Abstract
Anaerobic digestion (AD) is the primary technology for energy production from wet biomass under a limited oxygen supply. Various wastes rich in organic content have been renowned for enhancing the process of biogas production. However, several other intermediate unwanted products such as hydrogen sulfide, ammonia, carbon dioxide, siloxanes and halogens have been generated during the process, which tends to lower the quality and quantity of the harvested biogas. The removal of hydrogen sulfide from wastewater, a potential substrate for anaerobic digestion, using various technologies is covered in this study. It is recommended that microaeration would increase the higher removal efficiency of hydrogen sulfide based on a number of benefits for the specific method. The process is primarily accomplished by dosing smaller amounts of oxygen in the digester, which increases the system's oxidizing capacity by rendering the sulfate reducing bacteria responsible for converting sulfate ions to hydrogen sulfide inactive. This paper reviews physicochemical and biological methods that have been in place to eliminate the effects of hydrogen sulfide from wastewater treated anaerobically and future direction to remove hydrogen sulfide from biogas produced.
Keywords: Anaerobic digestion, Hydrogen sulfide, Methane
1. Introduction
Anaerobic digestion (AD) is a microbiological process widely used to produce biogas. This process is carried out under anaerobic conditions using organic waste as a raw material or substrate for anaerobic microorganisms [1]. Generally, biogas is a mixture of methane and carbon dioxide, the methane content being in the range of 50–70% [2]. Recently, the world has been turning from utilizing fossil fuels as an energy source for different purposes such as cooking, lighting and machinery operation [3,4]. Due to the increase in the costs of oil and natural gas on the global market as a result of fossil fuel depletion, there is a considerable increase in the demand for other alternative energy sources [5,6]. However, owing to the advancement of science and technology, it is recommended that other alternative sources of energy should be used as a direct replacement for these commonly used fossil fuels, which are not environmentally friendly considering the release of large amounts of carbon dioxide in the atmosphere when burnt.
Furthermore, the use of these alternative sources of energy is cost-effective due to sustainability and its contribution to clean energy generation and protecting the environment against global warming [7,8,9]. In order to provide alternate energy sources with little sludge generation, anaerobic digesters have been used all over the world. High-rate anaerobic digesters are commonly used in the treatment of sewage. This is due to the fact that high-rate anaerobic reactors, as opposed to conventional low-rate anaerobic digesters, are designed to operate at short hydraulic retention times (HRT) with higher solid retention times that can accommodate large amounts of biomass at once, improving sludge stabilization and increasing loading capacity [10]. The Upflow Anaerobic Sludge Blanket (UASB) reactor is one of the most widely utilized high rate digesters due to its effectiveness in removing organic matter and the high-quality amount of biogas it produces [11].
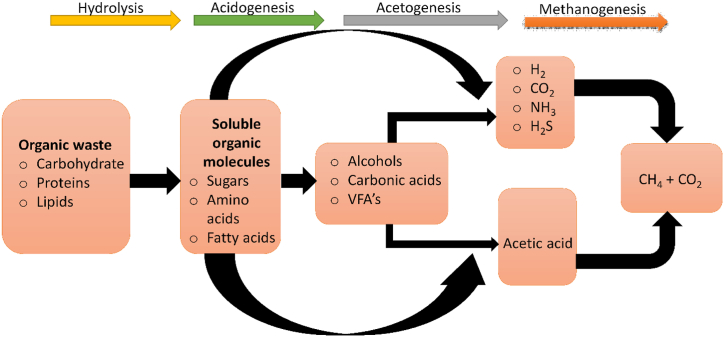
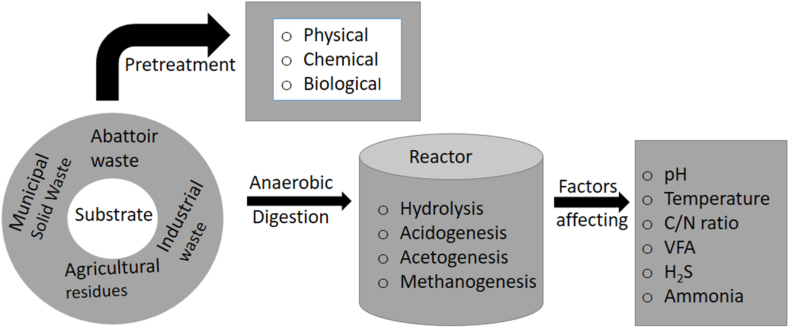
Biogas technology is among the ecofriendly processes in producing methane gas from various organic substrates such as cow dung [12,13], pig manure [14,15], industrial effluents [16,17], food waste [18], slaughterhouse waste [19,20] and agro-waste [21,22]. These wastes are plentiful and cheap to acquire since they are being discharged to the environment as a result of both domestic and industrial activities. However, the production of biogas is sometimes associated with other contaminants such as siloxanes [23], total ammonia nitrogen (TAN) [24], hydrogen sulfide [25,26], halogens [27,28] and other volatile organic compounds [29,30] which becomes inhibitory to methanogens and subjecting the whole digestion process to failure. Hydrogen sulfide (H2S) is mainly produced as an intermediate product during the AD process, in which excessive production significantly impacts the final stage of methanogenesis [31]. The produced H2S during acetogenesis tends to affect the growth of methanogens, which are required to initiate the methanogenesis process, as indicated in Fig. 1.
Fig. 1.
Anaerobic digestion process of organic waste.
In most cases, biogas production during anaerobic digestion of carbon-based substrate is highly influenced by sulfide deposits in the digester resulting from the protein breakdown process [32,33]. Generally, substrates rich in microbial sulfate and other sulfur-containing compounds are subjected to the release of hydrogen sulfide, which is noxious to humans and corrosive to various appliances and metal pipes, when treated both aerobically and anaerobically [34,35]. The aftermath of sulfide inhibition, which induces the antagonism between sulfate-reducing bacteria (SRB) and methane-producing archaea (MPA) throughout the AD process [36,37], releases hydrogen sulfide with a bad odour to the environment. Furthermore, when mixed in a higher amount with the biogas, the gas tends to corrode the surfaces of the cooking devices and other equipment utilizing biogas as fuel, for example, generator sets and several other automobile engines [38,39]. H2S can result in various specialized damages, such as corrosion-mechanical attacks, tool and equipment failures, and difficulties with tool joints (Table 4). Regardless of how the biogas will be used, hydrogen sulfide must be removed to variable degrees because its presence increases the cost of operations and maintenance and shortens the lifespan of gas handling equipment. Various studies have reported that SRB's effects on anaerobic processes go beyond reducing methane production due to consuming organic waste and creating hydrogen sulfide. For instance, Uberoi and Bhattacharya's research found that as the metabolic rate dropped with increased COD load, the ratio of chemical oxygen demand (COD) to sulfate (SO42−) had an impact on the SRB's effect on anaerobic digestion [40].
Table 4.
The effect of H2S on equipment and potential solutions.
| S/N | Specific damage | Explanation | Potential solution | Reference |
|---|---|---|---|---|
| 1. | Corrosion-Mechanical Attack | H2S can corrode metal surfaces and mechanically harm them, especially when moisture is available. Pipelines, valves, and other pieces of machinery may corrode, resulting in leaks, decreased structural integrity, and, eventually, failure. |
|
[41,42] |
| 2. | Failures in Tools and Equipment | H2S exposure can lead to malfunctions in the pumps, compressors, turbines, and electrical components involved in natural gas production. H2S's corrosive properties can damage gaskets, seals, and moving parts, which can cause equipment to break down or malfunction. |
|
[43] |
| 3. | Tool Joint Issues | In drilling operations, tool joints—connections between drill pipes—can also specifically pose problems. H2S can cause embrittlement and stress corrosion cracking in tool joints. |
|
[44,45] |
In practice, the resiliency of the acetoclastic methanogenesis pathway is crucial for effective methane synthesis during the switch from acidogenesis to methanogenesis. The initial stage of anaerobic digestion, known as acidogenesis (Fig. 1), involves the conversion of complex chemical compounds into simpler molecules such as acetate and volatile fatty acids [46]. These intermediary substances are transformed into methane during the process of methanogenesis, which comes after acetogenesis. Several factors, including the effects of mechanically mixing or agitating the anaerobic digestion system, can affect the stability of the acetoclastic methanogenesis pathway during this transition [47].
The ideal agitation conditions for the stability of the acetoclastic methanogenesis pathway may differ depending on the particular reactor design, feedstock properties, and the microbial population present [48]. To ensure the stability and effectiveness of the methanogenic process, rigorous monitoring and control of agitation parameters, such as mixing intensity and duration, is required.
On the other hand, temperature optimization is essential to produce biogas effectively in mesophilic AD. The activity of hydrolases and microbial metabolism can be increased by higher temperatures, hastening the breakdown of organic waste and increasing biogas output [12,49]. On the other hand, extremely high temperatures might stifle microbial activity and enzyme activity, resulting in unstable processes and reduced biogas generation [20].
Therefore, evaluating which method fits best in mitigating hydrogen sulfide is paramount during anaerobic digestion to produce high quality biogas.
This study has meticulously evaluated several approaches for easing the inhibitory process caused by sulfide levels forming in the digester and divided into two categories: physicochemical and biological methods.
2. Physicochemical procedures
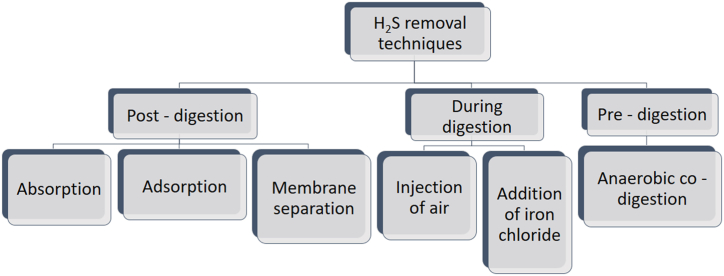
The physicochemical method for eliminating hydrogen sulfide from the biogas is a straightforward process that occurs in the reactor through diverse ways in which sulfide oxidizing bacteria is treated as a potential additive being added to oxidize sulfide to elemental sulfur. The post-digestion process in physicochemical procedures, however, involves using a variety of absorbents and adsorbents as a means of post-treatment, including activated carbon and wet scrubbers (Fig. 2). These materials directly remove hydrogen sulfide (H2S) through sorption interactions with the biogas.
Fig. 2.
A summary of different techniques for H2S removal [50].
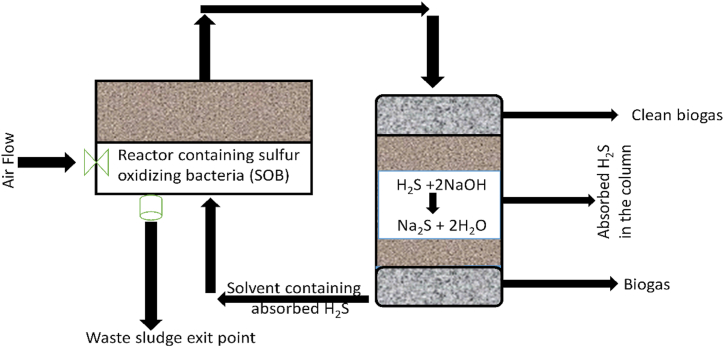
This procedure primarily uses the micro-aerobic process, precipitation, and scrubbing. However, biological desulfurization technology is a long-term method for removing sulfide from biogas. Its benefits include minimal chemical agent usage, little surplus sludge production, and effective elemental sulfur recycling. Hydrogen sulfide is initially transported to the liquid phase by the alkaline absorption solution in the current sulfide removal systems (Fig. 4), where sulfur-oxidizing bacteria (SOB) then oxidize it to sulfur or sulfate, resulting in a high sulfide removal efficiency [51].
Fig. 4.
Biological desulfurization process modified from reference (Okoro and Sun, 2019).
2.1. Micro-aerobic process
This method has been extensively used (as shown in Fig. 2 during air injection) to remove hydrogen sulfide from biogas because it is efficient, simple, and cost-effective [52]. The addition of air into an anaerobic digester is used in this technique [53]. The method enhances sulfide oxidation by introducing oxygen in the reactor containing sludge under micro-aerobic conditions [54]. This method uses oxygen as an electron acceptor during sulfide oxidation, in which excess sulfide is further transformed into elemental sulfur, as shown in equation (1).
