Abstract
Under increasing anthropogenic pressure, species with a previously contiguous distribution across their ranges have been reduced to small fragmented populations. The critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis), once commonly observed in the Yangtze River-Poyang Lake junction, is now rarely seen in the river-lake corridor. In this study, static passive acoustic monitoring techniques were used to detect the biosonar activities of the Yangtze finless porpoise in this unique corridor. Generalized linear models were used to examine the correlation between these activities and anthropogenic impacts from the COVID-19 pandemic lockdown and boat navigation, as well as environmental variables, including hydrological conditions and light levels. Over approximately three consecutive years of monitoring (2020–2022), porpoise biosonar was detected during 93% of logged days, indicating the key role of the corridor for finless porpoise conservation. In addition, porpoise clicks were recorded in 3.80% of minutes, while feeding correlated buzzes were detected in 1.23% of minutes, suggesting the potential existence of localized, small-scale migration. Furthermore, both anthropogenic and environmental variables were significantly correlated with the diel, lunar, monthly, seasonal, and annual variations in porpoise biosonar activities. During the pandemic lockdown period, porpoise sonar detection showed a significant increase. Furthermore, a significant negative correlation was identified between the detection of porpoise click trains and buzzes and boat traffic intensity. In addition to water level and flux, daylight and moonlight exhibited significant correlations with porpoise biosonar activities, with markedly higher detections at night and quarter moon periods. Ensuring the spatiotemporal reduction of anthropogenic activities, implementing vessel speed restrictions (e.g., during porpoise migration and feeding), and maintaining local natural hydrological regimes are critical factors for sustaining porpoise population viability.
Keywords: Yangtze finless porpoises, Yangtze River, Poyang Lake, Pandemic lockdown, Boat traffic, Hydrological regime, Light level
INTRODUCTION
Cetaceans, including whales, dolphins, and porpoises, are highly specialized aquatic mammals. As top predators in aquatic trophic networks, the size and distribution of cetacean populations are excellent indicators of habitat health (Echeverria et al., 2022; Würsig, 1989). In addition, cetaceans play key functions in ocean and freshwater ecosystems, including the facilitation of carbon and nutrient cycling (Savoca et al., 2021) and regulation of food webs (Springer et al., 2003).Cetaceans are also promising sources for cancer suppression research and can contribute to the development of future targets for human cancer therapies (Tollis et al., 2019) and are important subjects for the investigation of evolution (Goldbogen et al., 2019; Goswami et al., 2022) and bioinspired innovation (Capus et al., 2007; Vishnu et al., 2022).
The Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) is the only freshwater porpoise population in the world (Wang, 2009). Currently, its distribution is limited to the Yichang to Shanghai stretch of the Yangtze River, the longest river in China and third longest in the world, as well as the adjoining Poyang and Dongting lakes, the two largest freshwater lakes in China (Wang, 2009). The existing population of free-ranging Yangtze finless porpoises is currently classified as critically endangered by the IUCN Red List of Threatened Species (Wang et al., 2013), decreasing from approximately 1800 individuals in 2006 to 1012 individuals in 2017 (Huang et al., 2020; Zhao et al., 2008).
Conflicts between humans and wildlife have become increasingly common (McCauley et al., 2015). Many species, particularly freshwater cetaceans with once contiguous distributions, have been reduced to small fragmented populations due to increasing anthropogenic pressure (Robinson et al., 2019), including the Yangtze finless porpoise (Huang et al., 2020). Habitat fragmentation imposes considerable stress on species, not only reducing genetic variation but also threatening subpopulations with demographic collapse and potential extirpation, as observed in certain populations of the Indus River dolphin (Platanista gangetica minor) (Braulik et al., 2014).
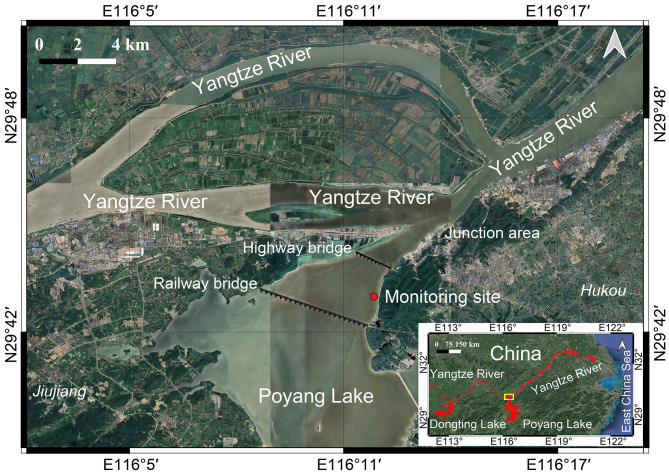
The main stream of the Yangtze River and Poyang Lake are vital habitats for the Yangtze finless porpoise, harboring approximately 44% and 45% of the total wild population, respectively (Huang et al., 2020). The confluence of the main trunk of the Yangtze River and Poyang Lake has consistently been identified as the river section with the highest rate of porpoise detection (Huang et al., 2020; Wei et al., 2003; Zhang et al., 1993). Historically, movement of finless porpoises in the junction area of Poyang Lake and the Yangtze River was relatively common (Wei et al., 2002; Zhang et al., 1993). However, the construction of two bridges in the Poyang Lake junction area has had an impact (Figure 1). Notably, the construction of a highway bridge in Poyang Lake, approximately 2.5 km above the Yangtze River and Poyang Lake junction , commenced in 1997 and finished in 2000. This bridge spans a total length of 3 799 m, featuring a four-span arrangement (65 m+123 m+318 m+130 m) and side spans of either 50 or 30 m. Similarly, the construction of the railway bridge in Poyang Lake, approximately 6 km above the Yangtze River and Poyang Lake junction, commenced in 2005 and finished in 2008. This bridge spans a total length of 5 378 m, also featuring a four-span arrangement (120 m+120 m+120 m+120 m) and side spans of either 41.8 or 32.7 m. Between 2006 and 2007, short-term static passive acoustic monitoring was conducted, ranging from several to 80 days, between the two bridges. During that time, finless porpoises were detected on more than 70% of the monitoring days (Dong et al., 2012; Li et al., 2010). From 2007 to 2010, a regular seasonal towed acoustic survey was conducted near the Yangtze River and Poyang Lake junction, showing fewer porpoise detections near the bridge compared to non-bridge areas (Kimura et al., 2012). Recent investigations, including towed acoustic surveys and visual line transect surveys, revealed rare porpoise detections between the two bridges (Zhi-Tao Wang, unpublished data). Considering the narrow water width, small bridge pier span, and busy traffic on the highway and railway bridges, which generates and transmits vibration and noise into the water near the Poyang Lake and Yangtze River junction, we hypothesized that the two bridges may act as barriers, hindering the movement of porpoise migration between the Yangtze River and Poyang Lake.
Figure 1.
Map locations of static acoustic monitoring in the Yangtze River and Poyang Lake junction area
Red area in lower right inset shows distribution area of Yangtze finless porpoise and yellow box marks the Yangtze River and Poyang Lake junction area.
The visual observation of free-ranging finless porpoises is challenging due to their brief surfacing to breathe, small body size, and absence of a dorsal fin, as well as external factors such as boat navigation and turbidity (Wang et al., 2014a). Toothed whales have evolved a sophisticated biosonar system for navigation, communication, and foraging (Au, 1993; Würsig, 1989). For navigation safety, Yangtze finless porpoises frequently employ biosonar to survey their surroundings at distances of up to 77 m, before proceeding quietly within a shorter distance (less than 20 m) (Akamatsu et al., 2005). Free-ranging finless porpoises emit species-specific echolocation signals almost continuously in the form of click trains, with an inter-click-train interval of 6.4 s (Akamatsu et al., 2007; Au, 1993). These echolocation clicks possess distinctive acoustic features that facilitate their identification (Wang et al., 2015a). Notably, during concurrent visual and acoustic surveys, the detection rate for solitary finless porpoises using acoustic methods is five times higher compared to visual methods (Akamatsu et al., 2008).
Therefore, in the current study, we employed passive acoustic monitoring to investigate the temporal biosonar activity of Yangtze finless porpoises at the Yangtze River and Poyang Lake junction to determine whether (and to what extent) the two bridges act as barriers for porpoise migration. Harbor porpoises (Phocoena phocoena), belonging to one of the three genera in the porpoise family, exhibit nearly continuous foraging behavior day and night (Wisniewska et al., 2016). Furthermore, the availability of prey is widely recognized as a primary factor influencing the habitat use of finless porpoises (Kimura et al., 2012; Wang et al., 2015a, 2014a). Therefore, we further explored finless porpoise feeding activity in the study area. In addition, we analyzed anthropogenic variables related to the COVID-19 pandemic lockdown and boat navigation, as well as environmental variables, including hydrological conditions and illumination, to investigate their potential impacts on the temporal presence of this native species in the region.
