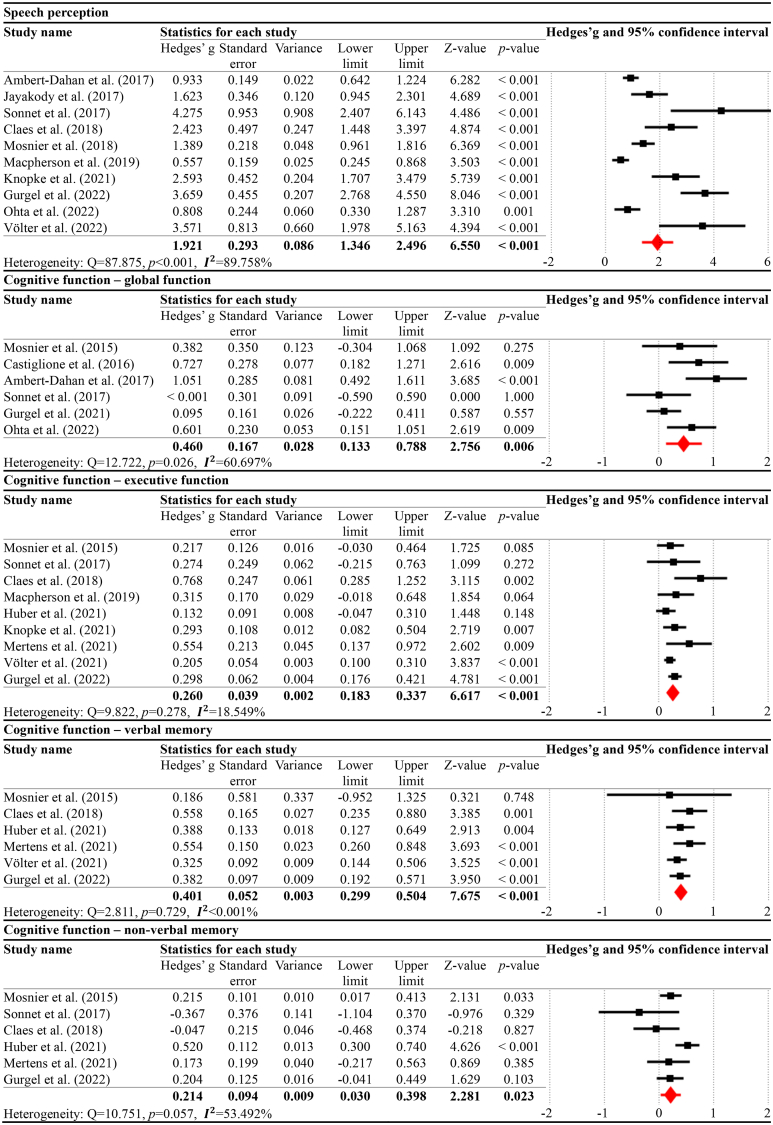
In the pre-CI vs. 12 months post-CI comparison (Table 4), the results indicated that speech perception significantly increased at 12 months post-CI compared to pre-CI, with a large effect size (g = 1.921, p < 0.001, = 89.758%). For the cognitive domains, all the following subtypes exhibited significant improvements: global function (g = 0.460, p = 0.006, = 60.697%), executive function (g = 0.260, p < 0.001, = 18.549%), verbal memory (g = 0.401, p < 0.001, <0.001%), and non-verbal memory (g = 0.214, p = 0.023, = 53.492%).
Table 4.
Meta-analysis results for pre-CI vs. 12 months post-CI.
The publication bias evaluation results for each analysis are provided in the appendix (pp 10–18).
5. Discussion
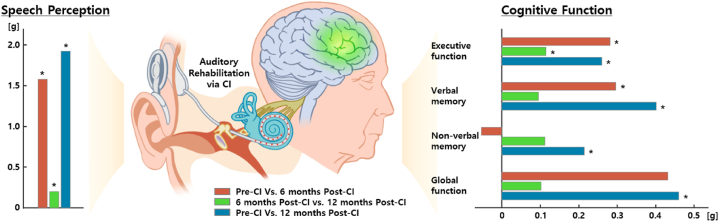
The current systematic review and meta-analysis demonstrated that auditory rehabilitation via CIs could enhance cognitive function as well as speech perception among those in the older population with SPHL. Considering that the cognitive abilities of older adults are on the trajectory of progressive decline with age [48], the significant results for cognitive improvement are critical. In particular, the results showing that such improvement is maintained not only at six months after CIs but also at 12 months, or even more enhanced at 12 months, considering the subtype analyses, are quite promising (Fig. 2).