Essentially, when sulfide is oxidized to elemental sulfur () or sulfate () forms, the process is regarded as the basic principle for hydrogen sulfide elimination [55]. Meanwhile, thiosulfate () may be produced during the process, with sulfide as the terminal electron acceptor throughout [56]. Sulfur is the primary end-product of sulfide oxidation under a limited supply of oxygen (microaerobic) conditions, especially when the oxygen level is less than 0.1 mg/L, as shown in the equation below:
| (1) |
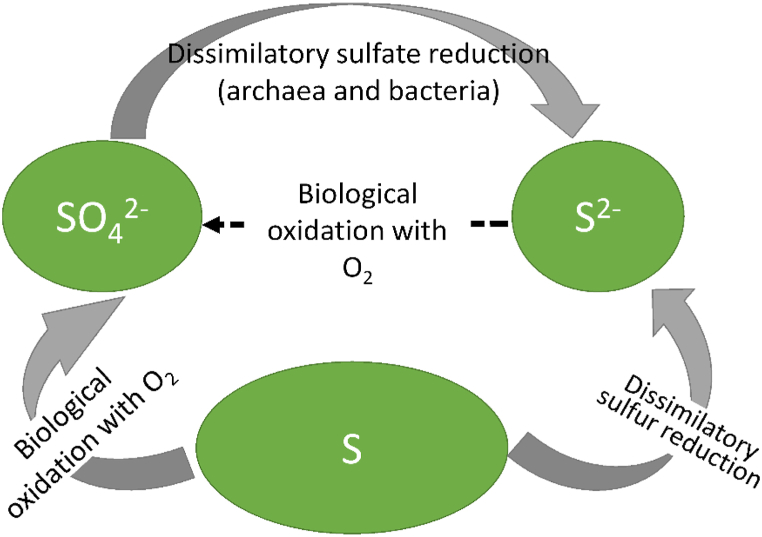
In general, controlling the production of sulfur and sulfate depends on the quantity of oxygen supplied in the reactor (Fig. 3) for the sulfide oxidation process in which elemental sulfur is regarded as an intermediate product undergoing disproportionation [57,58]. For example, 0.5 mol O2/mol S2− is thought to be necessary for the oxidation process of sulfide to elemental sulfur (Eqn. (1)). Nevertheless, 2 mol O2/mol s2− is considered to be required for the oxidation process of sulfide to sulfate (Eqn. (2)). The formation of sulfate depends on the limited conditions of sulfide in which the higher amount of oxygen consumed per 1 mol of sulfide will enhance sulfate formation [55].
| (2) |
Fig. 3.
The cycle of microbial sulfur during anaerobic digestion [59].
Meanwhile, Jung and Mora, in their study [60,61], reported that at oxygen levels more than 25 μmol O2/0.04 mol S2−, sulfide was predominantly absorbed and oxidized to elemental sulfur. This trend is similar to the findings of Tilahun and fellow researchers [62], who discovered that dissolved oxygen (DO) concentrations significantly impacted the formation of S0. However, there are additional important aspects to consider for an operative micro-aeration approach, such as oxygen transfer rate (OTR) and oxygen utilization rate (OUR), in addition to the quantity of oxygen prerequisite to augment the micro aeration course [63]. The two processes are considered the limiting components since they are essential in feeding the reactor sufficient oxygen for sulfide removal. Reactor conformation, micro-aeration method (i.e., usage of air or oxygen, bubble size, injection in aqueous or gaseous phase), Total solids (TS) concentration of substrate in the reactor, and other parameters all influence the OTR throughout the AD process [64]. Once the dispersion of air/oxygen in the reactor was inadequate, for example, the micro-aeration process failed to eliminate sulfide in the liquid phase [49]. On the other hand, the inoculum deployed and the substrate injected into the reactor during the AD process affects the OUR in micro-aeration. Initially, it was revealed that the microaeration process requires a particular oxygen dosing method for substrates with an increasing rate of hydrolysis, such as lignocellulosic biomass [63].
Meanwhile, the conversion of organic materials such as agricultural residues, municipal solid waste, abattoir waste, and others into methane has been fueled partly by the inoculum to substrate ratio (ISR) [65]. The optimal ISR in the reactor enables the presence of responsible bacteria for each stage involved during the AD process [66]. Initially, the inoculum used for optimal biomethane conversion has a number of characteristics, such as fewer pollutants from external sources that could interfere with the entire process. To ensure that the microbial species are acclimated, it is suggested that the inoculum be similar to the substrate being fed into the reactor. However, to attain higher levels of methane yield, the inoculum should be fresh and include the minimum viable inhibitors, such as hydrogen sulfide, ammonia, and heavy metals [67]. Generally, the highest ISR level often ensures a larger methane yield because it inhibits the acidification process and maintains the nutritional balance for microbes [68]. Nevertheless, as the inocula dosage increases, the reactor's performance improves (Table 1). By optimizing these factors, it is possible to enhance the efficiency of the AD process and generate higher methane content yields [69].
Table 1.
The impact of inoculum to substrate ratio on methane yield for selected substrates.
| S/N | Substrate | Inoculum | Inoculum to substrate ratio (ISR) | Methane yield (ml CH4/g VS added) | Average number of days | Reference |
|---|---|---|---|---|---|---|
| 1 | Ball milled straw (BMS) | Cattle manure | 10 | <50 | 150 | [70] |
| 30 | >200 | |||||
| 70 | >300 | |||||
| 2 | Kitchen waste | Industrial sludge | 0.5 | 220 | 25 | [71] |
| 1.0 | 180 | |||||
| 1.35 | 200 | |||||
| 2.3 | 220 | |||||
| 3 | Maize | Digester sludge from a municipal wastewater treatment plant | 3 | 7023 | 20 | [72] |
| 2 | 11,356 | |||||
| 1.5 | 14,921 | |||||
| 1 | 23,635 |
2.2. Precipitation process
The precipitation method, which employs metals with a strong affinity for sulfur, such as iron and zinc, has been one of the most common techniques for treating sulfur-containing wastewater. Metal salts such as iron, zinc, lead, and copper are added to precipitate sulfide. For instance, adding iron chloride during digestion (Fig. 2) results in the formation of highly insoluble metallic sulfide precipitates [73,74]. The metal sulfide plays a major role in reducing the solubility product and lowering sludge volume compared to hydroxide precipitation. However, sulfide precipitation is preferred over hydroxide precipitation because of its low solubility and lower sensitivity to pH changes. Because the solubility of metal sulfides is not as sensitive to pH changes as that of metal hydroxides, in addition, it is easier to recover metal from metal sulfides than hydroxides since sulfide precipitation results in lower effluent concentrations and less interference from chelating agents [75].
When treated with metal-containing wastewater, sulfate-reducing bacteria (SRB) display potential application over other chemical treatments. In the liquid phase, Fe3+ is quite effective at reducing sulfide. While being reduced into Fe2+, Fe3+ oxidizes sulfide to elemental sulfur, which precipitates with sulfide to generate ferrous sulfide precipitants [76,77,78].
| (3) |
| (4) |
For example, the study which was done by Zhao and other co-authors [79] discovered that Ferric iron (Eqn. (3)) is normally used for sulfide precipitation in sewers, thus conquering corrosion and odour control. According to the study, monitoring the sulfide level during precipitation enhances the evaluation of the performance of sulfate-reducing bacteria and methanogens in anaerobic sewer biofilms. Sewer biofilms were shown to be severely inhibited in reducing sulfate and producing methane when Fe3+ was added during sulfur precipitation in the liquid phase. These inhibiting effects are caused by the development of iron sulfide (FeS) precipitates (Eqn. (4)) and modifications to the dynamics of microbial communities, which an optimized dosage of Fe3+ addition can minimize.
However, sewer biofilms are affected by several factors, including pH elevation, which can promote the growth of some bacteria while inhibiting the growth of others, thereby causing changes in the composition of the microbial community [80].
On the other hand, time-based succession, which describes the slow changes in microbial populations over time, occurs in biofilms in sewer systems. As a result of factors like nutrition availability, organic substrate composition, and ambient circumstances, various microbial communities flourish at various phases.
In addition, by adjusting the sulfur cycle processes in the biofilm, ferric iron can help reduce sulfide concentrations and manage odours [81]. This is because some microorganisms engaged in sulfur metabolism can use ferric iron as an electron acceptor. It could foster the growth of sulfur-oxidizing bacteria, which use ferric iron as a source of energy to convert sulfide to sulfate.
Meanwhile, due to the precipitation process generated by the Fe3+ dose, the effluent emitted from the reactor produced a more significant amount of sulfate at the end of the process. The sulfide concentration in the reactor was reduced by about 60%, indicating that consistent Fe3+ addition would be considered a viable solution to lower the inhibition process due to sulfide. In other situations, it has also been ascertained that ferric iron has little effect on the precipitation reaction in acidic conditions. For instance, the sulfide elimination process to the level of 60% can be attained at a reduced time proportion of 1.5 s when the pH for wastewater is set at 7 [82]. The analysis of the significance of pH through precipitation reaction displays that the acidic condition can induce sulfide precipitation when the excess ferrous iron of about 40% is used, according to the study. At an alkaline medium (pH > 8), however, the result reveals that all of the additional iron (II) salts can be precipitated completely [83]. A 1:1 ratio of iron (III) to iron (II) should be perfected by mixing ferric chloride and ferrous sulfate to maximize the efficacy of the precipitation reaction. This is due to the fact that a combination of Fe(II) and Fe(III) has been proven to improve the recovery process of sulfide from wastewater rather than relying on either salt alone [73].
However, aromatic and sulfur functional groups in wastewater can effectively increase the precipitation process of metal sulfide by providing additional sites for metals to attach to sulfide [84]. In practice, the delocalized electrons in benzene rings and other aromatic compounds stabilize the bonding process with metal ions [85]. As a result, these metal ions form strong complexes with functional groups containing sulfur and sulfide. Since the generated metal-sulfur interactions are often insoluble, they can additionally be precipitated during the wastewater treatment process and serve to lower the sulfide concentration in wastewater.
Therefore, the augmentation of aromatic and sulfur functional groups in wastewater plays a crucial role in enhancing the affinity of metal – sulfur interaction, forming an insoluble curdle in the reactor. Sulfur can be removed from wastewater more effectively by precipitating out the insoluble metal complex that forms when metal ions like zinc or iron bind with sulfur.
Nevertheless, the addition of distinctive coagulants such as partial precipitants of ferric chloride and ferrous sulfate together with the hydroxides and carbonates of calcium in this manner being treated as coagulant-aids enriched the higher sulfide removal effectiveness in the range of 96–99% when the physicochemical technique was employed [86].
2.3. Adsorption of hydrogen sulfide (H2S) on activated carbon
Adsorption is a regular process for pollutant removal using physical and chemical procedures to eliminate adsorbed material in the form of adsorbate [87,88]. Adsorption is the process through which a material (adsorbate or sorbate) builds up on the surface of a solid (adsorbent or sorbent) [89]. The adsorbate might exist in a liquid or gaseous phase. Unsaturated forces at the solid surface that have the potential to form bonds with the adsorbate are the driving force behind adsorption [90]. For example, when iron metal is impregnated with an adsorbent such as biochar, H2S removal efficiency is enhanced from biogas using a biogas scrubbing column. The reactive oxygen and metal oxides in biochar can favour the conversion of sulfide into elemental sulfur and sulfates, which will further increase the H2S removal capacity [91]. During the AD process, this phenomenon is done after breaking down the biomass into biogas, as shown in Fig. 2 (Post digestion).
Meanwhile, Nguyen-Thanh and Bandosz investigated the effects of bentonite clay binders containing copper, zinc, or iron in the interlayer gaps on the adsorbents' ability to remove H2S. The adsorbent capacity of AC was then reported to be improved by surface modification with a binder containing copper, indicating how the oxygenated surface groups were aiding the adsorption process. For instance, an increase in surface acidity of about half a pH unit was found for carbon with binders [92]. Additionally, it has been investigated whether adding phosphates during AD can increase the effectiveness of H2S elimination. Trisodium phosphate (TSP) and monosodium phosphate (MSP) are examples of phosphates that can react with H2S to produce precipitates of insoluble metal sulfide [93]. Compared to gaseous H2S, these precipitates are easier to capture or remove from the gas phase. Phosphates can lower the potential of H2S release into the biogas and enhance overall H2S removal effectiveness.
The study by Ansari and others [94] demonstrated the importance of composite materials mixed in various amounts, which examined the impact of the concerted outcome of the modified surface properties of sewage sludge attained from materials bestowed with carbon. Compared to the adsorbents generated from pure precursors, the findings demonstrated that combining the polymer with sludge enhances the adsorption of hydrogen sulfide by 50%. The material's improved performance is due to the oxidation process of hydrogen sulfide influenced by catalytic centres from the sewage sludge. At the same time, a carbonaceous phase depicts a key role in boosting catalytic centre distribution by providing greater space for storage in its micropores. Due to the increased surface area and chemical interactions between the polymer and the sludge, this combination can be successful at adsorbing various sulfur-containing compounds, such as polysulfides and selenyl sulfides, despite the fact that its main focus is frequently on hydrogen sulfide (H2S) adsorption [95].
Conversely, sludge with a variety of binding sites and polymers with a high affinity for sulfur compounds are able to capture and immobilize these pollutants, lowering their concentration in the treated media, similarly to the adsorption of H2S. However, it's important to understand that the sorbent's precise design and functionalization methods will depend on the intended application, the H2S concentration, and the operating circumstances. Extensive testing and optimization studies are necessary to achieve the desired performance in removing H2S.
2.4. Stripping method
The technology is useful since it enhances energy recovery through biogas during the AD process while producing minimal sludge [96]. This method entails the creation of a stripper during anaerobic digestion capable of extracting sulfide from wastewater while maintaining the effluent's chemical properties. For example, an inventive stripper constructed in the laboratory with regulated factors encompassed during the design and set-up, such as airflow rate, liquid flow rate, liquid-to-air ratio, and pH profile, was claimed to have a 60–70% sulfide removal efficacy. The design imposed the stripper from the bottom, and the sulfide wastewater was passed from the top [97]. During stripper designing, adjusting the incoming air entailed per amount of sulfide in the particular experiment is critical. The amount of air fed into the stripper is one of the critical design control points. When the air is not properly controlled, sulfur dioxide (SO2) can be produced through hydrogen sulfide (H2S) oxidation. In addition, since it impacts the reaction kinetics of H2S oxidation, the temperature of the stripper needs to be carefully controlled [98]. However, to ensure sufficient contact between H2S and the oxidizing agent, it is also necessary to monitor the residence time of the gas stream inside the stripper. Controlling these critical components is essential for minimizing the risks that could result from poor designing of the stripper. Prioritizing risks include H2S release, SO2 generation and corrosion [99].