MATERIALS AND METHODS
Static passive acoustic monitoring was conducted at the Yangtze River and Poyang Lake junction. The monitoring site was at a fairway buoy (N29°43′14″, E116°11′39″), located at approximately an equal distance (1.6 km) from the highway and railway bridges (Figure 1). The distances between the buoy and downstream and upstream navigation channels were approximately 200 and 400 m, respectively.
Noninvasive passive acoustic monitoring methods were adopted, and acoustic data were collected by remote sensing without causing harm to the local porpoises. Permits for underwater acoustic recorder deployment at the buoy were obtained from the Jiangxi Provincial Port and Navigation Administration and Jiangxi Provincial Fisheries Administration in 2019.
Acoustic data collection
Instead of high-frequency full-bandwidth digital sound recorders, autonomous acoustic data loggers (C-POD) were applied in our research, which have been widely used in studies on fine-scale distribution, activity, and abundance of the harbor porpoise (Amundin et al., 2022; Carlén et al., 2018; Sveegaard et al., 2015), vaquita (Phocoena sinus) (Jaramillo-Legorreta et al., 2017), and bottlenose dolphin (Tursiops truncatus) (Nuuttila et al., 2013a; Pirotta et al., 2014). All devices were moored with a hydrophone deployed at approximately 1.5 m below the water surface. Each C-POD device was retrieved at intervals of one to three months to change batteries and memory cards. However, battery depletion and equipment failure due to weather-related delays in servicing resulted in some reductions in recording time. Consequently, data obtained on the days the C-POD was deployed or retrieved were discarded to avoid interference from servicing activities. As such, only data comprising a complete 24 h recording period were selected for further analysis.
Acoustic data analysis
Acoustic data were processed using the pattern recognition software C-POD.exe v2.048 (Chelonia, UK). The C-POD devices have a detection threshold of 114.5±1.2 dB re 1 µPa peak-to-peak at 130 kHz (Dähne et al., 2013) and monitor ultrasound at a frequency band between 20 and 160 kHz, encompassing the echolocation frequencies of most odontocete species, except for sperm whales. The C-PODs use online digital waveform characterization to register the time of detection of click events. For each click, various parameters are stored, including time of occurrence (5 µs resolution), click duration, peak amplitude, frequency (based on zero-crossing interval measurement to 200 ns resolution), bandwidth, and envelopes. The collected data are processed using the KERNO classifier, an offline automated click train detection and classification algorithm, which extracts coherent click trains and assigns each train to one of the following four source categories (narrow band high frequency (NBHF) porpoise, other cetaceans, boat sonar, and unclassified) and one of the following four levels of confidence (high, moderate, low, and doubtful). Most cetacean clicks occur in trains, and KERNO requires at least five clicks to classify a click sequence as a porpoise click train.
As the Yangtze River dolphin (Lipotes vexillifer, also named Baiji) is considered as likely extinct in the wild since 2006 (Turvey et al., 2007), the Yangtze finless porpoise is currently the only cetacean species in the Yangtze River, and species identification was not necessary for this study. Buzz signals, trains of echolocation clicks with a minimum inter-click interval shorter than 10 ms, have been widely used in cetacean research as a proxy of feeding activity (Todd et al., 2009; Wang et al., 2014a, 2015b). Finless porpoises also emit buzz signals during prey capture (Akamatsu et al., 2010; Wang et al., 2015a, 2014a), which were used in the current study to infer patterns of foraging activity. A conservative approach was taken, with only high- and moderate-quality NBHF trains and other cetaceans extracted as porpoise biosonar activity and used for further analysis. Detailed detection information on clicks and click trains were exported to text files and further aggregated into 1 min and 1 h periods with custom-made Matlab script (Mathworks, USA). Sonar activity was exported as porpoise click detection-positive rate per minute (DPRM), number of porpoise click trains per minute, number of porpoise buzzes DPRM, and number of porpoise buzzes per minute. To assess the false positive detection rate of porpoise sonar signals, a validation exercise was conducted involving the visual inspection of a subset of raw C-POD data files to identify false positive detections of click trains following the C-POD designer guidelines (https://www.chelonia.co.uk/index.html).
Anthropogenic and environmental data
The potential impacts of anthropogenic activities, hydrological regime, and illumination conditions on the temporal patterns of porpoise sonar activity were investigated. Anthropogenic activities included the COVID-19 lockdown and boat traffic, hydrological regime included water level and water flux, illumination conditions included diel and lunar cycles, and temporal scale included month, season, and year.
To stem the spread of COVID-19, the Poyang Lake junction area was locked down continuously from 1800h on 1 February to 2400h on 23 February 2020, with all commercial activities and roads, bridges, and shipping lanes closed, except for law enforcement vessels. Boat traffic was derived from the C-POD data, in which click trains with high and moderate confidence assigned to the boat sonar category were grouped into minutes and exported as boat sonar detection-positive minutes and number of boat sonar detections per minute.
Hydrological regime data of water level and flux at the acoustic monitoring site were obtained from the official database of the Water Resources Department of Hubei Province (https://slt.hubei.gov.cn/sjfb/). The water level was further divided into five consecutive phases: wet level (water level above 17 m), wet to normal (WN) transitional level (water level between 16.01 and 17 m), normal level (water level between 13.01 and 16 m), normal to dry (ND) transitional level (water level between 12.01 and 13 m), and dry level (water level lower than 12 m) (Fang et al., 2022).
To explore diel patterns, each day was split into three light periods based on solar elevation: night (sun altitude less than –12°), twilight (including dawn and dusk, sun altitude between 0° and –12°), and day (sun altitude greater than 0°) (Español-Jiménez & Van Der Schaar, 2018; Homfeldt et al., 2022; Kowarski et al., 2018; Mussoline et al., 2012; Ryan et al., 2019). The time division points between different light periods were obtained from time and data website (https://www.timeanddate.com/).
To explore lunar patterns, the study period was divided into the three phases: new moon period, quarter moon period (including first and last quarter moon periods), and full moon period (Wang et al., 2015b). The time points for the new, first quarter, full, and last quarter moon phases were obtained from the time and data website (https://www.timeanddate.com/). To further explore seasonal patterns, the entire study period was divided into the local city’s astronomical seasons as defined by equinoxes and solstices (https://www.timeanddate.com/calendar/seasons.html).
Statistical analysis
The generalized linear model (GLM) is a statistical modeling approach that characterizes the dependent variable as a linear combination of independent variables, incorporating an error term, and serves as a valuable tool for modeling the correlation between a response variable and a set of explanatory variables. In this study, GLM was applied to analyze porpoise biosonar parameter patterns and their correlation with the pandemic lockdown, boat traffic, water level, water flux, diel, month, season, and year. No evidence of collinearity was observed among the temporal factors (pairwise linear correlation coefficient<0.2). Full-factorial eight-way analysis of variance (ANOVA) was conducted using the porpoise biosonar parameters as dependent variables, with pandemic, boat traffic, water level, diel, month, season, and year allocated as fixed factors, water flux allocated as a covariate, and interaction terms included in the model. When significant differences were found for either fixed factor (main factor), post hoc multiple comparison tests were conducted using Tukey’s HSD (equal variances by Levene statistic, P>0.05) or Tamhane’s T2 method (variances not equal by Levene statistic, P<0.05). Interaction terms not reaching significance were excluded from the final model. Subset data of the biosonar parameters at night were extracted for analysis of lunar patterns by one-way ANOVA when the datasets were normally distributed (Kolmogorov-Smirnov test, P>0.05). Alternatively, when not normally distributed (Kolmogorov-Smirnov test, P<0.05), the Kruskal-Wallis test was used for overall lunar pattern differences and further analyzed using pairwise comparisons to determine how biosonar parameters varied across different lunar periods where applicable (Kruskal-Wallis test, P<0.05) (Zar, 1999). Spearman’s rho correlation was employed to investigate the correlation between porpoise biosonar detections and boat traffic conditions. Data analysis was performed using SPSS v26.0 (IBM SPSS Statistics for Windows, USA) and Matlab R2018b (Mathworks, USA), with the critical significance level (α) defined as 0.05.
RESULTS
Passive acoustic monitoring was conducted between 19 January 2020 and 30 September 2022 in 12 trials ranging in duration from 39 to 89 days (Table 1). The validation exercise showed that the false positive detection rate for porpoise biosonar was less than 0.1%. In total, 834 days with full 24 h recordings were extracted for further analysis (Table 1; Figure 2). Porpoise biosonar was detected on 773 days, accounting for 93% of the total 834 analyzed days. Of the analyzed 1 200 960 min, 45 684 min contained porpoise clicks and 14 735 min contained buzzes, accounting for 3.80% and 1.23%, respectively (Figure 2). A combined total of 175 225 click trains, 2 146 756 porpoise clicks, and 39 563 porpoise buzzes were detected (Table 1). Porpoise buzz signals accounted for approximately 22.6% of all porpoise biosonar click trains.
