Time estimation is a necessary mechanism for most cognitive functions. Common theories of temporal cognition therefore position the sense of time as part of central cognitive processing, influenced by perception, memory, and affective state, and used for timing judgements and timed responses. In contrast to stages of processing models, action-perception theory argues that action-representations alter perception and cognition. Here, we propose that the dynamic, sensorimotor relationship between intention and action outcomes determine time perception. This hypothesis was tested in three experiments (total N = 94, 57 female, 34 male, 3 non-binary, age 28.6, SD 7.3 years) that combined a motor control task with a temporal reproduction paradigm. Within a timed interval (T1), participants tracked a linear moving target using a pointing device, then matched T1 by manually reproducing it. Despite the tracking being independent of T1's objective duration, the experiments consistently showed subjective time was biased by action and perception properties. Experiment 1 showed distance of tracking target and sensorimotor resistance increased temporal estimates bias while delay of visual action outcomes reduced these bias. Experiment 2 replicated these findings in an online sample, while Experiment 3 partially extended them towards temporal anticipation. Across studies, experimental manipulation of cognitive demands and use of passive control conditions show that neither attentional mechanisms nor perceptual differences can account for the observed effects on time estimation. Instead, we argue that action representations alter time perception and that our sense of time is shaped by the intentions behind, motions during, and consequences of our behaviour in time and space.
1. Introduction
Whether we are boiling an egg, waiting for a traffic light, or playing an instrument in an orchestra, estimating the duration of time is an important capability that enables us to function in a world that is constantly changing. Traditionally, cognitive scientists have explained this ability by inferring a central time-keeping mechanism that works similarly to a natural biorhythm or internal clock; a hypothetical, sensory organ of time. The principal model, scalar expectancy theory [1,2], involves a pacemaker, an ‘internal clock’ [3,4] ticking at a variable but relatively consistent speed, and an accumulator, collecting ticks if an attentional switch requires a temporal judgement. Despite its simplicity, the model accounts for a variety of empirical findings of distortions in time perception [5,6]. For example, temporal dilation, or overestimating objective time subjectively, may occur both as a result of arousal speeding up the pacemaker (e.g. [7]) and from novel, deviant stimuli increasing attention, causing more ticks to be accumulated [8].
Given the positioning of the pacemaker and accumulator as central cognitive mechanisms, it is perhaps understandable that scarce attention has historically been given to the role of action planning and execution in perception of time. After all, in the classic, cognitivist stimulus-processing paradigm, temporal effects of perception and attention will ultimately change how time is reported, but how time is reported should not change its perception1. Thus, while action control and motor execution are necessary for any time estimation task, these are typically understood as process-pure indicators of how timing is affected by the perceptual or central variables under investigation. Consequently, while time perception clearly affects action, relatively scant attention has been paid to the question of whether action affects time perception.
If we take perception, however, as a cognitive function with a behavioural goal of steering action towards important stimuli [10], then it becomes plausible to assume action is as central to perception of time as it is to other modalities. Here, we argue on the basis of two theories that perception of the consequences of our actions determines subjective time perception. First, according to the sensorimotor account of visual consciousness [11], perception entails the knowledge of how movements result in sensory consequences. Given that movement is necessarily temporally defined, our experience of time may derive from the dynamic sensorimotor unfolding of action. Second, according to ideomotor theory, voluntary action results from the anticipation of the perceptual consequences of action, while we have otherwise no conscious access to the motor system itself (c.f. James, 1890, p. 501–502, [12]). Temporal cognition is only implicit in the theory, but is essential to observing an effect like intentional binding, in which sensory consequences to voluntary action are estimated as occurring earlier in time than those following involuntary motion [13]. Taking these theories together, we hypothesize that the dynamic, sensorimotor relationship between intention and action outcomes defines the subjective perception of time.
One source of evidence in support of an integrated action-perception theory of time comes from work on action timing and imaginary action. Indeed, the body of evidence related to action simulation theory [14] underlines how action itself, including its planning, execution, and motor control, necessarily involves temporal cognition to exercise dynamic control. For example, Decety et al. [15] studied the time used to imagine moving from one place to another and observed that this time strongly correlated with the movement time if the same participant were to actually move to the position. Moreover, motor parameters that affect action execution, such as physical abilities and environmental constraints, similarly alter imaginary action [16]. Action simulation theory thus makes a strong point that motor processes, for example represented by subliminal activation of the motor cortex, are used to estimate the duration of actions, whether imaginary or real. Importantly, however, although the theory involves both time and action, it makes no claim on action affecting perception of external (i.e. non-imagined) stimuli, their timing, or the perceived duration of actions.
There are, however, indications that the subjective durations of perceptual stimuli are affected by imaginary actions. Using two temporal cognition tasks, we previously [17] investigated whether action imagery would alter the estimated durations of videos. In one task, participants were asked to either directly report the duration of visual stimuli, in the other to keep a constant pace of tapping while watching the stimuli. Critically, while doing so, participants were requested to imagine running faster and faster or walking slower and slower. These two conditions resulted in respectively temporal overestimation and underestimation of the video duration and a related gradual increasing or decreasing tapping speed as a function of mental imagery. It therefore appears that imaginary action affects the timing of stimuli as well as the timing of action, but this does not answer whether actual action alters time perception.
The second source of evidence comes from the recent body of work investigating the subjective time of sensory intervals within periods of motor activity or inactivity (for a recent review see [18]). For example, Yokosaka et al. [19] showed visual intervals presented while participants made unrelated movements, and in comparing these against probes (without movements) using a method of constant stimuli, found relative temporal compression for fast movements. Interestingly, not only does movement itself alter interval timing, but various parameters of movements have been shown to affect temporal judgements in surprising ways. For example, auditory intervals presented while manipulating a robotic arm showed temporal compression as a function of the amount of viscosity introduced to the arm, which in turn reduced the distance of the movement [20]. Duration of movements likewise affects subjective timing: executing short and long movements bias judgements of auditory intervals towards congruent responses, suggesting a type of cross-modal integration [21]. On the other hand, the direction of hand movements as either pointing towards or away from the body has been shown to influence timing, showing that time perception is affected by even relatively subtle, configural motor parameters [22]. Finally, irrelevant movements have been shown to not only bias temporal judgements, but also affect their precision. For example, restricting movements has been shown to reduce accuracy of timing in bisection and temporal reproduction tasks [23,24].
The evidence thus shows the timing of visual and auditory intervals is affected by ongoing movement, although the degree to which this due to decisional effects as opposed to a purely perceptual effect of action on time can be hard to determine. Bisection tasks (as used by Refs. [20,23]) in particular make the assumption that episodic traces are retained over a long period of time for comparison against novel stimuli. Cue-probe comparisons suffer to a lesser extent of a reliance on memory, yet as this paradigm still invokes response categories (as long or short), which may be easily primed by an ideomotor effect (c.f [25]), a contamination of judgement by interference from long or short action categorisations. To avoid such problems, some studies used computational models to separate perceptual from decisional effects and explicitly compared outcomes from the bisection task with the temporal reproduction task, since the latter eliminates or at least reduces requirements related to the use of response categories [20].
A further limitation of most of the studies mentioned is the degree to which movements are dissociated from perceptual events. Seeing fast moving stimuli has long been known to alter their subjective timing [26,27], while the spatial distance between two visual cues increases the perceived temporal asynchrony, a phenomenon known as the kappa-effect [28]. Careful steps need therefore be taken to separate motoric from perceptual events, for example by hiding the hand movements [19]. However, this omits the fact that hiding visually does little to reduce tactile sensations accumulating as a hand glides over a glass plate or manipulates a robotic arm. Strictly speaking therefore, much of the literature may have shown only that haptic feedback resulting from actions alter cross-modal interval judgements. As perception-action theories tend to view action and perception as by their nature integrated, we believe a more fruitful approach may be therefore by to take it as a given that action involves sensory elements, and to investigate how the relation between motor movements and sensory consequences define time estimation.
2. Present study
To investigate the causal role of actions in time perception, we used a time reproduction task, in which a standard interval was marked by two visual cues and then manually reproduced using a key press. Critically, in between the two visual markers, participants engaged in a motor control task based on Spapé & Serrien (2010), during which they tracked a target moving from the centre of the screen to the periphery using a cursor controlled by drawing with a digital pen on a tablet. The key question was whether the time reproduction would be influenced by the task characteristics of the motor control task, even though the latter was experimentally independent from the time perception task. Four factors were manipulated during the motor control task to determine the role of the three essential aspects of action: its goal, the execution, and its related effects. First, the goal of the action was defined by the tracking distance between start and destination of tracking (Fig. 1, I). Second, the execution was defined by the motor resistance (II), or the inverse of the pen's sensitivity. Third, the action effect was operationalised as a sensorimotor delay between movement and visual feedback (III). To disentangle action-effects from cognitive effects, such as attentional requirements, we furthermore implemented a perturbation using an angular tracking distortion of pen movements (IV), requiring significant adjustment to stay on target [29]. Finally, to dissociate perceptual effects from action-related effects, a pure ‘observation’ condition was included, in which recorded tracking motions were visually replayed while no actual movements were performed.