Before being released into the atmosphere, hydrogen sulfide-contaminated air must be cleaned using an essential tool such as a biofilter [100]. Initially, the process relied on air recycling, with recycled air emitting traces of hydrogen sulfide into the atmosphere. Recycling stripper wastewater, which may include a lower percentage of free H2S, might be considered a sulfide concentration control strategy in the anaerobic reactor. When monitoring low concentrations of H2S, passive samplers consisting of polyethylene adsorbing cartridges loaded with zinc acetate are considered simple, portable, and economical to use [101]. However, it has been reported that air recycling and air injection into wastewater effectively reduce H2S [102]. The process of pumping air into the reactor often raises the dissolved oxygen concentration, favouring the aerobic process and reducing the growth of the anaerobic bacteria that produce H2S. On the other hand, recycling the air in the reactor under limited pressure substantially lowers the release of H2S by improving the treatment systems through optimization of the aeration system efficiency.
2.5. Wet scrubbing
Wet or chemical scrubbing or absorption involves the mass transfer of H2S in the liquid phase aided by acidic, basic, or oxidant reagents. The sodium hypochlorite solution (NaClO) oxidizes hydrogen sulfide (H2S) to sodium sulfate (Na2SO4) in the alkaline media (pH > 9), as indicated in the equation below (Eqn. (5))[103].
| H2S + 4NaOCl + 2NaOH → Na2SO4 + 4NaCl + 2H2O | (5) |
From the study done by Biard et al. [103] on the enforcement of the scrubber with a contactor where solvent and gas flow co-currently is at a high velocity greater than 12 m s−1, it was reported that hydrogen sulfide suppression efficacy of 95% could be achieved. This high removal efficiency is complemented by the residence time of the solvent in the scrubber being shortened to 30 m s (microsecond) using sodium hypochlorite (NaOCl) solution. This study used a single factor research approach for process parameter optimization, which meant that while one process parameter or factor was examined, all other parameters or factors remained unchanged [104]. According to these findings, the maximum H2S removal effectiveness was 98.2% under ideal experimental circumstances. Furthermore, it was reported that as UV-light intensity and solution pH were improved, the H2S removal efficiency increased. In this experiment, whereby hydrogen peroxide (H2O2) was employed as one of the oxidizing agents and urea as an alkaline media, the change of the H2O2 concentration from 0 to 0.2 mol/L stimulated the rise in H2S removal efficiency firstly at 38.7%–98.2% and then lessened to 98.2%–90.1%. Because of the high solubility of sulfur dioxide (SO2) in water, various studies indicate that the removal effectiveness of SO2 in the wet-scrubbing method was about 100% [105,106,107,108]. Furthermore, this method is extensively utilized due to its ease of use and great efficiency. However, using various absorbers necessitates a high regeneration energy input and non-corrosive equipment [109,110]. On the other hand, chemical scrubbing in packed towers may entail some running costs, as it necessitates building high and big wet scrubbers [111].
3. Biological methods
Biological desulfurization technology is a long-term method for removing sulfide from biogas. Its benefits include minimal chemical agent usage, little surplus sludge production, and effective elemental sulfur recycling. Hydrogen sulfide is initially transported to the liquid phase by the alkaline absorption solution in the current sulfide removal systems (Fig. 4), where sulfur-oxidizing bacteria (SOB) then oxidize it to sulfur or sulfate, resulting in a high sulfide removal efficiency [51].
In order to evacuate hydrogen sulfide from wastewater treated for the AD process, physicochemical processes such as absorption have been used [112,113], scrubbing [114], and using water-containing chemicals [115,116]. Nonetheless, these procedures are expensive and can result in chemical waste accumulation. Biological treatment procedures such as autotrophic denitrification [117] and biological desulfurization [118], on the other hand, have been preferred over other techniques due to the method's moderate operating conditions and low cost.
Two major techniques under this method are involved during the process; aerobic and anaerobic (or anoxic), depending on various electron acceptors. The electron acceptor in the aerobic technique is usually oxygen, whereas, in an anoxic approach, the electron acceptor is often nitrate or nitrite [119].
3.1. Aerobic biological technology
The microaerobic process involves using a low concentration of dissolved oxygen (DO) for microorganisms to facilitate hydrolysis acidification. However, the aerobic process is referred to as extended aeration, in which plenty of aeration is needed for energy derivation from carbohydrates (sugars). In practice, the microbes will start feeding on each other under the deprived aeration process [120]. Through enzymatic processes, microbial hydrolysis transforms complex chemicals into volatile fatty acids that microbes can use in subsequent stages of the AD process [46]. In order to produce an environment suitable for various microbial activities, the level of DO is often kept low (Fig. 4). However, several factors, including temperature, pH, nutrient availability, and substrate characteristics, impact the hydrolysis process [121]. Different hydrolysis settings are preferable depending on the particular microorganisms involved and the nature of the substrate being used. The control of the acidification process in the reactor depends critically on optimizing these factors for the hydrolysis progression (Fig. 5).
Fig. 5.
Factors affecting microbial hydrolysis during the anaerobic digestion process.
The quantity of air flow or (DO)in the reactor comprising wastewater for the removal of sulfide was examined in various experiments with this approach. For example, in their study, Guerrero and other co-authors [122] ascertained that the ratio of oxygen to sulfide, determined by the chemical reactions below (Eqns. (7), (8)), affects sulfide removal effectiveness under aerobic conditions.:
| (6) |
| (7) |
| (8) |
This method revealed that a maximum of 90% sulfide removal could be attained at optimal dissolved oxygen levels. The findings show that the minimum amount of oxygen can produce the highest sulfide removal efficiency. These results agree with the study of other scholars, such as Doğan and coworkers [123]. They run biological sulfide oxidation by operating an airlift reactor under oxygen-limited conditions (0.2–1.0 mg/L), as shown in Table 2. It was discovered in this investigation that as the volumetric sulfide loading rate was improved, elemental sulfur generation rose as well, with sulfide removal exceeding 93%.
Table 2.
A summary of the strategies used to control sulfide inhibition by the AD system and the conditions under which they were employed.
| Method/Technique | Working Principle | Appropriate condition(s) | Reference |
|---|---|---|---|
| Micro-aerobic process | The method uses oxygen as an electron acceptor during sulfide oxidation, in which excess sulfide is further transformed into elemental sulfur. |
|
[124,63,125,52] |
| Precipitation process | The method employs metals with a strong affinity for sulfur, such as iron and zinc. |
|
[126,127,128] |
| Adsorption process | A material (adsorbate or sorbate) builds up on the surface of a solid (adsorbent or sorbent). |
|
[129,130,131] |
| Stripping | A physical system in which air and wastewater flow in opposite directions under mass transfer. |
|
[132,133] |
| Wet scrubbing | The method uses water as an adsorbent since H2S is more soluble in water than methane. |
|
[134,135] |
| Aerobic biological technology | The process involves using a low concentration of (DO) for microorganisms to facilitate hydrolysis acidification. |
|
[136,137,60] |
| Biofiltration | Contaminated gases are carried into the biofilm and through the biofilter, where microbes use them as carbon or energy source. The H2S is further broken down to produce sulfate or sulfuric acid. |
|
[138,139,140] |
The mass transfer of H2S molecules during removal can be effectively controlled by adjusting the pressure, airflow rate, and silicone membrane design [141]. However, silicone membranes and silicon carbide nanocages utilized in gas sensing have multiple applications and work based on various mechanisms. While silicon membranes use selective permeability to separate gases, silicon carbide nanocages use adsorption or interaction with gas molecules as the basis for operation [142]. Generally, silicone membranes are designed to provide high separation efficiency and eliminate particular gases from gas streams, such as H2S [143]. On the other hand, the detection and measurement of gas molecule concentrations, including H2S, are the main objectives of silicon carbide nanocages [144]. Both have distinct capabilities that suit them for particular gas treatment and analysis applications.
In a combined anaerobic/microaerobic reactor with no significant sulfate production, this technique increased sulfide removal efficiency to around 96% [119].
However, biogas to air ratio increases and retention time (RT) were crucial features analyzed and proposed to influence sulfide removal efficiency. It was discovered that RT and increasing air mix ratio improved H2S removal efficiency. According to Chaiprapat and co-authors' research, the biogas-to-air ratio of 1:4 was adequate for H2S removal, with average reductions of 94.7%, 87.3%, and 85.6% for the biofiltration system reactor at retention times of 160, 80, and 40 s, respectively [145].
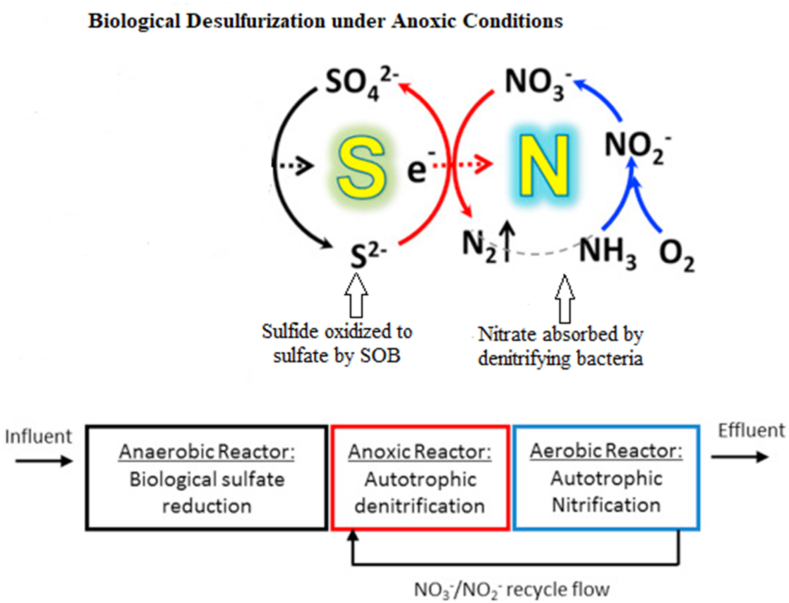
3.2. Anaerobic (anoxic) biological technology
The anoxic method describes a route in which denitrifying bacteria absorb nitrate to remove sulfide through sulfide oxidation (Fig. 6). Because nitrite and nitrate concentrations in wastewater are small, these quantities are intentionally fed into the digester during the sulfide removal process [146]. Even though the nitrate-reducing bacteria overwhelm the sulfate-reducing bacteria when competing, inducing nitrate in the digester augments sulfide reduction [147]. During the desulfurization process, autotrophic denitrification has been frequently used, in which sulfide oxidizing bacteria employ sulfide as an electron donor to link with the nitrate-lessening process [148]. When sulfide is oxidized to sulfate, elemental sulfur is generated as a transitional product. The process is enhanced by some bacteria species, such as Thiobacillus denitrificans which can also reduce nitrogenous species to dinitrogen apart from oxidizing sulfide to elemental sulfur. Below is a summary of the reactions (Eqns. (9), (10), (11), (12)) that influence the anoxic technique [149]:
| (9) |
| (10) |
| (11) |
| (12) |
Fig. 6.
Biological (Anoxic) Desulfurization Process modified from reference [150].
Yang and other colleagues [151] presented one of the studies looking at the efficacy of anoxic techniques in reducing sulfide concentrations in wastewater. Various tests were developed to investigate chemical and biological sulfide oxidation through nitrate at the water level. The findings revealed that sulfide oxidation was biologically controlled in anoxic settings, with elemental sulfur being retrieved as a byproduct. However, as an intermediary product, nitrite was deposited in the effluent. Furthermore, Yong Zeng and other co-authors [152], according to their findings, the loading rate can affect sulfide removal efficiency. However, the results show that the removal efficiencies plummeted substantially as the loading rate was reduced. For example, nearly 62% and 50% of efficacies were recounted when biogas slurry was recycled. This exclusion capability is compliant with the outcomes from the experiment, which was done by Mouna and companion researchers to assess the impact of several other parameters, such as the amount of H2S, Empty Bed Residence Time (EBRT), and the molar ratio of nitrogen to sulfide (N/S), in conjunction with the performance of the biofilter under anoxic conditions.
However, when sufficient is present, H2S is entirely oxidized into sulfate [153,58]. This incident shows how the sulfide to nitrate (S/N) molar ratio is fundamental throughout H2S oxidation. Referring to the research which was done by Li and other colleagues [154] to investigate the influence of the molar proportion of S/N on biogas desulfurization performance, it was revealed that the expulsion effectiveness of H2S upgraded from about 66 to 100% upon lessening the S/N ratios from 3.6 to 0.7. In this study, it was then agreed that different approaches for infusing nitrate in wastewater, i.e., intermittent and continuous, did not affect the exclusion of H2S considerably, while the intermittent inclusion of nitrate wastewater elevated the percentages of sulfate and denitrification performance.
In addition to anoxic bioreactors used for desulfurization, the carbon to nitrogen (C/N) ratio is vital to the AD process. A balanced C/N ratio ensures an appropriate supply of carbon to SRB, which is responsible for converting sulfate to sulfide during desulfurization [155]. Therefore, the C/N ratio not only controls microbial activity but also controls the availability of essential nutrients.
According to Guillermo and other co-authors' study [156], the anoxic desulfurization process improved methane content from 60.0% in the raw biogas to 61.7–63.5% in the purified biogas, increasing the effectiveness of H2S removal from 92–97% to 92–97%. However, the C/N ratio imbalances may result in process instability due to the accumulation of hazardous intermediates such as volatile fatty acids and an unbalanced pH, which slows the desulfurization process [157,158].