Table 1. Statement of acoustic monitoring time and porpoise biosonar and boat sonar detection results.
| Deploy date | Retrieval date | Analyzed days | No. days with porpoise sonar detection (daily positive rate, %) | No. porpoise click DPM | No. porpoise click trains | No. buzz DPM | No. buzzes | No. porpoise clicks | No. boat sonar DPM | No. boat sonars |
| DPM: Detection positive minute. Daily positive rates were obtained by dividing the number of porpoise click-positive days by analyzed days. | ||||||||||
| 01/19/2020 | 03/17/2020 | 57 | 57 (100) | 12 719 | 45 146 | 3 699 | 8 689 | 585 701 | 377 | 1 019 |
| 05/04/2020 | 07/09/2020 | 65 | 48 (74) | 332 | 1 276 | 52 | 133 | 14 444 | 167 | 373 |
| 07/26/2020 | 09/25/2020 | 60 | 46 (77) | 2 079 | 7 715 | 398 | 778 | 83 345 | 163 | 470 |
| 09/28/2020 | 12/16/2020 | 78 | 77 (99) | 5 334 | 17 122 | 1 411 | 4 279 | 214 443 | 979 | 2 698 |
| 12/27/2020 | 03/13/2021 | 75 | 73 (97) | 1 800 | 5 976 | 593 | 1 616 | 75 683 | 1 467 | 5 089 |
| 03/13/2021 | 06/03/2021 | 81 | 73 (90) | 1 094 | 2 418 | 192 | 332 | 24 436 | 214 | 516 |
| 06/20/2021 | 08/24/2021 | 64 | 50 (78) | 355 | 642 | 48 | 71 | 5 977 | 389 | 1 549 |
| 09/14/2021 | 11/28/2021 | 74 | 74 (100) | 8 674 | 37 906 | 2 840 | 7 962 | 417 960 | 898 | 2 693 |
| 11/28/2021 | 02/24/2022 | 87 | 86 ((99) | 4 585 | 18 978 | 1 695 | 4 786 | 253 342 | 1 597 | 4 633 |
| 03/18/2022 | 06/11/2022 | 84 | 84 (100) | 2 498 | 7 014 | 1 122 | 1 998 | 81 456 | 1 118 | 3 284 |
| 06/11/2022 | 08/23/2022 | 72 | 68 (94) | 1 670 | 8 099 | 671 | 2 014 | 87 455 | 815 | 2 369 |
| 08/23/2022 | 09/30/2022 | 37 | 37 (100) | 4 547 | 22 933 | 2 014 | 6 905 | 302 514 | 904 | 2 484 |
| Sum | 834 | 773 (93) | 45 687 | 175 225 | 14 735 | 39 563 | 2 146 756 | 9 088 | 27 177 | |
Figure 2.
Porpoise click detection-positive rate per minute (DPRM) (A) and porpoise buzz DPRM (B) as a function of time of day (X-axis) and date (Y-axis)
Results are given for each minute. Light gray horizontal boxes indicate periods without a full 24 h recording or periods of no recordings not included in this study. Solid black lines indicate start of dawn and end of dusk. Dashed broken lines indicate end of dawn and start of dusk. Line plots on top of a and b denote diel pattern of porpoise DPRM and porpoise buzz DPRM, respectively, over the analyzed period. Line plot on right side of A and B denotes summed porpoise click DPRM and porpoise buzz DPRM daily. DPM: Detection positive minute.
COVID-19 lockdown and boat traffic
The GLM results indicated that the COVID-19 lockdown was significantly correlated with all four biosonar parameters (Table 2; Figure 3A; Supplementary Tables S1–S3). Specifically, porpoise biosonar activity (for all measured parameters) during the lockdown period was significantly higher than that during the non-lockdown period (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3A).
Table 2. Eight-way ANOVA of effects of eight parameters (pandemic, boat traffic, water level, water flux, diel, month, season, year) on porpoise click detection-positive rate per minute.
| Source | Type III sum of squares | df | Mean square | F | P |
| Corrected model | 4 057.57 | 185 | 21.93 | 660.22 | 0.00 |
| Intercept | 5.63 | 1 | 5.63 | 169.53 | 0.00 |
| Pandemic | 2.46 | 1 | 2.46 | 73.99 | 0.00 |
| Boat traffic | 7.89 | 1 | 7.89 | 237.61 | 0.00 |
| Water level | 1.74 | 4 | 0.44 | 13.10 | 0.00 |
| Water flux | 4.20 | 1 | 4.20 | 126.35 | 0.00 |
| Diel | 4.59 | 2 | 2.29 | 69.04 | 0.00 |
| Month | 31.61 | 11 | 2.87 | 86.51 | 0.00 |
| Season | 35.61 | 3 | 11.87 | 357.31 | 0.00 |
| Year | 11.07 | 2 | 5.53 | 166.59 | 0.00 |
| Pandemic×boat traffic | 5.00 | 1 | 5.00 | 150.35 | 0.00 |
| Boat traffic×diel | 1.43 | 2 | 0.71 | 21.46 | 0.00 |
| Boat traffic×month | 3.18 | 11 | 0.29 | 8.69 | 0.00 |
| Water level×diel | 10.34 | 8 | 1.29 | 38.91 | 0.00 |
| Water level×month | 20.55 | 15 | 1.37 | 41.24 | 0.00 |
| Water level×year | 0.50 | 7 | 0.07 | 2.14 | 0.04 |
| Diel×month | 225.53 | 22 | 10.25 | 308.59 | 0.00 |
| Diel×season | 26.53 | 6 | 4.42 | 133.11 | 0.00 |
| Diel×year | 1.63 | 4 | 0.41 | 12.23 | 0.00 |
| Month×season | 29.81 | 1 | 29.81 | 897.28 | 0.00 |
| Month×year | 138.48 | 13 | 10.65 | 320.64 | 0.00 |
| Season×year | 186.70 | 6 | 31.12 | 936.68 | 0.00 |
| Water level×diel×month | 114.38 | 40 | 2.86 | 86.08 | 0.00 |
| Water level×diel×year | 3.84 | 16 | 0.24 | 7.23 | 0.00 |
| Diel×month×season | 16.39 | 2 | 8.19 | 246.62 | 0.00 |
| Error | 39 890.48 | 1 200 774 | 0.03 | ||
| Total | 45 686.00 | 1 200 960 | |||
| Corrected total | 43 948.05 | 1 200 959 |
Figure 3.
Bar graphs of GLM and nonparametric analysis of differences in porpoise biosonar parameters as a function of pandemic lockdown (A), boat traffic (B), water level (C), diel (D), lunar (E), month (F), season (G), and year (H)
Results are expressed as mean±standard error of the mean (SE). Error bars with different letters refer to post hoc multiple comparison tests that yielded significant results (P<0.05).
Boat traffic was detected every day (Supplementary Figure S1). The GLM results indicated that boat traffic was significantly correlated with all four biosonar parameters (Table 2; Figure 3B; Supplementary Tables S1–S3). Specifically, porpoise biosonar activity (for all measured parameters) was significantly higher during the time without boat traffic than that with boat traffic (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3B; Supplementary Tables S1–S3). A significant correlation was observed between porpoise and boat sonar detections (Spearman’s rho, P<0.001, n=1 200 960) (Figure 4). Porpoise click train detections were positively correlated with porpoise buzz detections (Spearman’s rho, P<0.001, n=1 200 960). In contrast, porpoise click train and buzz detections were significantly negatively correlated with boat traffic (Spearman’s rho, P<0.001, n=1 200 960) (Figure 4).
Figure 4.
Heatmap of Spearman’s rho correlation between porpoise biosonar and boat traffic detections
DPRM: Detection-positive rate per minute.
Water level and flux
Periodic changes in local water level and water flux were observed (Supplementary Figure S2). The GLM results indicated that water level was significantly correlated with all four biosonar parameters (Table 2; Figure 3C; Supplementary Tables S1–S3). Both the number of porpoise click trains and buzzes per minute differed significantly under different water levels (Tamhane’s T2 post hoc multiple comparison test; P<0.01). The numbers of porpoise click trains and buzzes per minute were significantly higher in the WN transitional level and dry level than in the other water levels (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3C). The GLM results also indicated that water flux was significantly correlated with all four biosonar parameters (Table 2; Supplementary Tables S1–S3).
Diel and lunar patterns
The GLM results indicated significant differences in diel patterns in all four biosonar parameters (Table 2; Figure 3C; Supplementary Figures S3–S8). In particular, porpoise click DPRM, number of porpoise click trains per minute, porpoise buzz DPRM, and number of porpoise buzzes per minute were significantly higher at night than at twilight and daytime (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figures 3D, 5).