Fig. 2.
Longitudinal effect of auditory rehabilitation via CIs on speech perception and cognitive function among an aging population.
The current study has great significance, as it is the first attempt to systematically analyze the longitudinal effects of CIs by encompassing the outcomes of both speech perception and cognitive function. Regardingthe speech perception domain, the results revealed that speech perception abilities substantially improved after CIs, which are consistent with previous findings [14,15,28]. The effects of CIs on the speech perception domain seem to carry significant and consistent clinical implications across the studies, with few contradictory findings, given that CIs are designed to directly improve hearing-related functions.
It is clinically meaningful and critical that significant long-term improvements were identified in the comparisons between the six months post-CI and 12 months post-CI, even though the effect size was small. Results concerning the long-term effects of CIs on speech perception abilities have been controversial across the studies. Some studies have reported that speech perception abilities substantially improved within six months after CIs but entered into a plateau phase without further improvement or deterioration [49,50]. By contrast, other studies have reported that speech perception abilities consistently improved, even after six months and as far advanced as seven to 10 years following CIs [11,51]. Studies that reported a relative plateau performance after CIs mainly included subjects with a wide range of ages at implantation (e.g., 18–90 [49]; 20.6–88.9 [50]). Meanwhile, in studies that reported consistent improvements after CIs, the age range at the time of CIs was somewhat limited to older adults aged 50 to 85. To date, very few studies have systematically investigated the effects of age at CI on the long-term effects among adults [52]. Despite limited evidence, it is interesting to note that people who underwent CIs after middle-age demonstrated greater long-term positive effects of CIs.
The effects of CIs on the cognitive domain were different for each subtype (Fig. 2). Executive function showed a significant improvement until 12 months post-CI, even though the effect size slightly decreased after six months. Verbal memory was enhanced, with a small-to-medium effect size, at six months post-CI, and this improvement was maintained until 12 months post-CI. Interestingly, non-verbal memory demonstrated no noticeable change at six months post-CI, but a significant improvement was demonstrated at 12 months post-CI compared to pre-CI. Overall, the gain from the first six months post-CI was greater than that from the last half months (six to 12 months post-CI), indicating that the first six months after implantation are a critical indicator of functional recovery. This is in line with earlier research that showed the functional re-organization would be most obvious in the first six months following implantation [26]. However, the trajectory of improvement seems to be differentiated depending on the cognitive subdomains [23,47].
These subtype analyses indicate that executive function or verbal memory, which is directly associated with auditory processing [53], begins improving along with a rapid enhancement of speech perception, whereas other relatively non-relevant cognitive functions, such as non-verbal memory, start to improve after the recovery of speech perception is maintained to some extent. These results suggest that the impact of auditory rehabilitation via CIs on cognitive function may be induced as a long-term effect compared with speech perception. This finding aligns with a previous study comparing the cognitive outcomes of CI recipients and CI candidates [47], in which the CI recipients showed significant improvements in spatial working memory and reaction time at six months post-CI compared to CI candidates, and further significant improvements in cognitive flexibility and paired associative learning (that did not show any change at six months post-CI) at 12 months post-CI.
Our meta-analysis results demonstrate that CIs may induce positive effects on maintaining and improving cognitive abilities over time. The link between the effects of CIs and cognitive improvement can be accounted for by the hypothesis that CIs may contribute to reducing the “listening effort” required for the processing and comprehension of auditory signals, leading to greater cognitive resources available for higher cognitive processing [54]. In fact, many previous studies have presented neuroimaging evidence supporting the use of additional cognitive resources in the brain when hearing challenge have emerged due to hearing loss [55,56] or environmental reasons (e.g., degraded speech) [57]. These studies commonly showed that the activity of the auditory cortical area is reduced and that the frontal cortical area is recruited in response to speech stimuli when there is a hearing challenge. These adaptations indicate increased “listening effort”. In addition, cross-modal plasticity has been identified, in which the auditory cortical area, which has lost its original function due to reduction or degradation of auditory input, is re-allocated to process other stimuli, such as visual, vibrotactile, and somatosensory input [58,59]. Recent neuroimaging studies have demonstrated that such brain re-organization following hearing loss is partially or completely restored by auditory rehabilitation through CIs or HAs [55,58,59].
