Abstract
Rationale
Routine spontaneous awakening and breathing trial coordination (SAT/SBT) improves outcomes for mechanically ventilated patients, but adherence varies. Understanding barriers to and facilitators of consistent daily use of SAT/SBT (implementation determinants) can guide the development of implementation strategies to increase adherence to these evidence-based interventions.
Objectives
We conducted an explanatory, sequential mixed-methods study to measure variation in the routine daily use of SAT/SBT and to identify implementation determinants that might explain variation in SAT/SBT use across 15 intensive care units (ICUs) in urban and rural locations within an integrated, community-based health system.
Methods
We described the patient population and measured adherence to daily use of coordinated SAT/SBT from January to June 2021, selecting four sites with varied adherence levels for semistructured field interviews. We conducted key informant interviews with critical care nurses, respiratory therapists, and physicians/advanced practice clinicians (n = 55) from these four sites between October and December 2021 and performed content analysis to identify implementation determinants of SAT/SBT use.
Results
The 15 sites had 1,901 ICU admissions receiving invasive mechanical ventilation (IMV) for ⩾24 hours during the measurement period. The mean IMV patient age was 58 years, and the median IMV duration was 5.3 days (interquartile range, 2.5–11.9). Coordinated SAT/SBT adherence (within 2 h) was estimated at 21% systemwide (site range, 9–68%). ICU clinicians were generally familiar with SAT/SBT but varied in their knowledge and beliefs about what constituted an evidence-based SAT/SBT. Clinicians reported that SAT/SBT coordination was difficult in the context of existing ICU workflows, and existing protocols did not explicitly define how coordination should be performed. The lack of an agreed-upon system-level measure for tracking daily use of SAT/SBT led to uncertainty regarding what constituted adherence. The effects of the COVID-19 pandemic increased clinician workloads, impacting performance.
Conclusions
Coordinated SAT/SBT adherence varied substantially across 15 ICUs within an integrated, community-based health system. Implementation strategies that address barriers identified by this study, including knowledge deficits, challenges regarding workflow coordination, and the lack of performance measurement, should be tested in future hybrid implementation-effectiveness trials to increase adherence to daily use of coordinated SAT/SBT and minimize harm related to the prolonged use of mechanical ventilation and sedation.
Keywords: spontaneous awakening trial, spontaneous breathing trial, implementation, coordination, telecritical care
Invasive mechanical ventilation (IMV) with sedation is a lifesaving measure for patients in respiratory failure (1). However, the prolonged use of IMV and continuous sedation can also cause harm, including lung injury, pneumonia, and delirium, contributing to lasting impairment known as post–intensive care syndrome (2–11). Daily interruptions in sedation (spontaneous awakening trials [SATs]) and daily spontaneous breathing trials (SBTs) separately decrease the duration of IMV and intensive care unit (ICU) length of stay without compromising patient comfort or safety (12, 13). Coordinating SAT and SBT (SAT/SBT) further increases ventilator-free days, decreases ICU length of stay, improves mortality, and reduces ventilator-associated events (14, 15). SAT and SBT are included in the ICU Liberation Bundle (ABCDEF Bundle), a collection of evidence-based interventions designed to hasten liberation from the ICU (16, 17).
Although Girard and colleagues demonstrated the efficacy of a paired SAT/SBT protocol in the Awakening and Breathing Controlled Trial in 2008, attempts to implement the protocol in real-world settings have yielded variable results (15, 18–22). Recent surveys of SAT/SBT implementation in the context of the ABCDEF Bundle suggest widespread uptake remains suboptimal (23, 24). A recent systematic literature review highlighted the need to examine the barriers to and facilitators (implementation determinants) of ABCDEF bundle components separately (25). The purpose of this study was to measure variation in adherence to consistent daily coordinated SAT/SBT use across 15 ICUs in an integrated, community- based health system and to identify implementation determinants through key informant interviews with ICU clinicians that might explain site-level differences in adherence.
Methods
Study Design and Setting
In preparation for a type II hybrid implementation-effectiveness trial (TEACH [Telehealth-Enabled, Real-Time Audit and Feedback for Clinician Adherence]; ClinicalTrials.gov identifier NCT05141396), we conducted a sequential, explanatory mixed-methods investigation of implementation context from January 2021 to March 2022 at 15 ICUs in Utah and Idaho that are part of Intermountain Health (Intermountain), a not-for-profit, integrated, community-based health system in the western United States. The study coincided with periods of strained ICU capacity during the coronavirus disease (COVID-19) pandemic with approximately 4,700 adult patients receiving IMV in 2021 (26). This study was approved by Intermountain’s institutional review board (IRB 1051681).
