Abstract
The surface activity of γ-oryzanol was evaluated by the pendant drop method (PDM), and its self-stabilizing properties were investigated by high-pressure homogenization (HPH) and solvent displacement method (SDM). Emulsions prepared by HPH were highly unstable due to the poor surface-active character of γ-oryzanol as identified by the PDM. In contrast, solid dispersions fabricated by SDM had comparable particle size to those prepared using Tween 80 (T80) as surfactant, and were stable up to 30 days of storage at 4 °C. The self-stabilizing properties of γ-oryzanol were attributed to the mechanism of spontaneous particle formation in SDM and to the ability of γ-oryzanol molecules to prevent particles aggregation by electrostatic repulsion. The outcome of this study indicates the potential of encapsulating selected bioactive compounds, such as γ-oryzanol, in stable colloidal systems by SDM without adding emulsifier(s), regardless of their surface-active character.
Keywords: γ-Oryzanol, Encapsulation, Emulsion, Dispersion, High-pressure homogenization, Solvent displacement method
1. Introduction
Synthetic emulsifiers may represent an appealing choice for many food manufacturers, because they are relatively cheap, industrially viable and often have good emulsifying properties compared to natural emulsifiers [1]. Several researchers have discussed, however, the potential of using bioactive surface-active compounds of dual function (i.e., emulsification, bioactivity) for the preparation and stabilization of food emulsions. Kregiel et al. [2], for example, introduced different saponin-based bioactive surfactants that can be used as emulsifiers, while McClements & Decker [3] published a systematic review about the utilization of interfacial antioxidants in emulsions. In their works, they cited several synthetic compounds, such as rosmarinate alkyl esters and alkyl gallates [4,5], and concluded that the future studies should focus primarily on identifying naturally occurring bioactive emulsifiers [3].
γ-Oryzanol has various biological functions, such as antioxidant and lipid lowering effect, that can have potential applications in nutraceuticals and functional food products. Its utilization faces, however, major challenges because of its hydrophobic character. Several studies have attempted to encapsulate γ-oryzanol in various systems, including monodisperse emulsions, nanoemulsions and zein nanoparticles, using different emulsifiers, such as Tween 20, Tween 80 or lecithin [[6], [7], [8]]. None of these studies presented, however, evidence about the self-stabilizing potential of γ-oryzanol in emulsifier-free systems.
The chemical structure of γ-oryzanol resemble those of typical surfactants, hence the incentive of this work to investigate its surface-active character and self-encapsulating performance in oil-in-water (O/W) emulsions and solid dispersions. The emulsions were fabricated by high-pressure homogenization (HPH), which depends importantly on interfacial tension (IT) reduction for successful preparation [9]. On the other hand, the dispersions were fabricated by solvent displacement (SDM) due to the limited effect that IT have on this method [10]. Our objective is to fabricate stable colloidal systems, encapsulating γ-Oryzanol, without emulsifier addition. We also want to investigate its self-stabilizing mechanism in the absence of emulsifiers.
2. Materials and methods
2.1. Materials
All products were purchased from Fujifilm Wako Pure Chemical Co. (Osaka, Japan) unless stated differently. According to the manufacturer, γ-oryzanol (97%+) consists of a mixture of triterpene alcohols and ferulic acid esters of phytosterols from rice bran oil and rice germ oil. The exact composition was not provided in the Product Specification Sheet. However, it is known that cycloartenyl ferulate, 24-methylcycloartenyl ferulate and campesteryl ferulate are the three major components of γ-oryzanol [11], accounting for approximately 80% of its composition [12]. Polyoxyethylene (20) sorbitan monooleate, Tween 80 (T80) (0.1 or 1%, w/w) was dissolved in double distilled water and filtered by a 0.45 μm PTFE membrane before utilization, while γ-oryzanol (0.1–1%, w/w) was dissolved in Long Chain Triglyceride oil, LCT (sunflower), Medium Chain Triglyceride oil, MCT (Taiyo Kagaku Co., Ltd., Tokyo, Japan), Small Chain Triglyceride oil, SCT (tributyrin) or ethanol (99.5%) overnight at room temperature.
2.2. Interfacial tension
Interfacial Tension (IT) was measured by the pendant-drop method (PD-W, Kyowa Interface Science Co., Ltd., Saitama, Japan), based on the optical determination of changes in the shape and size of pendant drops using an integrated visualization system. Briefly, the oil phase was injected into water using a 22-gauge stainless steel syringe needle, and a high-resolution camera captured then the images of the droplets to determine its dimensions, followed by interfacial tension calculation using the Young-Laplace equation. LCT and MCT oils have a lower density than water (917, 948 and 997 kg m−3, respectively), thus an upward inverted needle was used to immerse the oil phase into water. In contrast, SCT oil is denser than water (1032 and 997 kg m−3, respectively) and, therefore, a standard straight needle was used to generate the droplets.
2.3. ζ-potential
The ζ-potential of γ-oryzanol as a function of pH was determined following the protocol of Zembyla et al. [13]. Comprehensively, 0.1% (w/w) of γ-oryzanol was suspended in doubled distilled water and homogenized using a high-shear rate rotor-stator homogenizer at 10000 rpm for 5 min (Polytron PT-3000, Kinematica Inc., Luzern, Switzerland). The suspensions were then adjusted to the target pH (±0.1), using 0.1 or 1 M HCl or NaOH, and transferred immediately into folded capillary cells (DTS 1070, Malvern Instruments Ltd., Worces-tershire, UK) to measure their ζ-potential. The measurements were performed at room temperature using a scattered light angle of 173° (non-invasive backscatter detection), an equilibration time of 60 s, and refractive indexes of 1.467 and 1.330 for γ-oryzanol and water, respectively, and were recorded as an intensity-based average of 10–100 runs per sample. All measurements met the internal quality criteria set by the Malvern Zetasizer (Worces-tershire, UK).
