Abstract
Hypoxia is a common environmental stress factor in aquatic organisms, which varies among fish species. However, the mechanisms underlying the ability of fish species to tolerate hypoxia are not well known. Here, we showed that hypoxia response in different fish species was affected by lipid catabolism and preference for lipid or carbohydrate energy sources. Activation of biochemical lipid catabolism through peroxisome proliferator-activated receptor alpha (Pparα) or increasing mitochondrial fat oxidation in tilapia decreased tolerance to acute hypoxia by increasing oxygen consumption and oxidative damage and reducing carbohydrate catabolism as an energy source. Conversely, lipid catabolism inhibition by suppressing entry of lipids into mitochondria in tilapia or individually knocking out three key genes of lipid catabolism in zebrafish increased tolerance to acute hypoxia by decreasing oxygen consumption and oxidative damage and promoting carbohydrate catabolism. However, anaerobic glycolysis suppression eliminated lipid catabolism inhibition-promoted hypoxia tolerance in adipose triglyceride lipase (atgl) mutant zebrafish. Using 14 fish species with different trophic levels and taxonomic status, the fish preferentially using lipids for energy were more intolerant to acute hypoxia than those preferentially using carbohydrates. Our study shows that hypoxia tolerance in fish depends on catabolic preference for lipids or carbohydrates, which can be modified by regulating lipid catabolism.
Keywords: Hypoxia tolerance, Catabolic preference, Lipid, Carbohydrate, Oxidative damage
INTRODUCTION
Oxygen (O2) is an essential element for living organisms, necessary for various metabolic processes. However, factors such as ocean warming, eutrophication, day-night cycle, power failure, and high-density culture cause hypoxia in water (Karim et al., 2002; Keeling et al., 2010; Phan-Van et al., 2008), resulting in sudden massive fish mortality in wild or aquaculture environments (Landman et al., 2005; Wu, 2002). Despite these challenges, fish species exhibit extraordinary diversity in hypoxia tolerance. For instance, freshwater fish species such as the southern catfish (Silurus meridionalis), channel catfish (Ictalurus punetaus), rice field eel (Monopterus albus), and grass carp (Ctenopharyngodon idellus) exhibit asphyxiation points ranging from 0.15 to 0.39 mg/L (Yu et al., 2017), while certain seawater species such as the humpback grouper (Cromileptes altivelis) and leopard coral grouper (Plectropomus leopardus) exhibit ranges from 0.75 to 1.21 mg/L (Chen et al., 2015). Notably, species such as the Russian sturgeon (Acipenser gueldenstaedti) and Atlantic salmon (Salmon salar) demonstrate asphyxiation points ranging from 2.35 to 3.82 mg/L, thus highlighting high oxygen consumption and low hypoxia tolerance (Liu et al., 2013; Shi et al., 2018). The response of fish to hypoxia involves significant alterations in their behavior and physiology, including reduced swimming and food intake, aerial surface respiration (ASR), changes in the branchial system, increased red blood cell count, and enhanced antioxidant and anaerobic glycolysis capacities (Braz-Mota & Almeida-Val, 2021). Interestingly, the tolerance of fish to hypoxia can be influenced and potentially improved through nutritional supplementation (Magnoni et al., 2017; Yu et al., 2020). Studies have shown that dietary supplementation of vitamin C and carotenoids can enhance fish tolerance to acute hypoxia by improving antioxidant capacity (Chagas & Val, 2006; Henrique et al., 1998; Niu et al., 2014). However, the precise mechanisms and roles of nutrient metabolism and energy homeostasis in relation to hypoxia tolerance in fish remain unclear.
Hypoxia reduces electron transport efficiency in the respiratory chain and hinders adenosine triphosphate (ATP) synthesis (Wheaton & Chandel, 2011). Additionally, it induces incomplete utilization of oxygen, resulting in the generation of excessive reactive oxygen species (ROS) that can be harmful to organisms (Goda & Kanai, 2012; Majmundar et al., 2010). In response, mammals exposed to hypoxia activate anaerobic glycolysis and glycogen catabolism to reduce their oxygen dependence (Gudi et al., 1995; Kim et al., 2006). These adaptative processes are regulated by the hypoxia-inducible factor (HIF) signaling pathway, with HIF1A and HIF2A central in mammals (Koukourakis et al., 2001, 2002) and Hif3α (Hif1al) fulfilling a similar role in zebrafish (Zhang et al., 2012, 2014). Lipid metabolism also contributes to hypoxia adaptation. For example, in cancer cells, lipid synthesis is markedly elevated, while lipid catabolism is suppressed as an adaptive strategy to hypoxia (de la Rosa Rodriguez et al., 2021; Metallo et al., 2012; Zhang et al., 2017). However, the specific mechanisms by which lipid metabolism facilitates hypoxia adaptation are still not well known in fish.
Previous studies have indicated that Nile tilapia (Oreochromis niloticus) exposed to acute hypoxia show an increase in liver glycogen utilization (Li et al., 2018). Similarly, zebrafish (Danio rerio) fed a high carbohydrate diet exhibit improvement in survival during acute hypoxia stress due to activation of glycolysis activity (Ma et al., 2020). These studies suggest that promoting glucose utilization may improve hypoxia tolerance in fish. Unlike mammals, most teleost species derive energy from protein catabolism and exhibit limited efficiency in carbohydrate utilization (Wilson, 1994), as reflected in the rarity of carbohydrates as a staple food for many wild fish species. Nevertheless, we recently found that moderate inhibition of lipid catabolism can enhance glucose utilization by elevating glycolysis activity in omnivorous fish (Li et al., 2020a, 2020b). Consequently, we proposed that the regulation of lipid catabolism may modulate metabolic balance between glucose and lipids, thereby controlling hypoxia tolerance in fish. In addition, we hypothesized that fish in the wild with lower lipid catabolic preference than glycolysis activity may exhibit higher hypoxia tolerance.
In the current study, we investigated lipid catabolism in Nile tilapia and zebrafish, modified through biochemical inhibitors and gene editing techniques, as a potential mechanism for hypoxia tolerance. We collected 14 fish species representing different cladograms and trophic levels to explore the relationship between energy preference sources and hypoxia tolerance. Our findings revealed a strong correlation between the lipid/glucose catabolic activity ratio and hypoxia tolerance in fish. Furthermore, we observed that fish with a preference for lipid catabolism exhibited greater intolerance to acute hypoxia than those favoring carbohydrate utilization for energy. These findings offer valuable insights for improving hypoxia tolerance in cultured fish and understanding the diverse hypoxia adaptations observed in different fish species in nature.
MATERIALS AND METHODS
Animal ethics
All experiments were conducted strictly under the Guidance Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China. The animal experiments and gene editing of zebrafish were permitted by the Committee on the Ethics of Animal Experiments of East China Normal University (Approval Nos.: F20190101 and F20201002).