Given the findings of the literature, the long-term effect of CIs on cognitive domains could be interpreted that as “listening effort” decreases via auditory rehabilitation, cognitive resources are re-allocated to other cognitive processes, and functional re-organization of the brain is induced accordingly [53,60],thereby enhancing overall cognitive function. Moreover, such a cognitive improvement may have been driven by other underlying mechanisms. The reinstatement of acoustic stimulation to non-auditory brain areas due to auditory rehabilitation may have enhanced non-auditory functions, particularly the ability to process and perceive multimodal sensory signals, by reinforcing or maintaining the corresponding afferent pathways [[61], [62], [63]]. In addition, the restoration of auditory-limbic connectivity, which is related to emotional reactivity and emotion regulation [[64], [65], [66]], may have improved cognitive function by relieving depression or anxiety. Lastly, it may have had a positive impact on cognitive function as social interactions that were previously impaired by hearing loss became activated and normalized [67,68]. In this way, because the improvement in cognitive abilities due to auditory rehabilitation is induced by the brain re-organization due to plasticity changes resulting from the recovery of the auditory pathway, it may occur in the long-term after intervention, although this may vary across the subtypes of cognitive abilities.
Although the current meta-analysis provides crucial outcomes for the long-term effects of CIs among an older population, it still has several limitations. First, it relied on a small sample size of 20 studies. This is likely due to population bias; few studies have examined the longitudinal effects of CIs, especially among older generations. As recent research focuses greater attention on the relations between aging-related hearing loss and dementia, we expect more studies to report the long-term effects of CIs among aging populations. Furthermore, in some analyses, we found a relatively moderate-to-high level of heterogeneity across the studies. This is likely due to the individual variability of the participants along with the limited sample size.
Participants who were included in the current meta-analysis varied in terms of their age, duration of deafness, education level, language background, and baseline hearing and cognitive levels, as well as modality of CIs (unilateral or bilateral) and post-CI status (CI only or CI + contralateral HA). Previous studies have reported that such individual factors correlate with CIs outcomes. A recent meta-analysis revealed that a longer duration of deafness prior to CIs is associated with worse CI performance, especially in terms of speech perception, even though such characteristics are mitigated by more experience with CIs [69]. Regarding the modality of CIs, several studies have demonstrated that bilateral CIs outperform unilateral CIs regarding the understanding of speech in noise and the localization of sounds [70,71]. Gordon et al. further indicated that unilateral and bilateral CIs can induce different brain re-organizations [71]; In unilateral CIs, asymmetric strengthening from the stimulated ear to the contralateral auditory cortex occurs and the balance of activity between the two hemispheres in the auditory brainstem is disrupted, resulting in deterioration of binaural processing and lower speech segregation ability compared to bilateral CIs. Regarding such individual factors, the individuals included in the current meta-analysis exhibited diverse characteristics, which may have influenced the outcomes.
Given that individual variability with respect to speech perception and cognitive function increases among an older population [72], and is even greater among clinical populations with diseases [73], heterogeneity may to some extent be inevitable. However, more studies are needed to systematically control for various participant characteristics to minimize those influences on outcome measures by matching the demographic features in group comparisons of interest.
The current results indicate that auditory rehabilitation via CIs may be beneficial in preventing cognitive decline among the aging population. However, comparative studies with longitudinal data in a control group with SPHL but not receiving interventions such as CIs or HAs are essential to draw a definitive conclusion on this. Due to the nature of longitudinal follow-up studies targeting the clinical population, few studies have involved a control group undergoing no therapeutic intervention. To date, only two studies have defined CI candidates [47] or patients with SPHL who were not scheduled for CIs [19] as control groups and have compared them with the intervention groups; the studies demonstrated that at follow-up, the intervention group performed significantly better in cognitive tests than the control group. The sample size of the studies, including the control group, was too small to evaluate statistical significance; therefore, those comparison results were excluded from the current analysis. If more comparative studies including control groups are reported and a systematic meta-analysis including the corresponding results is conducted in the future, the efficacy of CIs will be able to be evaluated more clearly.