3.3. Biofiltration of hydrogen sulfide (H2S)
In this process, polluted gas (biogas) is carried into the biofilm and through the biofilter, where microbes use polluting gases such as H2S and methane (CH4) as a carbon or energy source. The most common microbial species in H2S biofiltration is Thiobacillus sp., which can break down H2S for energy and generate sulfate or sulfuric acid [159]. For microbial growth and proper distribution of both air and water, an ideal biofilter media should have a large surface area. As a result, microbial oxidation enables the biofilter to rapidly capture air that is odorous and contains H2S [102]. Normally, microbial oxidation makes it easier for H2S in the air to permeate into the biofilm and be broken down by the sulfur oxidizing bacteria (SOB). These microorganisms use H2S as an energy source during the process to transform H2S into sulfate (SO42−) or elemental sulfur [160].
However, biofiltration consistently contributes to decreased volatile fatty acids (VFA) accumulation after H2S treatment. Depending on the amount and composition of VFA and microorganisms present in the biofilter, the SOB may utilize VFA as a carbon source during the oxidation of H2S, hence reducing the amount of VFA produced [161,162].
In practice, the efficacy of energy or resource recovery in a treatment system is dependent upon the characteristics of the influent wastewater and process design, such as the capacity of a specific process or material to separate and selectively capture CO2 and H2S from a gas mixture, primarily containing CO2, CH4 (methane), and H2S [163]. Since methane can pass through and be recovered as an energy source, activated carbon and zeolites with improved porosity can improve the selectivity of both CO2 and H2S [164].
This technique has been considered superior to other conventional methods for eviction of H2S recently due to its effectiveness in treating lethal pollutants. However, the biofiltration process (as summarized in Table 2) is considered an effective and convenient approach to removing H2S since it can treat contaminants with very high flow rates regardless of concentration [165].
4. Conclusion and future prospects
4.1. Future prospects of hydrogen sulfide (H2S) removal
Existing techniques have been modified to enhance biogas production to reduce the impact of H2S emissions during anaerobic digestion and promote the sustainable utilization of organic waste for energy recovery. Typically, a post-treatment method has been widely employed, which tends to lower the quality of biogas during the process. Nonetheless, this process incurs additional costs, necessitating using absorbent and adsorbent materials to clean the biogas [166]. Among the methods examined in this study, microaeration emerges as a viable and cost-effective alternative, supported by various researchers (Table 3). This method offers advantages over other techniques since it only requires a small amount of oxygen to be introduced into the digester, effectively deactivating sulfate-reducing bacteria that play a key role in the conversion of sulfate ions (SO42−) to hydrogen sulfide through assimilation and dissimilation pathways (Fig. 3).
Table 3.
Advantages and disadvantages of various sulfur control approaches.
| Method | Advantages | Disadvantages | Economic considerations based on capital costa | References |
|---|---|---|---|---|
| Micro-aerobic process |
|
|
Lower operational cost equivalent to 0.0019 EUR/m3 of biogas treated | [167,168,55] |
| Precipitation process |
|
|
No data | [169,60] |
| Adsorption process |
|
|
Relatively expensive, with an estimated cost of US$2.3 per m3 of biogas | [170,2,171] |
| Stripping |
|
|
No data | [172,173] |
| Wet scrubbing |
|
|
When employing NaOH, it costs roughly US$2.38/m3. | [174,171,134] |
| Aerobic biological technology |
|
|
No data | [175,176] |
| Biofiltration |
|
|
High treatment cost nearly US$1.5/m3 of biogas | [145,165] |
Cost data based on estimates for the year 2015–2019 with currency conversion of 1EUR to US $1.12.
However, for the sustainable and effective implementation of the microaeration method, pretreatment is advisable to achieve satisfactory outcomes in removing H2S from the digester. In contrast to post-treatment, pretreatment is a relatively straightforward and cost-effective approach. It involves using chemicals to facilitate the precipitation of sulfide (S2−) and sulfate (SO42−) ions in the substrate before introducing them into the digester. Recent studies have demonstrated promising results for pretreatment in laboratory-scale applications [177]. However, further research is required to investigate the cost estimation and optimization of chemical addition for desulfurization when applied at a larger, full-scale level.
4.2. Conclusion
This review examines different approaches to controlling H2S by considering various factors, including operating principles, suitable conditions, economic viability, and the advantages and disadvantages of each technique. Post-treatment methods like biofiltration and wet scrubbers offer highly effective removal of H2S, particularly for large-scale applications, although with increased costs. Nevertheless, given the increasing demand for alternative energy sources like biogas, the micro aeration method is considered a straightforward and economical approach to enhance desulfurization while treating sulfur-containing materials for anaerobic digestion. In order to mitigate the risk of excessive aeration, the information presented also highlights the significance of a thorough design framework and the sharing of operational experiences.
Funding
This work was supported by the Office of the Deputy Vice Chancellor – Academic, Research and Consultancy (DVC - ARC), The University of Dodoma, Tanzania.
Author contribution statement
All authors listed have significantly contributed to the development and writing of this article.
1) conceived and designed the experiments.
2) performed the experiments.
3) analyzed and interpreted the data.
4) contributed reagents, materials, analysis tools or data.
5) wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the Office of the Deputy Vice Chancellor – Academic, Research and Consultancy (DVC - ARC), University of Dodoma, Tanzania, for both material and financial support. Also, the authors would like to thank colleagues from the Department of Chemistry, College of Natural and Mathematical Sciences, The University of Dodoma, for their assistance in the final compilation of this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19768.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Atelge M., Krisa D., Kumar G., Eskicioglu C., Nguyen D.D., Chang S.W., Atabani A., Al-Muhtaseb A.H., Unalan S. Biogas production from organic waste: recent progress and perspectives. Waste and Biomass Valoriz. 2020;3(11):1019–1040. [Google Scholar]
- 2.Mutegoa E., Malima N., Hilonga A., Njau K. Effect of mixing ratios of natural inorganic additives in removing ammonia and sulfide in the liquid phase during anaerobic digestion of slaughterhouse waste. Mater. Today Chem. 2021;(20) doi: 10.1016/j.mtchem.2020.100415. [DOI] [Google Scholar]
- 3.Battista F., Camacho Y.M., Hernández S., Bensaid S., Herrmann A., Krause H., Trimis D., Fino D. LCA evaluation for the hydrogen production from biogas through the innovative BioRobur project concept. Int. J. Hydrogen Energy. 2017;19(42):14030–14043. [Google Scholar]
- 4.Rasimphi T., Tinarwo D. Relevance of biogas technology to Vhembe district of the Limpopo province in South Africa. Biotechnology Reports. 2020;(25) doi: 10.1016/j.btre.2019.e00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafiee S., Topal E. When will fossil fuel reserves be diminished? Energy Pol. 2009;1(37):181–189. [Google Scholar]
- 6.Shah M.H., Salem S., Ahmed B., Ullah I., Rehman A., Zeeshan M., Fareed Z. Nexus between foreign direct investment inflow, renewable energy consumption, ambient air pollution, and human mortality: a public health perspective from non-linear ardl approach. Front. Public Health. 2022;(9) doi: 10.3389/fpubh.2021.814208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Amjith L., Bavanish B. A review on biomass and wind as renewable energy for sustainable environment. Chemosphere. 2022;(293) doi: 10.1016/j.chemosphere.2022.133579. [DOI] [PubMed] [Google Scholar]
- 8.Paolini V., Petracchini F., Segreto M., Tomassetti L., Naja N., Cecinato A. Environmental impact of biogas: a short review of current knowledge. J. Environ. Sci. Health, Part A. 2018;10(53):899–906. doi: 10.1080/10934529.2018.1459076. [DOI] [PubMed] [Google Scholar]
- 9.Sarkodie S.A., Strezov V., Weldekidan H., Asamoah E.F., Owusu P.A., Doyi I.N.Y. Environmental sustainability assessment using dynamic autoregressive-distributed lag simulations—nexus between greenhouse gas emissions, biomass energy, food and economic growth. Sci. Total Environ. 2019;(668):318–332. doi: 10.1016/j.scitotenv.2019.02.432. [DOI] [PubMed] [Google Scholar]
- 10.Gunes B., Stokes J., Davis P., Connolly C., Lawler J. Pre-treatments to enhance biogas yield and quality from anaerobic digestion of whiskey distillery and brewery wastes: a review. Renew. Sustain. Energy Rev. 2019;(113) [Google Scholar]
- 11.Pu Y., Tang J., Zeng T., Hu Y., Yang J., Wang X., Huang J., Abomohra A. Pollutant removal and energy recovery from swine wastewater using anaerobic membrane bioreactor: a comparative study with up-flow anaerobic sludge blanket. Water. 2022;15(14):2438. [Google Scholar]
- 12.Achinas S., Li Y., Achinas V., Euverink G.J.W. Influence of sheep manure addition on biogas potential and methanogenic communities during cow dung digestion under mesophilic conditions. Sustain. Environ. Res. 2018;5(28):240–246. [Google Scholar]
- 13.Adekunle A., Ibitoye S., Omoniyi P., Jilantikiri L., Sam-Obu C., Yahaya T., Mohammad B., Olusegun H. Production and testing of biogas using cow dung, jatropha and iron filins. J. Biores. Bioprod. 2019;3(4):143–148. [Google Scholar]
- 14.Juárez J.M., Pastor E.R., Sevilla J.M.F., Torre R.M., García-Encina P.A., Rodríguez S.B. Effect of pretreatments on biogas production from microalgae biomass grown in pig manure treatment plants. Bioresour. Technol. 2018;(257):30–38. doi: 10.1016/j.biortech.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 15.Pu C., Liu H., Ding G., Sun Y., Yu X., Chen J., Ren J., Gong X. Impact of direct application of biogas slurry and residue in fields: in situ analysis of antibiotic resistance genes from pig manure to fields. J. Hazard Mater. 2018;(344):441–449. doi: 10.1016/j.jhazmat.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Francese A., Aboagye-Mathiesen G., Olesen T., Cordoba P., Siñeriz F. Feeding approaches for biogas production from animal wastes and industrial effluents. World J. Microbiol. Biotechnol. 2000;2(16):147–150. [Google Scholar]
- 17.Patricio J., Kalmykova Y., Rosado L. A method and databases for estimating detailed industrial waste generation at different scales–With application to biogas industry development. J. Clean. Prod. 2020;(246) [Google Scholar]
- 18.Mirmohamadsadeghi S., Karimi K., Tabatabaei M., Aghbashlo M. Biogas production from food wastes: a review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019;(7) [Google Scholar]
- 19.Ali M.M., Ndongo M., Bilal B., Yetilmezsoy K., Youm I., Bahramian M. Mapping of biogas production potential from livestock manures and slaughterhouse waste: a case study for African countries. J. Clean. Prod. 2020;(256) [Google Scholar]
- 20.Fatima B., Liaquat R., Farooq U., Jamal A., Ali M., Liu F.-J., He H., Guo H., Urynowicz M., Huang Z. Enhanced biogas production at mesophilic and thermophilic temperatures from a slaughterhouse waste with zeolite as ammonia adsorbent. Int. J. Environ. Sci. Technol. 2020:1–10. [Google Scholar]
- 21.Aziz N.I.H.A., Hanafiah M.M., Ali M.Y.M. Renewable Energy.; 2019. Sustainable Biogas Production from Agrowaste and Effluents–A Promising Step for Small-Scale Industry Income; pp. 363–369. 132. [Google Scholar]
- 22.Bolzonella D., Battista F., Mattioli A., Nicolato C., Frison N., Lampis S. Biological thermophilic post hydrolysis of digestate enhances the biogas production in the anaerobic digestion of agro-waste. Renew. Sustain. Energy Rev. 2020;(134) [Google Scholar]