Experimental fish cultivation and diets
Zebrafish (Danio rerio, 0.3 to 0.4 g, AB line) were purchased from the Chinese National Zebrafish Resource Center (Wuhan, China). Juvenile Nile tilapia (Oreochromis niloticus), snakehead (Channa argus), largemouth bass (Micropterus salmoides), topmouth culter (Erythroculter ilishaeformis), pumpkinseed sunfish (Lepomis gibbosus), yellow catfish (Pelteobagrus fulvidraco), black carp (Mylopharyngodon piceus), white amur bream (Parabramis pekinensis), Bulatmai barbel (Luciobarbus capito), tench (Tinca tinca), bighead carp (Aristichthys nobilis), and crucian carp (Carassius auratus) (initial weights 2–40 g) were purchased from a farm (Huzhou, China), while Japanese medaka (Oryzias latipes, weighing 0.2–0.3 g) were purchased from an aquarium (Shanghai, China). Before the experiments, all fish were acclimatized in a recirculating aquaculture system and fed a commercial diet (protein 48%, fat 8%, fiber 3%, ash 16.5%, moisture 12%, Shengsuo Fishery Culture Feed Research Center, China) for two weeks. During the trials, small-sized zebrafish and Japanese medaka were kept in 50 L tanks, while all other large-sized fish were maintained in 150 L tanks.
For biochemical lipid catabolism inhibition, Nile tilapia were selected and randomly distributed into four treatment groups (three tanks per treatment, 20 fish per tank), fed a control (C), fenofibrate (Fen), L-carnitine (Car), or mildronate diet (Mil), respectively. To suppress anaerobic glycolysis and promote glucose oxidative, zebrafish lacking atgl (atgl−/−) were selected and randomly distributed into two treatment groups (three tanks per treatment, 60 fish per tank), fed either a C or dichloroacetate sodium diet (DCA), respectively. Fenofibrate, L-carnitine, mildronate and dichloroacetate sodium were provided by Sigma-Aldrich (purity>98%, USA). The dietary formulations are shown in Supplementary Table S1. The experimental fish were fed at 4% of their body weight twice daily (at 0800h and 1800h) for 4 to 12 weeks. All fish in each tank were weighed every two weeks to adjust feed amount. Water temperature was maintained at a stable level using an automatic heating system. The water ammonia nitrogen level was measured every two days, and once it exceeded 0.02 mg/L, one third of the water was replaced by clean water. During the trial, water temperature, dissolved oxygen (DO), and pH were maintained at 27±1 °C, 7.0±0.5 mg/L, and 7.5±0.5, respectively.
Establishment of gene knockout (KO) zebrafish line
Adipose triglyceride lipase (atgl), carnitine palmitoyltransferase 1B (cpt1b), and peroxisome proliferator-activated receptor alpha b (pparab) zebrafish mutants were generated using CRISPR/Cas9 gene KO technology (target design: http://zifit.partners.org). After spawning, zebrafish embryos were collected in Petri dishes and injected with a 400 ng/μL mixture containing the small-guide RNA target sequence, Cas9 protein, and buffer. Three months later, the F0 generation were mated with wild-type (WT) zebrafish to generate heterozygous F1 offspring, which were then allowed to self-cross to produce homozygous F2 mutants. The homozygous F2 mutants were allowed to self-cross to generate mass homozygous F3 mutants. Compared to WT zebrafish, the atgl−/−, cpt1b−/−, and pparab−/− zebrafish lacked 5, 11, and 43 base pairs in the target sites, encoding only 71, 80, and 34 amino acid residues, respectively (Supplementary Figure S5A, E, I).
Hypoxia equipment, acute hypoxia challenge, and fish sampling
After each feeding experiment, 10–20 fish per tank (30–60 fish per treatment) were fasted for 6 h and transferred to a customized hypoxic chamber (patent No.: ZL202020482406.9) to induce acute hypoxia. The fish were directly transferred from 7.0±0.5 mg/L to 0.5±0.1 mg/L DO and maintained at this level for 12 h by injecting nitrogen gas or air into the water. The DO levels were decreased continuously to 0.01 mg/L by prolonging the hypoxic period. Throughout this process, the dead fish were counted and recorded every 6 h, with a fish regarded as dead when its gills stopped moving for at least 1 h (Lu et al., 2019). After 12 h of acute hypoxia (0.5±0.1 mg/L), sample under hypoxic and normoxic conditions (7.0±0.5 mg/L) were collected simultaneously. All fish were anesthetized with MS-222 (20 mg/L), and liver and serum samples were collected and frozen for further analysis. Growth, organ index, and survival rate were calculated using the following formulae:
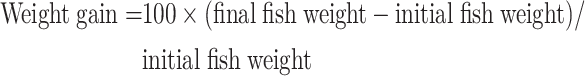 |
1 |
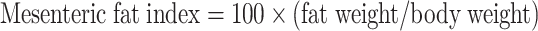 |
2 |
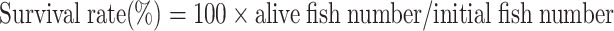 |
3 |
Biochemical assays and histological analysis
Serum and liver triglyceride (TG), free fatty acid (FFA), malondialdehyde (MDA), total protein (BCA method), glucose, glycogen, and lactate contents, and total antioxidant capacity (T-AOC), lactate dehydrogenase (LDH), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) activities were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, China) based on the manufacturer’s protocols. Liver ROS, Atgl, Cpt1b, Pparα, glucokinase (Gk), and pyruvate dehydrogenase E1 alpha 1 (Pdha1) levels were assessed using enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Hengyuan Biological Technology, China; Shanghai Enzyme-linked Biotechnology, China) following the manufacturer’s instructions. Liver lipid droplets were stained red with Oil red O stain, while glycogen was stained fuchsia with periodic acid and Schiff’s stain by Wuhan Servicebio Technology Co., Ltd. (China). The oxygen consumption rate (OCR) was measured using a Strathkelvin Instruments 782 Oxygen Meter system (UK). The free L-carnitine content in the liver was measured using liquid chromatography-mass spectrometry (LC-MS, Agilent, USA), as described in our previous study (Li et al., 2020a).
RNA extraction, transcriptome sequencing, and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from the tilapia and zebrafish livers using RNAiso Plus reagent (Takara, Japan). Only samples with an absorbance ratio (260/280 nm) ranging from 1.9 to 2.0 were used. Transcriptome sequencing was conducted using an Illumina NovaSeq 6000 sequencer by Majorbio Bio-Pharm Technology Co., Ltd. (China). We used Oreochromis niloticus (ASM185804v2) from the NCBI database as the reference genome and |log2FC|≥1 was performed to identify differentially expressed genes (DEGs) between normoxia and hypoxia treatments after 12 h (P-adjusted<0.05). All sequencing data were uploaded to the NCBI platform (GenBank accession: PRJNA891768, ID: 891768) and analyzed using the Majorbio bioinformation cloud platform (http://www.i-sanger.com). The qPCR steps and conditions were similar to those described in our previous study (Ma et al., 2020). The primers used for qPCR are shown in Supplementary Table S2. The amplification efficiency of the reference genes (ef1α and β-actin) and target genes was between 90% and 110%. Relative mRNA expression of genes was calculated using the 2−ΔΔCt method (Livak & Schmittgen, 2001).
Protein extraction and western blot analysis
Protein was extracted from the liver samples (30–50 mg) of tilapia and zebrafish using RIPA lysis buffer (Beyotime Biotechnology, China). The proteins were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After incubation with secondary antibodies, the membranes were scanned using an Odyssey CLX Imager (Li-Cor, USA). The glyceraldehyde-3-phosphate dehydrogenase (Gapdh), protein kinase AMP-activated catalytic subunit alpha (Ampk), v-akt murine thymoma viral oncogene homolog (Akt), pyruvate dehydrogenase kinase 2a (Pdk2), Pdha1, and Hif protein family levels were measured. The antibodies used in our study were specific for fish tissue and are detailed in Supplementary Table S3.