Fig. 1.
Experiment procedure. Top: Following a directional cue (a), the green circle indicated standard interval (T1) onset (b). During T1 (c), a stylus was used to draw (unfilled circle) continuously from the centre to the shown destination (asterisk) while following a target (filled circle) for 1500, 2500, or 3500 ms. The red circle (d) then indicated the end of T1, which was followed by an inter-stimulus interval (e) of 500–1000 ms). The same sequence of events then played out (f, g, and h being respectively Start T2, T2, and End T2), but now participants self-timed T2 by pressing a key when T2 subjectively matched T1. Bottom: schematic illustration of how actual movement (M, straight arrows) translates to visual feedback (dashed arrows) during T1 as a function of (I) distance of destination (in pixels), (II) resistance of movement (physical movement (M)/resistance = visualised movement), (III) delay of movement (in ms), and (IV) perturbation (in degrees).
Note that apart from these movement parameters and the reproduction task itself – cue-probe and bisection tasks being more common – there were two critical differences with the discussed literature. First, the movement period occurred within the timed period, as opposed to having a longer period of movement within which the timed interval occurs. Essentially, we therefore investigate whether the narrow period of movement affects the wider context of the visually presented interval as opposed to the other way around. Second, while the movement execution and temporal task were physically independent, they were connected within the same visual modality. Rather than strictly separating and hiding congruent action, we aimed to instead manipulate sensory, motoric, and sensorimotor parameters of the task and relate these to temporal cognition.
3. Experiment 1
In Experiment 1, we tested whether actions performed during interval timing affected estimated time. If time perception is independent from actions made during the motor control task, none of the factors related to action goals, executions, or outcomes should affect time perception. Secondly, if any effect would still be observed, but merely be related to visual differences, it should affect temporal reproduction both during the real, ‘performance’ conditions and observation-only conditions. Specifically, if actions do affect time perception then we would predict both distance and resistance should increase reproduced times, while delay should reduce it (predictions pre-registered in more detail at https://osf.io/8dc3b/wiki/Predictions/). The effects of resistance and delay should furthermore critically depend on motor activity and therefore not replicate in the observation-only task. Finally, if the effect of motor control on attention determines time perception, then perturbation should affect time perception in similar direction to resistance and delay.
4. Methods
4.1. Participants
A pilot study was conducted to determine expected effect sizes for all effects under consideration, following which G*Power was used to determine the number of participants required to attain sufficient (95%) power for each. This suggested a sample between N = 13 and N = 23. To accommodate for fallout due to technical errors and human performance, a sample of N = 26 volunteers was recruited via opportunity sampling. Of these, 16 were female, 10 male, their age was between 19 and 59 (M = 30.27, SD = 9.26) years, 25 reported being right-handed, 1 left-handed, and the majority were undergraduate or postgraduate students from the University of Helsinki. Participants received full instructions regarding the experimental procedure and were made aware of their rights, including the right to withdraw from the study at any point in time during the study or two months after, without fear of negative consequences. The study was in accordance with the guidelines set out by the University of Helsinki's Ethical Committee in Social and Behavioural Sciences (#422021).
4.2. Stimuli and apparatus
The experiment made use of a motor control task, a time reproduction task, and a sense of agency questionnaire, presented using E-Prime 3.0.3.82 running on Windows 10 PC. Visual stimuli were presented using a 21” LCD screen running at 60 Hz and a resolution of 1920 × 1080 px, while responses were captured using a USB keyboard and a Wacom Intuos 5 Touch tablet (27.5 × 19.0 cm) with stylus. During the motor control task, this tablet was used to move a ring-shaped cursor (r = 15 px) from the centre of a large, grey circle (r = 350 px) towards a destination asterisk (r = 10 px) placed 150 or 300 px off centre at an angle of 45⁰, 135⁰, 225⁰ or 315⁰. Participants were instructed to follow a tracking target, a black circle (radius = 5 px), which moved at a linear velocity to reach the destination within 1500, 2500, or 3500 ms, keeping it surrounded by the cursor as much as possible. To do this, they drew with the stylus from the centre of the tablet in the cued, diagonal direction for a distance of ca. 1.80 cm (for destinations of 150 px) or 3.61 cm (for 300 px).
As illustrated in Fig. 1 (lower panels), four aspects of the task were independently manipulated. First, the distance of the destination was either 150 or 300 px. This distance corresponded to the visual goal of the movement, but not necessarily the amount of movement, since this was defined by both the degree to which a participant stayed on target, and the resistance. Second, this resistance redefined the degree of physical movement on the tablet to visual movement on the screen by a factor 1 (normal speed) or 2 (half speed) in a continuous manner. That is, if the participant moved the pen on the tablet within one frame of the sampling frequency (at ca 30 Hz) to a distance normally corresponding to 2 px, a resistance of 2 would reduce the length of movement to 1 px2. Consequently, a destination distance of 150 px, which would normally require 1.80 cm of stylus movement needed ca. 3.61 cm to complete the same trajectory with a resistance of 2. Third, the delay was implemented by recording the visual movements, and playing these back 0 (no delay), 3 (100 ms delay) or 10 (333 ms) frames later. Note that this does not mean a shorter period of manual movement: participants still started their movement on cue, but their cursor only followed these movements later. Nor did it mean a longer period of visual feedback: both tracking target and cursor were removed from view following the signal ending T1. Fourth, the perturbation was a directional distortion of visual feedback, causing movements to appear rotated by an angle of 0⁰, 10⁰, or 25⁰. A video demonstration of the task is provided in the supplementary material (SI 1).
Parameters and movements were recorded for use in observation trials, during which participants did not use the tablet, instead passively viewing the drawings. The time reproduction task (Fig. 1, f-h) that immediately followed the motor control task used the same visual presentation, except without tracking target or cursor.
4.3. Procedure
Following signing of informed consent, participants were given more detailed task instructions, watched a demonstration video and undertook training during a short block of 8 trials before starting the experiment. Training trials were similar to the normal experiment, but with longer trial durations (1500–6000 ms), and without any experimental manipulations (no delay or perturbation and distance and resistance halfway between the experimental parameters). After completing this, participants undertook three experimental blocks of 108 trials each, of which one was a regular motor control block, one was a recorded motor control block, and one was an observation block. These were arranged in one of three orders (counter-balanced): regular-recording-observation, recording-observation-regular, recording-regular-observation.
During regular motor control blocks, participants used the stylus to follow the tracking target (as shown in Fig. 1) during the motor control task between two visual indicators marking the first temporal interval T1 (Fig. 1: a-d). After an inter-stimulus interval (ISI), randomized between 500 and 1000 ms, the interval onset cue was repeated, indicating the start of interval T2 (Fig. 1: f). Participants were requested to passively wait and press the X key with their left hand as soon as the duration of T2 matched T1. Doing this caused the ending signal of T2 to appear for 100 ms, followed by performance feedback. If T2 was more than twice or less than half the length of T1, they were informed that their trial was repeated due to their error. If T2 was 50% longer or 33% shorter than T1, they were urged to improve their performance and requested to press space to continue the experiment (the trial was not repeated). With lower errors, a simple smiley emoticon was presented for 750 ms before the next trial started automatically. During recorded motor control blocks, the trial sequence was exactly the same as during regular motor control blocks, but with motor control performances being recorded. These were then displayed during observation blocks, during which participants were requested not to use the stylus but simply observe as the drawings appeared on the screen.
In a subset of 8 trials, a short questionnaire appeared after the end of a trial to obtain experienced levels of sense of agency based on Longo & Haggard (2009[30]). During these, participants indicated their agreement with three statements on a Likert-type scale (1: not at all agree, 5: very much agree). In addition to statements regarding control (“It felt like I was in control of the cursor”) and predictability (“It felt like the cursor went exactly where I wanted it to go”), we added one pertaining to effort (“It was easy to stay on target”). Each statement was presented randomly in its negative or positive form. The entire experiment took ca. 1 h to complete.
4.4. Design
Each experimental block consisted of 108 trials obtained by orthogonal presentation of five randomized variables: trial duration (1500, 2500, or 3500 ms), distance (300 or 600), resistance (low or high), delay (0 ms, 100 ms or 333 ms), and perturbation (0⁰, 10⁰ or 25⁰). These experimental manipulations were also logged during the recorded motor control block, their order randomized during display in the observation block.
The primary outcome measure was the subjective time, as estimated by the reproduced time (RT, ms). Note that this marks a departure from the preregistered analysis, which suggested proportional accuracy (T2/T1) to partially control for the main effect of duration and the concomitant increase in variance for longer than shorter intervals (roughly in accordance with the Weber-Fechner law). However, we chose the more direct analysis due to its more obvious interpretation of a shorter or longer subjective time, as opposed to a more or less accurate estimation. The originally conducted analysis on temporal bias (i.e. proportional accuracy) is still provided in the supplementary information SI 2 for readers who wish to confirm that the results are similar if not identical.