- 23.Escudero M., Maffiotte C., Serrano J. Long-term operation of a solid oxide fuel cell with MoNi–CeO2 as anode directly fed by biogas containing simultaneously sulphur and siloxane. J. Power Sources. 2021;(481) [Google Scholar]
- 24.Yan L., Liu C., Zhang Y., Liu S., Zhang Y. Effects of C/N ratio variation in swine biogas slurry on soil dissolved organic matter: content and fluorescence characteristics. Ecotoxicol. Environ. Saf. 2021;(209) doi: 10.1016/j.ecoenv.2020.111804. [DOI] [PubMed] [Google Scholar]
- 25.Andreides M., Pokorná-Krayzelová L., Ambrožová J.Ř., Volcke E., Bartáček J. Key parameters influencing hydrogen sulfide removal in microaerobic sequencing batch reactor. Biochem. Eng. J. 2021 [Google Scholar]
- 26.González-Cortés J., Almenglo F., Ramírez M., Cantero D. Simultaneous removal of ammonium from landfill leachate and hydrogen sulfide from biogas using a novel two-stage oxic-anoxic system. Sci. Total Environ. 2021;(750) doi: 10.1016/j.scitotenv.2020.141664. [DOI] [PubMed] [Google Scholar]
- 27.Maurya R., Tirkey S.R., Rajapitamahuni S., Ghosh A., Mishra S. Elsevier; 2019. Recent Advances and Future Prospective of Biogas Production Advances In Feedstock Conversion Technologies For Alternative Fuels And Bioproducts; pp. 159–178. [Google Scholar]
- 28.Papurello D., Lanzini A. SOFC single cells fed by biogas: experimental tests with trace contaminants. Waste Manag. 2018;(72):306–312. doi: 10.1016/j.wasman.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Randazzo A., Asensio-Ramos M., Melián G., Venturi S., Padrón E., Hernández P., Pérez N., Tassi F. Volatile organic compounds (VOCs) in solid waste landfill cover soil: chemical and isotopic composition vs. degradation processes. Sci. Total Environ. 2020;(726) doi: 10.1016/j.scitotenv.2020.138326. [DOI] [PubMed] [Google Scholar]
- 30.Santos-Clotas E., Cabrera-Codony A., Boada E., Gich F., Muñoz R., Martín M.J. Efficient removal of siloxanes and volatile organic compounds from sewage biogas by an anoxic biotrickling filter supplemented with activated carbon. Bioresour. Technol. 2019;(294) doi: 10.1016/j.biortech.2019.122136. [DOI] [PubMed] [Google Scholar]
- 31.Ma J., Frear C., Wang Z.-w., Yu L., Zhao Q., Li X., Chen S. A simple methodology for rate-limiting step determination for anaerobic digestion of complex substrates and effect of microbial community ratio. Bioresour. Technol. 2013;(134):391–395. doi: 10.1016/j.biortech.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Arif Y., Hayat S., Yusuf M., Bajguz A. Hydrogen sulfide: a versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021;(158):372–384. doi: 10.1016/j.plaphy.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Y., Cheng H., Chen F., Zhang Y., Xu X., Huang C., Chen C., Liu W., Ding C., Li Z. Enhanced methane production by alleviating sulfide inhibition with a microbial electrolysis coupled anaerobic digestion reactor. Environ. Int. 2020;(136) doi: 10.1016/j.envint.2020.105503. [DOI] [PubMed] [Google Scholar]
- 34.Haouzi P., Sonobe T., Judenherc-Haouzi A. Hydrogen sulfide intoxication induced brain injury and methylene blue. Neurobiol. Dis. 2020;(133) doi: 10.1016/j.nbd.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Thatai S., Verma R., Khurana P., Goel P., Kumar D. Springer; 2019. Water Quality Standards, its Pollution and Treatment Methods A New Generation Material Graphene: Applications in Water Technology; pp. 21–42. [Google Scholar]
- 36.McCartney D., Oleszkiewicz J. Sulfide inhibition of anaerobic degradation of lactate and acetate. Water Res. 1991;2(25):203–209. [Google Scholar]
- 37.Piffer M.A., Zaiat M., do Nascimento C.A.O., Fuess L.T. Dynamics of sulfate reduction in the thermophilic dark fermentation of sugarcane vinasse: a biohydrogen-independent approach targeting enhanced bioenergy production. J. Environ. Chem. Eng. 2021;5(9) [Google Scholar]
- 38.Prathna T., Srivastava A. Ferric chloride for odour control: studies from wastewater treatment plants in India. Water Pract. Technol. 2021;1(16):35–41. [Google Scholar]
- 39.Verma C., Quadri T.W., Ebenso E.E., Quraishi M. Handbook of Polymer Nanocomposites for Industrial Applications. 2021. Polymer nanocomposites as industrially useful corrosion inhibitors: recent developments; pp. 419–435. [Google Scholar]
- 40.Uberoi V., Bhattacharya S.K. Interactions among sulfate reducers, acetogens, and methanogens in anaerobic propionate systems. Water Environ. Res. 1995;3(67):330–339. [Google Scholar]
- 41.Edyvean R.G., Videla H.A. Biological corrosion. Interdiscipl. Sci. Rev. 1991;3(16):267–282. [Google Scholar]
- 42.Kumari A., Chugh B., Sheetal, Singh G., Singh A.K. ACS Publications; 2021. Corrosion Inhibitors for Sweet (CO2 Corrosion) and Sour (H2S Corrosion) Environments Sustainable Corrosion Inhibitors I: Fundamentals, Methodologies, and Industrial Applications; pp. 189–205. [Google Scholar]
- 43.Chaghouri M., Gennequin C., Tidahy L.H., Cazier F., Abi–Aad E., Veignie E., Rafin C. Environmental technology; 2022. Low Cost and Renewable H2S-Biofilter Inoculated with Trichoderma harzianum; pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 44.Tumuluru M. Sulfide stress corrosion cracking in low-alloy steel inertia friction welds. Weld. J. 1987;3(66):61. [Google Scholar]
- 45.Zhang Z., Li J., Zeng D., Hu J., Hou D., Zhang L., Shi T. Stress corrosion cracking of high-strength drill pipe in sour gas well. J. Wuhan Univ. Technol.-Materials Sci. Ed. 2014;4(29):813–816. [Google Scholar]
- 46.Wainaina S., Lukitawesa Kumar, Awasthi M., Taherzadeh M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: a critical review. Bioengineered. 2019;1(10):437–458. doi: 10.1080/21655979.2019.1673937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu S., Selvam A., Wong J.W. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014;2(34):363–369. doi: 10.1016/j.wasman.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z., Hu Y., Wang S., Wu G., Zhan X. A critical review on dry anaerobic digestion of organic waste: characteristics, operational conditions, and improvement strategies. Renew. Sustain. Energy Rev. 2023;(176) [Google Scholar]
- 49.Sheets J.P., Ge X., Li Y. Effect of limited air exposure and comparative performance between thermophilic and mesophilic solid-state anaerobic digestion of switchgrass. Bioresour. Technol. 2015;(180):296–303. doi: 10.1016/j.biortech.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad W., Sethupathi S., Kanadasan G., Lau L.C., Kanthasamy R. A review on the removal of hydrogen sulfide from biogas by adsorption using sorbents derived from waste. Rev. Chem. Eng. 2021;3(37):407–431. [Google Scholar]
- 51.Li W., Zhang M., Kang D., Chen W., Yu T., Xu D., Zeng Z., Li Y., Zheng P. Environment International.; 2020. Mechanisms of Sulfur Selection and Sulfur Secretion in a Biological Sulfide Removal (BISURE) System. 137. [DOI] [PubMed] [Google Scholar]
- 52.Zhang R.-C., Xu X.-J., Chen C., Shao B., Zhou X., Yuan Y., Lee D.-J., Ren N.-Q. Bioreactor performance and microbial community analysis of autotrophic denitrification under micro-aerobic condition. Sci. Total Environ. 2019;(647):914–922. doi: 10.1016/j.scitotenv.2018.07.389. [DOI] [PubMed] [Google Scholar]
- 53.Girotto F., Peng W., Rafieenia R., Cossu R. Effect of aeration applied during different phases of anaerobic digestion. Waste and Biomass Valorization. 2018;2(9):161–174. [Google Scholar]
- 54.Muller C., Guevarra K., Summers A., Pierce L., Shahbaz P., Zemke P.E., Woodland K., Hollingsworth V., Nakhla G., Bell K. A review of the practical application of micro-aeration and oxygenation for hydrogen sulfide management in anaerobic digesters. Process Saf. Environ. Protect. 2022;(165):126–137. [Google Scholar]
- 55.Krayzelova L., Bartacek J., Díaz I., Jeison D., Volcke E.I., Jenicek P. Microaeration for hydrogen sulfide removal during anaerobic treatment: a review. Rev. Environ. Sci. Biotechnol. 2015;4(14):703–725. [Google Scholar]
- 56.Díaz I., Lopes A., Perez S., Fdz-Polanco M. Determination of the optimal rate for the microaerobic treatment of several H2S concentrations in biogas from sludge digesters. Water Sci. Technol. 2011;1(64):233–238. doi: 10.2166/wst.2011.648. [DOI] [PubMed] [Google Scholar]
- 57.Jung H., Baek G., Lee C. Magnetite-assisted in situ microbial oxidation of H2S to S0 during anaerobic digestion: a new potential for sulfide control. Chem. Eng. J. 2020;(397) [Google Scholar]
- 58.Manconi I., Carucci A., Lens P., Rossetti S. Simultaneous biological removal of sulphide and nitrate by autotrophic denitrification in an activated sludge system. Water Sci. Technol. 2006;12(53):91–99. doi: 10.2166/wst.2006.410. [DOI] [PubMed] [Google Scholar]
- 59.Tang K., Baskaran V., Nemati M. Bacteria of the sulphur cycle: an overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009;(44):73–94. [Google Scholar]
- 60.Jung H., Kim D., Choi H., Lee C. A review of technologies for in-situ sulfide control in anaerobic digestion. Renew. Sustain. Energy Rev. 2022;157 [Google Scholar]
- 61.Mora M., López L.R., Lafuente J., Pérez J., Kleerebezem R., van Loosdrecht M.C., Gamisans X., Gabriel D. Respirometric characterization of aerobic sulfide, thiosulfate and elemental sulfur oxidation by S-oxidizing biomass. Water Res. 2016;(89):282–292. doi: 10.1016/j.watres.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 62.Tilahun E., Sahinkaya E., Çalli B. A hybrid membrane gas absorption and bio-oxidation process for the removal of hydrogen sulfide from biogas. Int. Biodeterior. Biodegrad. 2018;(127):69–76. [Google Scholar]
- 63.Nguyen D., Khanal S.K. A little breath of fresh air into an anaerobic system: how microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018;7(36):1971–1983. doi: 10.1016/j.biotechadv.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Ochoa F., Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol. Adv. 2009;2(27):153–176. doi: 10.1016/j.biotechadv.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Sembera C., Macintosh C., Astals S., Koch K. Benefits and drawbacks of food and dairy waste co-digestion at a high organic loading rate: a Moosburg WWTP case study. Waste Manag. 2019;(95):217–226. doi: 10.1016/j.wasman.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Eskicioglu C., Ghorbani M. Effect of inoculum/substrate ratio on mesophilic anaerobic digestion of bioethanol plant whole stillage in batch mode. Process Biochem. 2011;8(46):1682–1687. [Google Scholar]
- 67.Wikandari R., Sanjaya A.P., Millati R., Karimi K., Taherzadeh M.J. Elsevier; 2019. Fermentation Inhibitors in Ethanol and Biogas Processes and Strategies to Counteract Their Effects Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; pp. 461–499. [Google Scholar]
- 68.Braguglia C.M., Gallipoli A., Gianico A., Pagliaccia P. Anaerobic bioconversion of food waste into energy: a critical review. Bioresour. Technol. 2018;(248):37–56. doi: 10.1016/j.biortech.2017.06.145. [DOI] [PubMed] [Google Scholar]
- 69.Jun W., Jing Y. Enhanced hydrolysis and methane yield by alying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manage. (Tucson, Ariz.) 2013;4(33):813–819. doi: 10.1016/j.wasman.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto A.G. Effect of inoculum/substrate ratio on methane yield and production rate from straw. Biol. Waste. 1989;4(28):247–255. [Google Scholar]
- 71.Neves L., Oliveira R., Alves M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem. 2004;12(39):2019–2024. [Google Scholar]
- 72.Raposo F., Banks C., Siegert I., Heaven S., Borja R. Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem. 2006;6(41):1444–1450. [Google Scholar]
- 73.Padival N.A., Kimbell W.A., Redner J.A. Use of iron salts to control dissolved sulfide in trunk sewers. J. Environ. Eng. 1995;11(121):824–829. [Google Scholar]
- 74.Poulton S.W., Krom M.D., Van Rijn J., Raiswell R. The use of hydrous iron (III) oxides for the removal of hydrogen sulphide in aqueous systems. Water Res. 2002;4(36):825–834. doi: 10.1016/s0043-1354(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 75.Kaksonen A.H., Riekkola-Vanhanen M.-L., Puhakka J. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res. 2003;2(37):255–266. doi: 10.1016/s0043-1354(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 76.Dohnalek D.A., FitzPatrick J.A. The chemistry of reduced sulfur species and their removal from groundwater supplies. J. Am. Water Works Assoc. 1983;6(75):298–308. [Google Scholar]