Cell culture, apoptotic cell flow detection, and intracellular oxygen assay
The culture processes for Nile tilapia primary hepatocytes and zebrafish hepatocytes were conducted as described in our previous study (Li et al., 2020a). Tilapia primary hepatocytes under normoxia or hypoxia conditions (12 h) were incubated using phospholipid binding protein (Annexin V-FITC) and nucleic acid dye 7-aminoactinomycin D (7-AAD-PC5.5). The hepatocyte apoptosis rate was detected using a flow cytometry kit (Vazyme Biotech Co., Ltd., China) following the manufacturer’s instructions. Zebrafish hepatocytes pretreated with Fen or L-carnitine were incubated using a NanO2 fluorescent probe (Agilent MitoXpress Intra, USA). The intracellular oxygen level represented by fluorescence values in 96-well plates was detected continuously for 30 min using a fluorescent microplate reader (BMG Labtech, Germany).
14C-labeled nutrient tracing and mitochondrial fatty acid β-oxidation (FAO) assay
Each of the 14 fish species underwent a 12 h fasting period. For each species, three fish were intraperitoneally injected with a nutrient mixture containing 14C-labeled palmitic acid (PA, labeled 0.05 μCi/g body weight and unlabeled 0.5 mg/g body weight), unlabeled D-glucose (GLU, 0.5 mg/g body weight), and unlabeled bovine serum albumin (BSA, 1 mg/g body weight). Another three fish for each species were intraperitoneally injected with a mixture containing 14C-labeled GLU (labeled 0.05 μCi/g body weight and unlabeled 0.5 mg/g body weight), unlabeled PA (0.5 mg/g body weight), and unlabeled BSA (1 mg/g body weight). The radioactive CO2 released from each fish injected with 14C-labeled PA or 14C-labeled GLU was collected and measured using a liquid scintillation counter (Tri-Carb 4910TR, PerkinElmer, USA), as described in our previous study (Li et al., 2020a).
Mitochondrial DNA sequencing and phylogenetic analyses
Muscle DNA samples from the 14 fish species were extracted using a TIANamp Marine Animals DNA Kit (Tiangen, China). Amplification of mitochondrial 12S rRNA, 16S rRNA, and cytochrome c oxidase subunit I (cox1) segments was performed using universal primers 12S1–12S2, 16SarL–16SbrH, and FishF1–FishR1, respectively (Cawthorn et al., 2012; Ward et al., 2005). All nucleotide sequences were analyzed using MEGA v6.0. Bayesian inference (BI) analyses were conducted with MrBayes v3.1.2. Trees and posterior probabilities were visualized using FIGTREE v1.4.2.
Statistical analyses
All data obtained were tested for normality and homogeneity of variances using the Shapiro-Wilk and Levene’s tests, respectively. Independent-samples t-test was used to evaluate significant differences (* or #) in measured parameters between each two treatments. The relationship between the ratio of 14CO2 from fatty acid to 14CO2 from glucose for each fish species and hypoxia tolerance was established using Pearson correlation. All statistical analyses were performed using SPSS Statistics v19.0 software (IBM, USA). Results with P≤0.05 were considered significant. All obtained results are presented as means±standard error of the mean (SEM, n=3–7).
RESULTS
Acute hypoxia induced cell death and reprogramed lipid and carbohydrate metabolism
Results showed that the mortality rate of Nile tilapia increased as DO decreased from 7.0 to 0.01 mg/L within 36 h of hypoxic stress (Figure 1A). Furthermore, exposure to hypoxia for 12 h resulted in increased hepatocyte apoptosis (Figure 1B; Supplementary Figure S1A) and up-regulation of hif1α and hif3α (hif1al) mRNA expression and Hif3α and Vegfa protein expression (Figure 1C). In addition, a total of 4603 DEGs were identified in the Nile tilapia after 12 h of hypoxia exposure (Supplementary Figure S1D), with distinct gene patterns observed between the control and hypoxia-treated fish (Supplementary Figure S1B, E). Notably, metabolism pathway enrichment analysis revealed significant impacts on lipid (209 genes) and carbohydrate (173 genes) metabolism (Figure 1D). Hypoxia (12 h) in Nile tilapia also resulted in the up-regulation of genes related to lipid synthesis (e.g., fasn, dgat1, pparg, and srebf1), down-regulation of genes related to lipid catabolism (e.g., atgl, cpt1b, pparα, and acox1) (Figure 1E), and up-regulation of genes related to hif (hif1α and hif3α) and anaerobic glycolysis (Figure 1E), and the stress, inflammation, and apoptosis signaling pathways (Supplementary Figure S1F). Furthermore, following exposure to hypoxia, the Nile tilapia showed an increase in liver lipid droplet (oil red staining) and TG contents (Figure 1F, G), and serum TG (Supplementary Figure S1C) and lactate contents (Figure 1I), as well as a decrease in serum (Figure 1G) and liver (Supplementary Figure S1C) FFA content, liver mitochondrial FAO activity (Figure 1G), and glycogen content (PAS staining, Figure 1H, I). Collectively, these results indicate that acute hypoxia mainly affected lipid metabolism by inhibiting lipid catabolism and increasing lipogenesis and carbohydrate metabolism by enhancing glycogen consumption in Nile tilapia.
Figure 1.
Effects of acute hypoxia on apoptosis and metabolism in tilapia
A: Survival rate of Nile tilapia during acute hypoxia (n=6 for three replicates). B: Proportion of early and late apoptosis in hepatocytes after exposure to normoxic (C) or hypoxic conditions for 12 h (12 h) in Nile tilapia primary hepatocytes. C: mRNA and protein expression of hif1α, hif2α, and hif3α; protein expression of Hif3α and Vegfa. D: Significantly changed metabolic KEGG pathways from RNA-seq. E: Differentially expressed genes (DEGs) related to lipid and glucose metabolism. F: Oil red staining. G: Triglyceride (TG) content in liver, free fatty acid (FFA) content in serum, mitochondrial fatty acid oxidation (FAO) in liver. H: Periodic acid-schiff (PAS) staining. I: Glycogen content in liver, glucose, and lactate in serum. *, **, and *** indicate significant differences (P<0.05), (P<0.01), and (P<0.001), respectively, between C and hypoxia 12 h groups.