2.4. High-pressure homogenization
The oil phase (5%, w/w), containing γ-Oryzanol, was added to distilled water or T80 solution and homogenized at 10 000 rpm for 5 min (Polytron PT-3000, Kinematica Inc., Luzern, Switzerland). The coarse emulsion was then homogenizer at 100 MPa for four cycles (NanoVater NV200, Yoshida Kikai Co., Ltd., Nagoya, Japan), and stored at 4 °C until analysis.
2.5. Solvent displacement method
The dispersions were fabricated by SDM, following the protocol of Shu et al. [14]. Briefly, the organic phase (1 mL), containing γ-Oryzanol, was injected into the aqueous phase (9 mL), consisting of distilled water or T80 solution, via a 26-gauge disposable syringe needle at 1 mL min−1 and 500 rpm for 6 min. The organic solvent (i.e., ethanol) was then eliminated by rotary evaporation at 60 hPa and 35 °C for 15 min, and the volume of the dispersions was completed to 10 mL with double distilled water or T80 solution to normalize γ-oryzanol concentration. The dispersions were stored immediately at 4 °C until analysis.
2.6. Particle characterization
The particle size characterization was performed by LS 13 320 Beckman Coulter (Brea, USA) or Malvern NanoZS Zetasizer (Worces-tershire, UK). The Beckman Coulter covers a particle diameter detection range from 40 nm to 2000 μm, while the Zetasizer covers a narrower range from 0.4 nm to 10 μm, so combined they allowed to characterize the different particles contained in the studied systems. The microstructures were observed by optical microscopy for the O/W emulsions (DM IRM, Leica Microsystems Pty., Ltd., Wetzlar, Germany) or transmission electron microscopy (Tecnai G2 F20, FEI Company, Oregon, USA) for the solid dispersions. For the emulsions, the samples (24 h of storage at 4 °C) were placed directly on the glass slides and observed with a ×40 magnification objective. For the dispersions, the samples were observed after 1 month of fabrication following the protocol of Tan et al. [15], using 1% phosphotungstic acid as staining agent and 120 KV as electron acceleration voltage. The ζ-potential characterization was performed using the same experimental settings presented in section 2.4, except that the emulsions were diluted with double distilled water (1/100) before measurement, while the dispersions were measured directly without dilution. All measurements performed by the Malvern Zetasizer met the internal quality criteria set by the equipment, unless stated differently.
2.7. Data analysis
All measurements were repeated 6 times on at least two independent samples and were analyzed, if applicable, by Tukey Post Hoc test (p-value ≤0.05), using Statistix analytical software ver. 8.1. (Tallahassee, FL, USA).
3. Results and discussion
3.1. Surface-active character
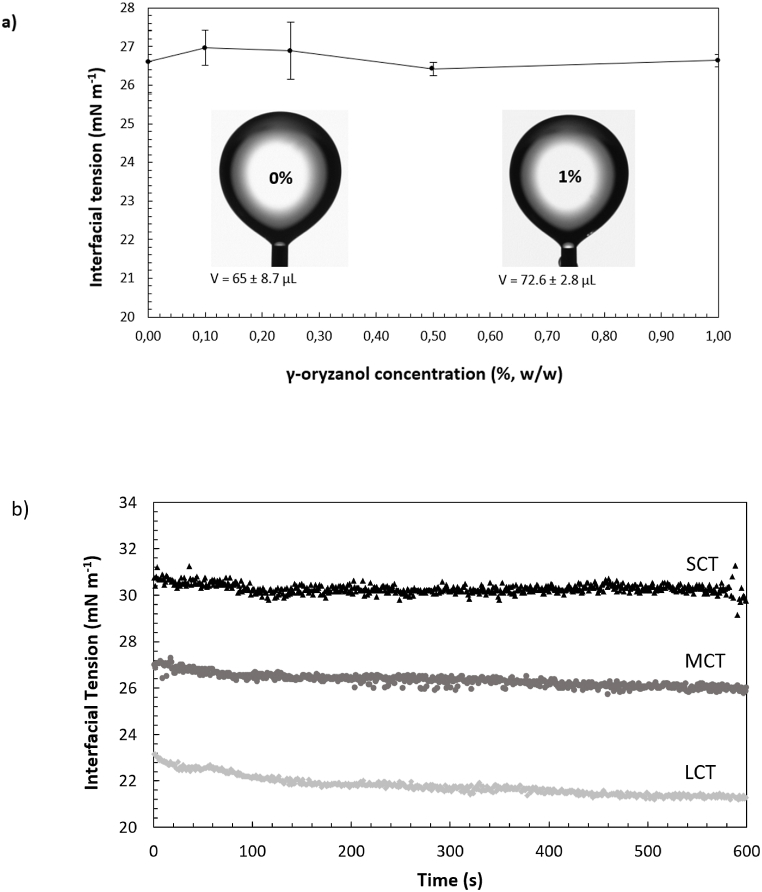
The IT did not reduce in the concentration range of 0.1–1% (w/w) (Fig. 1a). The IT was similar to that of the MCT oil/distilled water system (26.6 ± 0.8 mN m−1), indicating that γ-oryzanol was present exclusively in the oil phase rather than the oil/water interface. The pendant drop method is based on the optical determination of small changes in the shape and size of pendant drops using an integrated visualization system. Comprehensively, the elongation or shrinkage of droplets during the deposition of surface-active compounds at the liquid/liquid interface is converted into numerical values of IT by the Young-Laplace equation [16]. As shown in Fig. 1a, the morphology of the pendant droplets used for IT measurements remained fairly the same in the absence or presence of γ-oryzanol. Moreover, the volume of the droplets did not change significantly (p < 0.05) in both systems (Fig. 1a), confirming that this compound is not surface-active under the measurement conditions (i.e., room temperature and atmospheric pressure). Typical emulsifiers, such as polysorbates and phospholipids, are often surface-active at these concentrations and conditions. The critical micellar concentration of T80, for example, is less than 0.01% (w/w) [17], while soy lecithin can reduce the IT by up to 50% at concentrations as low as 0.1% (w/w) [18]. Selected bioactive compounds, such as glycyrrhizin and oleuropein, have been shown previously to reduce the IT at relatively low concentrations (< 1%, w/w) [19,20]. Moreover, crude emulsifying extracts from various plant origins, such as argan by-products, olive residue and bagasse, were very efficient in the presence of minor concentrations of bioactive surface-active compounds in their composition [[21], [22], [23]].