Critical care operations at Intermountain rely on an interprofessional team-based care model. In-scope ICUs were staffed by approximately 150 employed physicians and advanced practice providers, 750 critical care nurses, and 350 respiratory therapists (RTs). Larger ICUs were staffed by intensivists, and smaller ICUs were staffed by hospitalists. A remote telemedicine team of critical care physicians, nurses, and RTs supported bedside patient care by reviewing patient data, clarifying policies and procedures, and guiding bedside teams in developing evidence-based treatment plans with an emphasis on supporting smaller ICUs staffed by hospitalists. Critical care teams used a computerized ventilator protocol within the systemwide electronic health record (EHR) (Cerner Corporation) to support care for IMV patients (27).
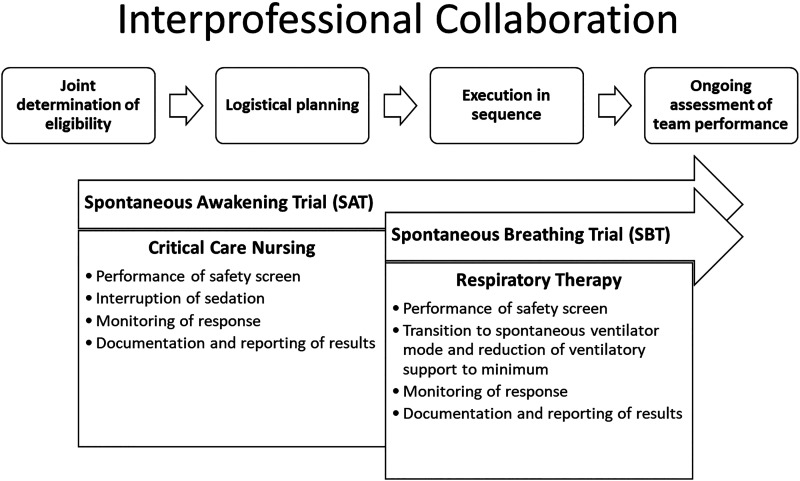
In 2018, Intermountain implemented the ABCDEF Bundle, including an SAT/SBT protocol adopted at all facilities (Figure 1) (17). The ABCDEF Bundle implementation strategies included didactic education, communications, and executive leadership emphasis. A workflow diagram job aid link was embedded with the EHR orders. Automated performance tracking was in place for other ventilator initiatives but not for SAT/SBT.
Figure 1.
Desired behaviors for a spontaneous awakening trial (SAT), a spontaneous breathing trial (SBT), and SAT/SBT coordination. Interprofessional collaboration between critical care nursing and respiratory therapy is needed to perform a coordinated SAT/SBT and includes 1) joint determination of eligibility, 2) logistical planning, 3) sequenced execution, and 4) ongoing assessment of team performance.
Measuring SAT/SBT Adherence
Site- and patient-level characteristics were summarized from January 1, 2021, to June 30, 2021. The patient study population included patients ⩾16 years old, excluding solid organ donors and patients receiving IMV for <24 hours. To measure site variation in adherence to coordinated SAT/SBT, we calculated the proportion of eligible patient ventilator-days associated with this population with an SAT, SBT, and SAT/SBT performed. SAT was considered performed if the nurse documented the performance or the patient was extubated. SBT was considered performed if the RT documented performance or the patient was extubated. Successful SAT/SBT coordination required SAT eligibility and performance of an SAT followed by an SBT within 2 hours (described in Table E1 and Figure E1 in the data supplement). Proportions were calculated for the measurement period, and sites were stratified by relative adherence level in tertiles: high, medium, and low. Sites were selected on the basis of tertile, geography, facility size, and site operational readiness as determined through conversations with local site ICU leaders.
Key Informant Interviews and Qualitative Data Analysis
To conduct key informant interviews, we developed tailored interview guides (Figure E2) for each professional role (nurse, RT, and physician/advanced practice provider) using a published methodology (28–33). We validated the interview guides through cognitive testing with content experts, and questions were refined on the basis of participant feedback (34). A field team of trained, experienced qualitative researchers (G.H.O., P.M.G., and A.J.K.) conducted semistructured interviews from October to December 2021 using a purposive sample of 10–20 key informants per site and a role-based criterion to ensure interprofessional representation. Individuals within each role varied in terms of years of experience and attitudes and beliefs regarding SAT/SBT. Local leaders recruited participants through e-mail or direct conversation. Participation was voluntary. Informed consent was obtained for interview participation and recording. Interviews were conducted in person, lasted 30 minutes, and were recorded for deidentified transcription. Interviews were continued at each site until thematic saturation was reached (35).
The field team analyzed the interview content using a hybrid deductive-inductive approach, incorporating both conventional and directed content analysis (36–38). Three experienced researchers trained in qualitative coding (G.H.O., P.M.G., and A.J.K.) independently coded interview content using open coding at the question level. Discrepancies were then discussed between coders until consensus was reached. Identified implementation determinants were summarized by domain, site, and clinical role using the Consolidated Framework for Implementation Research (CFIR) (39). The CFIR framework consists of five domains used to categorize determinants of implementation effectiveness, including individual (clinical characteristics), intervention (SAT/SBT protocol characteristics), inner setting (organization context), external setting, and implementation (prior implementation process).