Enhanced lipid catabolism reduced glucose breakdown and tolerance to acute hypoxia
Fen is a specific ligand that activates Pparα, a nuclear transcriptional factor for lipid catabolism (Varga et al., 2011). In our study, Nile tilapia were administered either a C or Fen diet (266.8 mg/kg/d) for six weeks (Figure 2A). Under normoxic conditions, the Fen diet significantly increased liver mitochondrial FAO activity (Figure 2B) but resulted in reduced weight gain (Supplementary Figure S2A), lower mesenteric fat index (Supplementary Figure S2B), and decreased intracellular oxygen levels (Figure 2C) compared to the C diet. The survival rates of the tilapia fed the Fen diet were also lower compared to the C group during acute hypoxia for 12–24 h (Figure 2D). The Fen diet also led to reduced levels of TG (Figure 2E), glycogen (Supplementary Figure S2C), and caspase3a mRNA expression (Supplementary Figure S2G), and phosphorylation of the Ampk (Thr172) and Akt (Ser473) proteins (Figure 2I), as well as an increase in lactate content (Supplementary Figure S2D) and Hif3α protein expression in the liver (Figure 2I). Under hypoxic conditions, tilapia on the Fen diet exhibited increased liver concentrations of FFA (Figure 2F) and MDA (Figure 2G), serum activities of GOT and GPT (Figure 2H), and liver expression of caspase3a mRNA (Supplementary Figure S2G) compared to the C diet. However, TG content (Figure 2E), T-AOC activity (Supplementary Figure S2F), and phosphorylation of Ampk (Thr172), Akt (Ser473), and Pdha1 proteins (Figure 2I) were all decreased. Of note, under hypoxic conditions, the Fen-fed tilapia demonstrated increased MDA (Figure 2G), GOT, and GPT levels (Figure 2H), ROS production (Supplementary Figure S2E), and caspase3a and chop mRNA expression (Supplementary Figure S2G), but down-regulated Hif3α and Akt (Ser473) phosphorylation compared to the Fen-fed fish under normoxic conditions. Interestingly, the above indices were statistically comparable between the Nile tilapia fed the C diet under normoxic and hypoxic conditions. These findings indicate that the Fen diet increased lipid catabolism and oxygen consumption in the Nile tilapia but reduced glucose utilization and health, thus compromising hypoxia tolerance.
Figure 2.
Effects of enhanced lipid catabolism (fenofibrate) on tolerance to acute hypoxia in Nile tilapia
A: Nile tilapia were fed control (C) or fenofibrate (Fen) diets for six weeks and sampled at normoxic and hypoxic conditions after 12 h. B: Mitochondrial fatty acid oxidation (FAO) in liver. C: Intracellular oxygen level in zebrafish hepatocytes treated with Fen. D: Survival rate of Nile tilapia during acute hypoxia. E: Triglyceride (TG) content in liver. F: Free fatty acid (FFA) content in liver. G: Malondialdehyde (MDA) content in liver. H: Glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) in serum. I: Protein expression of Hif3α, p-Ampk (t172), p-Akt (s473), p-Pdha1 (s293), and Gapdh in liver. *, **, and *** indicate significant differences (P<0.05), (P<0.01), and (P<0.001), respectively, between C and Fen diets. #, ##, and ### indicate significant differences (P<0.05), (P<0.01), and (P<0.001) between Nile tilapia under normoxic and hypoxic conditions.
To further investigate the impact of Fen on hypoxia tolerance, we supplemented the Nile tilapia with L-carnitine (16 mg/kg BW/d, 12 weeks), a known mitochondrial FAO promoter that enhances the entry efficiency of long-chain fatty acids into mitochondria (Supplementary Figure S3A). Interestingly, the Nile tilapia fed with dietary L-carnitine demonstrated a reduced survival rate under acute hypoxia (Supplementary Figure S3E), with comparable effects on lipid and carbohydrate metabolism, oxidative stress, and cellular damage as those observed in tilapia fed the Fen diet (Supplementary Figure S3B–J).
Suppression of lipid catabolism increased glucose breakdown and tolerance to acute hypoxia
Mil is an analogue of γ-butyrobetaine, a precursor of L-carnitine, and thus can inhibit the synthesis of endogenous L-carnitine and mitochondrial FAO activity (Simkhovich et al., 1988). Nile tilapia were fed with a C or Mil (1 000 mg/kg/d) diet for six weeks (Figure 3A). Under normoxic condition, the tilapia fed with the Mil diet showed decreased L-carnitine content (Figure 3B), reduced mitochondrial FAO (Figure 3C), and lower OCR (Figure 3D). As a result, the Mil diet led to an increased survival rate compared to the C diet during acute hypoxia (Figure 3E). Furthermore, the Mil-fed fish exhibited elevated TG (Figure 3F) and T-AOC levels (Supplementary Figure S4F), but reduced glycogen (Figure 3G), ROS (Figure 3H), MDA (Figure 3I), GOT (Supplementary Figure S4G), GPT (Supplementary Figure S4H), and Hif3α levels (Figure 3J) under both normoxic and hypoxic states in the liver. Under hypoxic conditions, the Mil diet led to increased lactate dehydrogenase (LDH) activity (Supplementary Figure S4D), ATP levels (Supplementary Figure S4E), and Akt (Ser473) phosphorylation (Figure 3J), but down-regulated grp78, tnfa, and caspase3a mRNA expression (Supplementary Figure S4I) compared to the C diet.
Figure 3.
Effects of biochemical lipid catabolism inhibition on tolerance to acute hypoxia in Nile tilapia
A: Nile tilapia were fed control (C) or mildronate (Mil) diets for six weeks and sampled under normoxic and hypoxic conditions after 12 h. B: L-carnitine (Car) content in liver. C: Mitochondrial fatty acid oxidation (FAO) in liver. D: Oxygen consumption rate (OCR) in Nile tilapia. E: Survival rate of Nile tilapia during acute hypoxia. F: Triglyceride (TG) content in liver. G: Glycogen content in liver. H: Reactive oxygen species (ROS) content in liver. I: Malondialdehyde (MDA) content in liver. J: Protein expression of Hif3α, p-Ampk (t172), p-Akt (s473), p-Pdha1 (s293), and Gapdh in liver.
The atgl, cpt1b, and pparab genes play crucial roles in lipolysis, mitochondrial FAO, and lipid catabolism stimulation, respectively (Li et al., 2020a, 2020b). To further validate the effects of lipid catabolism on hypoxia tolerance in fish, we used CRISPR/Cas9 to KO these three genes in zebrafish (Supplementary Figure S5A, B, E, F, I, J). Notably, the atgl−/−, cpt1b−/−, and pparab−/− zebrafish exhibited increased survival rates during acute hypoxia (Figure 4A, H, O) but lower OCR (Figure 4C, J, Q) compared to the WT zebrafish. Upon 14C-labeled PA injection, the atgl−/−, cpt1b−/−, and pparab−/− fish released less radioactive CO2 compared to the WT fish. Conversely, upon 14C-labeled GLU injection, the atgl−/−, cpt1b−/−, and pparab−/− fish released more radioactive CO2 compared to the WT fish (Figure 4B, I, P).
Figure 4.
Effects of lipid catabolism gene KO on tolerance to acute hypoxia in zebrafish
A, H, O: Survival rate of WT and gene KO zebrafish during acute hypoxia. B, I, P: Released radioactive CO2 by fish injected with 14C-labeled palmitic acid (PA) or 14C-labeled glucose (GLU). C, J, Q: Oxygen consumption rate (OCR). D, K, R: Triglyceride (TG) content in liver. E, L, S: Glycogen and lactate contents in liver. F, M, T: Reactive oxygen species (ROS) and total antioxidant capacity (T-AOC) in liver. G, N, U: Protein expression of Hif3α, p-Akt (s473), and Gapdh in liver.