Fig. 1.
(a) Static IT of γ-oryzanol (0.1–1%, w/w) at the MCT oil/water interface, and morphology and volume (v) of the pendant drops containing 0 or 1% (w/w) γ-oryzanol dissolved in MCT oil and used for IT measurements. (b) Dynamic IT of LCT, MCT or SCT oil, containing 1% (w/w) of γ-oryzanol, at the oil/water interface.
The viscosity of the carrier solvent can have a noticeable effect on the interfacial activity of surfactants by delaying their deposition at the oil-water interface [24]. LCT and MCT oils have relatively high viscosities at room temperature (25 and 50 mPa s, respectively) [25,26], while the viscosity of SCT oil is less than 20 mPa s [27]. As shown in Fig. 1b, the type of oil did not affect the dynamic interfacial activity of γ-oryzanol samples. The difference of IT at t = 0s is due to the difference of the density of the oils, rather than the adsorption of γ-oryzanol on the interfaces. Moreover, the IT at the MCT oil/water and SCT oil/water systems remained unchanged for up to 600 s after incubation of the droplets (Fig. 1b), indicating that there was no delay in the displacement of molecules to these interfaces. The slight decrease in IT at the LCT oil/water interface can be attributed to the presence of small amounts of surface-active impurities, such as mono- and diglycerides and free fatty acids, in the LCT oil.
3.2. Ionizable character
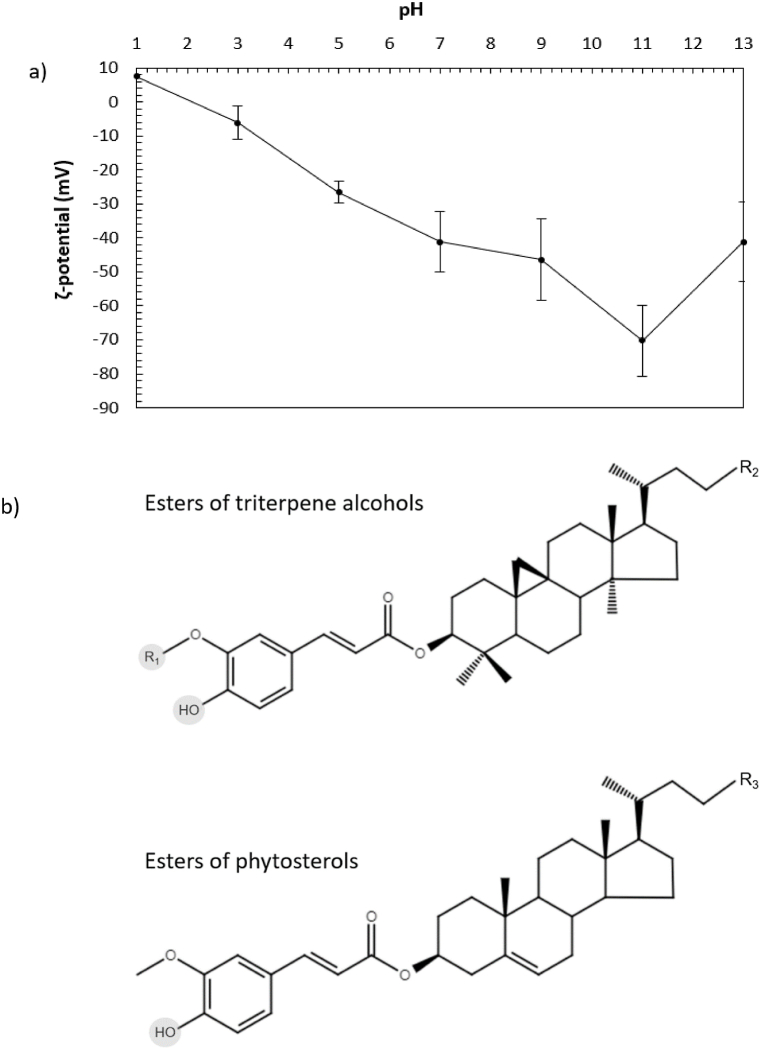
The absolute value of ζ-potential of γ-oryzanol suspensions gradually decreased when the pH of the system was lowered. Comprehensively, the particles had a minor positive charge at pH = 1, a fairly neutral charge at pH = 3 and strong negative charges in the pH range of 5–13 (Fig. 2a). ζ-Potential represent the surface charge of colloidal particles suspended in aqueous solutions. Therefore, the different charges reported hereafter originate potentially from the polar moieties of γ-oryzanol molecules, rather than their hydrophobic structures.
Fig. 2.