Results
From January 1 to June 30, 2021, the 15 ICUs had 9,305 patients ⩾16 years old, excluding solid organ donors, with 1,901 patients on IMV for ⩾24 hours (20%) (Figure E1). The patient population receiving IMV for ⩾24 hours was 63% male. The mean IMV patient age was 58 years, and the median IMV duration was 5.3 (interquartile range [IQR], 2.5–11.9) days. The median patient Acute Physiology and Chronic Health Evaluation Score was 18 (IQR, 13–24), with a median Charlson comorbidity index count per patient of 3 (IQR, 1–6) (Table 1). SAT/SBT adherence for the 15 sites was 21% (site range, 9–68%) (Table 2).
Table 1.
Characteristics of patients receiving invasive mechanical ventilation for ⩾24 h at study sites from January 1, 2021, to June 30, 2021
| Characteristics | Patients |
|---|---|
| (N = 1,901) | |
| Age, mean, yr (SD) | 58 (17) |
| Age groups, n (%) | |
| 16–17 yr | 6 (<1) |
| 18–54 yr | 704 (37) |
| 55–80 yr | 1,063 (56) |
| 80+ yr | 128 (7) |
| Male, n (%) | 1,206 (63) |
| Ethnicity, n (%) | |
| Hispanic | 254 (13) |
| Not Hispanic | 1,516 (80) |
| Unknown | 131 (7) |
| Race, n (%) | |
| Black | 32 (2) |
| White | 1,592 (84) |
| Other | 159 (8) |
| Unknown | 118 (6) |
| Duration of invasive mechanical ventilation | |
| Mean, d (range) | 9.6 (1–157) |
| Median, d (IQR) | 5.3 (2.5–11.9) |
| Charlson comorbidities, median (IQR) | 3 (1–6) |
| Acute Physiology and Chronic Health Evaluation Score, median (IQR) | 18 (13–24) |
Definition of abbreviations: IQR = interquartile range; SD = standard deviation.
Table 2.
Characteristics of study sites (N = 15) from January 1, 2021, to June 30, 2021
| Site | ICU Type | Hospital Designation | Staffed by | Beds | No. of ICU Patients* | No. of Patients Receiving IMV for ⩾24 h† | % ICU Patients Receiving IMV for ⩾24 h | Estimated % Patient Ventilator-Days with Performance of |
Performance Tertile | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinated SAT/SBT | SAT | SBT | |||||||||
| A | General | Community | I | 16 | 428 | 128 | 30% | 9% | 42% | 19% | 1-Low |
| B‡ | Respiratory | Level 1 trauma§ | I | 16 | 345 | 97 | 28% | 11% | 43% | 35% | 1-Low |
| C | General | Level 2 trauma | I | 16 | 1,047 | 260 | 25% | 16% | 24% | 49% | 1-Low |
| D‡ | General | Level 2 trauma | I | 36 | 1,584 | 390 | 25% | 17% | 42% | 37% | 1-Low |
| E | Thoracic | Level 1 trauma§ | I | 23 | 619 | 189 | 31% | 18% | 50% | 23% | 1-Low |
| F | Coronary | Level 1 trauma§ | I | 16 | 711 | 94 | 13% | 20% | 43% | 44% | 2-Medium |
| G‡ | General | Level 2 trauma | I | 32 | 1,443 | 258 | 18% | 22% | 44% | 48% | 2-Medium |
| H | General | Community | H-T | 4 | 107 | 3 | 3% | 25% | 50% | 25% | 2-Medium |
| I | Neurologic | Level 1 trauma§ | I | 16 | 598 | 99 | 17% | 32% | 44% | 74% | 2-Medium |
| J | General | Community | H-T | 6 | 181 | 11 | 6% | 33% | 50% | 100% | 2-Medium |
| K | Medical/surgical | Level 1 trauma§ | I | 25 | 1,118 | 291 | 26% | 39% | 71% | 70% | 3-High |
| L‡ | General | Community, level 3 trauma | H-T | 14 | 418 | 26 | 6% | 61% | 83% | 65% | 3-High |
| M | General | Community | H-T | 8 | 367 | 15 | 4% | 63% | 75% | 88% | 3-High |
| N | General | Community | H-T | 8 | 229 | 10 | 4% | 67% | 67% | 67% | 3-High |
| O | General | Community, level 3 trauma | H-T | 6 | 110 | 15 | 14% | 68% | 100% | 100% | 3-High |
| Total | 242 | 9,305 | 1,901 | 20% | 21% | 45% | 46% | ||||
Definition of abbreviations: H-T = hospitalist/telehealth; I = intensivist; ICU = intensive care unit; IMV = invasive mechanical ventilation; SAT/SBT = coordinated spontaneous awakening and breathing trials.