Results also showed that knocking out the three genes increased liver TG (Figure 4D, K, R) and phosphorylated Akt (Ser473) protein content (Figure 4G, N, U), but reduced glycogen (Figure 4L, S) and Hif3α protein content (Figure 4G, N, U) under both normoxic and hypoxic conditions. Under hypoxic conditions, the KO zebrafish demonstrated reduced ROS (Figure 4F, M, T) and MDA contents (Supplementary Figure S5D, H, L), as well as lower expression of stress and apoptosis-related genes (hsp90a, caspase3a, and chop). However, compared to the WT fish, the KO fish also showed increased T-AOC (Figure 4F, M, T) and LDH activity and up-regulated mRNA expression of glycolysis-related genes (pfk and pdk2) (Supplementary Figure S5C, G, K) in the liver. Under hypoxic conditions, the KO zebrafish displayed reduced glycogen content but increased lactate content (Figure 4E, L, S) in the liver compared to fish under normoxic conditions. These findings suggest that either biochemical inhibition of mitochondrial lipid catabolism or KO of key lipid catabolism genes (atgl, cpt1b, or pparab) led to increased glucose breakdown, reduced oxygen consumption, decreased oxidative damage, and ultimately improved tolerance to acute hypoxia in both Nile tilapia and zebrafish.
Suppression of glycolysis reduced acute hypoxia tolerance in fish with lipid catabolism inhibition
Dichloroacetate (DCA) is a specific inhibitor of pyruvate dehydrogenase kinase (PDK) protein, which suppresses anaerobic glycolysis and promotes glucose oxidative phosphorylation (Strum et al., 2013). To further explore whether the heightened hypoxia tolerance observed in fish with inhibited lipid catabolism is dependent on the activation of anaerobic glycolysis, we fed zebrafish lacking atgl (atgl−/−), a critical gene for initiating triglyceride lipolysis and representing lipid catabolism, either a C or DCA diet for four weeks (Figure 5A). The atgl−/− zebrafish fed the two different diets exhibited similar growth performance (Supplementary Figure S6A). However, those zebrafish fed the DCA diet showed increased OCR (Figure 5B) and decreased survival rate (Figure 5C) under acute hypoxia compared to the C group. Under normoxic conditions, the DCA-fed atgl−/− zebrafish exhibited reduced levels of Pdk2 and phosphorylated Pdha1 (Ser293) proteins, as well as a reduced Pdha1 (Ser293)/total Pdha1 ratio (Figure 5G), glycogen and TG content (Supplementary Figure S6D), and LDH activity (Figure 5E) in the liver. Conversely, the DCA-fed atgl−/− zebrafish exhibited increased mRNA expression of gk, pfk (Figure 5E), and hif3α (Supplementary Figure S6G), as well as higher levels of ROS (Figure 5F) and Hif3α protein (Figure 5G). under hypoxic conditions, the atgl−/− zebrafish fed with the DCA diet demonstrated increased gk (Figure 5E), hif3α, and caspase3a mRNA expression (Supplementary Figure S6G, H), MDA content (Figure 5F), and total Pdha1, Hif3α, and phosphorylated Akt (Ser473) protein levels (Figure 5G). However, these zebrafish also showed reduced pfk mRNA expression (Figure 5E), ATP (Supplementary Figure S6C), TG (Supplementary Figure S6D), T-AOC (Supplementary Figure S6F), and Pdk2 protein levels (Figure 5G). These findings indicate that DCA-induced inhibition of anaerobic glycolysis significantly diminished acute hypoxia tolerance in atgl−/− zebrafish by increasing oxygen consumption, impairing antioxidant capacity, and reducing energy supply from glycolysis. These results also confirm the essential roles of energy supply from lipid catabolism and glycolysis in tolerance to acute hypoxia in fish.
Figure 5.
Effects of suppressing glycolysis on tolerance to acute hypoxia in atgl−/− zebrafish
A: atgl−/− zebrafish were fed control (C) or dichloroacetate (DCA) diets for four weeks and sampled under normoxic and hypoxic conditions after 12 h. B: Oxygen consumption rate (OCR). C: Survival rate during acute hypoxia. D: Glycogen content in liver. E: Lactate dehydrogenase (LDH) activity and mRNA expression of glycolysis genes in liver. F: Reactive oxygen species (ROS) and malondialdehyde (MDA) contents in liver. G: Protein expression of Hif3α, p-Akt (s473), p-Pdha1 (s293), total Pdha1, Pdk2, and Gapdh in liver.
Fish with lower lipid/glucose catabolism ratios showed higher hypoxia tolerance
To verify whether the regulation of lipid/glucose catabolism is a common mechanism governing hypoxia tolerance in nature, we collected 14 fish species with diverse cladograms and trophic levels to investigate the correlation between their hypoxia tolerance capacities and energy supply preferences. Results showed that the survival rates of the 14 fish species varied significantly under acute hypoxia (Figure 6A), with Carassius auratus (Car) showing the strongest tolerance, surviving for 54 h in water containing less than 0.01 mg O2/L. The hypoxia tolerance ability of the 14 species was in the order Channa argus (Cha)<Micropterus salmonids (Mic)<Erythroculter ilishaeformis (Ery)<Oryzias latipes (Ory)≈Lepomis gibbosus (Lep)<Pelteobagrus fulvidraco (Pel)≈Parabramis pekinensis (Par)≈Mylopharyngodon piceus (Myl)≈Danio rerio (Dan)<Luciobarbus capito (Luc)<Tinca tinca (Tin)<Aristichthys nobilis (Ari)<Oreochromis niloticus (Ore)< Carassius auratus (Car) (Figure 6A, Supplementary Figure S7A). The median lethal DO levels for the 13 fish species were Cha (1.5), Mic (0.625), Ery (0.5), Pel (0.4), Lep (0.34), Myl (0.45), Ory (0.5), Dan (0.4), Par (0.24), Luc (0.2), Tin (0.1), Ari (0.035), and Ore (0.015) (Supplementary Figure S7B). Among the 14 fish species studied, the 14CO2 released from the ingested 14C-labeled glucose was normalized to the 14CO2 released from the ingested 14C-labeled palmitic acid (Figure 6B). Results showed that Cha, Mic, Ery, Pel, and Lep preferentially used fatty acids, while Car, Ore, Ari, and Tin preferentially used glucose for energy supply (Figure 6B), corresponding to their high hypoxia tolerance. We calculated the ratio of 14CO2 from fatty acid to 14CO2 from glucose for each fish species (Supplementary Figure S7C) and found an inverse correlation to hypoxia tolerance (r=0.812; Figure 6C).
Figure 6.
Effects of lipid/glucose catabolism ratio on hypoxia tolerance in 14 fish species
A: Survival rates of 14 fish species under acute hypoxia. B: Expired radioactive CO2 by 14 fish species injected with 14C-labeled glucose or 14C-labeled palmitic acid. C: Correlation analysis between survival time (h) and palmitic acid-CO2/glucose-CO2 in 14 fish species. D: Liver content of key enzymes of lipid metabolism (Atgl, Cpt1b, and Pparα) and glycolysis (Gk, Pdha1, and Ldh) in 14 fish species under normoxic and hypoxic conditions. E: Evolutionary tree (Bayesian inference) of 14 fish species by mitochondrial DNA sequencing.
