Abstract
The secondary use of tyre rubber is a potentially sustainable environmental solution. However, the sorption properties of used-tyre rubber have not yet been fully investigated. In this study, the rubber type (vulcanised or devulcanised part-worn tyre rubber) that can sorb phosphate phosphorus from aqueous solutions or wastewater more effectively is determined. The capacity of granules (0.3–1.0 mm in diameter) of non-devulcanised ground tyre rubber and uniquely chemically devulcanised rubber to adsorb phosphorus is evaluated under laboratory conditions. The results show that under the filtration of an aqueous solution or biologically treated wastewater at a flow rate of 0.75 m/h (1.2 L/h), 1 g of the devulcanised rubber medium accumulates 5.16 mg of phosphorus, which is five times more than that accumulated by the non-devulcanised rubber medium. The surface structure of the non-devulcanised rubber medium is more suitable for the sorption of devulcanised rubber granules. The sorption capacity and effectiveness of non-devulcanised rubber for phosphorus removal are more favourable compared with those of the tested natural and waste-prepared sorbents. Further research into this material as a medium for filter layers and for accumulating drainage should be conducted. The findings of this study are important for addressing issues associated with the secondary use of tyre rubber.
Keywords: Tyre rubber, Non-devulcanised, Devulcanised, Sorption, Phosphorus
Graphical abstract
Highlights
-
•
The aqueous solution was filtered at a flow rate of 0.75 m/h (1.2 L/h).
-
•
Devulcanised rubber efficiently (70–97% efficiency) removed P from wastewater.
-
•
1 g of the devulcanised rubber accumulates 5.16 mg of phosphorus.
-
•
1 g of the non-devulcanised rubber accumulates 1.05 mg of phosphorus.
1. Introduction
The increasing number of cars and trucks worldwide has resulted in the accumulations of billions of part-worn tyres in warehouses and landfills, which poses a severe threat to the environment [1]. Used tyres are typically discarded. The incineration of used tyres reduces the amount of waste; however, the process results in severe health and environmental problems caused by toxic, carcinogenic, and mutagenic pollutants, such as polycyclic aromatic hydrocarbons, volatile organic compounds, CO, CO2, SOX, and NOX [2]. This is a severe environmental issue because only a small proportion of part-worn tyres are recycled into value-added products, such as floor mats, tyre fuel, and hopper inserts for road pavements [3]. Various tyre-rubber shapes can be used as filter layers, media, drainage sealants, wetland and plant-bearing layer media, and lightweight media in island greenery and soft foundation areas (Park and Ye, 2016). The application of ground tyre rubber as an infill material in artificial football fields or playgrounds may be significantly limited because of restrictions on United States Environmental Protection Agency emissions [4]. The secondary use of tyre rubber is a potentially sustainable solution to environmental problems caused by tyre waste [5]. Old, unfit for use tires are an excellent source of secondary raw materials, until now they are managed in rather primitive ways. Of the 28,100 tons of tires delivered to the Lithuanian market in 2018, 52% of all sorted tire waste was exported, 22% was recycled, 13% was used for energy extraction, and 3.5% was processed (i.e. used, ready for use or disposal, burned in a fire) [6]. In Europe in 2018, recycling dominated as a way of managing tire waste. Sweden, Latvia and Lithuania were the only countries where other alternatives for the use of waste tires were more popular than recycling. Recycling tires is energetically twice as expensive as making a new product from original materials. Meanwhile, about twice as much synthetic rubber is produced and consumed as natural rubber. Oil prices also dictate rubber prices, which have been rising recently. A ton of rubber now costs around 2100 euros [6]. The need for recycling and secondary use of tire rubber will only grow in the future. Vulcanised rubbers cannot be reprocessed easily and can only be used another time [7,8]. Valorisation is key to the sustainable and widespread use of part-worn tyres because it decomposes the tyre rubber into components that can be recycled or further processed thermochemically [9]. Rubber is difficult to process because it comprises many components and has a three-dimensional (3D) molecular structure [10]. During devulcanization, the pieces of tires are crushed, their surface becomes rough, and the sulphur cross-links are broken in the inner parts, thus returning the rubber compounds to a rubbery state. The most widely used rubber grades are natural rubber and styrene–butadiene rubber, and the most typical vulcanisation technique is sulphur vulcanisation [11]. Over the recent decades, several rubber recycling processes have been developed, including devulcanisation [10,12]. Devulcanisation, grinding, and pyrolysis are the three most typically used valorisation methods [12,13]. The devulcanisation of rubber is a process in which the poly-, di-, and monosulfide bonds formed during vulcanisation are completely or partially broken. Breaking sulphur–sulphur (S–S) and carbon–sulphur (Csingle bondS) bonds requires 1.5 and 1.3 times less energy than breaking the main rubber chain, i.e. carbon–carbon (Csingle bondC) bonds [10]. Several devulcanisation processes have been developed, including biological, chemical, ultrasound, microwave, mechano–chemical, and thermo–mechanical processes [11,14,15]. The most promising techniques are microwave and ultrasonic devulcanisation because they are dry and eco-friendly [11].
By performing devulcanisation, 90% of rubber polymers can be recycled. The devulcanisation process is clean, nontoxic, waste-free, and environmentally friendly because it does not require high-temperature treatments (low energy consumption and CO2 emissions). Devulcanised rubber (DR) is characterised by homogeneity and dispersion, which significantly improves its tensile strength and other important properties, thus rendering it suitable for construction, agriculture, and environmental protection [16,17]. Additionally, the rubber of part-worn tyres can be used as an adsorbent to remove organic matter or heavy metals from contaminated wastewater [18,19]. Media containing rubber granules, chips, and rubber ash have been used. Meanwhile, sorption studies pertaining to ethylbenzene [5], toluene [20], aromatic hydrocarbons, naphthalene [21], and lead ions [22] have been performed. Tyre rubber has been shown to sorb pesticides and nitrates and remove phosphorus from water via an iron precipitation process [23].