Despite several limitations, the current study conveys clinical and empirical importance. Given that currently, more than 60 million people worldwide suffer from SPHL, but less than 5% receive care services through interventions such as CIs, regularly monitoring hearing ability in midlife and providing early intervention when hearing loss is detected may help reduce the risk of developing either MCI or dementia with age.
Author contribution statement
Sora An: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Eunha Jo: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Sang Beom Jun; Jee Eun Sung: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our appreciation to Dr. Jeong Yee for her advice and many discussions. This research was supported by the National Research Council of Science & Technology (NST) grant by the Korean government (MSIT) (No. CAP21051-000), the Convergent Technology R&D Program for Human Augmentation through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2019M3C1B8090805), the Basic Science Research Program through the NRF funded by the Korea government (NRF–2022R1A2C2005062), the Basic Science Research Program through the NRF funded by the Ministry of Education (NRF-2022R1I1A4063209, NRF-2020R1I1A1A01073605) and RP-Grant 2020 of Ewha Womans University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19703.
Contributor Information
Sang Beom Jun, Email: juns@ewha.ac.kr.
Jee Eun Sung, Email: jeesung@ewha.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia
- 2.Landeiro F., Walsh K., Ghinai I., Mughal S., Nye E., Wace H., et al. Measuring quality of life of people with predementia and dementia and their caregivers: a systematic review protocol. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G., Sommerlad A., Orgeta V., et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 4.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong N.M., An Y., Doshi J., et al. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA otolaryngol Head Neck Surg. 2019;145(9):794–802. doi: 10.1001/jamaoto.2019.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giroud N., Pichora-Fuller M., Mick P., Wittich W., Al-Yawer F., Rehan S., et al. Hearing loss is associated with gray matter differences in older adults at risk for and with Alzheimer's disease. Aging Brain. 2021;1 doi: 10.1016/j.nbas.2021.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin F., Ferrucci L., An Y., Goh J., Doshi J., Metter E., et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucholc M., McClean P.L., Bauermeister S., Todd S., Ding X., Ye Q., et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimer's Dementia: Translational Research & Clinical Interventions. 2021;7(1) doi: 10.1002/trc2.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross B., Fujioka T., Tremblay K.L., Picton T.W. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J. Neurosci. 2007;27(42):11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gates G.A., Mills J.H., Presbycusis Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 11.Dillon M.T., Buss E., Adunka M.C., et al. Long-term speech perception in elderly cochlear implant users. JAMA otolaryngol Head Neck Surg. 2013;139(3):279–283. doi: 10.1001/jamaoto.2013.1814. [DOI] [PubMed] [Google Scholar]
- 12.Wick C.C., Kallogjeri D., McJunkin J.L., et al. Hearing and quality-of-life outcomes after cochlear implantation in adult hearing aid users 65 years or older: a secondary analysis of a nonrandomized clinical trial. JAMA otolaryngol Head Neck Surg. 2020;146(10):925–932. doi: 10.1001/jamaoto.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosnier I., Bebear J.-P., Marx M., et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA otolaryngol Head Neck Surg. 2015;141(5):442–450. doi: 10.1001/jamaoto.2015.129. [DOI] [PubMed] [Google Scholar]