We also assessed the content of key proteins for lipid catabolism (Atgl, Cpt1b, and Pparα) and glycolysis (Gk, Pdha1, and Ldh) in the liver of the 14 fish species under normoxic and hypoxic conditions (Figure 6D). Our findings demonstrated that fish species with lower hypoxia tolerance, such as Cha, Mic, Ery, Pel, Lep, and Ory, exhibited higher levels of lipid catabolism-related proteins but lower levels of glycolysis-related proteins (Figure 6D). In contrast, fish species with higher hypoxia tolerance, such as Car, Ore, Ari, Tin, and Luc, exhibited lower levels of lipid catabolism-related proteins but higher levels of glycolysis-related proteins. To ensure that the hypoxia tolerance ability was not dependent on taxonomic status, we constructed a cladogram of the 14 fish species by sequencing their mitochondrial genes (12S rRNA, 16S rRNA, and cox1) (Figure 6E). In the evolutionary tree of the studied species, members of the same family were clustered together, similar to current taxonomic classifications. Channa argus belonged to the Channidae family, Micropterus salmoides and Lepomis gibbosus belonged to the Sunfish family, Erythroculter ilishaeformis, Parabramis pekinensis, Mylopharyngodon piceus, Aristichthys nobilis, Tinca tinca, Danio rerio, Luciobarbus capito, and Carassius auratus belonged to the Cyprinidae family, Pelteobagrus fulvidraco belonged to the Bagridae family, Oryzias latipes belonged to the Oryziidae family, and Oreochromis niloticus belonged to the Cichlidae family. Therefore, the hypoxia tolerance of different fish was not completely related to their taxonomic status. These findings indicate that fish species preferentially utilizing lipids for energy supply were more intolerant to acute hypoxia than those preferentially using glucose.
DISCUSSION
Our research findings suggest that lowering lipid catabolism in fish promotes hypoxia tolerance by reducing cellular oxygen consumption and increasing glycolytic activity. Mitochondrial FAO and the tricarboxylic acid cycle require oxygen to generate energy, while anaerobic glycolysis generates two ATP molecules under anoxic conditions (Scott, 2012). Therefore, inhibiting mitochondrial FAO can conserve cellular oxygen under hypoxic conditions. In the present study, we observed a decrease in mitochondrial FAO activity and an increase in lipid accumulation in tissues after hypoxia exposure. We also verified that stimulating lipid catabolism using Fen or L-carnitine impaired hypoxia tolerance, resulting in reduced cellular oxygen, inhibited Akt signaling, and decreased glycolysis activity. Inhibition of lipid catabolism using Mil or atgl, cpt1b, and pparab KO significantly reduced cellular oxygen consumption, activated glycolysis, and ultimately improved hypoxia tolerance.
Inhibiting lipid catabolism improves the tolerance of cultured fish to hypoxia through two distinct mechanisms. Firstly, it reduces the generation of excessive ROS and oxidative damage. Hypoxia reduces electron transfer efficiency in the mitochondrial respiratory chain, leading to incomplete oxygen reduction and the production of harmful ROS (Goda & Kanai, 2012; Majmundar et al., 2010). ROS formation is determined by the proportion of electrons entering the respiratory chain via FADH2 or NADH (F/N ratio), with lipid breakdown increasing the ratio and subsequent ROS production (Speijer, 2016). Therefore, inhibition of lipid catabolism results in reduced ROS production and oxidative damage, thus improving survival under acute hypoxia. In contrast, lipid catabolism stimulation induces higher oxidative damage and apoptosis in the liver under hypoxia. Hence, under hypoxic conditions, the reduction in lipid catabolism conserves cellular oxygen content, which reduces ROS production in the electron transport chain (ETC) and oxidative damage, thereby promoting hypoxia tolerance (Figure 7).
Figure 7.
Summary diagram of full text (Red font represents uptrend and green font represents downtrend)
Secondly, inhibition of lipid catabolism improves hypoxia tolerance by activating anaerobic glycolysis. Elevated glycolysis regulated by the AMPK, HIF, and AKT signaling pathways (Hardie et al., 2012; Wang et al., 1995; Zeng et al., 2000) is essential for hypoxia resistance in mammals (Strum et al., 2013). In contrast, most fish species have a limited ability to regulate glucose metabolism to improve hypoxia tolerance as they are less efficient at utilizing carbohydrates compared to mammals (Moon, 2001; Wilson, 1994). In our previous research, we found that moderate lipid catabolism inhibition promotes glucose utilization by activating the Pi3k/Akt pathway in fish (Li et al., 2020a, 2020b). Here, inhibition of anaerobic glycolysis in atgl−/− zebrafish fed DCA resulted in an increase in Hif3α levels and a decrease in the survival rate under acute hypoxia. Conversely, in cancer cells, DCA has been shown to inhibit anaerobic glycolysis and lactate production and promote oxidative phosphorylation of pyruvate, leading to ROS production and tumor apoptosis (Stakišaitis et al., 2019). These findings confirm that increased hypoxia tolerance due to lipid catabolism inhibition is tightly associated with activation of anaerobic glycolysis (Figure 7).
In nature, various factors, such as habitat, genome size, breathing pattern, hemoglobin content, and oxygen affinity, can influence hypoxia tolerance in fish (Bickler & Buck, 2007). However, our study demonstrated that hypoxia tolerance ability in fish also depended on their preference for lipid or carbohydrate utilization, providing a scientific basis for the variations in hypoxia tolerance observed among fish species based on nutrient metabolism. Notably, among the 14 fish species studied, Channa argus, Micropterus salmoides, Erythroculter ilishaeformis, Oryzias latipes, and Pelteobagrus fulvidraco are carnivorous fish with distinct dietary lipid, carbohydrate, and protein requirements ranging from 9% to 15%, 10% to 20%, and 41% to 53%, respectively (Huang et al., 2017; Liu et al., 2012; Sagada et al., 2017; Zhang et al., 2018). Although the specific dietary nutritional requirements of Lepomis gibbosus have not been reported, the species is described as an aggressive carnivorous fish (Almeida et al., 2014). Interestingly, these carnivorous species exhibited lower hypoxia tolerance ability. Conversely, species such as Carassius auratus, Oreochromis niloticus, Aristichthys nobilis, Luciobarbus capito, Danio rerio, Mylopharyngodon piceus, and Parabramis pekinensis are omnivorous fish, with dietary lipid, carbohydrate, and protein requirements ranging from 3.7% to 7.7%, 23% to 35%, and 31% to 40%, respectively (Cai et al., 2020; Li et al., 2010; Wu et al., 2016, 2019; Zhou et al., 2009). Results showed that these omnivorous species exhibited higher hypoxia tolerance ability. Thus, our study demonstrated that carnivorous fish with higher dietary lipid requirements exhibited lower tolerance to hypoxia when compared to omnivorous species with a greater capacity for carbohydrate utilization.