The removal of phosphorus from wastewater is crucial because excess phosphorus causes the eutrophication of natural water bodies. In addition to insufficiently treated wastewater, phosphates can enter the environment [24,25]; therefore, methods to reduce the concentration of phosphates in water from wastewater treatment plants are being devised [26]. Urban wastewater treatment plants use activated sludge. Although activated sludge removes organics and suspended solids from wastewater efficiently (>90%), excessive amounts of nitrogen and phosphorus compounds typically remain in the treated water. These nutrients must be removed from effluents using tertiary treatment filters. Various sorbents can be used as filter media, some of which are extremely expensive and others are inefficient owing to their environmental friendliness [27]. Researchers have focused on identifying inexpensive materials suitable as an alternative to activated carbon, which is an expensive adsorbent [[28], [29], [30]]. To effectively clean and manage wastewater, one must identify the appropriate treatment technique. The main methods used are adsorption, flocculation, oxidation, membranes, and filtration, which are performed using polymeric materials such as adsorbents, flocculants, filters, membranes, and polymeric composites [31,32]. Chemically modified polished tyre rubber absorbs fluoride and nitrate ions from water and is suitable for the adsorption of boron, arsenite, arsenate, and organic pollutants [7]. Waste-tyre-based activated carbon produced from rubber effectively removes heavy metals from wastewater [33]. When generating biochar from rubber via pyrolysis, refractory wastewater is formed, which is detrimental to the environment if not discarded appropriately [34]. Information regarding the effectiveness of tyre-based adsorbents in removing phosphorus from water is scarce. The advantage of rubber granules of part-worn tyres over commercial sorbents is that they are readily available. Recently, the sorption properties of rubber have been investigated [1,13]. The capacity of DR particles to adsorb phosphorus from aqueous solutions, particularly when devulcanised tyre granules with a larger surface area are used, remains unclear.
The aim of this study is to evaluate the capacity of the granules of two types of part-worn tyre rubber (non-devulcanised and devulcanised) to adsorb phosphates from aqueous solutions and biologically treated wastewater. A unique patented chemical-mechanical method is applied to devulcanise tyre rubber. Additionally, the authors propose a hypothesis suggesting that DR, owing to its specific structure, exhibit better sorption properties than non-devulcanised rubber (VR). The findings of this study are important for addressing issues associated with the secondary use of tyre rubber.
2. Materials and methods
2.1. Selected materials
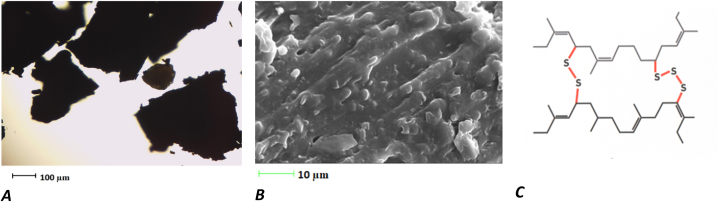
The first material selected was VR from end-of-life truck tyre tread buffers. Truck tyre tread buffings have a high devulcanised natural rubber content. The composition of the buffings used in this study was as follows: rubber content, 50.1% wt; organic additives, 12.7% wt; carbon black, 23.8% wt; and mineral substances and ash, 13.4% wt. The ground tyre rubber was composed of 7.0 ± 0.2 wt% volatile compounds (e.g. plasticisers, processing aids, curing additives, etc.), 56.3 ± 2.1 wt% rubbers, and 36.7 ± 2.2 wt% carbon black + ash [6]. Crushing rubber resulted in the formation of granules (Fig. 1, A–C).
Fig. 1.
Non-devulcanised rubber: A – image observed under an optical microscope (100× magnification); B – image observed under an electronic microscope; C – graphical representation of non-devulcanised crosslinks S–S and S–C of rubber matrix.
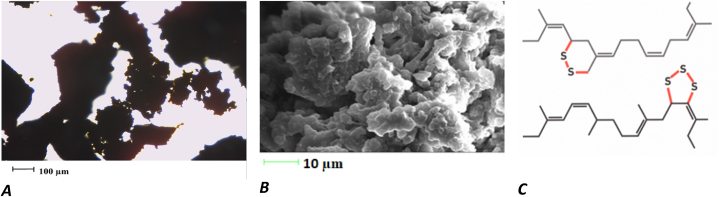
The second selected material was the DR of truck tyre tread buffings (Fig. 2, A–C). DR granules of part-worn tyres were obtained from manufacturers (JSC “Devulco”) using the patented method and material. The use of Devulco technology allows a high level of devulcanisation to be achieved without a significant deterioration in the quality of the polymers of the source rubber. During mechano–chemical processing, the devulcanising agent delocalises the S–S and S–C crosslinks in the rubber matrix, thus rendering the rubber polymers available for recycling. This technology is based on specially streamlined processing equipment, which completely eliminates the use of solvents and high temperatures. Hence, the process involved is economical and reliable. The devulcanisation modifier is protected by the Lithuanian patent LT 6053B “Devulcanization modifier for the production of regenerated rubber powder.” The devulcanization process does not require high-temperature processing; therefore, energy consumption and CO2 emission are low.
Fig. 2.
Devulcanised rubber: A – image observed under an optical microscope (100× magnification); B – image observed under an electronic microscope; C – graphical representation of delocalised crosslinks S–S and S–C of rubber matrix.
Fig. 2 shows that during the mechano–chemical treatment process, the devulcanising agent delocalised crosslinks S–S and S–C of the rubber matrix, the initial image of which is presented in Fig. 1, C. VR rubber presents a 3D structure, which is destroyed during devulcanisation. Subsequently, the S–S bridges are terminated, delocalised, or removed as H2S and SO2. The broken S–S bridges indicate that the DR is homogeneous and exhibits high dispersion (Fig. 2, C).
The rubber granules of the tyres were passed through sieves of appropriate mesh sizes to obtain the fractions of the tested materials (Table 1).
Table 1.