(a) ζ-Potential of γ-oryzanol particles dispersed in water as a function of pH. (b) Structural representation of γ-oryzanol main chemical species. R1 OH: ester caffeates. R1 CH3: ester ferulates. R2 = terminal radical of cycloartenol or methylenecycloartenol. R3 = terminal radical of campesterol or β-sistosterol. Shaded grey = ionizable groups. The structures were drawn using the online platform of Fisher Scientific by Marvin JS ChemAxon (https://www.fishersci.com).
While the pKa of the polar moieties of γ-oryzanol are known [28], the pKa of the entire molecules have not been reported yet in literature. Herrero-Martínez et al. [29] have determined the pKa of various molecules (catechin, epicatechin, resorcinol, kaempferol and quercetin) by capillary zone electrophoresis and compared them to the pKa values calculated by computational prediction. In general, the pKa of the hydroxyl groups of the quercetin nucleus ranged from 7.04 to 13.06. The pKa of kaempferol were about 7.11–13.26, while the pKa of the other flavonoids were all above 9. Hydroxyl groups of aromatic acids can undergo significant dissociation at relatively low pH, contributing potentially to the negative ζ-potential of their suspended particles. For example, the C7–OH of rutin undergoes significant dissociation from pH = 4 to pH = 8 (pKa ∼7.1), increasing the negative charge of rutin particles suspended in water as the pH is raised from 2 to 8 [13]. Similarly, the C3–OH group of the kaempferol nucleus of tiliroside dissociates appreciably between pH = 2 and pH = 8, resulting in a comparable profile of ζ-potential as a function of pH [30].
Highly charged particles are likely to be less surface-active, but higher charges can help forming and stabilizing fine dispersions [30]. Notably, glycyrrhizin was not surface-active at pH = 7, although it was possible to fabricate stable nanoemulsions by HPH, at neutral conditions, using this compound as a sole emulsifier. Comprehensively, increasing the pH > pKa increased the affinity of molecules for water and enhanced their electrostatic repulsion, leading to competitive adsorption at the oil/water interface when the IT was measured [20]. Therefore, it would be expected that colloidal systems containing γ-oryzanol can be maintained stable, without additives (i.e., emulsifiers, stabilizers), providing that small particles can be achieved during preparation.
3.3. Self-stabilizing character
IT reduction does not guarantee the ability of surfactants to fabricate stable emulsions or dispersions. Glycyrrhizin, for example, was efficient at producing stable nanosized droplets emulsions by HPH despite its poor affinity to the oil/water (pH = 7) interface at room temperature and atmospheric pressure [20]. In addition, several extracts that have been shown to reduce IT were unable to produce stable emulsions with HPH [22,31,32]. ζ-potential, on the other hand, is an important parameter to stabilize colloidal systems, including emulsions and dispersions, by generating electrostatic repulsive forces between the suspended particles [33].