- 77.Estay H., Barros L., Troncoso E. Metal sulfide precipitation: recent breakthroughs and future outlooks. Minerals. 2021;12(11):1385. [Google Scholar]
- 78.Singh P., Pal P., Mondal P., Saravanan G., Nagababu P., Majumdar S., Labhsetwar N., Bhowmick S. Kinetics and mechanism of arsenic removal using sulfide-modified nanoscale zerovalent iron. Chem. Eng. J. 2021;(412) [Google Scholar]
- 79.Zhao S., Feng S.J., Wu C.C., Zhang J., Chen K.P. A review on new ammonium oxidation alternatives for effective nitrogen removal from wastewater. J. Chem. Technol. Biotechnol. 2022;97(8):1911–2280. [Google Scholar]
- 80.Aqeel H., Weissbrodt D.G., Cerruti M., Wolfaardt G.M., Wilén B.-M., Liss S.N. Drivers of bioaggregation from flocs to biofilms and granular sludge. Environ. Sci. J. Integr. Environ. Res.: Water Res. Technol. 2019;12(5):2072–2089. [Google Scholar]
- 81.Talaiekhozani A., Bagheri M., Goli A., Khoozani M.R.T. An overview of principles of odor production, emission, and control methods in wastewater collection and treatment systems. J. Environ. Manag. 2016;(170):186–206. doi: 10.1016/j.jenvman.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 82.Kiilerich B., Van de Ven W., Nielsen A.H., Vollertsen J. Sulfide precipitation in wastewater at short timescales. Water. 2017;9(9):670. [Google Scholar]
- 83.Nielsen A.H., Hvitved‐Jacobsen T., Vollertsen J. Effects of pH and iron concentrations on sulfide precipitation in wastewater collection systems. Water Environ. Res. 2008;4(80):380–384. doi: 10.2175/106143007x221328. [DOI] [PubMed] [Google Scholar]
- 84.Deonarine A., Lau B.L., Aiken G.R., Ryan J.N., Hsu-Kim H. Effects of humic substances on precipitation and aggregation of zinc sulfide nanoparticles. Environ. Sci. Technol. 2011;8(45):3217–3223. doi: 10.1021/es1029798. [DOI] [PubMed] [Google Scholar]
- 85.Tsipis C.A. DFT study of “all-metal” aromatic compounds. Coord. Chem. Rev. 2005;24(249):2740–2762. [Google Scholar]
- 86.Altaş L., Büyükgüngör H. Sulfide removal in petroleum refinery wastewater by chemical precipitation. J. Hazard Mater. 2008;1–2(153):462–469. doi: 10.1016/j.jhazmat.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 87.Abdel Ghafar H.H., Ali G.A., Fouad O.A., Makhlouf S.A. Enhancement of adsorption efficiency of methylene blue on Co3O4/SiO2 nanocomposite. Desalination Water Treat. 2015;11(53):2980–2989. [Google Scholar]
- 88.Sadegh H., Ali G.A., Gupta V.K., Makhlouf A.S.H., Shahryari-ghoshekandi R., Nadagouda M.N., Sillanpää M., Megiel E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanost. Chem. 2017;1(7):1–14. [Google Scholar]
- 89.Aljerf L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J. Environ. Manag. 2018;(225):120–132. doi: 10.1016/j.jenvman.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 90.Zaera F. Chirality in adsorption on solid surfaces. Chem. Soc. Rev. 2017;23(46):7374–7398. doi: 10.1039/c7cs00367f. [DOI] [PubMed] [Google Scholar]
- 91.Choudhury A., Lansing S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J. Environ. Chem. Eng. 2021;1(9) [Google Scholar]
- 92.Nguyen-Thanh D., Bandosz T.J. Activated carbons with metal containing bentonite binders as adsorbents of hydrogen sulfide. Carbon. 2005;2(43):359–367. [Google Scholar]
- 93.Molins R.A. CRC Press; 1990. Phosphates in Food. [Google Scholar]
- 94.Ansari A., Bagreev A., Bandosz T.J. Effect of adsorbent composition on H2S removal on sewage sludge-based materials enriched with carbonaceous phase. Carbon. 2005;5(43):1039–1048. [Google Scholar]
- 95.Wilén B.-M., Jin B., Lant P. The influence of key chemical constituents in activated sludge on surface and flocculating properties. Water Res. 2003;9(37):2127–2139. doi: 10.1016/S0043-1354(02)00629-2. [DOI] [PubMed] [Google Scholar]
- 96.Kinidi L., Tan I.A.W., Abdul Wahab N.B., Tamrin K.F.B., Hipolito C.N., Salleh S.F. Recent development in ammonia stripping process for industrial wastewater treatment. Int. J. Chem. Eng. 2018:1–14. [Google Scholar]
- 97.Rao A.G., Prasad K.K., Naidu G.V., Rao N.C., Sarma P. Removal of sulfide in integrated anaerobic–aerobic wastewater treatment system. Clean Technol. Environ. Policy. 2003;1(6):66–72. [Google Scholar]
- 98.Lin Y., Feng L., Li X., Chen Y., Yin G., Zhou W. Study on ultrasound-assisted oxidative desulfurization for crude oil. Ultrason. Sonochem. 2020;(63) doi: 10.1016/j.ultsonch.2019.104946. [DOI] [PubMed] [Google Scholar]
- 99.Karne H., Mahajan U., Ketkar U., Kohade A., Khadilkar P., Mishra A. A review on biogas upgradation systems. Mater. Today: Proc. 2023;(72):775–786. [Google Scholar]
- 100.Furusawa N., Togashi I., Hirai M., Shoda M., Kubota H. Removal of hydrogen sulfide by a biofilter with fibrous peat. J. Ferment. Technol. 1984;6(62):589–594. [Google Scholar]
- 101.Nunes M., Kalinowski C., Godoi A., Gomes A., Cerqueira M. Hydrogen sulfide levels in the ambient air of municipal solid waste management facilities: a case study in Portugal. Case Studies in Chemical and Environmental Engineering. 2021;(4) [Google Scholar]
- 102.Easter C., Quigley C., Burrowes P., Witherspoon J., Apgar D. Odor and air emissions control using biotechnology for both collection and wastewater treatment systems. Chem. Eng. J. 2005;2–3(113):93–104. [Google Scholar]
- 103.Biard P.F., Couvert A., Renner C., Levasseur J.P. Wet scrubbing intensification applied to hydrogen sulphide removal in waste water treatment plant. Can. J. Chem. Eng. 2010;4(88):682–687. [Google Scholar]
- 104.Wang Y., Liu Y., Wang Y. Process Safety and Environmental Protection; 2020. Oxidation Absorption of Hydrogen Sulfide from Gas Stream Using Vacuum Ultraviolet/H2O2/Urea Wet Scrubbing System. [Google Scholar]
- 105.Hao R., Zhao Y., Yuan B., Zhou S., Yang S. Establishment of a novel advanced oxidation process for economical and effective removal of SO2 and NO. J. Hazard Mater. 2016;(318):224–232. doi: 10.1016/j.jhazmat.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 106.Sun W.-y., Ding S.-l., Zeng S.-s., Su S.-j., Jiang W.-j. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone. J. Hazard Mater. 2011;1(192):124–130. doi: 10.1016/j.jhazmat.2011.04.104. [DOI] [PubMed] [Google Scholar]
- 107.Yoon H.J., Park H.-W., Park D.-W. Simultaneous oxidation and absorption of NO x and SO2 in an integrated O3 oxidation/wet atomizing system. Energy Fuel. 2016;4(30):3289–3297. [Google Scholar]
- 108.Zhao Y., Hao R., Wang T., Yang C. Follow-up research for integrative process of pre-oxidation and post-absorption cleaning flue gas: absorption of NO2, NO and SO2. Chem. Eng. J. 2015;(273):55–65. [Google Scholar]
- 109.Chen Y. Zhejiang University of Technology; Hangzhou: 2017. Technical and Kinetic Study on Hydrogen Sulfide Removal by Chelated Iron in Oxidation Process; pp. 2–28. 1. [Google Scholar]
- 110.Wang Y., Wang Y., Liu Y. Removal of gaseous hydrogen sulfide using ultraviolet/Oxone-induced oxidation scrubbing system. Chem. Eng. J. 2020 [Google Scholar]
- 111.Couvert A., Sanchez C., Laplanche A., Renner C. Scrubbing intensification for sulphur and ammonia compounds removal. Chemosphere. 2008;8(70):1510–1517. doi: 10.1016/j.chemosphere.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 112.Boumnijel I., Amor H.B., Chekir H., Hajji N. Hydrogen sulphide removal from the effluents of a phosphoric acid production unit by absorption into chlorinated seawater under alkaline conditions. Compt. Rendus Chem. 2016;4(19):517–524. [Google Scholar]
- 113.Taheri M., Mohebbi A., Hashemipour H., Rashidi A.M. Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2–H2S–CH4 gas mixture using amine-based nanofluids in a wetted wall column. J. Nat. Gas Sci. Eng. 2016;(28):410–417. [Google Scholar]
- 114.Liu Y., Wang Y. Removal of gaseous hydrogen sulfide by a photo-Fenton wet oxidation scrubbing system. Energy Fuel. 2019;11(33):10812–10819. [Google Scholar]
- 115.Kang J.-H., Namgung H.-G., Cho J.-I., Yoo S.S., Lee B.-J., Ji H.W. Removal of hydrogen sulfide in septic tanks for treating black water via an immobilized media of sulfur-oxidizing bacteria. Int. J. Environ. Res. Publ. Health. 2020;3(17):684. doi: 10.3390/ijerph17030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li X., O'Moore L., Song Y., Bond P.L., Yuan Z., Wilkie S., Hanzic L., Jiang G. The rapid chemically induced corrosion of concrete sewers at high H2S concentration. Water Res. 2019;(162):95–104. doi: 10.1016/j.watres.2019.06.062. [DOI] [PubMed] [Google Scholar]
- 117.Vaiopoulou E., Melidis P., Aivasidis A. Sulfide removal in wastewater from petrochemical industries by autotrophic denitrification. Water Res. 2005;17(39):4101–4109. doi: 10.1016/j.watres.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 118.Cano P.I., Colon J., Ramírez M., Lafuente J., Gabriel D., Cantero D. Life cycle assessment of different physical-chemical and biological technologies for biogas desulfurization in sewage treatment plants. J. Clean. Prod. 2018;(181):663–674. [Google Scholar]
- 119.Cai J., Zheng P., Qaisar M., Zhang J. Elemental sulfur recovery of biological sulfide removal process from wastewater: a review. Crit. Rev. Environ. Sci. Technol. 2017;21(47):2079–2099. [Google Scholar]
- 120.Iannacone F., Di Capua F., Granata F., Gargano R., Esposito G. Simultaneous nitrification, denitrification and phosphorus removal in a continuous-flow moving bed biofilm reactor alternating microaerobic and aerobic conditions. Bioresour. Technol. 2020;(310) doi: 10.1016/j.biortech.2020.123453. [DOI] [PubMed] [Google Scholar]
- 121.Schnürer A. Biogas production: microbiology and technology. Anaerobes in Biotechnol. 2016:195–234. doi: 10.1007/10_2016_5. [DOI] [PubMed] [Google Scholar]
- 122.Guerrero L., Montalvo S., Huiliñir C., Campos J.L., Barahona A., Borja R. Advances in the biological removal of sulphides from aqueous phase in anaerobic processes: a review. Environ. Rev. 2016;1(24):84–100. [Google Scholar]
- 123.Doğan E.C., Türker M., Dağaşan L., Arslan A. Simultaneous sulfide and nitrite removal from industrial wastewaters under denitrifying conditions. Biotechnol. Bioproc. Eng. 2012;3(17):661–668. [Google Scholar]
- 124.Andreides M., Pokorná-Krayzelová L., Ambrožová J.Ř., Volcke E., Bartáček J. Key parameters influencing hydrogen sulfide removal in microaerobic sequencing batch reactor. Biochem. Eng. J. 2021;(168) [Google Scholar]
- 125.Yang Z., Liu Z., Sklodowska A., Musialowski M., Bajda T., Yin H., Drewniak L. Microbiological sulfide removal—from microorganism isolation to treatment of industrial effluent. Microorganisms. 2021;3(9):611. doi: 10.3390/microorganisms9030611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Georgiadis A.G., Charisiou N.D., Goula M.A. Removal of hydrogen sulfide from various industrial gases: a review of the most promising adsorbing materials. Catalysts. 2020;5(10):521. [Google Scholar]
- 127.Saravanan A., Kumar P.S., Jeevanantham S., Karishma S., Tajsabreen B., Yaashikaa P., Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: processes and applications towards sustainable development. Chemosphere. 2021;(280) doi: 10.1016/j.chemosphere.2021.130595. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., Lin H., Ding L., Hu B. Low-voltage electrochemical treatment to precipitate sulfide during anaerobic digestion of beet sugar wastewater. Sci. Total Environ. 2020;(747) doi: 10.1016/j.scitotenv.2020.141243. [DOI] [PubMed] [Google Scholar]
- 129.Han X., Chen H., Liu Y., Pan J. Study on removal of gaseous hydrogen sulfide based on macroalgae biochars. J. Nat. Gas Sci. Eng. 2020;(73) [Google Scholar]
- 130.Li Z.-Y., Akhtar M.S., Kwak D.-H., Yang O.-B. Improvement in the surface properties of activated carbon via steam pretreatment for high performance supercapacitors. Appl. Surf. Sci. 2017;(404):88–93. [Google Scholar]
- 131.Nowicki P., Skibiszewska P., Pietrzak R. Hydrogen sulphide removal on carbonaceous adsorbents prepared from coffee industry waste materials. Chem. Eng. J. 2014;(248):208–215. [Google Scholar]
- 132.Mutegoa E., Hilonga A., Njau K.N. Approaches to the mitigation of ammonia inhibition during anaerobic digestion–a review. Water Pract. Technol. 2020;3(15):551–570. doi: 10.2166/wpt.2020.047. [DOI] [Google Scholar]