- 14.Gaylor J.M., Raman G., Chung M., et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA otolaryngol Head Neck Surg. 2013;139(3):265–272. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 15.Boisvert I., Reis M., Au A., Cowan R., Dowell R.C. Cochlear implantation outcomes in adults: a scoping review. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambert-Dahan E., Routier S., Marot L., et al. Cognitive evaluation of cochlear implanted adults using CODEX and MoCA screening tests. Otol. Neurotol. 2017;38(8):e282–e284. doi: 10.1097/MAO.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 17.Mosnier I., Vanier A., Bonnard D., et al. Long‐term cognitive prognosis of profoundly deaf older adults after hearing rehabilitation using cochlear implants. J. Am. Geriatr. Soc. 2018;66(8):1553–1561. doi: 10.1111/jgs.15445. [DOI] [PubMed] [Google Scholar]
- 18.Buchman CA, Herzog JA, McJunkin JL, et al. Assessment of speech understanding after cochlear implantation in adult hearing aid users: a nonrandomized controlled trial. JAMA otolaryngol Head Neck Surg; 146(10): 916-924. [DOI] [PMC free article] [PubMed]
- 19.Mertens G., Andries E., Claes A.J., et al. Cognitive improvement after cochlear implantation in older adults with severe or profound hearing impairment: a prospective, longitudinal, controlled, multicenter study. Ear Hear. 2021;42(3) doi: 10.1097/AUD.0000000000000962. 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issing C., Baumann U., Pantel J., Stöver T. Impact of hearing rehabilitation using cochlear implants on cognitive function in older patients. Otol. Neurotol. 2021;42(8):1136–1141. doi: 10.1097/MAO.0000000000003153. [DOI] [PubMed] [Google Scholar]
- 21.Vasil K.J., Ray C., Lewis J., Stefancin E., Tamati T.N., Moberly A.C. How does cochlear implantation lead to improvements on a cognitive screening measure? J. Speech Lang. Hear. Res. 2021;64(3):1053–1061. doi: 10.1044/2020_JSLHR-20-00195. [DOI] [PubMed] [Google Scholar]
- 22.Sonnet M.-H., Montaut-Verient B., Niemier J.-Y., Hoen M., Ribeyre L., Parietti-Winkler C. Cognitive abilities and quality of life after cochlear implantation in the elderly. Otol. Neurotol. 2017;38(8):e296–e301. doi: 10.1097/MAO.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 23.Claes A.J., Van de Heyning P., Gilles A., Van Rompaey V., Mertens G. Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otol. Neurotol. 2018;39(9):e765–e773. doi: 10.1097/MAO.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 24.Anzivino R., Conti G., Di Nardo W., et al. Prospective evaluation of cognitive functions after rehabilitation with cochlear implant or hearing aids: preliminary results of a multicentric study on elderly patients. Am. J. Audiol. 2019;28(3S):762–774. doi: 10.1044/2019_AJA-HEAL18-18-0176. [DOI] [PubMed] [Google Scholar]
- 25.Gurgel R.K., Duff K., Foster N.L., Urano K.A., deTorres A. Evaluating the impact of cochlear implantation on cognitive function in older adults. Laryngoscope. 2022;132:S1–S15. doi: 10.1002/lary.29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Völter C., Götze L., Bajewski M., Dazert S., Thomas J.P. Cognition and cognitive reserve in cochlear implant recipients. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.838214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta Y., Imai T., Maekawa Y., et al. The effect of cochlear implants on cognitive function in older adults: a prospective, longitudinal 2-year follow-up study. Auris Nasus Larynx. 2022;49(3):360–367. doi: 10.1016/j.anl.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Schafer E.C., Miller S., Manning J., et al. Meta-analysis of speech recognition outcomes in younger and older adults with cochlear implants. Am. J. Audiol. 2021;30(3):481–496. doi: 10.1044/2021_AJA-20-00141. [DOI] [PubMed] [Google Scholar]
- 29.Hamerschmidt R., Santos V.M., Gonçalves F.M., et al. Changes in cognitive performance after cochlear implantation in adults and older adults: a systematic review and meta-analysis. Int. J. Audiol. 2022:1–12. doi: 10.1080/14992027.2022.2050823. [DOI] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 31.SourceForge. Plot digitizer. http://plotdigitizer.sourceforge.net/
- 32.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.Y., Park J.E., Lee Y.J., et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013;66(4):408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. John Wiley & Sons; 2021. Introduction to Meta-Analysis. [Google Scholar]
- 35.Bernard R., Borokhovski E., editors. Effect Size Calculation for Meta-Analysis. Campbell Colloquium; 2009. [Google Scholar]