Rubber pellets used in experimental investigation.
| No of the experiment | Devulcanised rubber (DR) pellets |
Non-devulcanised rubber (VR) pellets |
||||
|---|---|---|---|---|---|---|
| Fraction size, mm | Medium volume, mL | Medium mass, g | Fraction size, mm | Medium volume, mL | Medium mass, g | |
| I | 0.3–0.5 | 300 | 127 | 0.3–0.5 | 300 | 119 |
| II | 0.5–1.0 | 300 | 113 | 0.5–1.0 | 300 | 107 |
Qualitative elemental analysis of the rubber granules was performed using energy dispersive X-ray spectroscopy (EDS).
2.2. Research process
2.2.1. Salt and tap water solution K2HPO4 (I)
For the first phosphate removal experiment, an experimental stand was installed in the laboratory (Fig. 3).
Fig. 3.

Schematic illustration of experimental stand: 1 – container of phosphate solution, 2 – filtration columns, 3 – solution supply pump, 4 – solution sampling point (before filtration), 5 – filtrate acquisition point, 6 – overflow tube, 7 – retaining layer, 8 – filter media, 9 – solution distribution pipe to columns.
Container (1), as shown in Fig. 3, was filled with 100 L of potable tap water containing 15.4 g of K2HPO4, which resulted in a PO4–P solution with a concentration of 18.44 mg/L. Two filtration columns (2) with a 4.5 cm diameter were filled with filter media (8) of equal volume (300 mL). The height of the medium was 18 cm. A retaining layer (7) composed of small stones with a height of 3–4 cm was formed at the bottom of the columns. The stones were covered with a sorbing filter medium. The average dry weights of the DR and VR media were 127 and 119 g, respectively.
A pump (3) was applied to supply two filters with phosphate solution at a rate of 0.75 m/h (1.2 L/h). The experiment was performed for 8 h. Samples from the filtrate collection point (5) were obtained every 30 min to measure the PO4–P concentration. This experiment was repeated thrice. The temperatures and pH of the solutions were measured during the experiment. In particular, the temperatures of the prepared solutions were measured using a SevenGo pro SG6 meter (Mettler Toledo, Switzerland), whereas the pH was determined potentiometrically (LST EN ISO 10523:2012).
The PO4–P concentration in the filtrates was determined via MERCK Spectroquant® tests. The investigated samples were dispensed into cuvettes (Hellma) and analyzed using a Genesys 10 Ultraviolet–visible spectrophotometre. Additional measurements were performed by repeating the mixing experiments another three times within 10 min.
The effectiveness of phosphorus removal from the wastewater was calculated using formula (1).
| (1) |
Where:
Ei – effectiveness of removing phosphate phosphorus, %;
C1,i – PO4–P concentration before treatment, mg/L;
C2,i – PO4–P concentration after treatment, mg/L.
2.2.2. Phosphate sorption from biologically treated wastewater
A stand composed of two clear glass columns (4.5 cm diameter) was installed in the laboratory to remove phosphate from wastewater. A retaining layer comprising small stones measuring 3–4 cm in height was formed at the bottom of the columns. The stones were covered with a sorbing filter medium. The height of the medium was 18 cm. The volume of each medium was 300 mL. The average weight of the DR of the partially worn tyres was 113 g, whereas that of the VR was 107 g (Table 1).
One hundred litres of biologically (activated sludge)-treated wastewater from an individual wastewater treatment plant were transferred to the laboratory. The concentration of PO4–P in the wastewater was increased to 17–18 mg/L by adding K2HPO4 to accommodate the highest level of PO4–P in domestic wastewater. The effluent was filtered from top to bottom using a glass vial to such that the effluent is distributed proportionally across all columns and all filter media remain submerged. Each filtration experiment was performed for two days. The filters were operated for 3 h (first day) and 4 h (second day), respectively. There was a 19-h break between these periods. The filtration rate was controlled by fitting valves at the bottom of the columns and was calculated using the volumetric method. Wastewater was filtered at the same rate throughout the both columns. There was a total of three filtration stages at speeds of 0.25, 0.5 and 0.75 m/h, respectively. Filtration samples were obtained every 30 min from the filter outlet (branch pipes at the bottom of the columns). The effectiveness of phosphate removal from the wastewater was calculated using Formula (1). The phosphate phosphorus (PO4–P) concentration was determined spectrophotometrically using ammonium molybdate (LAND 58:2003). This experiment was repeated thrice. Absorbance measurements were performed by pouring the test samples into cuvettes (Hellma) and using a Genesys 10 UV–vis spectrophotometre (Thermo Fisher Scientific, USA) at the required wavelength. The indicator of COD was measured to estimate the concentration of organic matter in the wastewater. Chemical oxygen demand (COD) analysis was performed using the titrimetric method following the standard for wastewater examination (APHA, 2012). The device used was ECO 6 Thermoreactor (VELP Scientifica, Itally). The wastewater temperature and pH of the samples were determined during the experiments. The temperature was recorded using a SevenGo pro SG6 meter (Mettler Toledo, Switzerland), and the pH was determined potentiometrically (LST EN ISO 10523:2012). Qualitative elemental analysis of the rubber granules (used for phosphorus removal) was performed via EDS.
All measurements were performed in triplicate. The data were statistically processed using STATGRAPHICS (2018). One-way Analysis of variance (ANOVA) (at a significance level of p < 0.05) followed by Tukey's post-hoc test was performed to differentiate between the means of the samples.
3. Results and discussion
The results of the SEM and EDS analyses are shown in Fig. 4 (A–D) and Table 2.
Fig. 4.
Rubber granules: A – sample of devulcanised rubber; B – white dot in the sample of devulcanised rubber; C – sample of non-devulcanised rubber; D – white dot in the sample of non-devulcanised rubber.
Table 2.