In this section, we investigated the self-stabilizing performance of γ-oryzanol by HPH and SDM, aiming for stable particles, with an average (or mean) diameter < 200 nm; 200 nm is generally considered as the upper limit of nano-colloidal systems, as they are usually transparent below that level [34]. The volume mean droplet diameter (d4,3) and coefficient of variance (CV) of the emulsions were determined by laser diffraction due to the presence of large droplets that are undetectable by the Malvern Zetasizer (> 10000 nm), while the z-average particle size (dav) and the polydispersity index (PDI) of the dispersions were determined by dynamic light scattering as small particles (< 40 nm) are undetectable by the Beckman Coulter. For both instruments, the particle size was determined based on the Stokes-Einstein equation, which considers that the particles are spherical and do not interact [35]. Thus, we investigated the structures of the emulsions and the dispersions by optical microscopy and transmission electron microscopy, respectively, to confirm the accuracy of the particle size measurements.
3.3.1. O/W emulsions
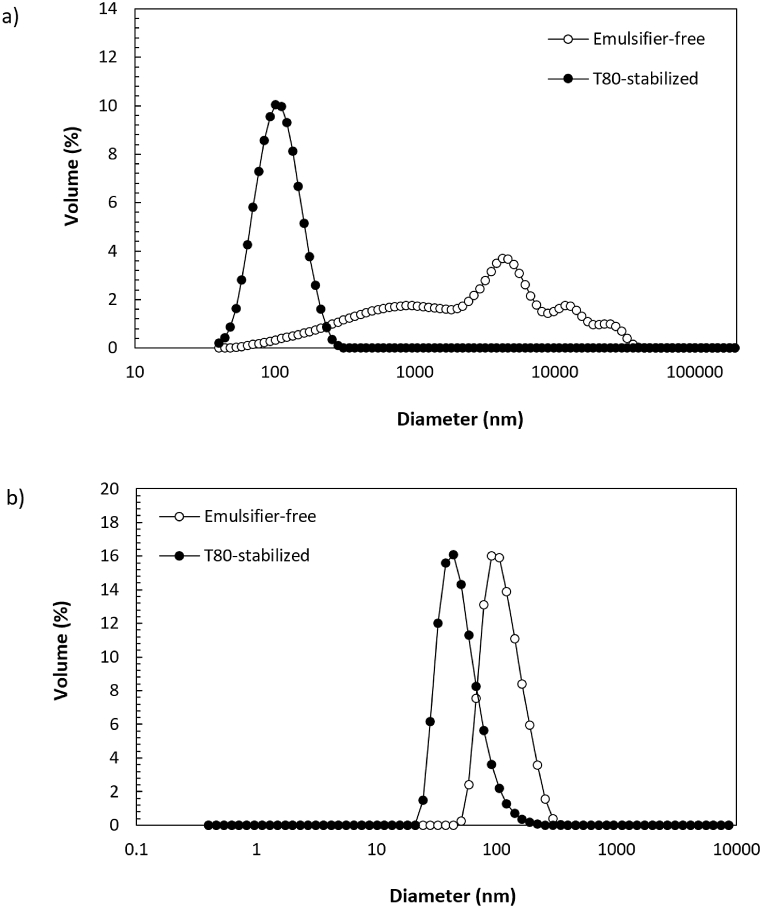
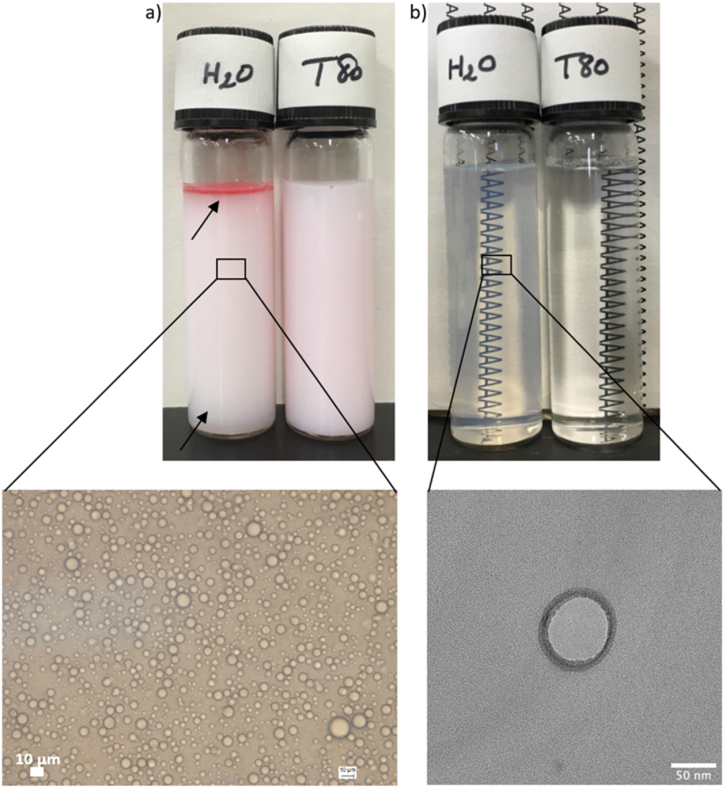
The target size of emulsions was achieved when using T80 as a model emulsifier (Table 1 and Fig. 3a), indicating that the processing conditions (100 MPa, 4 passes) that were used in these experiments were sufficient to fabricate small droplet size emulsions. In contrast, emulsions prepared using γ-oryzanol, without added emulsifier, showed larger particle sizes and broader size distributions at similar homogenization conditions (Table 1, Fig. 3a). Comprehensively, the d4,3 of emulsifier-free emulsions was approximately 4000 nm, while their average CV was higher than 80% (Table 1). Moreover, they were unstable after only 24 h of storage at 4 °C with evident oiling-off and phase separation (Fig. 4).
Table 1.
Volume mean droplet diameter (d4,3), z-average particle diameter (dav), coefficient of variance (CV) and polydispersity index (PDI) of γ-oryzanol O/W emulsions and solid dispersions fabricated using water (no added emulsifier) or aqueous phases of T80 as a model emulsifier.
| Emulsions |
Dispersions |
|||
|---|---|---|---|---|
| d4,3 (nm) | CV (%) | dav (nm) | PDI (−) | |
| Emulsifier-free | 3996 ± 953 | 82 ± 35 | 114 ± 10 | 0.09 ± 0.02 |
| T80 stabilized | 122 ± 5 | 27 ± 1 | 78 ± 8 | 0.18 ± 0.03 |
Fig. 3.
Particle size distribution of γ-oryzanol (a) O/W emulsions and (b) solid dispersions fabricated using purified water (no added emulsifier) or aqueous phases of T80 as a model emulsifier.
Fig. 4.
Visual appearance and microscopic observation of γ-oryzanol (a) O/W emulsions and (b) solid dispersions fabricated using purified water (no added emulsifier) or aqueous phases of T80 as a model emulsifier. For better visualization, 0.002% (w/w) of Sudan dye was added to MCT oil, containing 1% (w/w) of γ-oryzanol, before emulsion preparation. Emulsion's scale = 10 μm. Dispersion's scale = 50 nm.