The 14C-labeled glucose and palmitic acid tracking analyses provided further compelling evidence supporting the low hypoxia tolerance ability of carnivorous fish compared to omnivorous fish. The carnivorous species, such as Channa argus, Micropterus salmoides, Erythroculter ilishaeformis, Pelteobagrus fulvidraco, and Lepomis gibbosus, displayed a preference for using a higher level of fatty acids as their energy source, whereas the omnivorous species, such as Carassius auratus, Oreochromis niloticus, and Aristichthys nobilis, utilized higher amounts of glucose for energy. This difference was reflected in the elevated lipid catabolism protein expression levels in carnivorous fish and increased glycolysis protein levels in omnivorous fish. Tinca tinca, although primarily a carnivorous feeder with optimal dietary lipid, carbohydrate, and protein requirements of around 11%, 19%, and 48%, respectively (González-Rodríguez et al., 2014), displayed a high hypoxia tolerance similar to some omnivorous species, suggesting unique adaptation. In addition, this species demonstrated a higher capacity for glucose breakdown compared to fatty acid catabolism. Carassius auratus can survive for months in extreme anaerobic environments due to its distinct glucose metabolic mechanism, which can convert lactic acid (anaerobic product of glucose) into ethanol, which is then excreted into ambient water through the gills (Shoubridge & Hochachka, 1980). These findings support the notion that catabolic preference for lipids or glucose, rather than trophic level, is an important factor determining hypoxia tolerance in fish. Furthermore, the hypoxia tolerance observed in fish is not solely correlated with evolutionary position, as evidenced by the evolutionary tree of the 14 studied species. Therefore, environmental adaptation, such as food selection, rather than evolution, appears to play a more significant role in determining nutrient catabolic preference and hypoxia tolerance in fish. In short, our study presents a novel finding, suggesting that fish preferentially utilizing lipids as an energy source are more intolerant to acute hypoxia than those preferentially using carbohydrates.
This study makes an important contribution to our understanding of acute hypoxia tolerance in fish. Notably, our results showed that lowering lipid catabolism can improve acute hypoxia tolerance in cultured fish by reducing cellular oxygen consumption and elevating glycolysis activity. Furthermore, we showed that hypoxia tolerance in wild fish depends on catabolic preference for lipids or carbohydrates as an energy source. Thus, this study offers novel perspectives for devising potential strategies to enhance hypoxia tolerance in fish within aquaculture, and sheds light on the mechanisms underpinning the wide-ranging hypoxia tolerance observed among fish in nature.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
DATA AVAILABILITY
All sequencing data in the study were uploaded to the NCBI database under accession number SUB12161610/PRJNA891768, National Genomics Data Center under GSA accession number CRA011961 (https://bigd.big.ac.cn/gsa/browse/CRA011961), and Science Data Bank under the link https://doi.org/10.57760/sciencedb.09691.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Q.M. and Z.Y.D. designed and conducted the research. Q.M., Y.L., J.Z., and L.Y.L performed the experiments and analyzed the data. L.Q.C., F.Q., M.L.Z., and Q.L. provided essential materials and technical support. Q.M., Z.Y.D., and S.M.L wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Dong-Liang Li and Si-Lan Han for technical assistance in the study.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31830102, 32202950)
References
- Almeida D, Merino-Aguirre R, Vilizzi L, et al Interspecific aggressive behaviour of invasive pumpkinseed Lepomis gibbosus in iberian fresh waters. PLoS One. 2014;9(2):e88038. doi: 10.1371/journal.pone.0088038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Buck LT Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annual Review of Physiology. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- Braz-Mota S, Almeida-Val VMF Ecological adaptations of Amazonian fishes acquired during evolution under environmental variations in dissolved oxygen: a review of responses to hypoxia in fishes, featuring the hypoxia-tolerant Astronotus spp. Journal of Experimental Zoology Part A:Ecological and Integrative Physiology. 2021;335(9-10):771–786. doi: 10.1002/jez.2531. [DOI] [PubMed] [Google Scholar]
- Cai FF, Wang Y, Hu XQ, et al Effects of dietary lipid levels on growth performance, whole body composition and digestive enzyme activity of juvenile bighead carp (Aristichthys nobilis) Israeli Journal of Aquaculture-Bamidgeh. 2020;72:1200227. [Google Scholar]
- Cawthorn DM, Steinman HA, Witthuhn RC Evaluation of the 16S and 12S rRNA genes as universal markers for the identification of commercial fish species in South Africa. Gene. 2012;491(1):40–48. doi: 10.1016/j.gene.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Chagas EC, Val AL Ascorbic acid reduces the effects of hypoxia on the Amazon fish tambaqui. Journal of Fish Biology. 2006;69(2):608–612. doi: 10.1111/j.1095-8649.2006.01094.x. [DOI] [Google Scholar]
- Chen WQ, Wu HX, Wu L, et al Oxygen consumption rate and suffocation point of the juveniles for five species of mariculture fish. Journal of Marine Sciences. 2015;33(2):76–81. [Google Scholar]
- de la Rosa Rodriguez MA, Deng L, Gemmink A, et al Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Molecular Metabolism. 2021;47:101168. doi: 10.1016/j.molmet.2021.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda N, Kanai M Hypoxia-inducible factors and their roles in energy metabolism. International Journal of Hematology. 2012;95(5):457–463. doi: 10.1007/s12185-012-1069-y. [DOI] [PubMed] [Google Scholar]
- González-Rodríguez Á, Celada JD, Carral JM, et al Effects of varying protein level in practical diets on survival, growth, feed utilization and body composition of juvenile tench (Tinca tinca L. ) Aquaculture International. 2014;22(5):1723–1735. doi: 10.1007/s10499-014-9777-3. [DOI] [Google Scholar]
- Gudi R, Melissa MBK, Kedishvili NY, et al Diversity of the pyruvate dehydrogenase kinase gene family in humans. Journal of Biological Chemistry. 1995;270(48):28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique MMF, Gomes EF, Gouillou-Coustans MF, et al Influence of supplementation of practical diets with vitamin C on growth and response to hypoxic stress of seabream. Sparus aurata. Aquaculture. 1998;161(1-4):415–426. [Google Scholar]
- Huang D, Wu YB, Lin YY, et al Dietary protein and lipid requirements for juvenile largemouth bass. Micropterus salmoides. Journal of the World Aquaculture Society. 2017;48(5):782–790. doi: 10.1111/jwas.12417. [DOI] [Google Scholar]
- Karim R, Sekine M, Ukita M Simulation of eutrophication and associated occurrence of hypoxic and anoxic condition in a coastal bay in Japan. Marine Pollution Bulletin. 2002;45(1-12):280–285. doi: 10.1016/S0025-326X(02)00098-X. [DOI] [PubMed] [Google Scholar]
- Keeling RF, Körtzinger A, Gruber N Ocean deoxygenation in a warming world. Annual Review of Marine Science. 2010;2:199–229. doi: 10.1146/annurev.marine.010908.163855. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, et al HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. International Journal of Radiation Oncology, Biology, Physics. 2002;53(5):1192–1202. doi: 10.1016/S0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Skarlatos J, et al Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Research. 2001;61(5):1830–1832. [PubMed] [Google Scholar]
- Landman MJ, Van Den Heuvel MR, Ling N Relative sensitivities of common freshwater fish and invertebrates to acute hypoxia. New Zealand Journal of Marine and Freshwater Research. 2005;39(5):1061–1067. doi: 10.1080/00288330.2005.9517375. [DOI] [Google Scholar]
- Li LY, Li JM, Ning LJ, et al Mitochondrial fatty acid β-oxidation inhibition promotes glucose utilization and protein deposition through energy homeostasis remodeling in fish. The Journal of Nutrition. 2020a;150(9):2322–2335. doi: 10.1093/jn/nxaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Lv HB, Jiang ZY, et al Peroxisomal proliferator-activated receptor α-b deficiency induces the reprogramming of nutrient metabolism in zebrafish. The Journal of Physiology. 2020b;598(20):4537–4553. doi: 10.1113/JP279814. [DOI] [PubMed] [Google Scholar]
- Li MX, Wang XD, Qi CL, et al Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture. 2018;495:187–195. doi: 10.1016/j.aquaculture.2018.05.031. [DOI] [Google Scholar]
- Li XF, Liu WB, Jiang YY, et al Effects of dietary protein and lipid levels in practical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture. 2010;303(1-4):65–70. doi: 10.1016/j.aquaculture.2010.03.014. [DOI] [Google Scholar]
- Liu B, Xie J, Ge XP, et al Effect of high dietary carbohydrate on growth, serum physiological response, and hepatic heat shock cognate protein 70 expression of the top-mouth culter Erythroculter ilishaeformis Bleeker. Fisheries Science. 2012;78(3):613–623. doi: 10.1007/s12562-012-0486-4. [DOI] [Google Scholar]
- Liu LZ, Zhao W, Shi ZG The effects of body weight and time rhythm to the oxygen consumption rate and suffocation point of juvenile Acipenser gueldenstaedti Brandt. Journal of Biology. 2013;30(3):51–53,67. [Google Scholar]
-
Livak KJ, Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the
 method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar] - Lu DL, Ma Q, Wang J, et al Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. The Journal of Physiology. 2019;597(6):1585–1603. doi: 10.1113/JP277091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Hu CT, Yue JJY, et al High-carbohydrate diet promotes the adaptation to acute hypoxia in zebrafish. Fish Physiology and Biochemistry. 2020;46(2):665–679. doi: 10.1007/s10695-019-00742-2. [DOI] [PubMed] [Google Scholar]
- Magnoni LJ, Martos-Sitcha JA, Queiroz A, et al Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata) Biology Open. 2017;6(6):897–908. doi: 10.1242/bio.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, et al Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon TW Glucose intolerance in teleost fish: fact or fiction? Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology. 2001;129(2-3):243–249. doi: 10.1016/S1096-4959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Niu J, Wen H, Li CH, et al. 2014. Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture, 422–423: 8–17.