Includes all ICU patients ⩾16 years old, excluding solid organ donors.
Includes all patients receiving IMV for ⩾24 hours, regardless of individual ventilator-day eligibility.
Interview site.
Single hospital with five separate ICUs.
Fifty-five ICU professionals participated in semistructured interviews at four sites (Table 3), representing 40% of ICU beds and more than half of eligible SAT/SBT patient ventilator-days. The four geographically distributed sites (average distance between sites, 200 miles; range, 38–382 miles) included a level 1 trauma and tertiary referral center, two level 2 trauma and regional referral centers, and a small rural level 3 trauma center. We did not observe meaningful differences in implementation determinants across interview sites based on adherence, apart from the small rural site, where low mechanical ventilation volumes and staff experience working together appeared to enable coordination. The implementation determinants are organized below according to the five CFIR domains.
Table 3.
Characteristics of clinician participants in key informant interviews (N = 55) from October 1 to December 31, 2021
| Characteristics | Clinician Participants |
|---|---|
| (N = 55), n (%) | |
| Male | 30 (55) |
| Hispanic | 5 (9) |
| Race | |
| White | 51 (93) |
| Asian | 3 (5) |
| Native Hawaiian/other Pacific Islander | 1 (2) |
| Role | |
| Critical care nurse | 24 (44) |
| Respiratory therapist | 20 (36) |
| Physician/advanced practice clinician | 11 (20) |
| ICU site | |
| Site B: level 1 trauma center | 16 (29) |
| Site D: level 2 trauma center | 17 (31) |
| Site G: level 2 trauma center | 15 (27) |
| Site L: level 3 trauma center | 7 (13) |
SAT/SBT Protocol Characteristics
Clinicians at all sites acknowledged that SAT/SBT minimized the duration of IMV and continuous sedation (Table E2).
“If we can wake them up and get them extubated …, then the less the chance for them to end up with some kind of a hospital acquired infection.” — Nurse (site B)
“The goal pretty much all the time is to get a patient off the ventilator. The longer they’re on the ventilator, the more complications arise.” — RT (site B)
Routine assessments were superior to clinical judgment alone for identifying readiness for extubation, and SAT/SBT was generally beneficial.
“If we don’t purposely do these tests, we can sometimes miss people who actually are able to breathe enough on their own [and are ready] to be extubated.” — Physician (site B)
“When it’s coordinated, it takes less time.… If there’s an issue at any point, we’re both going to see it, and we’re both going to do what we need to … to adjust.” — Nurse (site L)
However, conducting an SAT or SBT often required remaining with the patient for an extended, unpredictable time frame, which limited clinician ability to complete other required tasks.
“You can’t turn it off or half it and then leave. You have to stay in the room. You’ve got to keep your eyes on it.” — Nurse (site G)
“You can’t really put a timeframe on [an SBT] … realistically, timeframe wise…. I would say everything’s at least a half hour, and if they do bad, then it takes more time.” — RT (site G)
Some clinicians did not believe coordination was always necessary to achieve the clinical benefits and the interdependence it fostered was disruptive to routine care.
“In my experience, sometimes it doesn’t matter if a patient’s sedated or not. They can do just fine on a breathing trial.” — RT (site G)
“[Coordination] could slow down the process … if you’re not ready, or the nurse isn’t ready, then somebody is waiting, and … patient care is getting delayed.” — RT (site G)
Absent a systemwide standard for how to coordinate SAT/SBT, approaches varied by site.
“I make sure I know who my RTs are for the specific rooms … if they just so happen to be rounding at the same time as I am, then I’ll go in and tell them.” — Nurse (site B)
“There’s often times when the nurses don’t communicate or the doctor comes by and turns off the sedation, and I’m busy doing something else and don’t have time to get there immediately.” — RT (site G)
The current design of SAT and SBT documentation within the EHR slowed data entry and made information retrieval cumbersome.
“It’s just really confusing on the documentation because it says, ‘Was a sedation vacation performed?’ It’s a ‘yes’ or ‘no.’ And when the patient’s not on any sedation or a paralytic, there should be like a ‘not applicable.’” — Nurse (site B)
“Are SATs and SBTs documented in [the EHR]? Yes, I’ve seen them.… Now, do I go in to check it? No, it’s still too much of a hassle.” — Physician (site G)
ICU Clinician Characteristics
Nurses and RTs were generally familiar with the concept of waking patients and allowing them to breathe on their own and the process for accomplishing that (Table E3). However, nurses varied in how they defined an SAT.