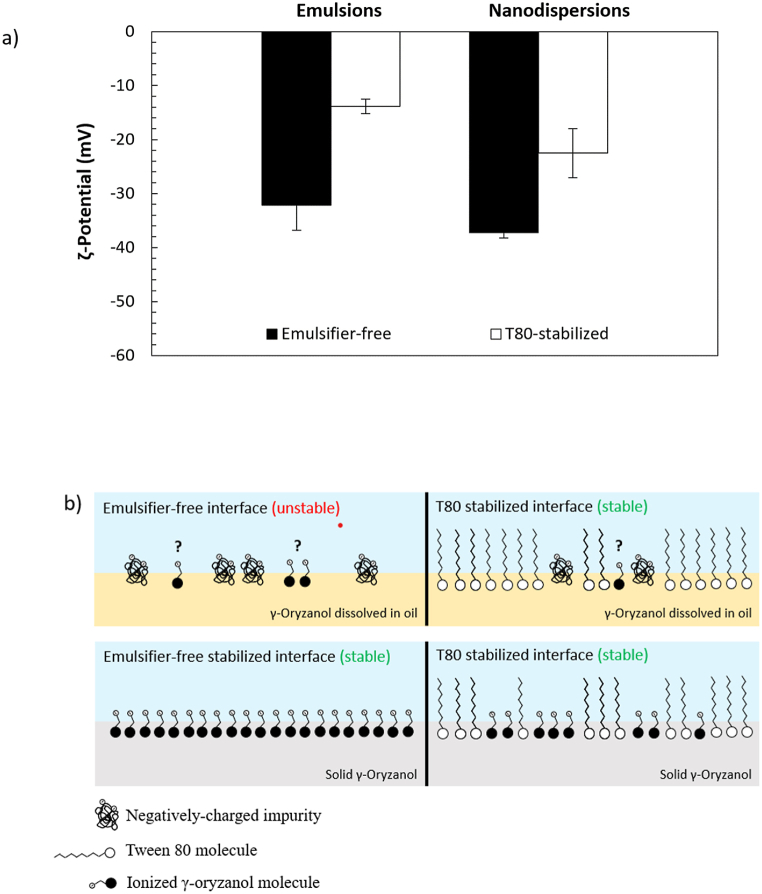
HPH can break up the oil phase into small spherical droplets of a few micrometres in diameter without the addition of emulsifiers [9]. The role of emulsifiers consists primarily of (i) decreasing the size of the droplets by reducing the IT and (ii) developing an interfacial layer around the formed droplets to protect them against recoalescence and/or aggregation [3]. Although the emulsions were unstable (Fig. 4), it is possible that γ-oryzanol adsorbed at the droplets' interface during homogenization, without necessarily providing adequate repulsive forces to prevent destabilization. We measured, therefore, the ζ-potential of γ-oryzanol-loaded emulsions and compared it to those fabricated with only MCT oil and purified water (control). Overall, the samples had a relatively higher ζ-potential (−32 mV) than the control emulsions (−24 mV). However, the ζ-potential of these later did not meet the internal quality criteria set by the Malvern Zetasizer; thus, it was not possible to draw conclusive remarks about its magnitude nor origin. For the of T80-stabilized emulsions, the ζ-potential was approximately −14 mV (Fig. 5a), contributed potentially by the presence of freely dissolved hydroxyl ions on the droplets’ interface [36]. The significant (p < 0.05) reduction of ζ-potential of these emulsions, compared to those fabricated without emulsifier, can be attributed to the coverage of the interfaces with uncharged polysorbate headgroups (Fig. 5b), which lead partially to the desorption of ionizable impurities from the interface.
Fig. 5.
(a) ζ-Potential of γ-oryzanol O/W emulsions and solid dispersions fabricated using purified water (no added emulsifier) or aqueous phases of T80 as a model emulsifier. (b) Illustration of the potential stabilization mechanisms of emulsifier-free and T80 stabilized γ-oryzanol O/W emulsions and solid dispersions fabricated by HPH and SDM, respectively.
3.3.2. Solid dispersions
The dav and the PDI of emulsifier-free γ-oryzanol dispersions were approximately 110 nm and 0.1 (−), respectively (Table 1). The particles were freely dispersed and spherical (Fig. 4), as estimated by the Stokes–Einstein equation. However, they showed relatively smaller diameters, compared to dav measurements, probably due to the formation of an extra solvent layer around the solvated particles, which was also considered by the Malvern Zetasizer for hydrodynamic diameter hypothetical estimation [37]. On the other hand, T80 produced smaller particles (dav = 78 nm), although higher PDI were obtained when using it as an emulsifier (Table 1). Nevertheless, both dispersions exhibited a monomodal size distribution (Fig. 3b) and showed no signs of aggregation or precipitation up to one month of storage at 4 °C (Fig. 4).
The particle formation mechanism by SDM is often explained the Gibbs-Marangoni effect or the Ouzo effect [38]. The Gibbs–Marangoni effect suggests that the particles are formed via interfacial tension turbulence at the organic/aqueous interface [39,40], while the ouzo effect suggests that the particles are formed simply due to spontaneous organic phase diffusion [10]. In both cases, interfacial repulsion between the generated particles is important for successful formulation by SDM. The emulsifier-free γ-oryzanol-loaded dispersions exhibited a ζ-potential of −37.35 mV (Fig. 5a); the surface charge of the particles stabilized with T80 was about −23 mV (Fig. 5a), suggesting that γ-oryzanol and polysorbate molecules coexisted simultaneously at their interface (Fig. 5b). These results suggest that stable γ-oryzanol dispersions can be successfully prepared by SDM without adding any type of stabilizer (e.g., emulsifier), due to the ability of γ-oryzanol molecules to generate electrostatic repulsion, preventing particles from aggregation.
4. Conclusions
γ-Oryzanol had poor interfacial activity as indicated by the PDM, resulting in unsuccessful emulsification by HPH due to the importance of IT reduction to fabricate stable emulsions by this method. In contrast, stable dispersions were prepared with γ-oryzanol by SDM, which can be attributed to the spontaneous particles’ formation and electrostatic repulsion. The results of this study highlight the potential of encapsulating bioactive compounds, such as γ-oryzanol, into stable particles without the addition of emulsifiers. Further studies can evaluate the self-encapsulating properties of other bioactive compounds by this method, regardless of their surface-active character.
Author contribution statement
Noamane Taarji: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Meryem Bouhoute: Lorena de Oliveira Felipe: Performed the experiments.
Mansour Sobeh: Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hasenhuettl G.L. In: Synthesis and Commercial Preparation of Food Emulsifiers Bt - Food Emulsifiers and Their Applications. Hasenhuettl G.L., Hartel R.W., editors. Springer International Publishing; Cham: 2019. pp. 11–39. [DOI] [Google Scholar]
- 2.D. Kregiel, J. Berlowska, I. Witonska, H. Antolak, C. Proestos, M. Babic, L. Babic, L.B. and B. Zhang, Saponin-based, Biological-Active Surfactants from Plants, in: 2021. doi:10.5772/68062.