Figure 5.
Daily (A) and diel patterns (B) of porpoise biosonar and boat sonar detections over the whole analyzed period. DPRM: detection-positive rate per minute
The Kolmogorov-Smirnov test indicated that the subset data of biosonar parameters during the night period were non-normally distributed (P<0.05), and thus nonparametric tests were adopted to analyze the lunar patterns of porpoise biosonar activity. Significant lunar patterns were detected in all four biosonar parameters (Porpoise click DPRM: Kruskal-Wallis χ2=142.04, df=2, P<0.001; Number of porpoise click trains per minute: Kruskal-Wallis χ2=144.92, df=2, P<0.001; Porpoise buzz DPRM: Kruskal-Wallis χ2=114.27, df=2, P<0.001; Number of porpoise buzzes per minute: Kruskal-Wallis χ2=115.1, df=2, P<0.001). Both porpoise click train DPRM and number of porpoise click trains per minute were significantly lower during the quarter moon period than during the new and full moon periods (multiple comparison test; P<0.01) (Figure 3E), whereas, both buzz DPRM and number of buzzes per minute were significantly higher during the quarter moon period than during the full and new moon periods (multiple comparison test; P<0.01) (Figure 3E).
Monthly, seasonal, and yearly patterns
The GLM results showed significant monthly differences in all four biosonar parameters (Table 2; Figure 3C). Notably, porpoise sonar activity across all parameters was significantly higher in February and September than in the other months (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3F).
The GLM results also showed significant seasonal differences in the four biosonar parameters (Table 2; Figure 3C), with porpoise activity showing a significant increase from spring to winter across all parameters (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3G).
The GLM results demonstrated significant annual differences in the four biosonar parameters (Table 2; Figure 3C). Notably, across all parameters, porpoise activity was significantly higher in 2020 than in 2022 and 2021 (Tamhane’s T2 post hoc multiple comparison test; P<0.01) (Table 2; Figure 3H).
Interaction effects
Analysis revealed significant interaction effects among anthropogenic activity, hydrological regime, and light level with respect to porpoise biosonar detections. Notably, significant interaction effects were observed between pandemic lockdown and boat traffic, boat traffic and different temporal scales, and water level and different temporal scales on porpoise biosonar detections, as well as between boat traffic and the diel phase (Table 2; Supplementary Tables S1–S3). In contrast, no significant correlation was found between boat traffic and water level, nor were the interaction effects of boat traffic and water level on porpoise biosonar detections significant (Spearman’s rho, P<0.001, n=1 200 960; Table 2; Supplementary Tables S1–S3).
DISCUSSION
Conservation management typically focuses on protecting wildlife habitats associated with key behavioral processes, such as resting, socializing, breeding, birthing, and feeding, while also minimizing possible anthropogenic impacts (Reynolds III et al., 2009; Wang et al., 2019, 2022, 2021c, 2014b). Knowing the timing and location of these vital behavioral processes is essential for mitigating anthropogenic impacts on endangered animal populations (Smith et al., 2016).
The C-POD method is a highly practical odontocete click monitoring approach (Carlén et al., 2018). In this study, of all detected porpoise biosonar click trains, buzz signals accounted for approximately 22.6%, comparable to that previously reported in wild harbor porpoises in West Wales, in which 27.3% of detected click trains were classified as buzz signals (Nuuttila et al., 2013a).
Pandemic lockdown and boat traffic
COVID-19 lockdown created a widespread reduction in human activity, including automobile and shipping traffic, leading to a profound reduction in air and water noise pollution (Derryberry et al., 2020; Loh et al., 2022; Thomson & Barclay, 2020). As a result, wildlife sightings in urban areas showed a worldwide increase (Loh et al., 2022). In this study, significantly higher porpoise sonar detection was observed during the pandemic lockdown period than during the non-lockdown period, with the reduced anthropogenic activity proving beneficial for the habitat use of local porpoises in the Poyang Lake junction area.
Vessel disturbance has been shown to cause both short-term impacts on critical dolphin behaviors (Lusseau, 2003) and disruption of dolphin habitat use through displacement (Lusseau & Higham, 2004), as well as long-term consequences on reproduction and population dynamics (Bejder et al., 2006). In river systems, boat traffic represents a major threat to dolphins, as observed for the Ganges River dolphin (Platanista gangetica gangetica) in the Turag River in Bangladesh (Baki et al., 2017), as well as the susu (P. g. gangetica) and bhulan (P. g. minor), two river dolphins in South Asia (Smith, 2002). The Yangtze River basin, characterized by heavy and persistent navigation and associated noise pollution, poses a significant threat to the finless porpoise (Wang et al., 2020, 2021a, 2021b). The findings of our study, indicating a significant negative correlation between porpoise sonar detections and boat traffic, are consistent with previous research conducted in the same area in 2007 (Dong et al., 2012). Substantial efforts can be made to reduce noise pollution by encouraging manufacturers to develop quieter vehicles. Spatial and temporal restrictions on vessel traffic, such as exclusion zones and speed limits, could also lower the incidence of vessel collisions and reduce the adverse effects of boat traffic on the viability of the local porpoise population. It is important to note that the static acoustic monitoring site in this study was located outside the main navigation channel, and thus the detected boat traffic flux and its impact on porpoises are likely underestimated. A more focused investigation of boat traffic in the Poyang Lake junction area is required to better understand the impact of local boat traffic on porpoises.
Water level and flux
Water level variations can affect the amount and type of aquatic habitats available to cetaceans and their prey (McGuire & Aliaga-Rossel, 2010). Previous studies have suggested that higher water levels may serve as the stimulus for increased Ganges River dolphin detection in the Turag River in Bangladesh from July to October, while reduced water flux, combined with adverse water quality, may contribute to the fewer dolphins encountered from December to July (Baki et al., 2017). In contrast, this study observed a significantly elevated rate of sonar detection for finless porpoises during dry-level periods than in wet-level periods, as observed for river dolphins boto (Inia geoffrensis geoffrensis) in Venezuela and bufeo (I. g. boliviensis) in Bolivia, where detection was higher during the falling water than rising water season (McGuire & Aliaga-Rossel, 2010). We speculate that during periods of high-water levels, the river basin widens, resulting in a more dispersed distribution of porpoises and a reduced detection rate in the wet season. Earlier preliminary studies conducted between the two bridges in Poyang Lake highlighted the impact of water flux on porpoise sonar detection (Li et al., 2010). Furthermore, studies conducted in the confluence area of Dongting Lake reported a strong positive correlation between water level and flux and capture abundance of migratory fish species (Liu et al., 2020). Flow velocity is also reported to influence the distribution of finless porpoises in Poyang Lake (Li et al., 2022). Artificial modifications in hydrological conditions can result in isolation between populations and a reduction in genetic variability (Pavanato et al., 2016). Therefore, the construction of water level control structures, such as hydraulic projects near corridors, should be carefully evaluated (Fang et al., 2016). Strong water flux in the barrage gate has been identified as a migration barrier for the susu and bhulan (Smith, 2002). Thus, maintaining local natural hydrological regimes is critical for porpoise migration in river-lake corridors and the viability of porpoise populations.
Diel and lunar patterns
In the Dongting Lake and Yangtze River junction, fish catches are consistently higher at night than during the day throughout the year (Liu et al., 2020). We speculate that a similar phenomenon may occur in Poyang Lake, producing higher porpoise sonar detections during the night.
Light levels, including daylight and moonlight, can impact the activity of various predators in both terrestrial and marine ecosystems (Bennie et al., 2014; Shaff & Baird, 2021). For instance, tagged rough-toothed dolphins (Steno bredanensis) exhibit their highest diving activity during dusk and night, while their lowest activity occurs at dawn and during the day, with the lunar phase further influencing diving patterns, leading to deeper and longer dives with increasing illumination (Shaff & Baird, 2021). Similarly, pantropical spotted dolphins (Stenella attenuata) (Baird et al., 2001) and melon-headed whales (Peponocephala electra) (West et al., 2018) in Hawaii display deeper, faster, and longer dives at night. Short-finned pilot whales (Globicephala macrorhynchus) around the main Hawaiian islands also exhibit deeper and longer dives during a full moon than during a new moon (Owen et al., 2019). Pantropical spotted dolphins in the pelagic waters of the eastern tropical Pacific Ocean travel more slowly but dive deeper and longer at nighttime (Scott & Chivers, 2009). Risso’s dolphins (Grampus griseus) in the Southern California Bight also show a significant diel pattern in echolocation clicks (Soldevilla et al., 2010). The diel movement patterns of prey, such as the vertical migration of zooplankton, which descend to deeper waters during the day to avoid visual predators and ascend to shallower waters at night to feed, are considered key factors for the diel activity of many marine predators (Shaff & Baird, 2021; Storrie et al., 2022). The diel cycle of animal behavior optimizes fitness by minimizing predation risk while optimizing physiological performance and foraging success (Benoit-Bird & Au, 2003; Papastamatiou et al., 2015). In this study, the porpoise sonar detection rate was significantly higher during the quarter moon phase than during the full moon. In contrast, previous studies have reported that the relative abundance of spinner dolphins (Stenella longirostris) in Hawaii and dusky dolphins (Lagenorhynchus obscurus) in New Zealand increases with increasing lunar illumination (Benoit-Bird et al., 2009). Although the dolphin response to the lunar phase is not yet clear, one possible explanation is that zooplankton, which are vertical migrants, tend to avoid the surface area during full moon periods (Pinot & Jansá, 2001). Thus, vertical migration of prey targeted by porpoises may also be suppressed, influencing their distribution.