Three analyses were conducted using linear mixed models designed in jamovi 2.3.24.0 (Jamovi Project, 2022[31]), using R 4.1[32], and the GAMLj package [33] to test effects of the experimental manipulations. Independent linear mixed models (with sum of squares estimation type I) were designed with duration, distance, resistance, delay, and perturbation as factors in full factorial composition3. Participant was used for clustering the variables, i.e. using the linear mixed model as a multilevel model by nesting the other factors within each single subject and using the participant as a random factor for estimating each intercept [34]. The model parameters were estimated using restricted maximum likelihood estimation and the denominator degrees of freedom were estimated using Satterthwaite's approximation [35]. First, we report the subjective time estimated during active blocks as our primary analysis. Second, to verify whether these effects were not merely due to visual effects, we repeat the analysis but with subjective time estimated during observational blocks. Third, to gain insight into the motor control accuracy, we repeated the analysis for active blocks, but with drawing error as dependent, measured as the root mean square over the minimum distance between the cursor and the least squares estimation between start and destination vector. Finally, we analysed sense of agency, computed as the average across three questionnaire items (negative items reverse scored), by means of more traditional repeated measures ANOVAs, since the questionnaire was taken relatively infrequently. Readers who are unfamiliar with linear mixed models may also refer to Supplementary Information 2, which presents also the outcomes of the other analyses using ANOVAs.
5. Results
As documented in the pre-registration, we analysed the reproduced times (RTs), including only trials with relatively accurate time reproductions (subjective/objective time >0.5 and <1.5), and during which participants moved as per instruction. This resulted in 1 participant with more than 33% data loss, and 2 subjects with missing cells within the analyses. The remaining sample was used to test whether increased distance and resistance causes temporal expansion, delay causes temporal compression, and perturbation causes temporal compression with a linear mixed model with temporal bias as dependent and duration, distance, resistance, delay, and perturbation as factors. To test whether these effects were found only for visual/motor conditions, or for both visual/motor and visual-only conditions, we then report the same analysis, but on the observational, control trials. We then analysed the effects of distance, delay, and perturbation on incidental questionnaire data for sense of agency with a single repeated measures ANOVA. As none of the analyses suggested effects of perturbation on timing or agency, we did not test preregistered hypothesis 5 (the effect of perturbation on temporal bias should reduce after controlling for sense of agency). However, we included an exploration on the relation between control and timing by analysing the drawing error using a linear mixed model with duration, distance, resistance, perturbation, and delay as factors.
5.1. Time estimation during active blocks
A total of 357 trials (7.2%) were removed due to low accuracy or failure to follow drawing instructions, resulting in 4611 trials remaining (200.5 per participant on average) for the analysis of time estimation during active blocks. Reproduction times (RTs) for these trials entered a linear mixed model analysis with objective duration (1500 vs 2500 vs 3500 ms), distance (150 vs 300 px), delay (0 vs 100 vs 333 ms), and perturbation (0 vs 10 vs 25 ⁰) as factors, and participant as random effect. The resulting model had a fit (AIC) of 68836.69, and the coefficient of determination (marginal R2) indicated it accounted for 57.9% of within-participant variance.
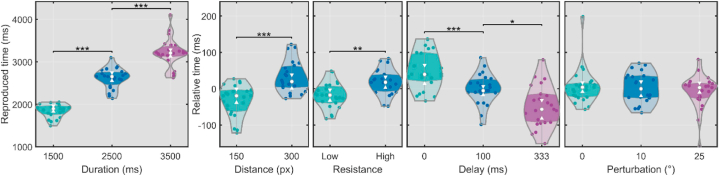
The results showed objective duration significantly affected RT, F (2, 4481.21) = 4052.11, p < .0001, suggesting that the intervals were clearly distinguishable, even though a central tendency could be observed: 1500 ms was estimated as 1855 (SE = 49.64), 2500 as 2615 (SE = 49.47), and 3500 ms as 3219 (SE = 49.46) ms. Increased distances had a significant effect on RT, F (1, 4481.05) = 35.47, p < .0001, Cohen's δ = 1.24 (95% CI [0.69, 1.78]), with increasing distance enlarging RTs by 73 (SE = 12.13) ms. Likewise, a higher resistance resulted in significantly longer (34 ms, SE = 12.20) RTs, F (1, 4481.04) = 7.59, p = .006, Cohen's δ = 0.57 (95% CI [0.13, 1.02]). Conversely, increased delay shortened RTs, F (2, 4481.04) = 20.04, p < .0001: a delay of 100 ms reduced RTs by 57 ms (SE = 14.94), Cohen's δ = 0.79 (95% CI [0.32, 1.26]) versus the 0 delay condition, while a delay of 333 ms reduced RTs (vs 0) by 94 (SE = 14.94) ms, Cohen's δ = 1.31 (95% CI [0.74, 1.87]). Perturbation, however, had no significant effect, F (2, 4481.05) = 0.50, p = .61. The only significant interaction observed was between objective duration and distance, F (2, 4481.05) = 18.76, p < .0001. A simple effects analysis with Bonferroni corrections applied showed that the effect of distance was mainly found for objective durations of 2500 (mean difference (D): 97 ms, SE = 20.9 ms, p < .0001) and 3500 (D = 151, SE = 20.57, p < .0001) ms, but not for 1500 ms (D = −31, SE = 22.16, p = .17).
Since few interactions were observed, the results could be adequately summarized as in Fig. 2: Distance and resistance increased RTs, delays reduced it, and perturbation had no clear effect.
Fig. 2.
Main effects of duration, distance, resistance, delay, and perturbation on estimated time. Presented successively across five panels, the effects of duration (ms), distance (pixels), resistance (low vs high), delay (ms) and perturbation (degrees of distortion) refer to the experimental manipulations occurring during the standard interval (T1), while estimated time was measured by reproducing this interval during T2. The results are shown in violins displaying distributions of scores with circles representing participant averages for each condition. Note: these are presented without adjustment for the first panel (duration), but with baseline timing error subtracted for all other panels. Shaded areas in violins display first and third quartiles, whiskers show standard errors of marginal means, and asterisks summarize post-hoc tests (Bonferroni-adjusted *: <0.05; **: <0.01; ***: <0.001).
5.2. Time estimation during passive blocks
The same type of linear mixed model was used to analyse temporal bias during trials in which participants merely observed drawing before reproducing the intervals, the results of which are presented in Fig. 3. Here, objective duration again significantly affected temporal bias, F (2, 2153.73) = 2207.62, p < .0001, in a manner analogous to that observed for active blocks. Likewise repeating the pattern of active blocks, distance had a significant effect, F (1, 2153.05) = 39.06, p < .0001, with longer distances increasing RTs by 101.89 (SE = 16.27), Cohen's δ = 1.31 (95% CI [0.74, 1.86]). This effect was again moderated by trial duration, F (2, 2153.05) = 10.06, p < .0001, with an effect of distance observed for 2500 ms (mean difference (D) = 88, SE = 27.13, p = .001) and 3500 ms (D = 198, SE = 26.80, p < .0001), but not for 1500 ms (D = 20, SE = 30.48, p = .52). However, neither resistance, F (2153.08) = 0.01, p = .93, nor delay, F (2, 2153.05) = 0.53, p = .59, significantly affected bias. Perturbation again had no significant effect, F (2, 2153.07) = 0.45, p = .64. Surprisingly, a significant four-way interaction was observed between delay, distance, resistance, and perturbation, F (4, 2153.06) = 2.79, p = .025, although we did not find a clear source of this interaction and considered it a false positive that was not replicated in any of the other analyses or experiments.
Fig. 3.
Main effects of duration, distance, resistance, delay, and perturbation on estimated time during passive blocks. Violins show distribution of scores with circles representing participant averages for each condition, being presented without adjustment for the first panel (duration), but with baseline timing error subtracted for the panels presenting the effects for distance, resistance, delay, and perturbation. Shaded areas within violins show first and third quartiles, and whiskers show standard errors of marginal means.
5.3. Sense of agency
Three repeated measures ANOVAs were conducted to test the effect of distance, delay, and perturbation on sense of agency separately. Sense of agency was not significantly affected by distance, F (1, 21) = 0.01, p = .93, = 0.00, or by perturbation, F (2, 42) = 0.44, p = .65, = 0.021, although it was affected by delay, F (1.72, 36.17) = 7.34, p = .003, = 0.26. As is summarized in Fig. 4, delay showed a linear, negative effect on sense of agency, F (1, 21) = 12.34, p = .002, = 0.37, with mean sense of agency decreasing from 3.24 (at 0 ms) on a scale of 1–5, to 2.78 (at 100 ms), and 2.54 (at 333 ms). To further examine how sense of agency was affected by delay, we undertook an exploratory analysis, in which we separated the mean sense of agency score into the three subscales control, effort, and predictability. This showed an effect of delay on control, F (1.76, 36.90) = 5.24, p = .01, = 0.20, and on effort, F (1.84, 38.64) = 8.54, p = .001, = 0.29, but not on predictability, F (2, 42) = 2.99, p = .06, = 0.13.