“I usually will turn off that sedation cold turkey.” — Nurse (site B)
“I will usually just halve their sedation.… I’ll keep an eye on them for half hour, 45 minutes.… Then, I’ll go down more.” — Nurse (site B)
Many RTs conflated an SBT with other spontaneous breathing modes, including weaning.
“So, we’ll push a couple buttons on the ventilator, and we’ll switch them to spontaneous mode and give them pressure support to start with…. As long as they’re breathing fine [and] … all their numbers on the ventilator are good, we’re kind of set for a couple hours until we need to make another change.” — RT (site G)
Many nurses and RTs demonstrated limited understanding of the other’s professional role knowledge domain, potentially impeding the development of a shared mental model for SAT/SBT performance.
“That I don’t know, because RTs come in to do their magical adjustments.” — Nurse (site G)
Awake and spontaneously breathing patients required more attention than sedated patients receiving IMV, which influenced motivations.
“Obviously, a sedated patient is easier to take care of than a non-sedated patient…. I mean it’s a dream to have a vented sedated patient that doesn’t have any family, right?” — Nurse (site D)
“It’s easier to care for the patient when they’re snowed.… And I hate to even say it that way. It’s not good for their mental state. It’s not good for anything after the ICU or their home life afterwards. But it’s much easier to care for them here for your 12 hours.” — RT (site G)
External Environment Characteristics
The COVID-19 pandemic was the dominant external characteristic and influenced patient acuity at all sites, requiring more intensive care and added activities, such as prone positioning (Table E4). Workforce constraints resulted in increased patient-to-clinician ratios. These capacity strains resulted in deprioritization of SAT/SBT for some patients. In addition, many patients with COVID-19 with severe acute respiratory distress syndrome remained ineligible for SAT/SBT for extended periods, limiting clinician experience with SAT/SBT and extubation.
“Pre-COVID, I feel like we were really big into our ABCDEF bundle, which included SAT and SBT to help produce the best outcomes for our patients.” — Nurse (site B)
“Especially in these COVID times where I have sometimes quite a few ventilators at one time, sometimes it’s hard to get to every single one because some [patients] are maybe more critical than others.… [Y]ou kind of have to triage at that point.” — RT (site B)
Organizational Context
Clinicians indicated that consistent daily SAT/SBT performance was challenging, given existing workflows (Table E5). Patient assignments did not always overlap across roles, leaving nurses and RTs to coordinate with multiple counterparts, with each additional individual’s schedule increasing logistical complexity. Nurses cared for 1–3 patients in close proximity, whereas RTs cared for up to 12 patients, sometimes spread across multiple inpatient settings. Nurses structured their days around ICU rounds and medication administration. RTs structured their days around scheduled patient-ventilator assessments required by the computerized ventilator protocol.
“It’s hard. In my world, there’s three or four different nurses, three different doctors.” — RT (site G)
“It’s so hard that we all have different schedules.… It’s just a lot of moving parts…. We don’t just have a set schedule that we can just follow to a book, that’s not real world.” — RT (site G)
Clinicians described many competing priorities that could take precedence over SAT/SBT and disrupt coordination plans.
“We’re flipping people over, back and forth, morning and night. And between those hours we’re doing physical therapy and occupational therapy and we’re helping them out with that. And if we have any transfers in the middle of the day, it’s very difficult to get in and do SBTs.” — RT (site B)
Telecommunication devices, including cellular phones and wireless communication badges, facilitated informal communication. Clinicians working in hospitalist-staffed units described how the remote telemedicine team facilitated care for mechanically ventilated patients.
“It’s a good thing … to have some extra eyes in critical care.… They help prompt us to do things for the care of patient.… They do help identify if we’re falling out of protocol, and they’ll call and communicate that with us.” — RT (site G)
Staffing and workload issues impacted routine performance of SAT/SBT, fueled in part by the pandemic. As a result of staffing shortages, Intermountain began employing traveling nurses during the pandemic, impacting knowledge and buy-in to institutional protocols.
“One thing recently that’s been an issue is acuity and how thin we are staffing wise. So, it’s very dangerous to be tripled and try and do a sedation vacation on a patient. Because you can’t have eyes on them as often as you would need to.” — Nurse (site B)
“Our workloads prohibit us from doing some of those things that we want to be doing…. If you have three or four really, really sick patients, you’re having to triage.” — RT (site G)
Prior Implementation Process Characteristics
The previous implementation of the ICU Liberation Bundle (ABCDEF Bundle) raised awareness of SAT/SBT through education efforts, but the success or failure of past implementation experiences influenced their engagement (Table E6).