Elemental composition of rubber granules.
| Element | Weight % |
|||
|---|---|---|---|---|
| DR (average) | DR (white dot) | VR (average) | VR (white dot) | |
| Carbon | 72.4 | 68.027 | 78.36 | 45.149 |
| Oxygen | 20.07 | 8.382 | 15.8 | 26.169 |
| Aluminium | – | – | – | 0.703 |
| Silicon | 3.79 | 2.947 | 0.79 | 0.426 |
| Sulphur | 1.15 | – | 1.36 | 1.009 |
| Calcium | 0.578 | – | 0.675 | – |
| Zinc | 1.37 | 3.488 | 1.812 | 2.287 |
| Iron | 0.66 | 17.155 | 1.212 | 24.256 |
| SUM | 100.1 | 100.0 | 100.0 | 100.0 |
Fig. 4 show the higher dispersion of the DR surface compared with that of the vulcanised case. Table 2 lists the average elemental composition of the DR and VR granules. In all the samples examined samples, carbon was the dominant element. On average, the VR granules contained 6% more carbon and 4% less oxygen than the DR granules. Low amounts (<5%) of silicon, sulphur, calcium, zinc and iron were detected in both types of rubbers. Fig. 4 (B, D) shows the tested material covered with ‘white dots’ containing 14 and 20 times more iron compared with the case of the average DR and VR samples (Table 2). The oxides of aluminium, iron, magnesium, and calcium have been reported to significantly affect the removal of phosphate from water [35]. Furthermore, zinc can attract and sustain phosphorus [36]. The negatively charged phosphate ions are attracted towards the surface, as shown in the reaction M-OH2+ + H2PO4−→ M-H2PO4 +H2O [35].
DR has a larger surface area and higher porosity (Fig. 2, B) than VR; therefore, it retains phosphorus in the micropores more effectively.
The results of the first experiment are shown in Fig. 5, Fig. 6, Fig. 7.
Fig. 5.
PO4–P removal efficiency: 1 – in DR medium; 2 – in VR medium.
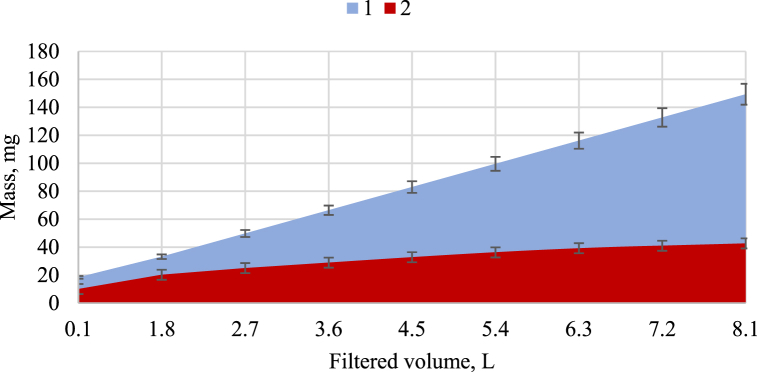
Fig. 6.
Mass of phosphorus entering devulcanised rubber (DR) medium and accumulated in the medium: 1 – mass of phosphorus entering the filter; 2 – mass of phosphorus accumulated in the filter.
Fig. 7.
Mass of phosphorus entering non-devulcanised rubber (VR) medium and accumulated in the medium: 1 – mass of phosphorus entering the filter; 2 – mass of phosphorus accumulated in the filter.
Fig. 5 shows that the filtrate of the DR medium had a lower concentration of PO4–P compared with that of the VR medium, thus rendering the DR medium more effective. Thirty minutes after filtration commenced, the effectiveness of the DR and VR media in removing PO4–P from the aqueous solution reached 93% and 39%, respectively. Thus, the effectiveness of the DR medium was 2.4 times higher. A further increase in the filtration time increased the PO4–P concentration in the filtrates. After 300 min of filtration, the filtration rate decreased to 0.38 m/h owing to clogging in the media. Consequently, the PO4–P removal efficiency deteriorated.
Fig. 6, Fig. 7 show the content of phosphorus accumulated in the VR and DR media during filtration.
The DR rubber medium required 450 min to sorb phosphorus: 13.4 L of the solution was filtered, and 655.3 mg of phosphorus was removed (Fig. 6). The capacity of the medium to accumulate phosphorus was 5.16 mg/g. The VR medium sorbed phosphorus for only 240 min: 8.1 L of the solution was filtered, and 125.3 mg of phosphorus was removed (Fig. 7). The capacity of the medium to accumulate phosphorus was 1.05 mg/g. The ongoing filtration experiment demonstrated that phosphorus desorption began after 270 min and that the phosphorus concentration in the filtrate outlet from the VR medium was higher than that in the filtrate inlet (Fig. 5). Thus, the capacity of the DR to sorb phosphorus was approximately five times higher than that of the VR.
A comparison of the capacity of other sorbents to sorb phosphorus from aqueous solutions is shown as follows: Waste concrete (modified by thermal stress method) indicated a sorption capacity of 1.1–5.0 mg/g [37], pelleted sewage sludge biochar (measuring 1–2 mm) indicated a maximum adsorption capacity of 1.04 mg/g for P [38], 1 g of quartz sand grains covered with an oxide-coated filter medium accumulated 0.84 mg of PO4–P [39], and ground burnt patties (solid waste generated from cooking fuel) indicated a sorption capacity of 0.41 mg/g [35]. Filtration occurred at a relatively high flow rate of 0.75 m/h (1.2 L/h) in this study, and the solution remained in the filter media for only 15 min. This filtration rate was 7–10 times higher than that reported in the literature [35,40]. The lower the filtration rate of the solution and the longer the residence time of the solutions in the filter media, the more complete was the sorption process and the higher was the sorption capacity of the materials. The conditions selected for this study corresponded to the actual filtration parameters in cases where the treated water flowed at high rates. The investigated media, particularly the DR, proved to be sufficiently effective in removing phosphorus from water.
Subsequently, a second experiment using wastewater from an individual wastewater treatment plant was performed [41], where activated sludge was used in the treatment process. The indicators for the wastewater transferred to the laboratory are presented in Table 3.
Table 3.