Fig. 4.
Main effects of distance, delay, and perturbation on sense of agency. Successively presented across four panels, the effects of distance (pixels), delay (ms) and perturbation (degrees of distortion) refer to experimental manipulations occurring during the immediately preceding trial. Sense of agency was calculated as the average across three Likert-type self-report items pertaining to ease, predictability, and control, with a range from 1 to 5. Violins show distribution of scores with circles representing participant averages for each condition (no adjustment for any panel). Shaded areas within violins show first and third quartiles, and whiskers show standard errors of marginal means.
5.4. Exploratory analyses
To determine how motor control during the standard interval was influenced by distance, resistance, perturbation, and delay, we conducted the same linear mixed model analysis as presented for RTs, but with error (px) as dependent. This showed significant effects for distance, F (2, 4481.32) = 177.02, p < .0001, distance, F (1, 4481.07) = 212.57, p < .0001, resistance, F (1, 4481.07) = 332.33, p < .0001, delay, F (2, 4481.06) = 344.18, p < .0001, and perturbation, F (2, 4481.07) = 38.47, p < .0001. These results, as summarized in Fig. 5, show an interesting similarity to those of temporal bias: as with temporal bias, errors were reduced with increasing duration but increased with longer distances. On the other hand, while temporal bias was increased for higher resistance but reduced for increased delay, errors show a reversed pattern, with lower error observed for higher resistance, and higher for increased delay. Perturbation showed an effect on errors, unlike on temporal bias, with 25 ⁰ of perturbation increasing errors by 5.04 px vs 0 ⁰ (SE = 0.65, p < .0001, Cohen's δ = 1.55 (95% CI [0.98, 2.23]), while 10 ⁰ did not have a significant effect (if anything, it reduced error by 0.22 px, SE = 0.65, p = 1.0). Unlike timing estimation, error was affected by several two (duration x delay, duration x distance, delay x distance, duration x resistance, delay x resistance, distance x resistance, all ps < .003), three-way (duration x delay x distance, duration x delay x resistance, duration x distance x resistance, delay x distance x resistance, all ps < .0001), and four-way (duration x delay x distance x resistance, p < .0001) interactions, but since none of these patterns repeated effects observed for the timing task, we did not further analyse their specific direction.
Fig. 5.
Main effects of duration, distance, resistance, delay, and perturbation on error during T1. Presented successively across five panels, the effects of duration (ms), distance (pixels), resistance (low vs high), delay (ms) and perturbation (degrees of distortion) refer to the experimental manipulations occurring during the standard interval. Error is the root mean square across recordings during this interval between cursor and nearest point on the optimal path from start to destination. Violins show distribution of scores with circles representing participant averages for each condition (no adjustment for any panel). Shaded areas within violins show first and third quartiles, and whiskers show standard errors of marginal means. Note that the outliers in the upper tails of each figure concern all concern the same participant. We repeated the main analysis reported under ‘Time estimation during active blocks’, but did not find the results were negatively affected by removing the individual.
6. Conclusions
In an experiment in which a motor control task was combined with a temporal reproduction task, the effects of perceived and enacted motion on time perception were investigated. Longer perceived distances resulted in longer time reproductions, as did longer executed distances, while longer visuomotor delays resulted in shorter time reproductions. However, in trials during which participants merely observed recordings of their motor performance, only the distance was found to alter time reproduction, while neither resistance nor delay had such an effect. Thus, the effect of distance can be attributed to visual perception, as the other effects were removed in visually identical but motorically passive control conditions.
The results thus argue in favour of a remarkably simple explanation: Time was proportionally overestimated whenever more motion occurred, whether the motion was visual, motoric, or visual-motoric. Thus, as longer distances increased time estimates, so too did longer motoric distances - in terms of the pen's drawing on the tablet – yield longer time estimates. A delay between drawing action and visual perception is tantamount to a shorter distance in visual-motoric distance, so the finding that delays elicit shorter time estimates is entirely consistent with this explanation.
Alternative explanations of the findings might, however, focus on critical differences between the conditions in terms of attentional mechanisms. Firstly, resistance might increase motor control demands and increase attentional focus, consequently dilating subjective time. This seems unlikely since increased attentional demands would more obviously move towards the motor control task and away from the timing task, reducing time estimates. More importantly, a control task involving a directional perturbation – which is known to strongly increases motor control requirements [29,36] – did not produce similar effects on time estimations, nor did erroneous movement in its own right.
A second alternative explanation involves the known effect of novelty on subjective time. For example, in the rapid serial visual presentation paradigm, infrequent task-relevant ‘oddball’ stimuli are often perceived as having a longer duration, even if their physical display time is exactly equal to the frequent, task-irrelevant ‘standard’ stimuli amongst which they are interspersed [37]. Thus, perhaps the drawing ‘not behaving as expected’ incurred an oddball effect [38] resulting in generally longer estimates. In order then to 1) replicate our own findings, 2) more strongly delineate the boundaries of the observed effects, and 3) to ascertain that the novelty of visuomotor resistance could not explain the results, we conducted Experiment 2.
7. Experiment 2
Experiment 2 was designed to replicate and extend the findings of Experiment 1 by including more levels of each of the two factors purely involving movement: perceived distance, and visuomotor resistance. Of critical importance was the inclusion of a resistance condition that was lower than normal. If novelty caused the experiment 1 effect of resistance, then reduced resistance (i.e. increased sensitivity) should cause an identical effect, being as ‘novel’. In other words, if a temporal oddball type of effect would account for the previous effects, then both increased and decreased resistance should produce increased time estimates. If, on the other hand, movement itself caused the observed effects, then decreased resistance should reduce subjective time.
Three further changes to the experimental setup were made. First, to keep the task from running overly long, one level was removed from the other two factors, of visuomotor delay and perturbation. Second, due to COVID-19, we conducted Experiment 2 in an online environment. This meant a drawing tablet was unavailable, so participants used either a laptop's touchpad or a desktop mouse for drawing. Third, since effects in temporal cognition are known to sometimes depend on the specific mode of response in the temporal reproduction task [39], we adjusted the task and requested participants to manually indicate both the start and the end of the interval, rather than just the end (as in Experiment 1). If this would provide similar results, it would indicate the effects were not due to the technical minutiae of the experimental paradigm.
7.1. Methods
7.1 1. Participants
Due to COVID-19 lockdown, the present study was conducted online using the E-Prime Go platform. E-Prime Go is an online version of the more familiar E-Prime environment [40], which participants can download and run on their local PC. As preregistered on aspredicted.org (https://aspredicted.org/x6u9m.pdf), we aimed to collect ca. 30 participants, with the added stopping rule to include those who had already signed up before the 30 was reached. Of the N = 38 thus participating in the experiment, 25 identified as female, 10 as male, and 3 as non-binary. They were between 18 and 47 years of age (mean = 26.8, SD = 6.33) and generally were Finnish residents (N = 35, the others from Czech Republic, Hungary, and The Netherlands), and most reported being right-handed (N = 33, 1 ambidextrous, 4 left-handed). As before, participants received full instructions regarding the experimental procedure and were made aware of their rights prior to providing their informed consent. Participants received €7 or €7,50 (if they also volunteered for an unrelated experiment) for their participation or could indicate a favourite charity to receive the money instead.
7.2. Stimuli and apparatus
Due to the experiment being conducted online, a variety of different hardware was used. While all PCs were using Microsoft Windows 7 or higher as an operating system, there was strong variation in resolution, with the largest group running the experiment at a resolution of 1920 × 1080 px. The remaining had either a lower (7 with 1366 × 768, 1 with 1600 × 900) or higher (2 with 2560 × 1440, 1 with 3200 × 1800) resolution, while of 3 participants, there was no data on display device used. All but three ran the experiment at a refresh rate of 60 Hz, the others at 40, 65, and 75 Hz. Since we expected few people to use a drawing tablet for regular interaction, we asked participants to report the device used in the experiment: 23 reported using a mouse, while 15 used a touchpad during the experiment.