Monthly, seasonal, and yearly patterns
Significant seasonal variation in dolphin sonar detection probability has also been observed in bottlenose dolphins in Cardigan Bay, Wales, with significantly higher sonar detection from June to August than from February to May (Nuuttila et al., 2013b). In contrast, after a one-year acoustic monitoring period in Cardigan Bay, previous research found that bottlenose dolphins show a detection peak in April-October and harbor porpoises show a detection peak in October–March (Simon et al., 2010). In Bunbury, Western Australia, the abundance of the Indo-Pacific bottlenose dolphin (Tursiops aduncus) is consistently lower during winter and higher during summer and autumn (Sprogis et al., 2018). Here, we observed significant seasonal and annual patterns in porpoise sonar activities. Notably, sonar detections were higher during the lockdown period, suggesting the importance of prolonged time recording, with data covering different seasons and ideally a time span longer than three years for analysis of habitat preference or identification of critical habitats (e.g., migration corridor) for cetaceans, especially endangered species (Van Parijs et al., 2009; Wang et al., 2015b). Our observation of consistently higher porpoise sonar detection in September during the three years of monitoring suggests more active biosonar activity or a higher number of porpoises in the region during that period, possibly linked to local fish migration, which warrants further investigation.
Migration of porpoise in the corridor
Infrastructure for transportation and power-generation, such as roads, bridges, and dams, can substantially influence migratory species movements (Moore & Berejikian, 2022; Shepard et al., 2008; Wilcove & Wikelski, 2008). For example, the Hood Canal Bridge in the northern outlet of the Hood Canal in Puget Sound, Washington (USA), interferes with steelhead (Oncorhynchus spp.) migration, leading to increased mortality (Moore et al., 2013; Moore & Berejikian, 2022). Similarly, construction of the John’s Pass Bridge in Florida (USA) led to a significant decline in sighting probabilities of female bottlenose dolphins (Weaver, 2015). Furthermore, a previous towed acoustic survey conducted in the Poyang Lake junction area found fewer porpoise detections near the bridge compared to non-bridge areas (Kimura et al., 2012). In the current study, although finless porpoises were still observed between the two bridges, indicating migration was not entirely blocked, the percentage of time in which porpoise sonar was detected was extremely low (approximately 3.80%), suggesting only temporary occupancy and that the bridge may act as a barrier to local porpoises, albeit with the possibility of small-scale river-lake migration.
Cetaceans have been observed to congregate in areas where water flows intersect. River dolphins in South Asia, such as the susu and bhulan, frequently occur in convergence areas (Smith, 2002). Confluences and small tributaries in the Amazon and Orinoco are critical habitats for the river dolphins boto and bufeo, as revealed by satellite tracking data (Mosquera-Guerra et al., 2018). The finless porpoise was once commonly observed in the convergence area of the Wanhe River, Yangtze River, Dongting Lake, and Poyang Lake (Wei et al., 2003; Zhang et al., 1993, 2015). Notably, high prey biomass and availability have been observed in the confluence areas of the Yangtze River (Zhang et al., 1993, 2015).
The joint areas between the Yangtze River and lakes, such as the confluence areas of Dongting Lake and Poyang Lake, serve as critical corridors for river-lake migration of fish species (Ru & Liu, 2013). Dominant fish in the Poyang Lake junction area include resident species, such as common carp (Cyprinus carpio), crucian carp (Carassius auratus), sharp belly (Hemiculter leucisculus), and short jaw anchovy (Coilia brachygnathus), and river-lake migration species, such as silver carp (Hypophthalmichthys molitrix) and bighead carp (Aristichthys nobilis) (Hu et al., 2011; Wang et al., 2016). The migratory fish species tend to migrate from the Yangtze River to the lakes during autumn and winter and vice versa during spring and summer (Liu et al., 2019, 2020; Wang et al., 2016), and account for more than 10% of local fish diversity and harvest (Hu et al., 2011; Wang et al., 2016). Fish are the primary prey item of finless porpoises and prey availability is a crucial factor in their habitat use (Kimura et al., 2012; Wang et al., 2015a, 2014a). In 2021, the Chinese Government implemented an emergency 10 year ban on fishing across the entire Yangtze River Basin, alleviating prey availability pressure, with the most recent survey indicating that the free-ranging Yangtze finless porpoise population has increased from 1 012 in 2017 to 1 249 in 2022.
Retention of gene flow among fragmented habitat patches is vital for the sustainability of populations sensitive to inbreeding (Keller & Waller, 2002). Small isolated populations often suffer reduced fitness from inbreeding depression due to increased homozygosity of strongly deleterious recessive mutations (Robinson et al., 2019). Migrants from adjacent populations and dispersal among populations are critical for population viability (Manlik et al., 2019; Wilcove & Wikelski, 2008), and protecting migration corridors that connect these habitats is vital for their conservation (Geijer & Jones, 2015). The maximum detection range of C-POD for bottlenose dolphins and harbor porpoises is approximately 1 512 m (range: 1 272–1 779 m) and 500 m, respectively (Nuuttila et al., 2013a, 2013b). The single hydrophone device used in this study precludes robust conclusions regarding the migration of porpoises in this area. Thus, further research on the migration of porpoises using an array of detectors, such as hydrophone arrays, over a larger spatial area and possible animal tagging should help increase our understanding of overall movement ecology and provide greater insight into habitat use.
Conservation and management implications
In contrast to other forms of environmental pollution, such as the oil spill in Barataria Bay, Louisiana (USA), which continues to cause persistent adverse effects in common bottlenose dolphins 18 years after exposure, including chronic disease, impaired stress response and reproduction, and increased mortality (Schwacke et al., 2022), noise pollution generally leaves no lasting traces once the source of pollution is removed. However, prolonged exposure may lead to enduring changes in physiological and cognitive traits (Schwacke et al., 2022). Therefore, implementing mitigation measures against noise pollution could yield immediate beneficial effects for wildlife (Halfwerk, 2020). For example, the songs of white-crowned sparrows (Zonotrichia leucophrys) in the San Francisco Bay area (USA) immediately changed in response to the substantially reduced noise level during the COVID-19 shutdown, resulting in increased acoustic signal efficacy and salience (Derryberry et al., 2020). In our study, the temporary pandemic lockdown immediately increased porpoise biosonar behavior, providing policy-makers with a potential solution to mitigate the detrimental effects of noise pollution by reducing exposure during critical life cycle stages, such as migration or feeding. The ecological navigation speed of ships and ecological flow in confluence areas should also be investigated. The seasonal control of bridge speed and traffic flow, or even temporary traffic prohibitions, during porpoise migration and feeding also warrant further investigation.
CONCLUSIONS
Migrants from adjacent populations are critical for cetacean population viability. The Yangtze River and Poyang Lake junction area serves as the sole corridor for the local river-lake migration of the Yangtze finless porpoise. Through passive acoustic monitoring, our study revealed the impact of anthropogenic activities and environmental variables on the diel, lunar, seasonal, and annual biosonar activity patterns of porpoises in the Poyang Lake junction area. The detection of porpoises for relatively short and concise periods on most days suggests that small-scale river-lake migration may still exist. Furthermore, the significantly higher porpoise sonar activity observed during the pandemic lockdown period indicates that mitigation measures against noise pollution could immediately benefit this species. The negative correlation between porpoise click train and buzz detections and boat traffic underscores the importance of implementing vessel speed restrictions during critical stages of the porpoise life cycle, such as migration and feeding. The hydrological regime also influenced porpoise biosonar activity, emphasizing the importance of maintaining local hydrological conditions in this river-lake corridor for porpoise migrants. This study provides a foundation for future work on porpoise migration in this unique corridor and for guiding better management of natural resources and conservation of the endemic finless porpoise.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.T.W. and K.X.W. contributed to project conception. Z.T.W. contributed to funding acquisition. P.X.D. and Z.T.W. conducted the experiments. P.X.D., Z.T.W., T.A., and N.T. analyzed the data and prepared the figures and manuscript. Z.T.W., T.A., N.T., G.Y.L, K.X.W., and D.W. provided technical support. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We gratefully thank the staff from the Aquatic Animal Conservation Group of the Institute of Hydrobiology of the Chinese Academy of Sciences for their assistance in data collection. We also acknowledge the cooperation of all crew members onboard the survey boat for their valuable assistance during the research period. We would like to express our gratitude to the academic editor, as well as to the three anonymous reviewers, for their valuable critiques of a prior iteration of this manuscript
Funding Statement
This work was supported by Science and Technology Service Network Initiative Program of the Chinese Academy of Sciences,the National Natural Science Foundation of China (41806197) and the Exploratory Program of the Natural Science Foundation of Zhejiang Province (ZX2023000154).