- 36.Abrams K.R., Gillies C.L., Lambert P.C. Meta‐analysis of heterogeneously reported trials assessing change from baseline. Stat. Med. 2005;24(24):3823–3844. doi: 10.1002/sim.2423. [DOI] [PubMed] [Google Scholar]
- 37.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duval S., Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 40.Castiglione A., Benatti A., Velardita C., et al. Aging, cognitive decline and hearing loss: effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol. Neurootol. 2016;21(Suppl. 1):21–28. doi: 10.1159/000448350. [DOI] [PubMed] [Google Scholar]
- 41.Castiglione A., Benatti A., Girasoli L., et al. Cochlear implantation outcomes in older adults. Hear. Bal. Commun. 2015;13(2):86–88. [Google Scholar]
- 42.Huber M., Roesch S., Pletzer B., Lukaschyk J., Lesinski-Schiedat A., Illg A. Can cochlear implantation in older adults reverse cognitive decline due to hearing loss? Ear Hear. 2021;42(6):1560–1576. doi: 10.1097/AUD.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 43.Knopke S., Schubert A., Häussler S.M., Gräbel S., Szczepek A.J., Olze H. Improvement of working memory and processing speed in patients over 70 with bilateral hearing impairment following unilateral cochlear implantation. J. Clin. Med. 2021;10(15) doi: 10.3390/jcm10153421. 3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macpherson E.A., Curca I.A., Scollie S., et al. Effects of bimodal and bilateral cochlear implant use on a nonauditory working memory task: reading span tests over 2 years following cochlear implantation. Am. J. Audiol. 2019;28(4):947–963. doi: 10.1044/2019_AJA-19-0030. [DOI] [PubMed] [Google Scholar]
- 45.Zhan K.Y., Lewis J.H., Vasil K.J., et al. Cognitive functions in adults receiving cochlear implants: predictors of speech recognition and changes after implantation. Otol. Neurotol. 2020;41(3):e322–e329. doi: 10.1097/MAO.0000000000002544. [DOI] [PubMed] [Google Scholar]
- 46.Völter C., Götze L., Haubitz I., Müther J., Dazert S., Thomas J.P. Impact of cochlear implantation on neurocognitive subdomains in adult cochlear implant recipients. Audiol. Neurootol. 2021;26(4):236–245. doi: 10.1159/000510855. [DOI] [PubMed] [Google Scholar]
- 47.Jayakody D.M., Friedland P.L., Nel E., Martins R.N., Atlas M.D., Sohrabi H.R. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otol. Neurotol. 2017;38(8):e289–e295. doi: 10.1097/MAO.0000000000001502. [DOI] [PubMed] [Google Scholar]
- 48.Lin F.R., Yaffe K., Xia J., Xue Q.-L., Harris T.B., Purchase-Helzner E., et al. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenarz M., Sönmez H., Joseph G., Büchner A., Lenarz T. Long-term performance of cochlear implants in postlingually deafened adults. JAMA otolaryngol Head Neck Surg. 2012;147(1):112–118. doi: 10.1177/0194599812438041. [DOI] [PubMed] [Google Scholar]
- 50.Häußler S.M., Knopke S., Wiltner P., Ketterer M., Gräbel S., Olze H. Long-term benefit of unilateral cochlear implantation on quality of life and speech perception in bilaterally deafened patients. Otol. Neurotol. 2019;40(4):e430–e440. doi: 10.1097/MAO.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 51.Ruffin C.V., Tyler R.S., Witt S.A., Dunn C.C., Gantz B.J., Rubinstein J.T. Long‐term performance of Clarion 1.0 cochlear implant users. Laryngoscope. 2007;117(7):1183–1190. doi: 10.1097/MLG.0b013e318058191a. [DOI] [PubMed] [Google Scholar]
- 52.Lenarz M., Sönmez H., Joseph G., Büchner A., Lenarz T. Cochlear implant performance in geriatric patients. Laryngoscope. 2012;122(6):1361–1365. doi: 10.1002/lary.23232. [DOI] [PubMed] [Google Scholar]
- 53.Peelle J.E. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2):204. doi: 10.1097/AUD.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uchida Y., Sugiura S., Nishita Y., Saji N., Sone M., Ueda H. Age-related hearing loss and cognitive decline—the potential mechanisms linking the two. Auris Nasus Larynx. 2019;46(1):1–9. doi: 10.1016/j.anl.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Pereira-Jorge M., Andrade K., Palhano-Fontes F., Diniz P., Sturzbecher M., Santos A., Araújo D.B.d. Anatomical and functional MRI changes after one year of auditory rehabilitation with hearing aids. Neural Plast. 2018 doi: 10.1155/2018/9303674. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell J. Sharma. Compensatory changes in cortical resource allocation in adults with hearing loss. Front. Syst. Neurosci. 