“I didn’t really understand it [SAT/SBT] fully until we did an ABCDE bundle [training], and that was maybe two or three years ago…. I feel like our team does really well when there’s a good, thorough roll out.” — RT (site G)
“Five years ago, the roll out [of the computerized ventilator protocol] was just a little bit weak.… We had to do a little damage control … and roll everything out again.” — RT (site G)
Discussion
This mixed-methods study, including quantitative data on 1,901 patients invasively mechanically ventilated for ⩾24 hours at 15 ICU sites and 55 key informant interviews across 4 sites, details implementation determinants of consistent daily SAT/SBT use, filling an identified gap in the literature (25). The results generally corroborate the findings of previous research regarding implementation of the ABCDEF Bundle, extend our knowledge of the specific determinants that influence consistent performance of SAT/SBT, and provide actionable insights for tailoring implementation strategies to increase SAT/SBT adherence as part of a larger implementation trial we are conducting (25, 40, 41).
Participants were generally familiar with routinely using SAT and SBT but differed in their detailed understanding of the protocol. Consistent with other studies, we found some nurses preferred to gradually reduce the rate of sedative infusion rather than pause it completely, because of perceived safety concerns (18, 40, 42). Some RTs conflated SBT with spontaneous breathing modes and weaning ventilatory support generally. This was due, at least in part, to the use of ventilator weaning protocols within our system that transition patients from a volume control mode to a pressure support mode, which the patient may continue for several days before extubation (43). Implementation strategies that evaluate existing field practices and provide education and training for frontline teams may be required to address this variation and clarify expectations.
Routinized interprofessional coordination was inherently difficult, even when the tasks requiring coordination were understood by the clinical team. The lack of overlapping patient assignments resulted in clinicians having to coordinate with multiple counterparts. Differences in daily work routines across roles resulted in scheduling conflicts. Unpredictable events frequently disrupted plans. This supports previous research linking the ability to anticipate team members’ behaviors with the ABCDEF Bundle implementation (44). Other institutions have proposed standard times for conducting SAT/SBT (21). Although this may enhance routinization, this approach may require frequent adaptation, given the unpredictability of patient needs, impacting staff availability.
Organizations can strengthen existing protocols by establishing clear definitions of SAT/SBT consistent with clinical standards, providing explicit instructions regarding coordination communication, and designating process ownership (45, 46). Limiting the number of SAT/SBT required per day to the minimum necessary to achieve the clinical benefit can minimize workflow disruption. Previous descriptions of SAT/SBT protocol implementation have noted the importance of tracking performance to achieve high adherence (15, 19). Most delivery systems rely on their existing EHR for data capture, in which documentation of SAT/SBT can vary meaningfully in both form and content. Implementation strategies may require redesigning specific fields to capture clinical activities in a format useful for performance measurement. A task force with clinical, technological, and implementation expertise may be required to develop feasible metrics that have systemwide agreement for measuring performance. Creation of an automated electronic dashboard offers advantages (22).
The effects of the COVID-19 pandemic impacted routine daily execution of SAT/SBT at all facilities, consistent with reports from other systems (47, 48). Sustained heavy workloads were associated with significant staff turnover, constraining nurse and RT labor supply, which led to the introduction of rotating travelers. Clinicians described adapting to these circumstances by making deliberate decisions regarding prioritization of daily work and clinical initiatives, shedding light on the practical limitations of routine daily SAT/SBT relative to other evidence-based practices. SAT/SBT can be labor-intensive to implement routinely. Efforts to implement may not acknowledge and address practical resource limitations in sustained daily use across all eligible patients. Appropriate adaptations may exist at times, given clinical circumstances, and should be considered in setting performance standards (49, 50). Virtual teaming through telemedicine is one additional approach for expanding workforce capacity. Telemedicine approaches have been shown to be effective for implementation of evidence-based practice initiatives (51).
Strengths and Limitations
This study has limitations. Although useful for implementation purposes, this study design cannot establish causal relationships. Our measurements of site-level adherence may have misestimated actual adherence behavior, given the reliance on clinical documentation within the EHR and the fact that measurements were taken during the COVID-19 pandemic, when patient volumes were at capacity systemwide. We did not examine implementation determinants at all sites, nor did we interview all frontline clinicians within selected units. However, site selection based on differences in size and location represented more than 50% of eligible SAT/SBT patient ventilator-days during the study period. Although we compiled a large sample of key informants, participants were selected by local site leaders on the basis of availability. We provided local leaders with specific instructions for identifying participants and allowed additional interviews after the single-day site visit to ensure thematic saturation. Although responses appeared frank, we cannot rule out the possibility that participant statements were influenced by the researchers’ institutional alignment as health system employees. The generalizability of study results may be influenced by system- and site-level characteristics, including patient volume, geographic reach, and the patient and clinician study population.