Contributor Information
Zhi-Tao Wang, Email: wangzhitao@nbu.edu.cn.
Ke-Xiong Wang, Email: wangk@ihb.ac.cn.
References
- Akamatsu T, Teilmann J, Miller LA, et al. 2007. Comparison of echolocation behaviour between coastal and riverine porpoises. Deep Sea Research Part II: Topical studies in Oceanography, 54(3–4): 290–297.
- Akamatsu T, Wang D, Wang K, et al Estimation of the detection probability for Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis) with a passive acoustic method. The Journal of the Acoustical Society of America. 2008;123(6):4403–4411. doi: 10.1121/1.2912449. [DOI] [PubMed] [Google Scholar]
- Akamatsu T, Wang D, Wang K, et al Scanning sonar of rolling porpoises during prey capture dives. Journal of Experimental Biology. 2010;213(1):146–152. doi: 10.1242/jeb.037655. [DOI] [PubMed] [Google Scholar]
- Akamatsu T, Wang D, Wang KX, et al Biosonar behaviour of free-ranging porpoises. Proceedings of the Royal Society B:Biological Sciences. 2005;272(1565):797–801. doi: 10.1098/rspb.2004.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundin M, Carlström J, Thomas L, et al Estimating the abundance of the critically endangered Baltic Proper harbour porpoise (Phocoena phocoena) population using passive acoustic monitoring. Ecology and Evolution. 2022;12(2):e8554. doi: 10.1002/ece3.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au WWL. 1993. The Sonar of Dolphins. New York: Springer.
- Baird RW, Ligon AD, Hooker SK, et al Subsurface and nighttime behaviour of pantropical spotted dolphins in Hawai'i. Canadian Journal of Zoology. 2001;79(6):988–996. doi: 10.1139/z01-070. [DOI] [Google Scholar]
- Baki MA, Bhouiyan NA, Islam S, et al Present status of Ganges river dolphins Platanista gangetica gangetica (Roxburgh, 1801) in the Turag river, Dhaka, Bangladesh. International Journal of Zoology. 2017;2017:8964821. [Google Scholar]
- Bejder L, Samuels A, Whitehead H, et al Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conservation Biology. 2006;20(6):1791–1798. doi: 10.1111/j.1523-1739.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- Bennie JJ, Duffy JP, Inger R, et al Biogeography of time partitioning in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(38):13727–13732. doi: 10.1073/pnas.1216063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Bird KJ, Au WWL Prey dynamics affect foraging by a pelagic predator (Stenella longirostris) over a range of spatial and temporal scales. Behavioral Ecology and Sociobiology. 2003;53(6):364–373. doi: 10.1007/s00265-003-0585-4. [DOI] [Google Scholar]
- Benoit-Bird KJ, Dahood AD, Würsig B Using active acoustics to compare lunar effects on predator-prey behavior in two marine mammal species. Marine Ecology Progress Series. 2009;395:119–135. doi: 10.3354/meps07793. [DOI] [Google Scholar]
- Braulik GT, Arshad M, Noureen U, et al Habitat fragmentation and species extirpation in freshwater ecosystems; Causes of range decline of the Indus River Dolphin (Platanista gangetica minor) PLoS One. 2014;9(7):e101657. doi: 10.1371/journal.pone.0101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capus C, Pailhas Y, Brown K, et al Bio-inspired wideband sonar signals based on observations of the bottlenose dolphin (Tursiops truncatus) The Journal of the Acoustical Society of America. 2007;121(1):594–604. doi: 10.1121/1.2382344. [DOI] [PubMed] [Google Scholar]
- Carlén I, Thomas L, Carlström J, et al Basin-scale distribution of harbour porpoises in the Baltic Sea provides basis for effective conservation actions. Biological Conservation. 2018;226:42–53. doi: 10.1016/j.biocon.2018.06.031. [DOI] [Google Scholar]
- Dähne M, Verfuß U K, Brandecker A, et al Methodology and results of calibration of tonal click detectors for small odontocetes (C-PODs) The Journal of the Acoustical Society of America. 2013;134(3):2514–2522. doi: 10.1121/1.4816578. [DOI] [PubMed] [Google Scholar]
- Derryberry EP, Phillips JN, Derryberry GE, et al Singing in a silent spring: Birds respond to a half-century soundscape reversion during the COVID-19 shutdown. Science. 2020;370(6516):575–579. doi: 10.1126/science.abd5777. [DOI] [PubMed] [Google Scholar]
- Dong SY, Dong LJ, Li SH, et al Effects of vessel traffic on the acoustic behavior of Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis) in the confluence of Poyang lake and the Yangtze River: using fixed passive acoustic observation methods. Acta Hydrobiologica Sinica. 2012;36(2):246–254. [Google Scholar]
- Echeverria A, Botta S, Marmontel M, et al Trophic ecology of Amazonian River dolphins from three rivers in Brazil and Bolivia. Mammalian Biology. 2022;102(5):1687–1696. [Google Scholar]
- Español-Jiménez S, Van Der Schaar M First record of humpback whale songs in Southern Chile: Analysis of seasonal and diel variation. Marine Mammal Science. 2018;34(3):718–733. doi: 10.1111/mms.12477. [DOI] [Google Scholar]
- Fang HW, Dai DC, Li SH, et al Forecasting Yangtze finless porpoise movement behavior using an Eulerian–Eulerian-diffusion method (EEDM) Ecological Engineering. 2016;88:39–52. doi: 10.1016/j.ecoleng.2015.12.008. [DOI] [Google Scholar]
- Fang SW, Wang SG, Ouyang QL Discussion on the definition standard of the wet season, normal season and dry season in Poyang lake. Journal of China hydrology. 2022;42(1):11–15. [Google Scholar]
- Geijer CKA, Jones PJS A network approach to migratory whale conservation: Are MPAs the way forward or do all roads lead to the IMO? Marine Policy. 2015;51:1–12. doi: 10.1016/j.marpol.2014.06.002. [DOI] [Google Scholar]
- Goldbogen JA, Cade DE, Wisniewska DM, et al Why whales are big but not bigger: Physiological drivers and ecological limits in the age of ocean giants. Science. 2019;366(6471):1367–1372. doi: 10.1126/science.aax9044. [DOI] [PubMed] [Google Scholar]
- Goswami A, Noirault E, Coombs EJ, et al Attenuated evolution of mammals through the Cenozoic. Science. 2022;378(6618):377–383. doi: 10.1126/science.abm7525. [DOI] [PubMed] [Google Scholar]
- Halfwerk W The quiet spring of 2020. Science. 2020;370(6516):523–524. doi: 10.1126/science.abe8026. [DOI] [PubMed] [Google Scholar]
- Homfeldt TN, Risch D, Stevenson A, et al Seasonal and diel patterns in singing activity of humpback whales migrating through Bermuda. Frontiers in Marine Science. 2022;9:941793. doi: 10.3389/fmars.2022.941793. [DOI] [Google Scholar]
- Hu ML, Wu ZQ, Liu YL Fish diversity and community structure in Hukou area of Lake Poyang. Journal of Lake Sciences. 2011;23(2):246–250. doi: 10.18307/2011.0213. [DOI] [Google Scholar]
- Huang J, Mei ZG, Chen M, et al Population survey showing hope for population recovery of the critically endangered Yangtze finless porpoise. Biological Conservation. 2020;241:108315. doi: 10.1016/j.biocon.2019.108315. [DOI] [Google Scholar]
- Jaramillo-Legorreta A, Cardenas-Hinojosa G, Nieto-Garcia E, et al Passive acoustic monitoring of the decline of Mexico's critically endangered vaquita. Conservation Biology. 2017;31(1):183–191. doi: 10.1111/cobi.12789. [DOI] [PubMed] [Google Scholar]
- Keller LF, Waller DM Inbreeding effects in wild populations. Trends in Ecology & Evolution. 2002;17(5):230–241. [Google Scholar]
- Kimura S, Akamatsu T, Li SH, et al Seasonal changes in the local distribution of Yangtze finless porpoises related to fish presence. Marine Mammal Science. 2012;28(2):308–324. doi: 10.1111/j.1748-7692.2011.00490.x. [DOI] [Google Scholar]
- Kowarski K, Evers C, Moors-Murphy H, et al Singing through winter nights: Seasonal and diel occurrence of humpback whale (Megaptera novaeangliae) calls in and around the Gully MPA, offshore eastern Canada. Marine Mammal Science. 2018;34(1):169–189. doi: 10.1111/mms.12447. [DOI] [Google Scholar]
- Li QY, Deng MM, Li WY, et al Habitat configuration of the Yangtze finless porpoise in Poyang Lake under a shifting hydrological regime. Science of the Total Environment. 2022;838:155954. doi: 10.1016/j.scitotenv.2022.155954. [DOI] [PubMed] [Google Scholar]
- Li SH, Dong SY, Kimura S, et al. 2010. Detection of Yangtze finless porpoises in the Poyang Lake mouth area via passive acoustic data-loggers. In: Shostell J, Ruiz-Garcia M. Biology Evolution and Conservation of River Dolphins Within South America and Asia. Nova Science Publishers Inc. , 343–355.