Wastewater indicators used in experiment (II).
| Sample No |
BOD5 |
COD |
SS |
NKj |
NO2–N |
NO3–N |
Ntot |
Ptot |
NH4–N |
pH |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | – | |||||||||
| 1 | 9.01 | 36.5 | 8.3 | 9.8 | 0.05 | 6.53 | 16.6 | 3.95 | 7.3 | 7.5 |
| 2 | 9.9 | 37.52 | 10.3 | 11.8 | 0.45 | 7.95 | 20.3 | 4.92 | 8.9 | 7.8 |
| Avg. | 9.46 | 37.01 | 9.3 | 10.8 | 0.25 | 7.24 | 18.45 | 4.44 | 8.1 | 7.65 |
Table 3 shows that the concentration of total phosphorus in wastewater did not reach 5 mg/L. However, such a concentration is only characteristic of that in modern facilities, as the phosphorus concentration at the effluent outlets of individual wastewater treatment plants is typically greater; for example, wastewater containing phosphorus concentrations exceeding 20 mg/L may be observed at the outlets of septic tanks [42]. Additionally, authors [35] reported that occasional failures in the system of treatment equipment containing highly polluted domestic wastewater may result in extremely high levels of phosphates (up to 15 mg/L) entering water bodies, which may be catastrophic to the aquatic ecosystem. To account for the unfavourable conditions in actual scenarios, the PO4–P concentration of wastewater used in this study was increased to 17.5 mg/L by adding K2HPO4.
The findings of the second experiment are presented in Fig. 8, Fig. 9.
Fig. 8.
Findings of experiment II: PO4–P concentration in the container and in the filtrate of the columns: 1 - primary PO4–P concentration in the container, 2 - filtrate from DR medium (v = 0.75 m/h), 3 - filtrate from DR medium (v = 0.5 m/h), 4 - filtrate from DR medium (v = 0.25 m/h), 5 - filtrate from VR medium (v = 0.75 m/h), 6 - filtrate from VR medium (v = 0.5 m/h), 7 - filtrate from VR medium (v = 0.25 m/h).
Fig. 9.
Findings of experiment II: COD concentration in the container and in the filtrate of the columns: 1 - primary COD concentration in the container, 2 - filtrate from DR medium (v = 0.75 m/h), 3 - filtrate from DR medium (v = 0.5 m/h), 4 - filtrate from DR medium (v = 0.25 m/h), 5 - filtrate from VR medium (v = 0.75 m/h), 6 - filtrate from VR medium (v = 0.5 m/h), 7 - filtrate from VR medium (v = 0.25 m/h).
As can be seen from Fig. 8, a lower concentration of PO4–P remained in the filtrate from DR medium than in the filtrate from VR medium. COD concentration was also lower after filtration through DR medium (Fig. 9). PO4–P and COD removal efficiency from wastewater depended on the filtration rate (Table 4).
Table 4.
Removal efficiency of PO4–P and COD.
| Tested material | Removal efficiency, % |
|||
|---|---|---|---|---|
| PO4–P |
COD |
|||
| v = 0.25 m/h | v = 0.75 m/h | v = 0.25 m/h | v = 0.75 m/h | |
| DR | 99.4 | 98.3 | 43.2 | 21.6 |
| VR | 47.3 | 22.7 | 15.9 | 0 |
The highest efficiency of phosphorus removal from wastewater (99.4%) was achieved using a DR material when the wastewater filtration rate was 0.25 m/h. The highest COD removal efficiency (43.2%) was also achieved using a DR material. When filtering the wastewater through the VR material at the highest speed (0.75 m/h), the COD concentration did not decrease. The efficiency of pollutant removal increased after a 19-h break, which was made after 3 h of filtration. At the beginning of filtration (v = 0.75 m/h) on the second day (after the break), the PO4–P removal efficiencies by the VR and DR were 2% and 17% higher, respectively, than those at the end of the first day. Thus, one can conclude that sorption occurred for a certain duration after the filtration stopped. During the break, the medium recovered partially and functioned more efficiently the following day.
The elemental composition of the media used is listed in Table 5.
Table 5.
Elemental composition of rubber used.
| Element | Weight % | Weight % σ | Weight % | Weight % σ |
|---|---|---|---|---|
| DR | DR | VR | VR | |
| Carbon | 75.37 | 0.764 | 74.65 | 0.742 |
| Oxygen | 18.57 | 0.675 | 20.51 | 0.734 |
| Sodium | 0.12 | 0.13 | – | – |
| Aluminium | 0.275 | 0.068 | 0.085 | 0.032 |
| Silicon | 0.884 | 0.072 | 0.423 | 0.045 |
| Phosphorus | 0.902 | 0.092 | 0.648 | 0.068 |
| Sulphur | 1.0 | 0.095 | 1.241 | 0.074 |
| Calcium | 1.79 | 0.113 | 1.202 | 0.087 |
| Zinc | 1.09 | 0.187 | 1.236 | 0.186 |
| SUM | 100 | 100 |
Table 5 shows that both media contained phosphorus (0.65%–0.90%). Additionally, the DR medium included sodium. Compared with the data provided in Table 2, the content of calcium present in DR and VR increased. Phosphorus, sodium, and calcium entered the filter media together with the treated wastewater and thus were adsorbed. A decrease in zinc and silicon was observed in DR and VR after wastewater filtration. A similar result was obtained by the authors [43] while simulating a conventional landfill leachate collection system. It was observed that arsenic, cobalt, lead and nickel concentrations were lower in the cell containing tire-chips than in the cell without tire-chips, except iron and zinc. Although leaching of zinc from fillers is undesirable, if tire-chips are used in areas where contamination levels are high, then they can be used as a sorbent for environmental clean-up [43]. On the other hand, activated carbon made from tire rubber has the ability to sorb zinc from wastewater: when the optimum pH is 6, its sorption capacity is 4.995 mg/g [44]. DR medium retained more organic pollutants from wastewater, which was shown by the COD index (Fig. 9, Table 4). According to the authors [45], the sorption to tire crumb rubber (as a composite sorbent) was interpreted as being a combination of adsorption onto carbon black and absorption into the rubber matrix. Tire materials have therefore been proposed as a cost-effective sorbent for the removal of organic pollutants from water [45]. Tire rubber pellets can be used as a potential alternative to conventional gravel or sand in the drainage layer of the collection and treating system of leachate [46]. The rubber used for creating fillers in columns provided for bigger porosity of the layer [47]. The use of rubber pellets would reduce the magnitude of the solid waste disposal problem with its recycling and convert it into a useful material for conserving the environment and reducing pollution.