2013;7 doi: 10.3389/fnsys.2013.00071. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peelle J.E. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2) doi: 10.1097/AUD.0000000000000494. 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosemann S., Gieseler A., Tahden M., Colonius H., Thiel C.M. Treatment of age-related hearing loss alters audiovisual integration and resting-state functional connectivity: a randomized controlled pilot trial. Eneuro. 2021;8(6) doi: 10.1523/ENEURO.0258-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma A., Glick H., Campbell J., Torres J., Dorman M., Zeitler D.M. Cortical plasticity and re-organization in pediatric single-sided deafness pre-and post-cochlear implantation: a case study. Otol. Neurotol.: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2016;37(2) doi: 10.1097/MAO.0000000000000904. e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park D.C., Bischof G.N. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci. 2013;15(1) doi: 10.31887/DCNS.2013.15.1/dpark. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meredith M.A., Allman B.L., Keniston L.P., Clemo H.R. Auditory influences on non-auditory cortices. Hear. Res. 2009;258(1–2):64–71. doi: 10.1016/j.heares.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H., Cho J., Kim Y.R., Song Y., Chun S.-I., Suh J.-Y., et al. Response of the primary auditory and non-auditory cortices to acoustic stimulation: a manganese-enhanced MRI study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preisig B.C., Riecke L., Hervais-Adelman A. Speech sound categorization: the contribution of non-auditory and auditory cortical regions. Neuroimage. 2022;258 doi: 10.1016/j.neuroimage.2022.119375. [DOI] [PubMed] [Google Scholar]
- 64.Rutherford B.R., Brewster K., Golub J.S., Kim A.H., Roose S.P. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatr. 2018;175(3):215–224. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cosh S., Helmer C., Delcourt C., Robins T.G., Tully P.J. Depression in elderly patients with hearing loss: current perspectives. Clin. Interv. Aging. 2019:1471–1480. doi: 10.2147/CIA.S195824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blazer D.G., Tucci D.L. Hearing loss and psychiatric disorders: a review. Psychol. Med. 2019;49(6):891–897. doi: 10.1017/S0033291718003409. [DOI] [PubMed] [Google Scholar]
- 67.Keesom S.M., Hurley L.M. Silence, solitude, and serotonin: neural mechanisms linking hearing loss and social isolation. Brain Sci. 2020;10(6) doi: 10.3390/brainsci10060367. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla A., Harper M., Pedersen E., Goman A., Suen J.J., Price C., et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngology-Head Neck Surg. (Tokyo) 2020;162(5):622–633. doi: 10.1177/0194599820910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernhard N., Gauger U., Romo Ventura E., Uecker F.C., Olze H., Knopke S., et al. Duration of deafness impacts auditory performance after cochlear implantation: a meta‐analysis. Laryngoscope investigative otolaryngology. 2021;6(2):291–301. doi: 10.1002/lio2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rana B., Buchholz J.M., Morgan C., Sharma M., Weller T., Konganda S.A., et al. Bilateral versus unilateral cochlear implantation in adult listeners: speech-on-speech masking and multitalker localization. Trends in hearing. 2017;21 doi: 10.1177/2331216517722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gordon K.A., Wong D.D., Papsin B.C. Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain. 2013;136(5):1609–1625. doi: 10.1093/brain/awt052. [DOI] [PubMed] [Google Scholar]
- 72.Haynes B.I., Bauermeister S., Bunce D. A systematic review of longitudinal associations between reaction time intraindividual variability and age-related cognitive decline or impairment, dementia, and mortality. J. Int. Neuropsychol. Soc. 2017;23(5):431–445. doi: 10.1017/S1355617717000236. [DOI] [PubMed] [Google Scholar]
- 73.Wagner A.E., Nagels L., Toffanin P., Opie J.M., Başkent D. Individual variations in effort: assessing pupillometry for the hearing impaired. Trends hear. 2019;23 doi: 10.1177/2331216519845596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.