7.3. Procedure
Following signing of informed consent, participants ran the experiment. The experimental task and the instructions were the same as before, except for the following changes. First, the design now included lower (factor 0.5) as well as higher resistance, and a medium distance (225 px). Second, owing to the additional design cells, there was a need to reduce the length of the experiment, especially due to it now being run online. For this reason, no observation block was included and questionnaire items were displayed only in one of the 3 blocks (randomized), and only after the 36 trials in which the duration was 2500 ms. Third, to keep performance standards high, we showed negative feedback if objective time was 43% longer than subjective time or subjective time was 40% longer than subjective time, and repeated trials with subjective/objective time <0.6 or >1.7. Fourth, T2 was indicated by pressing the X-key twice, the interval between designating the estimated time of T1 to be more consistent with the literature [41,42]. Fifth, the sense of agency questionnaire was shortened to only the three items in their positive statement: ‘I had to use almost no effort to stay on target’; ‘It felt like I was in control of the cursor’; and ‘It felt like the cursor went exactly where I wanted it to go’. Finally, some minor coding improvements were added to reduce CPU requirements and improve reliability across different computers. Full experimental source code is available at: https://osf.io/7hwm8/. The experiment took on average 60.0 (SD = 10.8) minutes to complete.
7.4. Design
Experiment 2, like Experiment 1, included three blocks of 108 trials. In each block, trial duration (1500, 2500, or 3500 ms), distance (150, 225, or 300 px), resistance (low, medium, or high), delay (33 or 200 ms), and perturbation (0⁰ or 20⁰) were randomized and repeated three times. We altered the analysis reported for Experiment 1 accordingly, conducting a linear mixed model analysis with trial duration, distance, resistance, delay, and perturbation as factors in a full factorial model with the reproduced times (RTs) in ms as dependent and participant as random effect. Note that traditional repeated measures ANOVAs as were originally preregistered are provided as supplementary material. All post-hoc analyses are conducted with Bonferroni adjustments.
8. Results
As described in the preregistration (https://aspredicted.org/x6u9m.pdf), trials were removed if fewer than 75 or more than 600 pixels were drawn; if less than 20% of the path was completed; if subjective time/objective time (temporal bias) was below 0.5 or above 1.5. Participants were removed from further analysis if fewer than 6 trials (ca. 33%) in any design cell remained after these procedures. The latter rule was rather liberal in hindsight: on average 16.5 trials (91.9%, SD = 8.1%) remained following filtering. Accordingly, all participants entered data analysis.
8.1. Main analysis
A total of 1015 trials (7.8%) were removed due to low accuracy or failure to follow drawing instructions, resulting in 11,914 trials remaining (330.9 per participant on average) for the analysis of time estimation. These trials were analysed using a linear mixed model analysis with objective duration (1500 vs 2500 vs 3500 ms), resistance (low vs medium vs high) distance (150 vs 225 vs 300 px), delay (33 vs 200 ms), and perturbation (0 vs 20 ⁰) as factors, and participant as random effect. The resulting model had a fit (AIC) of 176744.62, and the coefficient of determination (marginal R2) indicated it accounted for 53.4% of within-participant variance.
The results showed objective duration significantly increased reproduced times (RTs), F (2, 11771.28) = 9409.23, p < .0001. As with Experiment 1, the durations were clearly distinguished, but a central tendency effect was noticeable: objective durations of 1500 were reproduced as 1808 ms (SE = 42.42), of 2500 as 2515 (SE = 42.36), and of 3500 as 3046 (SE = 42.34). Both increased distance, F (2, 11771.09) = 9.40, p < .0001, and resistance, F (2, 11771.07) = 5.77, p = .003, resulted in significantly increased RTs, while increasing delay reduced RTs, F (1, 11771.09) = 28.76, p < .0001. Perturbation again had no significant effect, F (1, 11771.04) = 0.08, p = .77. Post-hoc analysis further specified the effect of distance as mainly concerning the difference between the 300 px and 150 px conditions (mean difference (D) = 39, SE = 8.97 ms, p < .0001, Cohen's δ = 0.70 (95% CI [0.35, 1.06])), rather than between 225 and 150 (D = 18, p = .14) or 300 and 225 (D = 21.28, p = .054), although a clear linear trend is visible (see Fig. 6). A similar pattern was observed for resistance, although less clearly so: medium resistance resulted in longer RTs than low resistance (D = 25, SE = 8.97, p = .02, Cohen's δ = 0.45 (95% CI [0.12, 0.78]), as did high resistance (D = 28, SE = 8.97, p = .006, Cohen's δ = 0.50 (95% CI [0.17, 0.84]). No significant difference was found between high and medium resistance (D = 3, SE = 8.96, p = 1.0). The effect of 200 ms of delay resulted in 39 ms longer RTs (SE = 7.32, p < .0001, Cohen's δ = 0.87 (95% CI [0.49, 1.23]). As with Experiment 1, distance interacted with duration, F (4, 11771.05) = 4.14, p = .002, with a simple effects analysis indicating that no significant distance effect occurred in 1500 ms durations, F (2, 11771.10) = 0.34, p = .71, while this effect was clearly significant in 2500 ms, F (2, 11771.02) = 7.73, p = .0004, and 3500 ms, F (2, 11771.04) = 10.88, p < .0001. Unlike Experiment 1, however, resistance here entered also in an interaction with duration, F (4, 11771.05) = 3.24, p = .011. Here, simple effects analysis showed significant effects across durations, Fs > 3.08, ps < .05, but in a dissociable manner: for 1500 ms and 2500 ms, medium resistance increased RTs (in 1500 ms: D = 40 ms, p = .013; in 2500 ms: D = 46 ms, p = .003), but high resistance did not differ from low resistance (in 1500 ms: D = 21, p = .18; in 2500 ms: D = 29, p = .058); while for 3500 ms intervals, only an effect of high vs low resistance was observed (D = 32, p = .03). Only one other interaction was observed, between delay and perturbation, F (1, 11771.05) = 3.90, p = .048, but as with the unanticipated 4-way interaction in Experiment, no clear pattern could be observed, and the finding was considered likely to be a false positive.
Fig. 6.
Main effects of duration, distance, resistance, delay, and perturbation on anticipatory reaction time in Experiment 3. Presented successively across five panels, the effects of duration (ms), distance (pixels), resistance (low or high), delay (ms) and perturbation (degrees of distortion) reflect experimental conditions. Error bars indicate within-subject standard errors.
8.2. Exploratory analysis
As will be discussed further on, one explanation of the findings regarding resistance involved a suspected influence of response device. Accordingly, we conducted an exploratory analysis to investigate whether the type of response device could potentially account for the differences between Experiments 1 and 2. This analysis used a similar linear mixed model to analyse the reproduced times (RTs) at the trial level with trial duration, distance, resistance, delay, and perturbation as factors, but now with response device (mouse vs trackpad) added as between subjects factor. Since this 6-factor model risked overfitting, we removed every interaction between the five within-subject factors, and only added response device in two-way interaction with the other factors. Interestingly, this showed only (in addition to the previously reported effects) a clear interaction effect between pointing device and trial duration, F (2, 11862.23) = 26.29, p < .0001, with mouse users showing less of a central tendency effect (1500 vs 2500 vs 3500 ms judged as 1799, 2533, 3090) than trackpad users (1819, 2486, 2977). Furthermore, given that the ad-hoc added between-subjects factor afforded low statistical power to compare the mouse-using majority (N = 23) with a trackpad-using minority (N = 15), we investigated the supposition that the response device played a role descriptively. This showed that while those using a mouse showed an effect in-line with Experiment 1 (resistance low vs medium vs high: 2453, 2476, 2492 ms), those with a trackpad had no linear effect of resistance (2416, 2442, 2425 ms).
9. Conclusions
A replication of Experiment 1 was conducted to determine the reliability of its findings and to investigate whether the effects of distance and resistance extended in both directions. The main observation from Experiment 1 was indeed replicated: despite the motor control task having no impact on the objective duration of T1, its characteristics affected estimated time. Tracking distance, sensorimotor resistance, and visual feedback delay, affected estimated time while perturbation again did not.. For tracking distance and delay, these were in the same direction, the former providing a clear, linear relationship between visual distance and estimated duration.
While decreased resistance thus indeed produced temporal underestimation, we did not replicate the effect of increased resistance: if anything, a slight underestimation was observed compared to medium amounts of resistance. Why would increased resistance no longer result in temporal overestimation? Notably, the online environment of Experiment 2 necessitated the use of more available pointing devices for drawing, generally computer mice and trackpads. Thus, instead of the use of a single device with a relatively large area of operation (of ca. 660 cm2), almost half of the sample pool in Experiment 2 used trackpads (ca. 60 cm2), requiring a multiplicity of strokes rather than single drawing movements once an edge is reached. Indeed, an exploratory analysis in the results of Experiment 2 suggested underestimation was particularly to touchpads.
More pertinent than the question of whether the effect of increased resistance could be replicated was whether the findings would extend beyond the confines of the reproduction task. If action alters time perception of an interval, then this should alter not merely retrospective timing, but also prospective timing. That is, if an interval is presented in pairs of equal length, then a subjectively increased or decreased first interval should result in the second interval to be anticipated as ending later or sooner respectively. Consequently, reaction times to the ending of the second interval should be selectively affected by subjective perception of the first, similar to the variable foreperiod task [43,44]. Thus, a minor adjustment of the experiment was made to verify whether action not only affected temporal perception, but also anticipation of future events.