The wastewater filtering results showed that the efficiency of the DR medium in removing PO4–P was to 5–7 times higher than that of the VR medium. This is because the DR surface structure is more suitable for sorption and has a larger particle surface area. The authors of [48] reported that a bond was formed between phosphorus and carbon during the amidoalkylation of phosphonic carboxylic acids. Compared with sorbents such as dolomite, limestone, opoka, burnt patties, sand, and alkaline P-filters investigated previously [35,42,49], DR proved to be sufficiently effective in removing phosphorus from wastewater. The highest sorption capacity of materials described in the literature was achieved under laboratory conditions using a high concentration of P in artificial solutions. The concentration of P used in other studies was typically higher than that in conventional domestic wastewater [49]. The authors of [50] discovered that polyethylene glycol/chitosan and polyvinyl alcohol/chitosan composites were effective in removing phosphate anions. The sorption capacity of the composites were 74.9 and 46.2 mg/g, respectively, but the ambient pH must be ∼3. In this study, the pH was approximately neutral, similar to the actual conditions for domestic wastewater treatment. The initial concentration of PO4–P in the wastewater was similar to the actual situation and thus not extremely high. Preliminary experiments regarding phosphorus removal from aqueous solutions and wastewater showed that DR is a promising sorbent. DR can be used as filtration layers, filler materials, drainage sealants, wetlands, or plant-bearing media. If wastewater filtered through a DR filter medium is released into nature, then the leaching of toxic substances from the filter medium must be investigated. Reusing part-worn tyre rubber (for P removal) is advantageous because part-worn tyre rubber is waste and its cost is lower compared with those of other raw materials used as adsorbents. Future work should focus on phosphorus recovery from eluates. Hence, further research on DR and the possibilities for its regeneration are recommended.
4. Conclusions
Two types of part-worn rubber tyres were investigated in this study. VR and DR granules measuring 0.3–1.0 mm in diameter successfully sorbed PO4–P from aqueous solution and biologically treated wastewater. Under the test conditions, the filtration of the aqueous solution at a flow rate of 0.75 m/h (1.2 L/h) showed that 1 g of the DR medium accumulated 5.16 mg of phosphorus, i.e. five times more than that accumulated by the VR medium (1.05 mg/g). In terms of PO4–P removal from wastewater at a flow rate of 0.75 m/h, the DR medium was 5–7 times more effective than the VR medium owing to its more suitable surface structure for sorption. The sorption capacity and effectiveness of the DR medium for phosphorus removal were higher compared with those of the tested natural and waste-prepared sorbents. Hence, further investigations into DR media as filter layer, backfill, and drainage-packing materials is recommended.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contribution statement
Tomas Januševičius; Julita Šarko; Aušra Mažeikienė: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors are unable or have chosen not to specify which data has been used.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Tomas Januševičius, Email: tomas.janusevicius@vilniustech.lt.
Julita Šarko, Email: julita.starenko@vilniustech.lt.
Aušra Mažeikienė, Email: ausra.mazeikiene@vilniustech.lt.
References
- 1.Jones I., Zhu M., Zhang J., Zhang Z., Preciado-Hernandez J., Gao J., Zhang D. The application of spent tyre activated carbons as low-cost environmental pollution adsorbents: a technical review. J. Clean. Prod. 2021;312 doi: 10.1016/J.JCLEPRO.2021.127566. [DOI] [Google Scholar]
- 2.Downard J., Singh A., Bullard R., Jayarathne T., Rathnayake C.M., Simmons D.L., Wels B.R., Spak S.N., Peters T., Beardsley D., Stanier C.O., Stone E.A. Uncontrolled combustion of shredded tires in a landfill – Part 1: characterization of gaseous and particulate emissions. Atmos. Environ. 2015;104:195–204. doi: 10.1016/J.ATMOSENV.2014.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad M., Beddu S., Hussain S., Manan A., binti Itam Z., Ahmad M., Beddu S., Hussain S., Manan A., binti Itam Z. Mechanical properties of hot-mix asphalt using waste crumber rubber and phenol formaldehyde polymer. AIMS Mater. Sci. 2019;6:1164–1175. doi: 10.3934/MATERSCI.2019.6.1164. 2019 61164. [DOI] [Google Scholar]
- 4.Formela K. Sustainable development of waste tires recycling technologies – recent advances, challenges and future trends. Adv. Ind. Eng. Polym. Res. 2021;4:209–222. doi: 10.1016/J.AIEPR.2021.06.004. [DOI] [Google Scholar]
- 5.Aisien F.A., Amenaghawon N.A., Akhidenor S.A. Adsorption of ethylbenzene from aqueous solution using recycled rubber from scrap tyre. J. Sci. Res. Reports. 2013;2:497–512. doi: 10.9734/JSRR/2013/4920. [DOI] [Google Scholar]
- 6.Ministry of the Environment of the Republic of Lithuania . 2021. Study of the Use of Tire Waste in the Production of Construction and Other Products, Construction or Other Activities (In Lithuanian: Padangų Atliekų Panaudojimo Statybos Ir Kitų Produktų Gamyboje, Statyboje Ar Kitose Veiklose Studija)https://am.lrv.lt/uploads/am/documents/files/atliekos/studijos-ataskaitos/2021-02-22 Galutine ataskaita.pdf [Google Scholar]