- 133.Silva A., Ricci B., Koch K., Weißbach M., Amaral M. Dissolved hydrogen sulfide removal from anaerobic bioreactor permeate by modified direct contact membrane distillation. Separ. Purif. Technol. 2020;(233) [Google Scholar]
- 134.Wang Y., Liu Y., Wang Y. Oxidation absorption of hydrogen sulfide from gas stream using vacuum ultraviolet/H2O2/urea wet scrubbing system. Process Saf. Environ. Protect. 2020;(140):348–355. [Google Scholar]
- 135.Wang Y., Wang Y., Liu Y. Removal of gaseous hydrogen sulfide using ultraviolet/Oxone-induced oxidation scrubbing system. Chem. Eng. J. 2020;(393) [Google Scholar]
- 136.Cui G., Bhat S.A., Li W., Ishiguro Y., Wei Y., Li F. H2S, MeSH, and NH3 emissions from activated sludge: an insight towards sludge characteristics and microbial mechanisms. Int. Biodeterior. Biodegrad. 2022;(166) [Google Scholar]
- 137.González-Cortés J.J., Torres-Herrera S., Almenglo F., Ramírez M., Cantero D. Hydrogen sulfide removal from biogas and sulfur production by autotrophic denitrification in a gas-lift bioreactor. ACS Sustain. Chem. Eng. 2020;28(8):10480–10489. [Google Scholar]
- 138.Ibrahim R., El Hassni A., Navaee-Ardeh S., Cabana H. Biological elimination of a high concentration of hydrogen sulfide from landfill biogas. Environ. Sci. Pollut. Control Ser. 2022;1(29):431–443. doi: 10.1007/s11356-021-15525-7. [DOI] [PubMed] [Google Scholar]
- 139.Jia T., Sun S., Zhao Q., Peng Y., Zhang L. Extremely acidic condition (pH< 1.0) as a novel strategy to achieve high-efficient hydrogen sulfide removal in biotrickling filter: biomass accumulation, sulfur oxidation pathway and microbial analysis. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.133770. [DOI] [PubMed] [Google Scholar]
- 140.Rolewicz-Kalińska A., Lelicińska-Serafin K., Manczarski P. Volatile organic compounds, ammonia and hydrogen sulphide removal using a two-stage membrane biofiltration process. Chem. Eng. Res. Des. 2021;(165):69–80. [Google Scholar]
- 141.Pabby A.K., Sastre A.M. State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J. Membr. Sci. 2013;(430):263–303. [Google Scholar]
- 142.Gao X., Da C., Chen C., Li Z., Gu X., Bhatia S.K. The induced orientation effect of linear gases during transport in a NaA zeolite membrane modified by alkali lignin. J. Membr. Sci. 2021;(620) [Google Scholar]
- 143.George G., Bhoria N., AlHallaq S., Abdala A., Mittal V. Polymer membranes for acid gas removal from natural gas. Separ. Purif. Technol. 2016;(158):333–356. [Google Scholar]
- 144.Yang X., Deng Y., Yang H., Liao Y., Cheng X., Zou Y., Wu L., Deng Y. Functionalization of mesoporous semiconductor metal oxides for gas sensing: recent advances and emerging challenges. Adv. Sci. 2023;1(10) doi: 10.1002/advs.202204810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chaiprapat S., Mardthing R., Kantachote D., Karnchanawong S. Removal of hydrogen sulfide by complete aerobic oxidation in acidic biofiltration. Process Biochem. 2011;1(46):344–352. [Google Scholar]
- 146.Soreanu G., Béland M., Falletta P., Edmonson K., Seto P. Laboratory pilot scale study for H2S removal from biogas in an anoxic biotrickling filter. Water Sci. Technol. 2008;2(57):201–207. doi: 10.2166/wst.2008.023. [DOI] [PubMed] [Google Scholar]
- 147.Kotu S.P., Mannan M.S., Jayaraman A. Emerging molecular techniques for studying microbial community composition and function in microbiologically influenced corrosion. Int. Biodeterior. Biodegrad. 2019;(144) [Google Scholar]
- 148.Cui Y.-X., Biswal B.K., Guo G., Deng Y.-F., Huang H., Chen G.-H., Wu D. Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 2019;15(103):6023–6039. doi: 10.1007/s00253-019-09935-4. [DOI] [PubMed] [Google Scholar]
- 149.Li W., Zhao Q.-l., Liu H. Sulfide removal by simultaneous autotrophic and heterotrophic desulfurization–denitrification process. J. Hazard Mater. 2009;2–3(162):848–853. doi: 10.1016/j.jhazmat.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 150.Wu D., Ekama G.A., Chui H.-K., Wang B., Cui Y.-X., Hao T.-W., van Loosdrecht M.C., Chen G.-H. Large-scale demonstration of the sulfate reduction autotrophic denitrification nitrification integrated (SANI®) process in saline sewage treatment. Water Res. 2016;(100):496–507. doi: 10.1016/j.watres.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 151.Yang W., Vollertsen J., Hvitved-Jacobsen T. Anoxic sulfide oxidation in wastewater of sewer networks. Water Sci. Technol. 2005;3(52):191–199. [PubMed] [Google Scholar]
- 152.Zeng Y., Xiao L., Zhang X., Zhou J., Ji G., Schroeder S., Liu G., Yan Z. Biogas desulfurization under anoxic conditions using synthetic wastewater and biogas slurry. Int. Biodeterior. Biodegrad. 2018;(133):247–255. [Google Scholar]
- 153.Jing C., Ping Z., Mahmood Q. Simultaneous sulfide and nitrate removal in anaerobic reactor under shock loading. Bioresour. Technol. 2009;12(100):3010–3014. doi: 10.1016/j.biortech.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 154.Li X., Jiang X., Zhou Q., Jiang W. Effect of S/N ratio on the removal of hydrogen sulfide from biogas in anoxic bioreactors. Appl. Biochem. Biotechnol. 2016;5(180):930–944. doi: 10.1007/s12010-016-2143-3. [DOI] [PubMed] [Google Scholar]
- 155.Yang B., Wang Y., Lu Y., Liu L., Huang S., Cheng F., Feng Z. Effects of simultaneous denitrification and desulfurization and changes of microbial community structure with corncob solid slow-release carbon source under different S/N ratios. J. Water Proc. Eng. 2022;(47) [Google Scholar]
- 156.Quijano G., Valenzuela E.I., Cantero D., Ramírez M., Figueroa-González I. Impact of an anoxic desulfurization process on methane content of the purified biogas. Fuel. 2021;(303) [Google Scholar]
- 157.Di Capua F., Spasiano D., Giordano A., Adani F., Fratino U., Pirozzi F., Esposito G. High-solid anaerobic digestion of sewage sludge: challenges and opportunities. Appl. Energy. 2020;(278) [Google Scholar]
- 158.Fuchs W., Wang X., Gabauer W., Ortner M., Li Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018;(97):186–199. [Google Scholar]
- 159.Xie P., Li C.-L., Shao B., Xu X.-J., Chen X.-D., Zhao L., Zhou X., Lee D.-J., Ren N.-Q., Chen C. Simultaneous removal of carbon dioxide, sulfur dioxide and nitric oxide in a biofilter system: optimization operating conditions, removal efficiency and bacterial community. Chemosphere. 2021;(276) doi: 10.1016/j.chemosphere.2021.130084. [DOI] [PubMed] [Google Scholar]
- 160.Syed M., Soreanu G., Falletta P., Béland M. Removal of hydrogen sulfide from gas streams using biological processes• a review. Can. Biosyst. Eng. 2006;(48):2. [Google Scholar]
- 161.Pachaiappan R., Cornejo-Ponce L., Rajendran R., Manavalan K., Femilaa Rajan V., Awad F. A review on biofiltration techniques: recent advancements in the removal of volatile organic compounds and heavy metals in the treatment of polluted water. Bioengineered. 2022;4(13):8432–8477. doi: 10.1080/21655979.2022.2050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramírez-Sáenz D., Zarate-Segura P.B., Guerrero-Barajas C., Garcia-Peña E.I. H2S and volatile fatty acids elimination by biofiltration: clean-up process for biogas potential use. J. Hazard Mater. 2009;2–3(163):1272–1281. doi: 10.1016/j.jhazmat.2008.07.129. [DOI] [PubMed] [Google Scholar]
- 163.Khanongnuch R., Abubackar H.N., Keskin T., Gungormusler M., Duman G., Aggarwal A., Behera S.K., Li L., Bayar B., Rene E.R. Bioprocesses for resource recovery from waste gases: current trends and industrial applications. Renew. Sustain. Energy Rev. 2022;(156) [Google Scholar]
- 164.Barelli L., Bidini G., Micoli L., Sisani E., Turco M. 13X Ex-Cu zeolite performance characterization towards H2S removal for biogas use in molten carbonate fuel cells. Energy. 2018;(160):44–53. [Google Scholar]
- 165.Vikrant K., Kailasa S.K., Tsang D.C., Lee S.S., Kumar P., Giri B.S., Singh R.S., Kim K.-H. Biofiltration of hydrogen sulfide: trends and challenges. J. Clean. Prod. 2018;(187):131–147. [Google Scholar]
- 166.Farghali M., Andriamanohiarisoamanana F.J., Ahmed M.M., Kotb S., Yamamoto Y., Iwasaki M., Yamashiro T., Umetsu K. Prospects for biogas production and H2S control from the anaerobic digestion of cattle manure: the influence of microscale waste iron powder and iron oxide nanoparticles. Waste Manag. 2020;(101):141–149. doi: 10.1016/j.wasman.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Becker C., Marder M., Junges E., Konrad O. Technologies for biogas desulfurization-An overview of recent studies. Renew. Sustain. Energy Rev. 2022;159 [Google Scholar]
- 168.Krayzelova L., Bartacek J., Díaz I., Jeison D., Volcke E.I., Jenicek P. Microaeration for hydrogen sulfide removal during anaerobic treatment: a review. Rev. Environ. Sci. Biotechnol. 2015;(14):703–725. [Google Scholar]
- 169.Edathil A.A., Kannan P., Haija M.A., Banat F. Sulfide remediation from wastewater using hydrothermally synthesized δ-MnO2/porous graphitic carbon as adsorbent. Environ. Res. 2021;196 doi: 10.1016/j.envres.2020.110429. [DOI] [PubMed] [Google Scholar]
- 170.Kailasa S.K., Koduru J.R., Vikrant K., Tsang Y.F., Singhal R.K., Hussain C.M., Kim K.-H. Recent progress on solution and materials chemistry for the removal of hydrogen sulfide from various gas plants. J. Mol. Liq. 2020;(297) [Google Scholar]
- 171.Vu H.P., Nguyen L.N., Wang Q., Ngo H.H., Liu Q., Zhang X., Nghiem L.D. Hydrogen sulphide management in anaerobic digestion: a critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022;(346) doi: 10.1016/j.biortech.2021.126634. [DOI] [PubMed] [Google Scholar]
- 172.Deng Z., Oraby E., Eksteen J. The sulfide precipitation behaviour of Cu and Au from their aqueous alkaline glycinate and cyanide complexes. Separ. Purif. Technol. 2019;(218):181–190. [Google Scholar]
- 173.Guo J., Jiao W., Qi G., Yuan Z., Liu Y. Applications of high-gravity technologies in gas purifications: a review. Chin. J. Chem. Eng. 2019;6(27):1361–1373. [Google Scholar]
- 174.Deyhim S., Ghorbani-Shahna F., Jaleh B., Tapak L. Development of scrubber with nano-TiO2 coated packing for H2S removal. Process Saf. Environ. Protect. 2021;(149):158–168. [Google Scholar]
- 175.Chen C., Zhang R.-C., Xu X.-J., Fang N., Wang A.-J., Ren N.-Q., Lee D.-J. Enhanced performance of denitrifying sulfide removal process at high carbon to nitrogen ratios under micro-aerobic condition. Bioresour. Technol. 2017;(232):417–422. doi: 10.1016/j.biortech.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 176.Saia F.T., Souza T.S., Duarte R.T.D., Pozzi E., Fonseca D., Foresti E. Microbial community in a pilot-scale bioreactor promoting anaerobic digestion and sulfur-driven denitrification for domestic sewage treatment. Bioproc. Biosyst. Eng. 2016;2(39):341–352. doi: 10.1007/s00449-015-1520-6. [DOI] [PubMed] [Google Scholar]
Further reading
- 177.Huertas J.K., Quipuzco L., Hassanein A., Lansing S. Comparing hydrogen sulfide removal efficiency in a field-scale digester using microaeration and iron filters. Energies. 2020;18(13):4793. [Google Scholar]
- 178.Albina P., Durban N., Bertron A., Albrecht A., Robinet J.-C., Erable B. Influence of hydrogen electron donor, alkaline pH, and high nitrate concentrations on microbial denitrification: a review. Int. J. Mol. Sci. 2019;20(20):5163. doi: 10.3390/ijms20205163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Apollon W., Rusyn I., González-Gamboa N., Kuleshova T., Luna-Maldonado A.I., Vidales-Contreras J.A., Kamaraj S.-K. Science of The Total Environment; 2022. Improvement of Zero Waste Sustainable Recovery Using Microbial Energy Generation Systems: A Comprehensive Review. [DOI] [PubMed] [Google Scholar]
- 180.Arulmani S.R.B., Dai J., Li H., Chen Z., Sun W., Zhang H., Yan J., Kandasamy S., Xiao T. Antimony reduction by a non-conventional sulfate reducer with simultaneous bioenergy production in microbial fuel cells. Chemosphere. 2022;(291) doi: 10.1016/j.chemosphere.2021.132754. [DOI] [PubMed] [Google Scholar]
- 181.Barton L.L., Fauque G.D. Biochemistry, physiology and biotechnology of sulfate‐reducing bacteria. Adv. Appl. Microbiol. 2009;(68):41–98. doi: 10.1016/S0065-2164(09)01202-7. [DOI] [PubMed] [Google Scholar]
- 182.Bashkova S., Armstrong T.R., Schwartz V. Selective catalytic oxidation of hydrogen sulfide on activated carbons impregnated with sodium hydroxide. Energy Fuel. 2009;3(23):1674–1682. [Google Scholar]
- 183.Batstone D.J., Puyol D., Flores-Alsina X., Rodríguez J. Mathematical modelling of anaerobic digestion processes: applications and future needs. Rev. Environ. Sci. Biotechnol. 2015;4(14):595–613. [Google Scholar]