9.1. Experiment 3
To determine whether action affects anticipation of future events through selective adjustments of time perception, we adjusted the experiment from a temporal reproduction task to a simple reaction time task. Intervals were presented in pairs. The first (T1) proceeded as with Experiments 1 and 2, with the adjustments as detailed in the Methods section. For the second interval (T2), participants were no longer required to accurately match its duration to T1, but merely to anticipate and make a speeded response to its ending. Since participants were aware through instruction and training that each T2 would be equal in length to T1, the subjective perception of T1 duration was expected to alter the anticipation of T2 ending.
10. Methods
10.1. Participants
Similar to experiment 1, a sample of N = 30 volunteers (2 left and 28 right-handed) were recruited via opportunity sampling using a recruitment mailing list. Sixteen reported their gender as female, fourteen as male, and they were between 19 and 50 years of age (M = 28.79, SD = 6.37).
10.2. Stimuli and apparatus
This experiment was again conducted offline, with the same stimuli and hardware as Experiment 1.
10.3. Procedure
After the participant read instructions, viewed a tutorial video, and signed informed consent, the experiment was started by the lab assistant. The experimental task and the instructions were the same as Experiment 1 except for the following changes. First, to reduce the variability of anticipatory reaction times, only two T1/T2 durations were used: 1500 and 2500 ms. Second, the amount of perturbation was increased slightly to include 0⁰, 10⁰, 25⁰, and 45⁰. The resistance (low vs high), distance (150 vs 300 px) and delay (0 vs 100 vs 333 ms) factors were unchanged. Third, no additional questionnaires were presented during or after the experiment.
Finally, and most importantly, T2 was no longer self-timed: participants were no longer expected to accurately reproduce T1 time during T2, but rather use their T1 timing to anticipate the ending of T2. The visual presentation duration of T2 was therefore always the same as T1, and the variable of interest was reaction time rather than reproduced time. To focus participants on anticipation, as opposed to passively reacting to the T2 end, any trial was repeated if reactions preceded T2 ending by 200 or more, or occurred more than 300 ms after it. The experiment took on average 73.0 (SD = 10.2) minutes to complete. Participants reacted half the time (varied by block) with their right hand and the other half with their left.
10.4. Design
Experiment 3 was longer in duration than Experiments 1 and 2, including four blocks of 96 trials. In each block, trial duration (1500 or 2500 ms), distance (150 or 300 px), resistance (low or high), delay (0, 100 or 333 ms), and perturbation (0⁰, 10⁰, 25⁰ or 45⁰) were crossed orthogonally and presented in random order. The analysis was this time not preregistered, but followed almost the same procedure as in Experiment 1 and 2, but now on anticipatory reaction times (only accurate reactions). These were analysed by means of a linear mixed model with trial duration, distance, resistance, delay, and perturbation in full factorial interaction with participant as random effect for determining model intercept. Note that traditional repeated measures ANOVAs are further provided as supplementary material.
11. Results
Inaccurate trials – with responses occurring earlier than 200 ms before or later than 300 ms after the ending of T2, or during which no T1 drawing was performed – occurred in approximately 14.9% of trials, leaving 9806 trials (326.9 on average per participant) in total for the analysis. Reaction times for these trials were analysed using a linear mixed model with objective duration (1500 vs 2500), distance (150 vs 300 px), resistance (low vs high), delay (0 vs 100 vs 333 ms), and perturbation (0 vs 10 vs 25 vs 45 ⁰) as factors, and participant as random effect. The resulting model had a fit (AIC) of 118161.56, and the coefficient of determination (marginal R2) indicated it accounted for 5.3% of within-participant variance.
This analysis showed significant main effects of duration, F (1, 9681.81) = 656.15, p < .0001, resistance, F (1, 9681.06) = 7.09, p = .008, while distance, F (1, 9681.10) = 0.37, p = .54, delay, F (1, 9681.05) = 0.72, p = .40, and perturbation, F (3, 9681.06) = 1.02, p = .38, were non-significant. As summarized in Fig. 7, T1 duration significantly reduced RTs by 51.47 ms on average (SE = 2.01), from 183.03 to 132.57 ms, Cohen's δ = 4.67 (95% CI [3.42, 5.92]). Increased resistance reduced RTs by 5.33 ms (SE = 2.00), Cohen's δ = 0.49 (95% CI [0.10, 0.86]). While – unlike in the previous experiments – distance had no main effect, it entered an interaction with duration, F (1, 9681.10) = 7.71, p = .006. A simple effects analysis with trial duration as moderator showed there was no significant effect at 1500 ms durations, t (9681.04) = −1.54, p = .12, while at 2500 ms durations, longer distances again increased RTs, t (9681.15) = 2.38, p = .018. During such trials, RTs were 6.77 ms later, Cohen's δ = 0.43 (95% CI [0.06, 0.81]). No other interaction was significant at p < .05.
Fig. 7.
Main effects of duration, distance, resistance, delay, and perturbation on anticipatory reaction time. Presented successively across five panels, the effects of duration (ms), distance (pixels), resistance (low vs high), delay (ms) and perturbation (degrees of distortion) refer to the experimental manipulations occurring during the standard interval. Anticipatory reaction time refers to the response speed (ms) to the cue signifying the end of T2.
12. Conclusions
Experiment 3 was conducted to determine whether actions executed during a standard interval (T1) affected the anticipation of the ending of a subsequent interval (T2) of the same duration. A main effect of duration was observed, replicating well-known findings of the variable foreperiod task [44]. More interestingly, the effect of T1 resistance replicated the pattern observed in Experiments 1 and 2, with greater resistances resulting in later anticipation.
However, distance only had a small effect at longer intervals, while delay had no reliable effect on reaction time at all. The larger effect of distance at longer interval replicates Experiment 2, suggesting perhaps a Weber-Fechner scaled effect, but the lack of an effect of delay was more surprising. Perhaps this argues for a dissociation between perception and anticipation, but simpler explanations should be considered first. First, Experiment 3 measured time perception more indirectly than Experiments 1 and 2: time perception was critical in Experiment 3 to correctly anticipate T2 ending, but not sufficient. Thus, for example, the reversed effect of duration does not indicate that 2500 ms was suddenly perceived as shorter than 1500 ms, but should mainly reflect the fact that the longer T2 lasts, the more likely is that it would end soon, with corresponding accumulation of preparatory activity, according to the foreperiod literature [44]. Second, reaction times in Experiment 3 were very short, with experimental differences in the single digits, with a drop in power accordingly. While such small effects are not uncommon in the variable foreperiod paradigm, experiments using it tend to have far more repetitions and much shorter durations (e.g., [45]) than those used here (4 per design cell).
Finally, the relationship between pen movement and visual feedback, as defined by resistance, was found to have carry-over effects from time perception to action anticipation. That is, a T1 subjectively experienced as longer through increased resistance (as estimated in Experiments 1 and 2) thus delayed the expectation of T2 ending, increasing reaction times. This suggests therefore that not only do sensorimotor contingencies alter the perceived duration of T1, but also that these affect prospective timing and action planning.
13. General discussion
The present work hypothesized that temporal cognition is not only a function of perception and attention, but is determined by action-related mechanisms. Specifically, we hypothesized that three essential aspects of action – goals, execution, and outcomes, determine time perception. These were investigated using a motor control task coupled with a time reproduction task (Experiments 1 and 2) and a foreperiod task (Experiment 3).
First, the intended result, or planned action outcome, defines the goal of an action [46]. Here, the goal was operationalised by providing a simple target to draw towards in the timed interval, and while the distance of this target was task-irrelevant, it nevertheless adjusted time perception. The effect was first observed in Experiment 1, while in Experiment 2, we showed target distance to linearly increase time reproduction, although more so for longer than shorter intervals. The latter effect was replicated in Experiment 3, with longer distances increasing anticipatory reaction times, at least for longer intervals. In general, this suggests that the interval between two temporal markers is perceived as longer if motions are made toward a more distant goal during this interval.
Second, the motoric aspect, or controlled biomechanic behaviour performed during movement, defines the execution of actions [47]. Here, execution was operationalised by altering manual motion in two ways, by adjusting the resistance (or sensitivity) and the perturbation (directional distortion) of the drawing during the timed interval. However, only the first parameter had a clear impact on timing. This is hard to align with a central timekeeping model of temporal cognition: Resistance in movement was hardly noticeable while the perturbation is known to increase motor control demands [36], which should distract from timing and therefore decrease time estimates. Only resistance affected timing, but by increasing time estimates in temporal reproduction (Experiments 1 and 2) and anticipation (Experiment 3). Notice, however, that while resistance has a clear link between action and perception (or sensorimotor contingency), perturbation does not: it requires an angular rotation of drawing but no additional movement other than error correction. It seems therefore that the action-perception relationship is what determines time perception, not perception or attention per se.