The study has several strengths, including the use of adherence data to evaluate performance and guide sampling, qualitative methodological rigor, a large sample size with diverse viewpoints across locations and roles, and a theory-informed instrument to facilitate questioning. This study follows an unsuccessful effort in 2018 to implement SAT/SBT with high adherence as part of the ABCDEF Bundle. The results of this study suggest that future implementation strategies to achieve high adherence should 1) ensure common definitions for SAT, SBT, and coordination; 2) optimize workflows across professional roles before implementation with potential for local adaptation; 3) implement a reliable and visible measurement system; and 4) consider the use of enabling technologies such as telemedicine to facilitate interprofessional coordination. These results are actionable and will inform a type II hybrid implementation-effectiveness trial (NCT05141396) to measure the effect of these implementation strategies on adherence to SAT/SBT and clinical outcomes.
Acknowledgments
Acknowledgment
G.H.O. acknowledges the support of the Stanford-Intermountain Fellowship in Population Health, Delivery Science, and Primary Care. The authors acknowledge the participation and support of site leaders and frontline care teams who work tirelessly to improve patients’ lives.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (U01HL159878); the National Center for Advancing Translational Sciences (KL2TR002539) of the National Institutes of Health; and the National Science Foundation Future of Work at the Human Technology Frontier (2026498). The views expressed in this article do not communicate an official position of Intermountain Health, the National Institutes of Health, or the National Science Foundation.
Author Contributions: G.H.O., P.M.G., L.L., I.D.P., C.K.G., S.J.S., S.M.A., R.S., and A.J.K. contributed to the conception and design of this study. G.H.O., P.M.G., J.R.J., A.J.K., C.W., L.C., C.J., and D.W., made specific contributions to data acquisition and analysis. All authors participated in the initial drafting and revisions of the work, approved the final revised version, and accept accountability for the overall integrity of the research process and the article.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Slutsky AS. Mechanical ventilation. Chest . 1993;104:1833–1859. doi: 10.1378/chest.104.6.1833. [DOI] [PubMed] [Google Scholar]
- 2. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis . 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med . 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 4. Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med . 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 5. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med . 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 6. American Thoracic Society, European Society of Intensive Care Medicine, Societé de Réanimation de Langue Française. International consensus conferences in intensive care medicine: ventilator-associated lung injury in ARDS. This official conference report was cosponsored by the American Thoracic Society, the European Society of Intensive Care Medicine, and the Societé de Réanimation de Langue Française, and was approved by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med . 1999;160:2118–2124. doi: 10.1164/ajrccm.160.6.ats16060. [DOI] [PubMed] [Google Scholar]
- 7. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest . 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 8. Samuelson K, Lundberg D, Fridlund B. Memory in relation to depth of sedation in adult mechanically ventilated intensive care patients. Intensive Care Med . 2006;32:660–667. doi: 10.1007/s00134-006-0105-x. [DOI] [PubMed] [Google Scholar]
- 9. Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MA, et al. Sedation Practice in Intensive Care Evaluation (SPICE) Study Group investigators Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med . 2013;39:910–918. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med . 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 11. Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med . 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med . 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 13. Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med . 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 14. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet . 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 15. Klompas M, Anderson D, Trick W, Babcock H, Kerlin MP, Li L, et al. CDC Prevention Epicenters The preventability of ventilator-associated events. Am J Respir Crit Care Med . 2015;191:292–301. doi: 10.1164/rccm.201407-1394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med . 2017;45:321–330. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality. Coordinated spontaneous awakening and breathing trials protocol. 2017 [accessed 2023 July 13]. Available from: www.ahrq.gov/hai/tools/mvp/modules/technical/sat-sbt-protocol.html.
- 18. Kher S, Roberts RJ, Garpestad E, Kunkel C, Howard W, Didominico D, et al. Development, implementation, and evaluation of an institutional daily awakening and spontaneous breathing trial protocol: a quality improvement project. J Intensive Care Med . 2013;28:189–197. doi: 10.1177/0885066612444255. [DOI] [PubMed] [Google Scholar]
- 19. Jones K, Newhouse R, Johnson K, Seidl K. Achieving quality health outcomes through the implementation of a spontaneous awakening and spontaneous breathing trial protocol. AACN Adv Crit Care . 2014;25:33–42. doi: 10.1097/NCI.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 20. Khan BA, Fadel WF, Tricker JL, Carlos WG, Farber MO, Hui SL, et al. Effectiveness of implementing a wake up and breathe program on sedation and delirium in the ICU. Crit Care Med . 2014;42:e791–e795. doi: 10.1097/CCM.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stollings JL, Foss JJ, Ely EW, Ambrose AM, Rice TW, Girard TD, et al. Pharmacist leadership in ICU quality improvement: coordinating spontaneous awakening and breathing trials. Ann Pharmacother . 2015;49:883–891. doi: 10.1177/1060028015582050. [DOI] [PubMed] [Google Scholar]