- Phan-Van M, Rousseau D, De Pauw N Effects of fish bioturbation on the vertical distribution of water temperature and dissolved oxygen in a fish culture-integrated waste stabilization pond system in Vietnam. Aquaculture. 2008;281(1-4):28–33. doi: 10.1016/j.aquaculture.2008.04.033. [DOI] [Google Scholar]
- Sagada G, Chen JM, Shen BQ, et al Optimizing protein and lipid levels in practical diet for juvenile northern snakehead fish (Channa argus) Animal Nutrition. 2017;3(2):156–163. doi: 10.1016/j.aninu.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CB Glucose and fat oxidation: bomb calorimeter be damned. The Scientific World Journal. 2012;2012:375041. doi: 10.1100/2012/375041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi KP, Dong SL, Zhou YG, et al Comparative evaluation of toleration to heating and hypoxia of three kinds of Salmonids. Journal of Ocean University of China. 2018;17(6):1465–1472. doi: 10.1007/s11802-018-3673-9. [DOI] [Google Scholar]
- Shoubridge EA, Hochachka PW Ethanol: novel end product of vertebrate anaerobic metabolism. Science. 1980;209(4453):308–309. doi: 10.1126/science.7384807. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Shutenko ZV, Meirēna DV, et al 3-(2, 2, 2-Trimethylhydrazinium)propionate(thp)-a novel γ-butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochemical Pharmacology. 1988;37(2):195–202. doi: 10.1016/0006-2952(88)90717-4. [DOI] [PubMed] [Google Scholar]
- Speijer D Being right on Q: shaping eukaryotic evolution. Biochemical Journal. 2016;473(22):4103–4127. doi: 10.1042/BCJ20160647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakišaitis D, Juknevičienė M, Damanskienė E, et al The importance of gender-related anticancer research on mitochondrial regulator sodium dichloroacetate in preclinical studies in vivo. Cancers. 2019;11(8):1210. doi: 10.3390/cancers11081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum SB, Adalsteinsson Ö, Black RR, et al Case Report: sodium dichloroacetate (DCA) inhibition of the "Warburg Effect" in a human cancer patient: complete response in non-Hodgkin's lymphoma after disease progression with rituximab-CHOP. Journal of Bioenergetics and Biomembranes. 2013;45(3):307–315. doi: 10.1007/s10863-012-9496-2. [DOI] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L. 2011. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1812(8): 1007–1022.
- Wang GL, Jiang BH, Rue EA, et al Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Zemlak TS, Innes BH, et al DNA barcoding Australia's fish species. Philosophical Transactions of the Royal Society B:Biological Sciences. 2005;360(1462):1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW, Chandel NS Hypoxia. 2. Hypoxia regulates cellular metabolism. American Journal of Physiology:Cell Physiology. 2011;300(3):C385–C393. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP Utilization of dietary carbohydrate by fish. Aquaculture. 1994;124(1-4):67–80. doi: 10.1016/0044-8486(94)90363-8. [DOI] [Google Scholar]
- Wu C, Shan JF, Feng JC, et al. 2019. Effects of dietary Radix Rehmanniae Preparata polysaccharides on the growth performance, immune response and disease resistance of Luciobarbus capito. Fish & Shellfish Immunology, 89: 641–646.
- Wu CL, Ye JY, Gao JE, et al. 2016. The effects of dietary carbohydrate on the growth, antioxidant capacities, innate immune responses and pathogen resistance of juvenile Black carp Mylopharyngodon piceus. Fish & Shellfish Immunology, 49: 132–142.
- Wu RSS Hypoxia: from molecular responses to ecosystem responses. Marine Pollution Bulletin. 2002;45(1-12):35–45. doi: 10.1016/S0025-326X(02)00061-9. [DOI] [PubMed] [Google Scholar]
- Yu HB, Zhang C, Zhang XT, et al Dietary nano-selenium enhances antioxidant capacity and hypoxia tolerance of grass carp Ctenopharyngodon idella fed with high-fat diet. Aquaculture Nutrition. 2020;26(2):545–557. doi: 10.1111/anu.13016. [DOI] [Google Scholar]
- Yu LN, Yang D, Liu HY, et al Correlation between hemoglobin and asphyxiation point in twelve species of freshwater fish. Chinese Journal of Zoology. 2017;52(3):478–484. [Google Scholar]
- Zeng GY, Nystrom FH, Ravichandran LV, et al Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–1545. doi: 10.1161/01.CIR.101.13.1539. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhao NN, Sharawy Z, et al Effects of dietary lipid and protein levels on growth and physiological metabolism of Pelteobagrus fulvidraco larvae under recirculating aquaculture system (RAS) Aquaculture. 2018;495:458–464. doi: 10.1016/j.aquaculture.2018.06.004. [DOI] [Google Scholar]
- Zhang P, Lu L, Yao Q, et al Molecular, functional, and gene expression analysis of zebrafish hypoxia-inducible factor-3α. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology. 2012;303(11):R1165–R1174. doi: 10.1152/ajpregu.00340.2012. [DOI] [PubMed] [Google Scholar]
- Zhang P, Yao Q, Lu L, et al Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Reports. 2014;6(6):1110–1121. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Saarinen AM, Hitosugi T, et al Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. eLife. 2017;6:e31132. doi: 10.7554/eLife.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XX, Wang YB, Gu Q, et al Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio) Aquaculture. 2009;291(1-2):78–81. doi: 10.1016/j.aquaculture.2009.03.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.