- 7.Formela K. Waste tire rubber-based materials: processing, performance properties and development strategies. Adv. Ind. Eng. Polym. Res. 2022;5:234–247. doi: 10.1016/J.AIEPR.2022.06.003. [DOI] [Google Scholar]
- 8.Fazli A., Rodrigue D. Recycling waste tires into ground tire rubber (GTR)/Rubber compounds: a review. J. Compos. Sci. 2020;4:103–104. doi: 10.3390/JCS4030103. [DOI] [Google Scholar]
- 9.Murillo R., Aylón E., Navarro M.V., Callén M.S., Aranda A., Mastral A.M. The application of thermal processes to valorise waste tyre. Fuel Process. Technol. 2006;87:143–147. doi: 10.1016/J.FUPROC.2005.07.005. [DOI] [Google Scholar]
- 10.Asaro L., Gratton M., Poirot N., Seghar S., Aït Hocine N. Devulcanization of natural rubber industry waste in supercritical carbon dioxide combined with diphenyl disulfide. Waste Manag. 2020;118:647–654. doi: 10.1016/J.WASMAN.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Dorigato A., Rigotti D., Fredi G. Recent advances in the devulcanization technologies of industrially relevant sulfur-vulcanized elastomers. Adv. Ind. Eng. Polym. Res. 2022 doi: 10.1016/J.AIEPR.2022.11.003. [DOI] [Google Scholar]
- 12.Bockstal L., Berchem T., Schmetz Q., Richel A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: a review. J. Clean. Prod. 2019;236 doi: 10.1016/J.JCLEPRO.2019.07.049. [DOI] [Google Scholar]
- 13.Kenawy S.H., Khalil A.M. Reclaiming waste rubber for a green environment. Biointerface Res. Appl. Chem. 2020;11:8413–8423. doi: 10.33263/BRIAC111.84138423. [DOI] [Google Scholar]
- 14.Seghar S., Asaro L., Rolland-Monnet M., Aït Hocine N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019;144:180–186. doi: 10.1016/J.RESCONREC.2019.01.047. [DOI] [Google Scholar]
- 15.Diaz R., Colomines G., Peuvrel-Disdier E., Deterre R. Thermo-mechanical recycling of rubber: relationship between material properties and specific mechanical energy. J. Mater. Process. Technol. 2018;252:454–468. doi: 10.1016/J.JMATPROTEC.2017.10.014. [DOI] [Google Scholar]
- 16.Lapkovskis V., Mironovs V., Goljandin D. Suitability of devulcanized crumb rubber for oil spills remediation. Energy Proc. 2018;147:351–357. doi: 10.1016/J.EGYPRO.2018.07.103. [DOI] [Google Scholar]
- 17.Irtiseva K., Lapkovskis V., Mironovs V., Ozolins J., Thakur V.K., Goel G., Baronins J., Shishkin A. Towards next-generation sustainable composites made of recycled rubber. Cenospheres, and Biobinder, Polym. 2021;13:574. doi: 10.3390/POLYM13040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Morales J., Perales-Pérez O., Román-Velázquez F. 2012. Sorption of Triclosan onto Tyre Crumb Rubber; pp. 831–845. 10.1260/0263-6174.30.10.831.30. [DOI] [Google Scholar]
- 19.Deivasigamani K., Nanjan J., Mani H.P. Recycling of waste gasket rubber granules by bulk CuCl2 and nano CuCl2: removal of Hg(II) ions by recycled rubber granules. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017;473 doi: 10.1098/RSPA.2016.0771. [DOI] [Google Scholar]
- 20.Amenaghawon A., Aisien F., Agho O. Kinetic and Isotherm Studies, Undefined. 2013. Adsorption of toluene by waste tyre rubber granules: effect of operating variables; pp. 427–438. [DOI] [Google Scholar]
- 21.Aisien F.A., Amenaghawon A.N., Adinkwuye A.I. Batch study, equilibrium and kinetics of adsorption of naphthalene using waste tyre rubber granules. J. Xenobiotics. 2014;4:2264. doi: 10.4081/xeno.2014.2264. [DOI] [Google Scholar]
- 22.Fadhil Nassar E., Zageer D., Khlaf M.S. Elimination of lead ions from aqueous environment by waste tires rubber, orient. J. Phys. Sci. 2017;2:81–87. doi: 10.13005/OJPS02.02.06. [DOI] [Google Scholar]
- 23.Park J.K., Ye C. Beneficial use of scrap tires for retardation of pesticide movement in golf courses. Adv. Recycl. Waste Manag. 2016;1 doi: 10.4172/2475-7675.1000106. [DOI] [Google Scholar]
- 24.Weedon C.M., Murphy C., Sweaney G. Establishing a design for passive vertical flow constructed wetlands treating small sewage discharges to meet British Standard EN 12566. Environ. Technol. 2017;38:220–229. doi: 10.1080/09593330.2016.1191549. [DOI] [PubMed] [Google Scholar]
- 25.Bustillo-Lecompte C.F., Mehrvar M. Treatment of actual slaughterhouse wastewater by combined anaerobic–aerobic processes for biogas generation and removal of organics and nutrients: an optimization study towards a cleaner production in the meat processing industry. J. Clean. Prod. 2017;141:278–289. doi: 10.1016/j.jclepro.2016.09.060. [DOI] [Google Scholar]
- 26.Hamisi R., Renman A., Renman G. Performance of an on-site wastewater treatment system using reactive filter media and a sequencing batch constructed wetland. Sustainability. 2019;11:1–16. [Google Scholar]
- 27.Whitton R., Ometto F., Pidou M., Jarvis P., Villa R., Jefferson B. Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015;4:133–148. doi: 10.1080/21622515.2015.1105308. [DOI] [Google Scholar]
- 28.Kasprzyk M., Gajewska M. Phosphorus removal by application of natural and semi-natural materials for possible recovery according to assumptions of circular economy and closed circuit of P. Sci. Total Environ. 2019;650:249–256. doi: 10.1016/J.SCITOTENV.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Kim S., Park Y.H., Lee J.B., Kim H.S., Choi Y.E. Phosphorus adsorption behavior of industrial waste biomass-based adsorbent, esterified polyethylenimine-coated polysulfone-Escherichia coli biomass composite fibers in aqueous solution. J. Hazard Mater. 2020;400 doi: 10.1016/J.JHAZMAT.2020.123217. [DOI] [PubMed] [Google Scholar]