- Liu F, Lin PC, Li MZ, et al Situations and conservation strategies of fish resources in the Yangtze river basin. Acta Hydrobiologica Sinica. 2019;43(S1):144–156. [Google Scholar]
- Liu YJ, Gao L, Zheng YH, et al Annual dynamics and migration characteristics of fish resources in the Chenglingji, in the channel connecting Dongting Lake and the Yangtze River. Resources and Environment in the Yangtze Basin. 2020;29(2):376–385. [Google Scholar]
- Loh HC, Looi I, Ch’ng ASH, et al Positive global environmental impacts of the COVID-19 pandemic lockdown: a review. GeoJournal. 2022;87(5):4425–4437. doi: 10.1007/s10708-021-10475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D Male and female bottlenose dolphins Tursiops spp. have different strategies to avoid interactions with tour boats in Doubtful Sound, New Zealand. Marine Ecology Progress Series. 2003;257:267–274. doi: 10.3354/meps257267. [DOI] [Google Scholar]
- Lusseau D, Higham JES Managing the impacts of dolphin-based tourism through the definition of critical habitats: the case of bottlenose dolphins (Tursiops spp. ) in Doubtful Sound, New Zealand. Tourism Management. 2004;25(6):657–667. doi: 10.1016/j.tourman.2003.08.012. [DOI] [Google Scholar]
- Manlik O, Chabanne D, Daniel C, et al Demography and genetics suggest reversal of dolphin source-sink dynamics, with implications for conservation. Marine Mammal Science. 2019;35(3):732–759. doi: 10.1111/mms.12555. [DOI] [Google Scholar]
- McCauley DJ, Pinsky ML, Palumbi SR, et al Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- McGuire TL, Aliaga-Rossel ER. 2010. Seasonal ecology of Inia in three river basins of South America (Orinoco, Amazon, and upper Madeira). In: Ruiz-Garcia M, Shostell JM. Biology, Evolution and Conservation of River Dolphins within South America and Asia. New York: Nova Science Publishers, Inc. , 29–54.
- Moore M, Berejikian BA, Tezak EP A floating bridge disrupts seaward migration and increases mortality of steelhead smolts in Hood Canal, Washington State. PLoS One. 2013;8(9):e73427. doi: 10.1371/journal.pone.0073427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ME, Berejikian BA Coastal infrastructure alters behavior and increases predation mortality of threatened Puget Sound steelhead smolts. Ecosphere. 2022;13(4):e4022. doi: 10.1002/ecs2.4022. [DOI] [Google Scholar]
- Mosquera-Guerra F, Trujillo F, Oliveira-Da-Costa M, et al. 2018. Movements and habitat use of river dolphins (Cetartiodactyla: Iniiidae) in the Amazon and Orinoco river basins, determined from satellite tagging. Page SC/67B/SM/14 in International whaling commission. Slovenia.
- Mussoline SE, Risch D, Hatch LT, et al Seasonal and diel variation in North Atlantic right whale up-calls: implications for management and conservation in the northwestern Atlantic Ocean. Endangered Species Research. 2012;17(1):17–26. doi: 10.3354/esr00411. [DOI] [Google Scholar]
- Nuuttila HK, Meier R, Evans PGH, et al Identifying foraging behaviour of wild bottlenose dolphins (Tursiops truncatus) and harbour porpoises (Phocoena phocoena) with static acoustic dataloggers. Aquatic Mammals. 2013a;39(2):147–161. doi: 10.1578/AM.39.2.2013.147. [DOI] [Google Scholar]
- Nuuttila HK, Thomas L, Hiddink JG, et al Acoustic detection probability of bottlenose dolphins, Tursiops truncatus, with static acoustic dataloggers in Cardigan Bay, Wales. The Journal of the Acoustical Society of America. 2013b;134(3):2596–2609. doi: 10.1121/1.4816586. [DOI] [PubMed] [Google Scholar]
- Owen K, Andrews RD, Baird RW, et al Lunar cycles influence the diving behavior and habitat use of short-finned pilot whales around the main Hawaiian Islands. Marine Ecology Progress Series. 2019;629:193–206. doi: 10.3354/meps13123. [DOI] [Google Scholar]
- Papastamatiou YP, Watanabe YY, Bradley D, et al Drivers of daily routines in an ectothermic marine predator: Hunt warm, rest warmer? PLoS One. 2015;10(6):e0127807. doi: 10.1371/journal.pone.0127807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanato HJ, Melo-Santos G, Lima DS, et al Risks of dam construction for South American river dolphins: a case study of the Tapajós River. Endangered Species Research. 2016;31:47–60. doi: 10.3354/esr00751. [DOI] [Google Scholar]
- Pinot JM, Jansá J Time variability of acoustic backscatter from zooplankton in the Ibiza Channel (western Mediterranean) Deep Sea Research Part I:Oceanographic Research Papers. 2001;48(7):1651–1670. doi: 10.1016/S0967-0637(00)00095-9. [DOI] [Google Scholar]
- Pirotta E, Thompson PM, Miller PI, et al Scale-dependent foraging ecology of a marine top predator modelled using passive acoustic data. Functional Ecology. 2014;28(1):206–217. doi: 10.1111/1365-2435.12146. [DOI] [Google Scholar]
- Reynolds III JE, Marsh H, Ragen TJ Marine mammal conservation. Endangered Species Research. 2009;7(1):23–28. [Google Scholar]
- Robinson JA, Räikkönen J, Vucetich LM, et al Genomic signatures of extensive inbreeding in Isle Royale wolves, a population on the threshold of extinction. Science Advances. 2019;5(5):eaau0757. doi: 10.1126/sciadv.aau0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru HJ, Liu XQ River-lake migration of fishes in the Dongting Lake area of the Yangtze floodplain. Journal of Applied Ichthyology. 2013;29(3):594–601. doi: 10.1111/jai.12116. [DOI] [Google Scholar]
- Ryan JP, Cline DE, Joseph JE, et al Humpback whale song occurrence reflects ecosystem variability in feeding and migratory habitat of the northeast Pacific. PLoS One. 2019;14(9):e0222456. doi: 10.1371/journal.pone.0222456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca MS, Czapanskiy MF, Kahane-Rapport SR, et al Baleen whale prey consumption based on high-resolution foraging measurements. Nature. 2021;599(7883):85–90. doi: 10.1038/s41586-021-03991-5. [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Marques TA, Thomas L, et al Modeling population effects of the Deepwater Horizon oil spill on a long-lived species. Conservation Biology. 2022;36(4):e13878. doi: 10.1111/cobi.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MD, Chivers SJ Movements and diving behavior of pelagic spotted dolphins. Marine Mammal Science. 2009;25(1):137–160. doi: 10.1111/j.1748-7692.2008.00241.x. [DOI] [Google Scholar]
- Shaff JF, Baird RW Diel and lunar variation in diving behavior of rough-toothed dolphins (Steno bredanensis) off Kauaʻi, Hawaiʻi. Marine Mammal Science. 2021;37(4):1261–1276. doi: 10.1111/mms.12811. [DOI] [Google Scholar]
- Shepard DB, Kuhns AR, Dreslik MJ, et al Roads as barriers to animal movement in fragmented landscapes. Animal Conservation. 2008;11(4):288–296. doi: 10.1111/j.1469-1795.2008.00183.x. [DOI] [Google Scholar]
- Simon M, Nuuttila H, Reyes-Zamudio MM, et al Passive acoustic monitoring of bottlenose dolphin and harbour porpoise, in Cardigan Bay, Wales, with implications for habitat use and partitioning. Journal of the Marine Biological Association of the United Kingdom. 2010;90(8):1539–1545. doi: 10.1017/S0025315409991226. [DOI] [Google Scholar]
- Smith BD. 2002. Susu and Bhulan: Platanista gangetica gangetica and P. g. minor. In: Perrin WF, Wursig B, Thewissen JGM. Encyclopedia of Marine Mammals. San Diego: Academic Press, 1208–1213.