- 3.McClements D.J., Decker E. Interfacial antioxidants: a review of natural and synthetic emulsifiers and coemulsifiers that can inhibit lipid oxidation. J. Agric. Food Chem. 2018;66:20–35. doi: 10.1021/acs.jafc.7b05066. [DOI] [PubMed] [Google Scholar]
- 4.Panya A., Laguerre M., Bayrasy C., Lecomte J., Villeneuve P., McClements D.J., Decker E.A. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions. J. Agric. Food Chem. 2012;60:2692–2700. doi: 10.1021/jf204848b. [DOI] [PubMed] [Google Scholar]
- 5.González M.J., Medina I., Maldonado O.S., Lucas R., Morales J.C. Antioxidant activity of alkyl gallates and glycosyl alkyl gallates in fish oil in water emulsions: relevance of their surface active properties and of the type of emulsifier. Food Chem. 2015;183:190–196. doi: 10.1016/j.foodchem.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Khalid N., Kobayashi I., Neves M.A., Uemura K., Nakajima M., Nabetani H. Encapsulation of β-sitosterol plus γ-oryzanol in O/W emulsions: formulation characteristics and stability evaluation with microchannel emulsification. Food Bioprod. Process. 2017;102:222–232. doi: 10.1016/j.fbp.2017.01.002. [DOI] [Google Scholar]
- 7.Zhong J., Liu X., Wang Y., Qin X., Li Z. γ-oryzanol nanoemulsions produced by a low-energy emulsification method: an evaluation of process parameters and physicochemical stability. Food Funct. 2017;8:2202–2211. doi: 10.1039/c7fo00023e. [DOI] [PubMed] [Google Scholar]
- 8.Rodsuwan U., Pithanthanakul U., Thisayakorn K., Uttapap D., Boonpisuttinant K., Vatanyoopaisarn S., Thumthanaruk B., Rungsardthong V. Preparation and characterization of gamma oryzanol loaded zein nanoparticles and its improved stability. Food Sci. Nutr. 2021;9:616–624. doi: 10.1002/fsn3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroën K., de Ruiter J., Berton-Carabin C. The importance of interfacial tension in emulsification: connecting scaling relations used in large scale preparation with microfluidic measurement methods. ChemEngineering. 2020;4 doi: 10.3390/chemengineering4040063. [DOI] [Google Scholar]
- 10.Ganachaud F., Katz J.L. Nanoparticles and nanocapsules created using the ouzo effect: spontaneous emulsification as an alternative to ultrasonic and high-shear devices. ChemPhysChem. 2005;6:209–216. doi: 10.1002/cphc.200400527. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z., Godber J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999;47:2724–2728. doi: 10.1021/jf981175j. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z., Godber J.S. Antioxidant activities of major components of γ-oryzanol from rice bran using a linoleic acid model. JAOCS (J. Am. Oil Chem. Soc.) 2001;78:645. doi: 10.1007/s11746-001-0320-1. [DOI] [Google Scholar]
- 13.Zembyla M., Murray B.S., Radford S.J., Sarkar A. Water-in-oil Pickering emulsions stabilized by an interfacial complex of water-insoluble polyphenol crystals and protein. J. Colloid Interface Sci. 2019;548:88–99. doi: 10.1016/j.jcis.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Shu G., Khalid N., Tan T.B., Zhao Y., Neves M.A., Kobayashi I., Nakajima M. Comparison of ergocalciferol nanodispersions prepared using modified lecithin and sodium caseinate: insights of formulation, stability and bioaccessibility. J. Funct.Foods. 2017;38:28–35. doi: 10.1016/j.jff.2017.08.047. [DOI] [Google Scholar]
- 15.Tan T.B., Yussof N.S., Abas F., Mirhosseini H., Nehdi I.A., Tan C.P. Forming a lutein nanodispersion via solvent displacement method: the effects of processing parameters and emulsifiers with different stabilizing mechanisms. Food Chem. 2016;194:416–423. doi: 10.1016/j.foodchem.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Bagalkot N., Hamouda A.A., Isdahl O.M. Dynamic interfacial tension measurement method using axisymmetric drop shape analysis. MethodsX. 2018;5:676–683. doi: 10.1016/j.mex.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y., Leser M.E., Sher A.A., McClements D.J. Formation and stability of emulsions using a natural small molecule surfactant: quillaja saponin (Q-Naturale®) Food Hydrocolloids. 2013;30:589–596. doi: 10.1016/j.foodhyd.2012.08.008. [DOI] [Google Scholar]
- 18.Chung C., Sher A., Rousset P., Decker E.A., McClements D.J. Formulation of food emulsions using natural emulsifiers: utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017;209:1–11. doi: 10.1016/j.jfoodeng.2017.04.011. [DOI] [Google Scholar]
- 19.Souilem S., Kobayashi I., Neves M.A., Jlaiel L., Isoda H., Sayadi S., Nakajima M. Interfacial characteristics and microchannel emulsification of oleuropein-containing triglyceride oil–water systems. Food Res. Int. 2014;62:467–475. doi: 10.1016/j.foodres.2014.03.049. [DOI] [Google Scholar]
- 20.Taarji N., Bouhoute M., Fainassi F., Hafidi A., Kobayashi I., Neves M.A., Tominaga K., Isoda H., Nakajima M. Interfacial and emulsifying properties of purified glycyrrhizin and non-purified glycyrrhizin-rich extracts from liquorice root (Glycyrrhiza glabra) Food Chem. 2021;337 doi: 10.1016/j.foodchem.2020.127949. [DOI] [PubMed] [Google Scholar]