- 30.Pap S., Kirk C., Bremner B., Turk Sekulic M., Shearer L., Gibb S.W., Taggart M.A. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent. Water Res. 2020;173 doi: 10.1016/J.WATRES.2020.115573. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z., Zhou Y., Feng Z., Rui X., Zhang T., Zhang Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers. 2019;11:1252. doi: 10.3390/POLYM11081252. 11 (2019) 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia K., Liu X., Wang W., Yang X., Zhang X. Synthesis of modified starch/polyvinyl alcohol composite for treating textile wastewater. Polymers. 2020;12:289. doi: 10.3390/POLYM12020289. 12 (2020) 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimpe K.M., Ngila J.C., Nomngongo P.N. Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater ABOUT THE AUTHORS. Cogent Eng. 2017;4 doi: 10.1080/23311916.2017.1330912. [DOI] [Google Scholar]
- 34.ran Shen X., Geng C.X., Lv B.Q., Xu W., Xu Y., Zhao H.Z. Tire pyrolysis wastewater treatment by a combined process of coagulation detoxification and biodegradation. Environ. Sci. Ecotechnol. 2021;8 doi: 10.1016/J.ESE.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rout P.R., Bhunia P., Dash R.R. Modeling isotherms, kinetics and understanding the mechanism of phosphate adsorption onto a solid waste: ground burnt patties. J. Environ. Chem. Eng. 2014;2:1331–1342. doi: 10.1016/j.jece.2014.04.017. [DOI] [Google Scholar]
- 36.Soltangheisi A., Rahman Z.A., Ishak C.F., Musa H.M., Zakikhani H. Interaction effects of Phosphorus and zinc on their uptake and 32P absorption and translocation in sweet corn (Zea mays var. Saccharata) grown in a tropical soil. Asian J. Plant Sci. 2014;13:129–135. doi: 10.3923/AJPS.2014.129.135. [DOI] [Google Scholar]
- 37.Liu D., Zhu H., Wu K., Wang F., Zhao X., Liao Q. Understanding the effect of particle size of waste concrete powder on phosphorus removal efficiency. Constr. Build. Mater. 2020;236 doi: 10.1016/J.CONBUILDMAT.2019.117526. [DOI] [Google Scholar]
- 38.Januševičius T., Mažeikienė A., Danila V., Paliulis D. The characteristics of sewage sludge pellet biochar prepared using two different pyrolysis methods. Biomass Convers. Biorefinery. 2022 doi: 10.1007/S13399-021-02295-Y. [DOI] [Google Scholar]
- 39.Mažeikienė A., Vaiškūnaitė R., Šarko J. Sand from groundwater treatment coated with iron and manganese used for phosphorus removal from wastewater. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142915. [DOI] [PubMed] [Google Scholar]
- 40.Rout P.R., Bhunia P., Dash R.R. Effective utilization of a sponge iron industry by-product for phosphate removal from aqueous solution: a statistical and kinetic modelling approach. J. Taiwan Inst. Chem. Eng. 2015;46:98–108. doi: 10.1016/j.jtice.2014.09.006. [DOI] [Google Scholar]
- 41.Biotechnology group . 2021. Wastewater Treatment Plants.https://www.biogroup.lt/ [Google Scholar]
- 42.Vidal B., Hedström A., Herrmann I. Phosphorus reduction in filters for on-site wastewater treatment. J. Water Process Eng. 2018;22:210–217. doi: 10.1016/j.jwpe.2018.02.005. [DOI] [Google Scholar]
- 43.Park J.K., Edil T.B., Kim J.Y., Huh M., Lee S.H., Lee J.J. Suitability of shredded tyres as a substitute for a landfill leachate collection medium. Waste Manag. Res. 2003;21:278–289. doi: 10.1177/0734242X0302100311. [DOI] [PubMed] [Google Scholar]
- 44.Cherono F., Mburu N., Kakoi B. Adsorption of lead, copper and zinc in a multi-metal aqueous solution by waste rubber tires for the design of single batch adsorber. Heliyon. 2021;7 doi: 10.1016/J.HELIYON.2021.E08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hüffer T., Wehrhahn M., Hofmann T. The molecular interactions of organic compounds with tire crumb materials differ substantially from those with other microplastics. Environ. Sci. Process. Impacts. 2020;22:121–130. doi: 10.1039/C9EM00423H. [DOI] [PubMed] [Google Scholar]
- 46.Balegh B., Sellaf H. Treatment of leachate of landfills using filters of ceramic waste and scrap rubber waste, water. Air. Soil Pollut. 2022;233:1–14. doi: 10.1007/S11270-022-06004-X/FIGURES/14. [DOI] [Google Scholar]
- 47.Bazienė K., Vasarevičius S., Siddiqui A.A. Clogging test of landfill leachate drainage using different fillers. J. Environ. Eng. Landsc. Manag. 2012;20:301–306. doi: 10.3846/16486897.2012.736865. [DOI] [Google Scholar]
- 48.Dmitriev M.E., Golovash S.R., Borodachev A.V., Ragulin V.V. Mechanism of phosphorus-carbon bond formation in the amidoalkylation of phosphonous carboxylic acids. J. Org. Chem. 2021;86:593–600. doi: 10.1021/ACS.JOC.0C02259. [DOI] [PubMed] [Google Scholar]
- 49.Johansson Westholm L. Substrates for phosphorus removal - potential benefits for on-site wastewater treatment? Water Res. 2006;40:23–36. doi: 10.1016/j.watres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Rajeswari A., Amalraj A., Pius A. Removal of phosphate using chitosan-polymer composites. J. Environ. Chem. Eng. 2015;3:2331–2341. doi: 10.1016/J.JECE.2015.08.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.