- 184.Bekmezci O.K., Ucar D., Kaksonen A.H., Sahinkaya E. Sulfidogenic biotreatment of synthetic acid mine drainage and sulfide oxidation in anaerobic baffled reactor. J. Hazard Mater. 2011;3(189):670–676. doi: 10.1016/j.jhazmat.2011.01.087. [DOI] [PubMed] [Google Scholar]
- 185.Cassini F., Scheutz C., Skov B.H., Mou Z., Kjeldsen P. Mitigation of methane emissions in a pilot-scale biocover system at the AV Miljø Landfill, Denmark: 1. System design and gas distribution. Waste Manag. 2017;(63):213–225. doi: 10.1016/j.wasman.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 186.Celis‐García L.B., Razo‐Flores E., Monroy O. Performance of a down‐flow fluidized bed reactor under sulfate reduction conditions using volatile fatty acids as electron donors. Biotechnol. Bioeng. 2007;4(97):771–779. doi: 10.1002/bit.21288. [DOI] [PubMed] [Google Scholar]
- 187.Chen Q., Wang Z., Long D., Liu X., Zhan L., Liang X., Qiao W., Ling L. Role of pore structure of activated carbon fibers in the catalytic oxidation of H2S. Ind. Eng. Chem. Res. 2010;7(49):3152–3159. [Google Scholar]
- 188.Chong S., Sen T.K., Kayaalp A., Ang H.M. The performance enhancements of upflow anaerobic sludge blanket (UASB) reactors for domestic sludge treatment–a state-of-the-art review. Water Res. 2012;11(46):3434–3470. doi: 10.1016/j.watres.2012.03.066. [DOI] [PubMed] [Google Scholar]
- 189.Cui H., Turn S.Q., Reese M.A. Removal of sulfur compounds from utility pipelined synthetic natural gas using modified activated carbons. Catal. Today. 2009;4(139):274–279. [Google Scholar]
- 190.Dai X., Hu C., Zhang D., Dai L., Duan N. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour. Technol. 2017;(245):598–605. doi: 10.1016/j.biortech.2017.08.208. [DOI] [PubMed] [Google Scholar]
- 191.Das J., Ravishankar H., Lens P.N. Biological biogas purification: recent developments, challenges and future prospects. J. Environ. Manag. 2022;(304) doi: 10.1016/j.jenvman.2021.114198. [DOI] [PubMed] [Google Scholar]
- 192.Deng Y.-F., Wu D., Huang H., Cui Y.-X., van Loosdrecht M.C., Chen G.-H. Exploration and verification of the feasibility of sulfide-driven partial denitrification coupled with anammox for wastewater treatment. Water Res. 2021;(193) doi: 10.1016/j.watres.2021.116905. [DOI] [PubMed] [Google Scholar]
- 193.Díaz I., Fdz-Polanco M. Robustness of the microaerobic removal of hydrogen sulfide from biogas. Water Sci. Technol. 2012;8(65):1368–1374. doi: 10.2166/wst.2012.013. [DOI] [PubMed] [Google Scholar]
- 194.Duangmanee T., Kumar S., Sung S. Micro-aeration for sulfide removal in anaerobic treatment of high-solid wastewater: a pilot-scale study. Proceed. Water Environ. Federat. 2007;16(2007):2748–2760. [Google Scholar]
- 195.Embaby M.A., Haggag E.-s. A., El-Sheikh A.S., Marrez D.A. Biosorption of Uranium from aqueous solution by green microalga Chlorella sorokiniana. Environ. Sci. Pollut. Control Ser. 2022 doi: 10.1007/s11356-022-19827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fdz-Polanco M., Diaz I., Perez S., Lopes A., Fdz-Polanco F. Hydrogen sulphide removal in the anaerobic digestion of sludge by micro-aerobic processes: pilot plant experience. Water Sci. Technol. 2009;12(60):3045–3050. doi: 10.2166/wst.2009.738. [DOI] [PubMed] [Google Scholar]
- 197.Fu S.-F., Wang F., Shi X.-S., Guo R.-B. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem. Eng. J. 2016;(287):523–528. [Google Scholar]
- 198.Gajda I., Greenman J., Melhuish C., Santoro C., Ieropoulos I. Microbial Fuel Cell-driven caustic potash production from wastewater for carbon sequestration. Bioresour. Technol. 2016;(215):285–289. doi: 10.1016/j.biortech.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 199.Giuffrè A., Vicente J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/6290931. 1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Glória R., Motta T., Silva P., Costa P.d., Brandt E., Souza C., Chernicharo C. Stripping and dissipation techniques for the removal of dissolved gases from anaerobic effluents. Braz. J. Chem. Eng. 2016;4(33):713–721. [Google Scholar]
- 201.Gómez-Borraz T.L., González-Sánchez A., Bonilla-Blancas W., Revah S., Noyola A. Characterization of the biofiltration of methane emissions from municipal anaerobic effluents. Process Biochem. 2017;(63):204–213. [Google Scholar]
- 202.Habeeb O.A., Kanthasamy R., Ali G.A., Sethupathi S., Yunus R.B.M. Hydrogen sulfide emission sources, regulations, and removal techniques: a review. Rev. Chem. Eng. 2018;6(34):837–854. [Google Scholar]
- 203.Jaber M.B., Couvert A., Amrane A., Le Cloirec P., Dumont E. Hydrogen sulfide removal from a biogas mimic by biofiltration under anoxic conditions. J. Environ. Chem. Eng. 2017;6(5):5617–5623. [Google Scholar]
- 204.Jenicek P., Celis C., Krayzelova L., Anferova N., Pokorna D. Improving products of anaerobic sludge digestion by microaeration. Water Sci. Technol. 2014;4(69):803–809. doi: 10.2166/wst.2013.779. [DOI] [PubMed] [Google Scholar]
- 205.Jeong S., Cho K., Jeong D., Lee S., Leiknes T., Vigneswaran S., Bae H. Effect of engineered environment on microbial community structure in biofilter and biofilm on reverse osmosis membrane. Water Res. 2017;(124):227–237. doi: 10.1016/j.watres.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 206.Kampschreur M.J., Temmink H., Kleerebezem R., Jetten M.S., van Loosdrecht M.C. Nitrous oxide emission during wastewater treatment. Water Res. 2009;17(43):4093–4103. doi: 10.1016/j.watres.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 207.Khanal S.K., Huang J.-C. Anaerobic treatment of high sulfate wastewater with oxygenation to control sulfide toxicity. J. Environ. Eng. 2003;12(129):1104–1111. [Google Scholar]
- 208.Khanal S.K., Huang J.-C. ORP-based oxygenation for sulfide control in anaerobic treatment of high-sulfate wastewater. Water Res. 2003;9(37):2053–2062. doi: 10.1016/S0043-1354(02)00618-8. [DOI] [PubMed] [Google Scholar]
- 209.Khoshnevisan B., He L., Xu M., Valverde-Pérez B., Sillman J., Mitraka G.-C., Kougias P.G., Zhang Y., Yan S., Ji L. From renewable energy to sustainable protein sources: advancement, challenges, and future roadmaps. Renew. Sustain. Energy Rev. 2022;(157) [Google Scholar]
- 210.Ko J.H., Xu Q., Jang Y.-C. Emissions and control of hydrogen sulfide at landfills: a review. Crit. Rev. Environ. Sci. Technol. 2015;19(45):2043–2083. [Google Scholar]
- 211.Kouzbour S., Gourich B., Gros F., Vial C., Stiriba Y. A novel approach for removing cadmium from synthetic wet phosphoric acid using sulfide precipitation process operating in batch and continuous modes. Miner. Eng. 2022;(187) [Google Scholar]
- 212.Lasocki J., Kołodziejczyk K., Matuszewska A. Laboratory-scale investigation of biogas treatment by removal of hydrogen sulfide and carbon dioxide. Pol. J. Environ. Stud. 2015;3(24):1427–1434. [Google Scholar]
- 213.Li H., Monnell J.D., Alvin M., Vidic R.D. Factors affecting activated carbon-based catalysts for selective hydrogen sulfide oxidation. Main Group Chem. 2008;3(7):239–250. [Google Scholar]
- 214.Liu D., Andreasen R.R., Poulsen T.G., Feilberg A. A comparative study of mass transfer coefficients of reduced volatile sulfur compounds for biotrickling filter packing materials. Chem. Eng. J. 2015;(260):209–221. [Google Scholar]
- 215.Lohwacharin J., Annachhatre A.P. Biological sulfide oxidation in an airlift bioreactor. Bioresour. Technol. 2010;7(101):2114–2120. doi: 10.1016/j.biortech.2009.10.093. [DOI] [PubMed] [Google Scholar]
- 216.Mahmood Q., Zheng P., Cai J., Wu D., Hu B., Li J. Anoxic sulfide biooxidation using nitrite as electron acceptor. J. Hazard Mater. 2007;1–2(147):249–256. doi: 10.1016/j.jhazmat.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 217.Marsh H., Reinoso F.R. Elsevier; 2006. Activated Carbon. [Google Scholar]
- 218.Merico E., Grasso F., Cesari D., Decesari S., Belosi F., Manarini F., De Nuntiis P., Rinaldi M., Gambaro A., Morabito E. Characterisation of atmospheric pollution near an industrial site with a biogas production and combustion plant in southern Italy. Sci. Total Environ. 2020;(717) doi: 10.1016/j.scitotenv.2020.137220. [DOI] [PubMed] [Google Scholar]
- 219.Mulu E., M'Arimi M.M., Ramkat R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev. 2021;(145) [Google Scholar]
- 220.Nguyen D., Wu Z., Shrestha S., Lee P.-H., Raskin L., Khanal S.K. Intermittent micro-aeration: new strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019;(166) doi: 10.1016/j.watres.2019.115080. [DOI] [PubMed] [Google Scholar]
- 221.Pudi A., Rezaei M., Signorini V., Andersson M.P., Baschetti M.G., Mansouri S.S. Hydrogen sulfide capture and removal technologies: a comprehensive review of recent developments and emerging trends. Separ. Purif. Technol. 2022 [Google Scholar]
- 222.Rahimnejad M., Adhami A., Darvari S., Zirepour A., Oh S.-E. Microbial fuel cell as new technology for bioelectricity generation: a review. Alex. Eng. J. 2015;3(54):745–756. [Google Scholar]
- 223.Ramos I., Díaz I., Fdz-Polanco M. The role of the headspace in hydrogen sulfide removal during microaerobic digestion of sludge. Water Sci. Technol. 2012;10(66):2258–2264. doi: 10.2166/wst.2012.457. [DOI] [PubMed] [Google Scholar]
- 224.Rene E.R., Sergienko N., Goswami T., López M.E., Kumar G., Saratale G.D., Venkatachalam P., Pakshirajan K., Swaminathan T. Effects of concentration and gas flow rate on the removal of gas-phase toluene and xylene mixture in a compost biofilter. Bioresour. Technol. 2018;(248):28–35. doi: 10.1016/j.biortech.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 225.Rodrigo M., Canizares P., Lobato J., Paz R., Sáez C., Linares J. Production of electricity from the treatment of urban waste water using a microbial fuel cell. J. Power Sources. 2007;1(169):198–204. [Google Scholar]
- 226.Sánchez-Andrea I., Sanz J.L., Bijmans M.F., Stams A.J. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard Mater. 2014;(269):98–109. doi: 10.1016/j.jhazmat.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 227.Seredych M., Bandosz T.J. Role of microporosity and nitrogen functionality on the surface of activated carbon in the process of desulfurization of digester gas. J. Phys. Chem. C. 2008;12(112):4704–4711. [Google Scholar]
- 228.Sonawane J.M., Ezugwu C.I., Ghosh P.C. Microbial fuel cell-based biological oxygen demand sensors for monitoring wastewater: state-of-the-art and practical applications. ACS Sens. 2020;8(5):2297–2316. doi: 10.1021/acssensors.0c01299. [DOI] [PubMed] [Google Scholar]
- 229.Sun J., Dai X., Liu Y., Peng L., Ni B.-J. Sulfide removal and sulfur production in a membrane aerated biofilm reactor: model evaluation. Chem. Eng. J. 2017;(309):454–462. [Google Scholar]
- 230.Tabatabaei M., Aghbashlo M., Valijanian E., Panahi H.K.S., Nizami A.-S., Ghanavati H., Sulaiman A., Mirmohamadsadeghi S., Karimi K. A comprehensive review on recent biological innovations to improve biogas production, part 1: upstream strategies. Renew. Energy. 2020;(146):1204–1220. [Google Scholar]
- 231.Van der Zee F., Villaverde S., Garcia P., Polanco F.F.-. Sulfide removal by moderate oxygenation of anaerobic sludge environments. Bioresour. Technol. 2007;3(98):518–524. doi: 10.1016/j.biortech.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 232.Zhang L., De Schryver P., De Gusseme B., De Muynck W., Boon N., Verstraete W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: a review. Water Res. 2008;1–2(42):1–12. doi: 10.1016/j.watres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 233.Zhou W., Imai T., Ukita M., Li F., Yuasa A. Effect of limited aeration on the anaerobic treatment of evaporator condensate from a sulfite pulp mill. Chemosphere. 2007;5(66):924–929. doi: 10.1016/j.chemosphere.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 234.Zitomer D., Shrout J. High‐sulfate, high‐chemical oxygen demand wastewater treatment using aerated methanogenic fluidized beds. Water Environ. Res. 2000;1(72):90–97. [Google Scholar]
- 235.Zulkefli N.N., Masdar M.S., Wan Isahak W.N.R., Md Jahim J., Md Rejab S.A., Chien Lye C. Removal of hydrogen sulfide from a biogas mimic by using impregnated activated carbon adsorbent. PLoS One. 2019;2(14) doi: 10.1371/journal.pone.0211713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.