- 21.Bouhoute M., Taarji N., Vodo S., Kobayashi I., Zahar M., Isoda H., Nakajima M., Neves M.A. Formation and stability of emulsions using crude extracts as natural emulsifiers from Argan shells. Colloids Surf. A Physicochem. Eng. Asp. 2020:591. doi: 10.1016/j.colsurfa.2020.124536. [DOI] [Google Scholar]
- 22.Fainassi F., Taarji N., Benkhalti F., Hafidi A., Neves M.A., Isoda H., Nakajima M. Emulsion formation and stabilizing properties of olive oil cake crude extracts. Processes. 2021;9 doi: 10.3390/pr9040633. [DOI] [Google Scholar]
- 23.Vodo S., Taarji N., Bouhoute M., de Oliveira Felipe L., Neves M.A., Kobayashi I., Uemura K., Nakajima M. Potential of bagasse obtained using hydrothermal liquefaction pre-treatment as a natural emulsifier. Int. J. Food Sci. Technol. 2020 doi: 10.1111/ijfs.14543. n/a. [DOI] [Google Scholar]
- 24.Lloyd D.M., Norton I.T., Spyropoulos F. Processing effects during rotating membrane emulsification. J. Membr. Sci. 2014;466:8–17. doi: 10.1016/j.memsci.2014.04.035. [DOI] [Google Scholar]
- 25.Diamante L.M., Lan T. Absolute viscosities of vegetable oils at different temperatures and shear rate range of 64.5 to 4835 s−1. Journal of Food Processing. 2014;2014 doi: 10.1155/2014/234583. [DOI] [Google Scholar]
- 26.Zhang N., Liu C., Jin L., Zhang R., Siebert H.-C., Wang Z., Prakash S., Yin X., Li J., Hou D., Sun B., Liu M. Influence of long-chain/medium-chain triglycerides and whey protein/tween 80 ratio on the stability of phosphatidylserine emulsions (o/w) ACS Omega. 2020;5:7792–7801. doi: 10.1021/acsomega.9b03702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eiteman M.A., Goodrum J.W. Density and viscosity of low-molecular weight triglycerides and their mixtures. JAOCS (J. Am. Oil Chem. Soc.) 1994;71:1261. doi: 10.1007/BF02540548. [DOI] [Google Scholar]
- 28.Aguilar-Hernández I., Afseth N.K., López-Luke T., Contreras-Torres F.F., Wold J.P., Ornelas-Soto N. Surface enhanced Raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib. Spectrosc. 2017;89:113–122. doi: 10.1016/j.vibspec.2017.02.002. [DOI] [Google Scholar]
- 29.Herrero-Martínez J.M., Sanmartin M., Rosés M., Bosch E., Ràfols C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis. 2005;26:1886–1895. doi: 10.1002/elps.200410258. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z., Murray B.S., Ross A.-L., Povey M.J.W., Morgan M.R.A., Day A.J. Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloids Surf. B Biointerfaces. 2012;92:84–90. doi: 10.1016/j.colsurfb.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Ralla T., Herz E., Salminen H., Edelmann M., Dawid C., Hofmann T., Weiss J. Emulsifying properties of natural extracts from panax ginseng l. Food Biophys. 2017;12:479–490. doi: 10.1007/s11483-017-9504-5. [DOI] [Google Scholar]
- 32.Bouhoute M., Taarji N., Vodo S., Kobayashi I., Zahar M., Isoda H., Nakajima M., Neves M.A. Formation and stability of emulsions using crude extracts as natural emulsifiers from Argan shells. Colloids Surf. A Physicochem. Eng. Asp. 2020;591 doi: 10.1016/j.colsurfa.2020.124536. [DOI] [Google Scholar]
- 33.Lu G.W., Gao P. In: Personal Care & Cosmetic Technology. T.-H V.S.B., Kulkarni N.-I.D.D.S., editors. William Andrew Publishing; Boston: 2010. Chapter 3 - emulsions and microemulsions for topical and transdermal drug delivery; pp. 59–94. [DOI] [Google Scholar]
- 34.Chuo S.C., Mohd Setapar S.H. In: Micro and Nano Technologies. Mohd Setapar S.H., Ahmad A., T.-N M.B., P, Jawaid C.U.P.-B.E., editors. Elsevier; 2022. 15 - application of nanoemulsion in cosmetics; pp. 355–371. [DOI] [Google Scholar]
- 35.Klang V., Matsko N.B., Valenta C., Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43:85–103. doi: 10.1016/j.micron.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y., Leser M.E., Sher A.A., McClements D.J. Formation and stability of emulsions using a natural small molecule surfactant: quillaja saponin (Q-Naturale ®) Food Hydrocolloids. 2013;30:589–596. doi: 10.1016/j.foodhyd.2012.08.008. [DOI] [Google Scholar]
- 37.Maguire C.M., Rösslein M., Wick P., Prina-Mello A. Characterisation of particles in solution - a perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018;19:732–745. doi: 10.1080/14686996.2018.1517587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora-Huertas C.E., Fessi H., Elaissari A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: critical comparison. Adv. Colloid Interface Sci. 2011;163:90–122. doi: 10.1016/j.cis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Quintanar-Guerrero D., Allémann E., Fessi H., Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev. Ind. Pharm. 1998;24:1113–1128. doi: 10.3109/03639049809108571. [DOI] [PubMed] [Google Scholar]
- 40.Galindo-Rodriguez S., Allémann E., Fessi H., Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharmaceut. Res. 2004;21:1428–1439. doi: 10.1023/b:pham.0000036917.75634.be. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.