Abstract
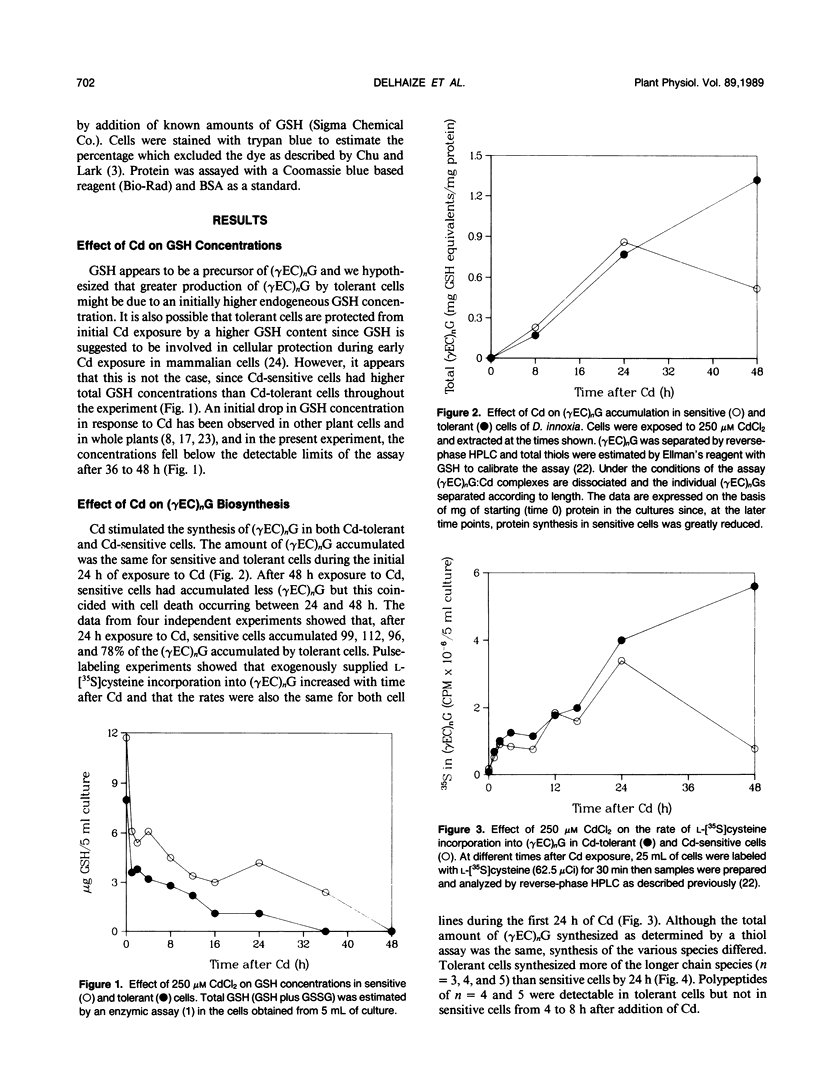
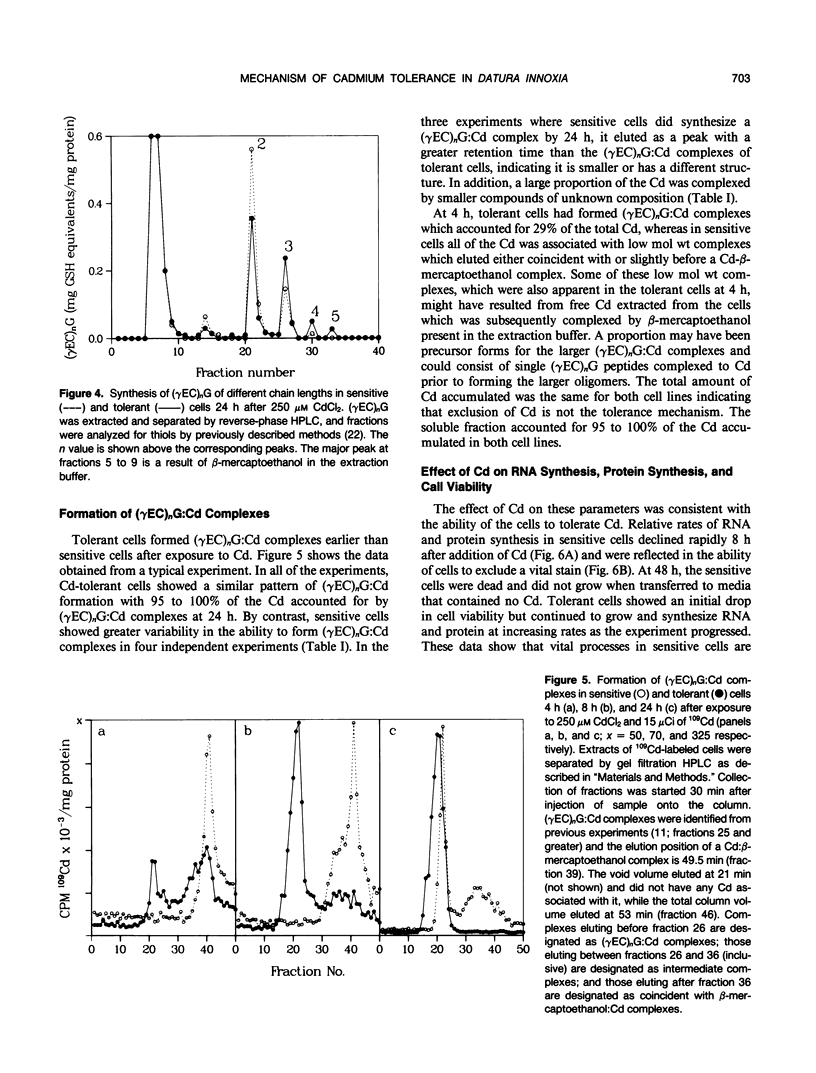
The effects of Cd on poly(γ-glutamylcysteinyl)glycine [(γEC)nG] biosynthesis and formation of (γEC)nG:Cd complexes were measured in two cell lines of Datura innoxia with differing Cd tolerance. In addition, RNA synthesis, protein synthesis, and GSH concentrations were measured during a 48 hour exposure to Cd. Exposure to 250 micromolar CdCl2 was toxic to the sensitive line, whereas the tolerant line survived and grew in its presence. Cd-sensitive cells synthesized the same amount of (γEC)nG as tolerant cells during an initial 24 hour exposure to 250 micromolar CdCl2. However, rates of (γEC)nG:Cd complex formation differed between the two cell lines with the sensitive cells forming complexes later than tolerant cells. In addition, the complexes formed by sensitive cells were of lower molecular weight than those of tolerant cells and did not bind all of the cellular Cd. Pulse-labeling of cells with l-[35S]cysteine resulted in equivalent rates of incorporation into the (γEC)nG of both cell lines during the initial 24 hours after Cd. Rates of protein and RNA synthesis were similar for both cell lines during the initial 8 hours after Cd but thereafter declined rapidly in sensitive cells. This was reflected by a decline in viability of sensitive cells. The GSH content of both cell lines declined rapidly upon exposure to Cd but was higher in sensitive cells throughout the experiment. These results show that the biosynthetic pathway for (γEC)nG synthesis in sensitive cells is operational and that relative overproduction of (γEC)nG is not the mechanism of Cd-tolerance in a Cd-tolerant cell line of D. innoxia. Rapid formation of (γEC)nG:Cd complexes that bind all of the cellular Cd within 24 hours appears to correlate with tolerance in these cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Bernhard W. R., Kägi J. H. Purification and characterization of atypical cadmium-binding polypeptides from Zea mays. Experientia Suppl. 1987;52:309–315. doi: 10.1007/978-3-0348-6784-9_27. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Jackson P. J., Roth E. J., McClure P. R., Naranjo C. M. Selection, Isolation, and Characterization of Cadmium-Resistant Datura innoxia Suspension Cultures. Plant Physiol. 1984 Aug;75(4):914–918. doi: 10.1104/pp.75.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. J., Unkefer C. J., Doolen J. A., Watt K., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine: its role in cadmium resistance in plant cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6619–6623. doi: 10.1073/pnas.84.19.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A., Wada C., Hayashi Y. Occurrence of acid-labile sulfide in cadmium-binding peptide 1 from fission yeast. J Biochem. 1983 Feb;93(2):661–664. doi: 10.1093/oxfordjournals.jbchem.a134222. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Hayashi Y. Isolation of mutants of Schizosaccharomyces pombe unable to synthesize cadystin, small cadmium-binding peptides. Biochem Biophys Res Commun. 1988 Feb 29;151(1):32–39. doi: 10.1016/0006-291x(88)90555-4. [DOI] [PubMed] [Google Scholar]
- Reese R. N., Mehra R. K., Tarbet E. B., Winge D. R. Studies on the gamma-glutamyl Cu-binding peptide from Schizosaccharomyces pombe. J Biol Chem. 1988 Mar 25;263(9):4186–4192. [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Effects of buthionine sulfoximine on cd-binding Peptide levels in suspension-cultured tobacco cells treated with cd, zn, or cu. Plant Physiol. 1987 Jul;84(3):574–577. doi: 10.1104/pp.84.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Properties of tobacco (Nicotiana tabacum) cadmium-binding peptide(s). Unique non-metallothionein cadmium ligands. Biochem J. 1987 Feb 1;241(3):641–647. doi: 10.1042/bj2410641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H. V., Huang B., Hatch E., Goldsbrough P. B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987 Dec;85(4):1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. K., Anderson M. E., Meister A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987 Sep;1(3):220–223. doi: 10.1096/fasebj.1.3.2887478. [DOI] [PubMed] [Google Scholar]
- Steffens J. C., Hunt D. F., Williams B. G. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem. 1986 Oct 25;261(30):13879–13882. [PubMed] [Google Scholar]