Third, according to proponents of ideomotor theories, actions are cognitively represented by their perceptual outcomes or action effects [48,49]. Here, action-effects were investigated by manipulating the consequences of drawing, specifically the delay between manual motions and visual feedback. In Experiments 1 and 2, this had a clear effect on time perception, with the amount of delay linearly reducing the time estimates of the interval. Experiment 3 showed no effect of delay on anticipation, suggesting delay-incurred perceptual changes of T1 time do not necessarily carry over to anticipatory reactions to T2.
At first glance, it might appear that a model involving cognitive resources or attention could provide a reasonable alternative to account for the observed findings. Manipulations causing attention to shift towards time estimation are indeed known to cause overestimation while distractions and additional cognitive load results in underestimation [6,8,50]. However, this explanation cannot account for the present findings. First, increased resistance hardly increased attentional demands, as measured in subjective reports and error rates. Second, increased resistance caused over-rather than underestimation. Third, underestimation was observed in delay, and although attention deficits due to delay may partially account for this effect, it seems unintuitive that even a barely noticeable (100 ms) delay would still cause an effect. Fourth, in cases were attentional deficits were a-priori expected due to perturbation increasing demands for motor control, no effect on timing was observed in any of the conducted experiments. We therefore do not consider attentional resources to be a strong candidate for accounting for the effects observed in the present study.
A more likely contender to at least partially account for the present findings would be the so-called kappa-effect. The kappa-effect refers to the psychophysical observation that the larger the spatial distance between two temporal markers, the larger the interval between them is judged [28,51]. Of course, here, the time between two stimuli presented at the same location was estimated, so it would be a novel finding that a goal cued at a farther distance could similarly cause a kappa-effect. Similarly, changes in features other than within visual space can define a distance (c.f. In frequency, [52]), so perhaps the effect of resistance might present a hitherto unknown motor kappa effect: increased manual movement distance increases the perceived temporal interval between two events. Indeed, this explanation is consistent with previous literature, which shows that reducing movement causes a relative underestimation of timed auditory intervals [20]. Note that this finding is only in seeming contradiction to the present work, as whereas De Kock et al. [20] increased resistance reduced movement, here the tracking task required an opposite reaction to resistance, increasing manual movement. Yet, manual movement is not sufficient an explanation to account for the inverse effect of delay and the lack of effect of perturbation – both of which similarly increase motor activity. We therefore argue the motor activity must be understood in relation to the observed effects in order to fully explain how action affects time perception.
Finally, some of the observed effects may be explained by intentional binding [13]. If we assume that participants failed to follow instructions precisely and estimated the interval as defined by the onset of their decision to move, until the end of the standard interval, then exactly the reported pattern of results would have followed. That is, the awareness of the decision to move would be temporally shifted towards the presentation of visual feedback (the voluntary consequence, see also [53]). However, this assumption requires participants to have approached the task as if it were an intentional binding task, repeatedly failing to understand instructions to the contrary. Furthermore, while the theory seems to plausibly account for the effects of delays, it does so by considering how actions cause perceptions [54], making almost identical claims to the suggested hypothesis, yet without the additional value of being able to account for the effects of distance and resistance.
Instead, a parsimonious account for both temporal compression resulting from delays, and temporal expansion from increased distance and resistance is by acknowledging the role of action-perception in temporal cognition. We show that more spatially distant goals, longer manual motions, and shorter intervals between manual motions result in shorter time estimations. Put differently, sensorimotor contingencies [11], or the degree to which actions translate into visual consequences, determine time perception. If more motion is required, because a goal is more distant, or because more manual motion translates into less visual feedback, an interval is overestimated. Conversely, when motions have a visual lack of consequences, such as with delayed feedback, this is discounted from the timed interval, resulting in temporal underestimation. Thus, by referring to the sensorimotor contingencies, all three effects can be accounted for by the same mechanism.
Finally, it should be noted that while the experiments indicate that the subjective timing between two perceptual events depends on action characteristics occurring within that interval, they cannot dissociate whether this is due to solely action-timing or timing of visual events. Given that action took place within the timed interval, we assume that the subjective duration of action was compressed or expanded depending on experimental characteristics, which then caused a temporal recalibration [55], shifting the visual events before and after in time. Future studies, however, might perform a direct test by requesting participants to explicitly engage in motor timing (e.g. using a continuous measure [56]), or by contrasting the present results with perceptual timing within an action interval. However, whether due to action-induced perceptual bias, or a type of source confusion in timing, the present study underlines that the relation between perception and action is integral to subjective timing of events.
Although the presented work comes from a tradition dominated by a theory-driven, model-based approach, we believe the results can have important, practical implications. Indeed, timing is essential to performance in the real world, so the knowledge that not only perceptions and actions, but the relation between the two affect time perception may be important to various fields from precision engineering to symphonic composition. The results indicate medium effects of even relatively subtle experimental action-perception manipulations on time perception, converging well with previous findings [20]. Furthermore, we provide evidence that the effect of resistance – although interestingly, neither distance nor delay – extends beyond estimating time, and towards temporal anticipation. In other words, this particular parameter not only affects judgements or perception of time, but also causes a carry-over effect towards actual performance. Since foreperiod tasks are understood to provide a more implicit measure of subjective time [57,58], this suggests an interesting dissociation between different action parameters.
In conclusion, time perception is not merely the logging of perceptual events, but has a critical role for action control. Since perceptual events are typically not passively encountered without prior cause, but often appear as the results of actions, we argue that the sense of time is the result of the dynamic interplay between motions and action-effects, or the unfolding of sensorimotor contingencies. The sense of a journey's duration, in other words, is determined not just by the ticking of a watch, but by the distance of the goal, the effort of travelling, and the relation between movement intentions and movement results.
Author contribution statement
Michiel M. Spapé;: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Deborah J. Serrien: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Niklas Ravaja: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express their gratitude towards Ville Harjunen and Markku Kilpeläinen for guidance on statistical analysis and two anonymous reviewers for providing expert commentaries on our work.
This research was funded by the EU; ERC Horizon 2020 VirtualTimes project. The authors declare no competing interest.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19728.
Of course, there are multiple different tasks used in the literature, and results of some experimental manipulation may vary depending on the task (e.g. [9]). However, such differences are generally understood as reflecting the validity of a paradigm in a deeper sense, not in whether, for example, time was reported using a touchpad or a keyboard.
This is similar to the inverse of the mouse pointer (Windows) or tracking (OSX) speed that can be adjusted in most PC operating systems. Given that there was relatively little manual movement (average 1.08 cm/s, compared to 5.45 cm/s on screen), the subjective experience of the manipulation was rather subtle, and never commented upon by any participant, as opposed to the more obtrusive effects of delay and perturbation.