- Smith H, Frère C, Kobryn H, et al Dolphin sociality, distribution and calving as important behavioural patterns informing management. Animal Conservation. 2016;19(5):462–471. doi: 10.1111/acv.12263. [DOI] [Google Scholar]
- Soldevilla MS, Wiggins SM, Hildebrand JA Spatial and temporal patterns of Risso’s dolphin echolocation in the Southern California Bight. The Journal of the Acoustical Society of America. 2010;127(1):124–132. doi: 10.1121/1.3257586. [DOI] [PubMed] [Google Scholar]
- Springer AM, Estes JA, Van Vliet GB, et al Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12223–12228. doi: 10.1073/pnas.1635156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprogis KR, Christiansen F, Wandres M, et al El Niño Southern Oscillation influences the abundance and movements of a marine top predator in coastal waters. Global Change Biology. 2018;24(3):1085–1096. doi: 10.1111/gcb.13892. [DOI] [PubMed] [Google Scholar]
- Storrie L, Hussey NE, MacPhee SA, et al Empirically testing the influence of light regime on diel activity patterns in a marine predator reveals complex interacting factors shaping behaviour. Functional Ecology. 2022;36(11):2727–2741. doi: 10.1111/1365-2435.14172. [DOI] [Google Scholar]
- Sveegaard S, Galatius A, Dietz R, et al Defining management units for cetaceans by combining genetics, morphology, acoustics and satellite tracking. Global Ecology and Conservation. 2015;3:839–850. doi: 10.1016/j.gecco.2015.04.002. [DOI] [Google Scholar]
- Thomson DJM, Barclay DR Real-time observations of the impact of COVID-19 on underwater noise. The Journal of the Acoustical Society of America. 2020;147(5):3390–3396. doi: 10.1121/10.0001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd VLG, Pearse WD, Tregenza NC, et al Diel echolocation activity of harbour porpoises (Phocoena phocoena) around North Sea offshore gas installations. ICES Journal of Marine Science. 2009;66(4):734–745. doi: 10.1093/icesjms/fsp035. [DOI] [Google Scholar]
- Tollis M, Robbins J, Webb AE, et al Return to the sea, get huge, beat cancer: An analysis of cetacean genomes including an assembly for the humpback whale (Megaptera novaeangliae) Molecular Biology and Evolution. 2019;36(8):1746–1763. doi: 10.1093/molbev/msz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey ST, Pitman RL, Taylor BL, et al First human-caused extinction of a cetacean species? Biology Letters. 2007;3(5):537–540. doi: 10.1098/rsbl.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Parijs SM, Clark CW, Sousa-Lima RS, et al Management and research applications of real-time and archival passive acoustic sensors over varying temporal and spatial scales. Marine Ecology Progress Series. 2009;395:21–36. doi: 10.3354/meps08123. [DOI] [Google Scholar]
- Vishnu H, Hoffmann-Kuhnt M, Chitre M, et al A dolphin-inspired compact sonar for underwater acoustic imaging. Communications Engineering. 2022;1(1):10. doi: 10.1038/s44172-022-00010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D Population status, threats and conservation of the Yangtze finless porpoise. Chinese Science Bulletin. 2009;54(19):3473–3484. [Google Scholar]
- Wang D, Turvey ST, Zhao X, et al. 2013. Neophocaena asiaeorientalis ssp. asiaeorientalis. In: The IUCN Red List of Threatened Species. Version 2013.
- Wang S, Duan XB, Chen WJ, et al Status and changes of fish resources in the Hukou area of Poyang Lake. Freshwater Fisheries. 2016;46(6):50–55. [Google Scholar]
- Wang ZT, Akamatsu T, Duan PX, et al Underwater noise pollution in China’s Yangtze River critically endangers Yangtze finless porpoises (Neophocaena asiaeorientalis asiaeorientalis) Environmental Pollution. 2020;262:114310. doi: 10.1016/j.envpol.2020.114310. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Akamatsu T, Mei ZG, et al Frequent and prolonged nocturnal occupation of port areas by Yangtze finless porpoises (Neophocaena asiaeorientalis): Forced choice for feeding? Integrative Zoology. 2015a;10(1):122–132. doi: 10.1111/1749-4877.12102. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Akamatsu T, Nowacek DP, et al Soundscape of an Indo-Pacific humpback dolphin (Sousa chinensis) hotspot before windfarm construction in the Pearl River Estuary, China: Do dolphin engage in noise avoidance and passive eavesdropping behavior? Marine Pollution Bulletin. 2019;140:509–522. doi: 10.1016/j.marpolbul.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Akamatsu T, Wang KX, et al The Diel rhythms of Biosonar behavior in the Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) in the port of the Yangtze river: The correlation between prey availability and boat traffic. PLoS One. 2014a;9(5):e97907. doi: 10.1371/journal.pone.0097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZT, Duan PX, Akamatsu T, et al Riverside underwater noise pollution threaten porpoises and fish along the middle and lower reaches of the Yangtze River, China. Ecotoxicology and Environmental Safety. 2021a;226:112860. doi: 10.1016/j.ecoenv.2021.112860. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Duan PX, Chen M, et al Vocalization of Bryde’s whales (Balaenoptera edeni) in the Beibu Gulf, China. Marine Mammal Science. 2022;38(3):1118–1139. doi: 10.1111/mms.12917. [DOI] [Google Scholar]
- Wang ZT, Duan PX, Wang KX, et al Noise pollution disrupts freshwater cetaceans. Science. 2021b;374(6573):1332–1333. doi: 10.1126/science.abf0222. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Nachtigall PE, Akamatsu T, et al Passive acoustic monitoring the diel, lunar, seasonal and tidal patterns in the biosonar activity of the Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary, China. PLoS One. 2015b;10(11):e0141807. doi: 10.1371/journal.pone.0141807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZT, Supin AY, Akamatsu T, et al Auditory evoked potential in stranded melon-headed whales (Peponocephala electra): With severe hearing loss and possibly caused by anthropogenic noise pollution. Ecotoxicology and Environmental Safety. 2021c;228:113047. doi: 10.1016/j.ecoenv.2021.113047. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Wu YP, Duan GQ, et al Assessing the underwater acoustics of the world's largest vibration hammer (OCTA-KONG) and its potential effects on the Indo-Pacific humpbacked dolphin (Sousa chinensis) PLoS One. 2014b;9(10):e110590. doi: 10.1371/journal.pone.0110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A Sex difference in bottlenose dolphin sightings during a long-term bridge construction project. Animal Behavior and Cognition. 2015;2(1):1–13. doi: 10.12966/abc.02.01.2015. [DOI] [Google Scholar]
- Wei Z, Wang D, Zhang XF, et al Population size, behavior, movement pattern and protection of Yangtze finless porpoise at Balijiang section of the Yangtze River. Resources and Environment in the Yangtze Basin. 2002;11(5):427–432. [Google Scholar]
- Wei Z, Zhang XF, Wang KX, et al Habitat use and preliminary evaluation of the habitat status of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) in the Balijiang section of the Yangtze River, China. Acta Zoologica Sinica. 2003;49(2):163–170. [Google Scholar]
- West KL, Walker WA, Baird RW, et al Stomach contents and diel diving behavior of melon-headed whales (Peponocephala electra) in Hawaiian waters. Marine Mammal Science. 2018;34(4):1082–1096. doi: 10.1111/mms.12507. [DOI] [Google Scholar]
- Wilcove DS, Wikelski M Going, going, gone: Is animal migration disappearing. PLoS Biology. 2008;6(7):e188. doi: 10.1371/journal.pbio.0060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska DM, Johnson M, Teilmann J, et al Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Current Biology. 2016;26(11):1441–1446. doi: 10.1016/j.cub.2016.03.069. [DOI] [PubMed] [Google Scholar]
- Würsig B Cetaceans. Science. 1989;244(4912):1550–1557. doi: 10.1126/science.2662403. [DOI] [PubMed] [Google Scholar]
- Zar JH. 1999. Biostatistical Analysis. 4th ed. Upper Saddle River: Prentice-Hall.
- Zhang XF, Liu RJ, Zhao QZ, et al The population of Finless porpoise in the middle and lower reaches of Yangtze river. Acta Theriologica Sinica. 1993;13(4):260–270. [Google Scholar]
- Zhang XK, Yu DP, Wang HL, et al Effects of fish community on occurrences of Yangtze finless porpoise in confluence of the Yangtze and Wanhe Rivers. Environmental Science and Pollution Research. 2015;22(12):9524–9533. doi: 10.1007/s11356-015-4102-x. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Barlow J, Taylor BL, et al Abundance and conservation status of the Yangtze finless porpoise in the Yangtze River, China. Biological Conservation. 2008;141(12):3006–3018. doi: 10.1016/j.biocon.2008.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.