Although the LMM provided an analysis at the trial level, the full factorial model involves few observations for each design cell at the higher (3+) levels, for which reason caution for type-I errors must be exercised especially for non-preregistered tests (i.e. any interaction).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psychol. Rev. 1977;84:279. [Google Scholar]
- 2.Wearden J.H., McShane B. Interval production as an analogue of the peak procedure: evidence for similarity of human and animal timing processes. Q. J. Exp. Psychol. 1988;40:363–375. [Google Scholar]
- 3.Treisman M. Temporal discrimination and the indifference interval: implications for a model of the" internal clock". Psychol. Monogr.: General and Applied. 1963;77:1. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- 4.Treisman M., Faulkner A., Naish P.L., Brogan D. The internal clock: evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception. 1990;19:705–742. doi: 10.1068/p190705. [DOI] [PubMed] [Google Scholar]
- 5.Grondin S. Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Atten. Percept. Psychophys. 2010;72:561–582. doi: 10.3758/APP.72.3.561. [DOI] [PubMed] [Google Scholar]
- 6.Zakay D., Block R.A. Advances in Psychology. Elsevier; 1996. The role of attention in time estimation processes; pp. 143–164. [Google Scholar]
- 7.Droit-Volet S., Fayolle S.L., Gil S. Emotion and time perception: effects of film-induced mood. Front. Integr. Neurosci. 2011;5:33. doi: 10.3389/fnint.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tse P.U., Intriligator J., Rivest J., Cavanagh P. Attention and the subjective expansion of time. Percept. Psychophys. 2004;66:1171–1189. doi: 10.3758/bf03196844. [DOI] [PubMed] [Google Scholar]
- 9.Gil S., Droit-Volet S. “Time flies in the presence of angry faces”depending on the temporal task used! Acta Psychol. 2011;136:354–362. doi: 10.1016/j.actpsy.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Prinz W. Perception and action planning. Eur. J. Cognit. Psychol. 1997;9:129–154. [Google Scholar]
- 11.O'Regan J.K., Noë A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 2001;24:939. doi: 10.1017/s0140525x01000115. [DOI] [PubMed] [Google Scholar]
- 12.James W. Dover Publications; New York: 1890. The Principles of Psychology. [Google Scholar]
- 13.Haggard P. Conscious intention and motor cognition. Trends Cognit. Sci. 2005;9:290–295. doi: 10.1016/j.tics.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- 15.Decety J., Jeannerod M., Prablanc C. The timing of mentally represented actions. Behav. Brain Res. 1989;34:35–42. doi: 10.1016/s0166-4328(89)80088-9. [DOI] [PubMed] [Google Scholar]
- 16.Jeannerod M., Decety J. Mental motor imagery: a window into the representational stages of action. Curr. Opin. Neurobiol. 1995;5:727–732. doi: 10.1016/0959-4388(95)80099-9. [DOI] [PubMed] [Google Scholar]
- 17.Spapé M.M., Harjunen V.J., Ravaja N. Time to imagine moving: simulated motor activity affects time perception. Psychonomic Bull. Rev. 2022;29:819–827. doi: 10.3758/s13423-021-02028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Kock R., Gladhill K.A., Ali M.N., Joiner W.M., Wiener M. How movements shape the perception of time. Trends Cognit. Sci. 2021;25:950–963. doi: 10.1016/j.tics.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokosaka T., Kuroki S., Nishida S., Watanabe J. Apparent time interval of visual stimuli is compressed during fast hand movement. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Kock R., Zhou W., Joiner W.M., Wiener M. Slowing the body slows down time perception. Elife. 2021;10 doi: 10.7554/eLife.63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yon D., Edey R., Ivry R.B., Press C. Time on your hands: perceived duration of sensory events is biased toward concurrent actions. J. Exp. Psychol. Gen. 2017;146:182. doi: 10.1037/xge0000254. [DOI] [PubMed] [Google Scholar]
- 22.Tomassini A., Morrone M.C. Perceived visual time depends on motor preparation and direction of hand movements. Sci. Rep. 2016;6 doi: 10.1038/srep27947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiener M., Zhou W., Bader F., Joiner W.M. Movement improves the quality of temporal perception and decision-making. eneuro. 2019;6 doi: 10.1523/ENEURO.0042-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Kock R., Zhou W., Datta P., Mychal Joiner W., Wiener M. The role of consciously timed movements in shaping and improving auditory timing. Proceedings of the Royal Society B. 2023;290 doi: 10.1098/rspb.2022.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin Y.K., Choe S., Kwon O.-S. Strong evidence for ideomotor theory: unwilled manifestation of the conceptual attribute in movement control. Front. Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1066839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown S.W. Time, change, and motion: the effects of stimulus movement on temporal perception. Percept. Psychophys. 1995;57:105–116. doi: 10.3758/bf03211853. [DOI] [PubMed] [Google Scholar]
- 27.Gavazzi G., Bisio A., Pozzo T. Time perception of visual motion is tuned by the motor representation of human actions. Sci. Rep. 2013;3:1–8. doi: 10.1038/srep01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price-Williams D.R. The kappa effect. Nature. 1954;173:363–364. doi: 10.1038/173363a0. [DOI] [PubMed] [Google Scholar]
- 29.Spapé M.M., Serrien D.J. Interregional synchrony of visuomotor tracking: perturbation effects and individual differences. Behav. Brain Res. 2010;213:313–318. doi: 10.1016/j.bbr.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Longo M.R., Haggard P. Sense of agency primes manual motor responses. Perception. 2009;38:69–78. doi: 10.1068/p6045. [DOI] [PubMed] [Google Scholar]
- 31.Project J. Jamovi (v 2.3.24.0)[Computer Software] 2022 [Google Scholar]
- 32.R Core Team . 2021. R: A Language and Environment for Statistical Computing. v4.1. [Google Scholar]
- 33.Gallucci M. 2019. GAMLj: General Analyses for Linear Models. [Google Scholar]
- 34.Hoffman L., Rovine M.J. Multilevel models for the experimental psychologist: foundations and illustrative examples. Behav. Res. Methods. 2007;39:101–117. doi: 10.3758/bf03192848. [DOI] [PubMed] [Google Scholar]
- 35.Satterthwaite F.E. An approximate distribution of estimates of variance components. Biometrics bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- 36.Serrien D.J., Spapé M.M. Motor awareness and dissociable levels of action representation. Neurosci. Lett. 2011;494:145–149. doi: 10.1016/j.neulet.2011.02.077. [DOI] [PubMed] [Google Scholar]
- 37.Birngruber T., Schröter H., Ulrich R. Duration perception of visual and auditory oddball stimuli: does judgment task modulate the temporal oddball effect? Attention, Perception. & Psychophysics. 2014;76:814–828. doi: 10.3758/s13414-013-0602-2. [DOI] [PubMed] [Google Scholar]
- 38.Pariyadath V., Eagleman D. The effect of predictability on subjective duration. PLoS One. 2007;2:e1264. doi: 10.1371/journal.pone.0001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mioni G., Stablum F., McClintock S.M., Grondin S. Different methods for reproducing time, different results. Atten. Percept. Psychophys. 2014;76:675–681. doi: 10.3758/s13414-014-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spapé M.M., Verdonschot R., Steenbergen H. van. second ed. Leiden Univerisity Press; 2019. The E-Primer: an Introduction to Creating Psychological Experiments in E-Prime. updated for E-Prime 3. 2nd ed. [Google Scholar]
- 41.Fortin C., Rousseau R. Interference from short-term memory processing on encoding and reproducing brief durations. Psychol. Res. 1998;61:269–276. doi: 10.1007/s004260050031. [DOI] [PubMed] [Google Scholar]
- 42.Isham E.A., Le C., Ekstrom A.D. Rightward and leftward biases in temporal reproduction of objects represented in central and peripheral spaces. Neurobiol. Learn. Mem. 2018;153:71–78. doi: 10.1016/j.nlm.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grondin S., Rammsayer T. Variable foreperiods and temporal discrimination. The Quarterly Journal of Experimental Psychology Section A. 2003;56:731–765. doi: 10.1080/02724980244000611. [DOI] [PubMed] [Google Scholar]
- 44.Niemi P., Näätänen R. Foreperiod and simple reaction time. Psychol. Bull. 1981;89:133. [Google Scholar]
- 45.Langner R., Steinborn M.B., Eickhoff S.B., Huestegge L. When specific action biases meet nonspecific preparation: event repetition modulates the variable-foreperiod effect. J. Exp. Psychol. Hum. Percept. Perform. 2018;44:1313. doi: 10.1037/xhp0000561. [DOI] [PubMed] [Google Scholar]
- 46.Ajzen I. Action Control. Springer; 1985. From intentions to actions: a theory of planned behavior; pp. 11–39. [Google Scholar]
- 47.Schack T. The cognitive architecture of complex movement. Int. J. Sport Exerc. Psychol. 2004;2:403–438. [Google Scholar]
- 48.Elsner B., Hommel B. Effect anticipation and action control. J. Exp. Psychol. Hum. Percept. Perform. 2001;27:229. doi: 10.1037//0096-1523.27.1.229. [DOI] [PubMed] [Google Scholar]
- 49.Koch I., Keller P., Prinz W. The ideomotor approach to action control: implications for skilled performance. Int. J. Sport Exerc. Psychol. 2004;2:362–375. [Google Scholar]
- 50.Block R.A., Gruber R.P. Time perception, attention, and memory: a selective review. Acta Psychol. 2014;149:129–133. doi: 10.1016/j.actpsy.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Kuroda T., Grondin S., Miyazaki M., Ogata K., Tobimatsu S. The kappa effect with only two visual markers. Multisensory Res. 2016;29:703–725. [Google Scholar]
- 52.Yoblick D.A., Salvendy G. Influence of frequency on the estimation of time for auditory, visual, and tactile modalities: the kappa effect. J. Exp. Psychol. 1970;86:157. doi: 10.1037/h0029935. [DOI] [PubMed] [Google Scholar]
- 53.Engbert K., Wohlschläger A., Thomas R., Haggard P. Agency, subjective time, and other minds. J. Exp. Psychol. Hum. Percept. Perform. 2007;33:1261–1268. doi: 10.1037/0096-1523.33.6.1261. [DOI] [PubMed] [Google Scholar]
- 54.Buehner M.J. Understanding the past, predicting the future: causation, not intentional action, is the root of temporal binding. Psychol. Sci. 2012;23:1490–1497. doi: 10.1177/0956797612444612. [DOI] [PubMed] [Google Scholar]
- 55.Bansal A., Weech S., Barnett-Cowan M. Movement-contingent time flow in virtual reality causes temporal recalibration. Sci. Rep. 2019;9:4378. doi: 10.1038/s41598-019-40870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda S., Shimoda S. Subjective time compression induced by continuous action. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coull J.T., Cotti J., Vidal F. Differential roles for parietal and frontal cortices in fixed versus evolving temporal expectations: dissociating prior from posterior temporal probabilities with fMRI. Neuroimage. 2016;141:40–51. doi: 10.1016/j.neuroimage.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 58.Coull J.T., Giersch A. The distinction between temporal order and duration processing, and implications for schizophrenia. Nature Reviews Psychology. 2022;1:257–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.