- 22. Anderson BJ, Do D, Chivers C, Choi K, Gitelman Y, Mehta SJ, et al. Clinical impact of an electronic dashboard and alert system for sedation minimization and ventilator liberation: a before-after study. Crit Care Explor . 2019;1:e0057. doi: 10.1097/CCE.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller MA, Govindan S, Watson SR, Hyzy RC, Iwashyna TJ. ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Ann Am Thorac Soc . 2015;12:1066–1071. doi: 10.1513/AnnalsATS.201501-066OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, et al. Worldwide survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) bundle. Crit Care Med . 2017;45:e1111–e1122. doi: 10.1097/CCM.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costa DK, White MR, Ginier E, Manojlovich M, Govindan S, Iwashyna TJ, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients. Chest . 2017;152:304–311. doi: 10.1016/j.chest.2017.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utah State Government. https://coronavirus-dashboard.utah.gov/hosp.html
- 27. Peltan ID, Knighton AJ, Barney BJ, Wolfe D, Jacobs JR, Klippel C, et al. Delivery of lung-protective ventilation for acute respiratory distress syndrome: a hybrid implementation-effectiveness trial. Ann Am Thorac Soc . 2023;20:424–432. doi: 10.1513/AnnalsATS.202207-626OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greene JC, Caracelli VJ, Graham WF. Toward a conceptual framework for mixed-method evaluation designs. Educ Eval Policy Anal . 1989;11:255–274. [Google Scholar]
- 29. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol . 2005;8:19–32. [Google Scholar]
- 30. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid-Based Healthc . 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 31. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health . 1984;74:979–983. doi: 10.2105/ajph.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Office for Civil Rights, U.S. Department of Health and Human Services. 2002. https://www.hhs.gov/sites/default/files/ocr/privacy/hipaa/understanding/special/research/research.pdf
- 33. Knighton AJ, Kean J, Wolfe D, Allen L, Jacobs J, Carpenter L, et al. Multi-factorial barriers and facilitators to high adherence to lung-protective ventilation using a computerized protocol: a mixed methods study. Implement Sci Commun . 2020;1:67. doi: 10.1186/s43058-020-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla JL, Leighton JP. In: Understanding and investigating response processes in validation research. Zumbo BD, Hubley AM, editors. Cham, Switzerland: Springer; 2017. Cognitive interviewing and think aloud methods; pp. 211–228. [Google Scholar]
- 35. Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health . 2010;25:1229–1245. doi: 10.1080/08870440903194015. [DOI] [PubMed] [Google Scholar]
- 36. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res . 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 37.Spencer L, Ritchie J, Lewis J, Dillon L.2003. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/498321/Quality-in-qualitative-evaulation_tcm6-38739.pdf
- 38.Hahn C. Doing qualitative research using your computer: a practical guide. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- 39. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci . 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Balas MC, Burke WJ, Gannon D, Cohen MZ, Colburn L, Bevil C, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med . 2013;41:S116–S127. doi: 10.1097/CCM.0b013e3182a17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carrothers KM, Barr J, Spurlock B, Ridgely MS, Damberg CL, Ely EW. Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med . 2013;41:S128–S135. doi: 10.1097/CCM.0b013e3182a2c2b1. [DOI] [PubMed] [Google Scholar]
- 42. Miller MA, Bosk EA, Iwashyna TJ, Krein SL. Implementation challenges in the intensive care unit: the why, who, and how of daily interruption of sedation. J Crit Care . 2012;27:218.e1–218.e7. doi: 10.1016/j.jcrc.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, et al. VENTILA Group Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med . 2008;177:170–177. doi: 10.1164/rccm.200706-893OC. [DOI] [PubMed] [Google Scholar]
- 44. Boltey EM, Iwashyna TJ, Hyzy RC, Watson SR, Ross C, Costa DK. Ability to predict team members’ behaviors in ICU teams is associated with routine ABCDE implementation. J Crit Care . 2019;51:192–197. doi: 10.1016/j.jcrc.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girard TD, Hargett KD, Singh J. In: ICU liberation: the power of pain control, minimal sedation, and early mobility. 2nd ed. Balas MC, Clemmer T, Hargett KD, editors. Mount Prospect, IL: Society of Critical Care Medicine; 2015. Spontaneous awakening and breathing trials; pp. 19–30. [Google Scholar]
- 46. Barr J, Ghaferi AA, Costa DK, Hedlin HK, Ding VY, Ross C, et al. Organizational characteristics associated with ICU Liberation (ABCDEF) bundle implementation by adult ICUs in Michigan. Crit Care Explor . 2020;2:e0169. doi: 10.1097/CCE.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu K, Nakamura K, Katsukawa H, Nydahl P, Ely EW, Kudchadkar SR, et al. Implementation of the ABCDEF bundle for critically ill ICU patients during the COVID-19 pandemic: a multi-national 1-day point prevalence study. Front Med (Lausanne) . 2021;8:735860. doi: 10.3389/fmed.2021.735860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu K, Nakamura K, Katsukawa H, Elhadi M, Nydahl P, Ely EW, et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explor . 2021;3:e0353. doi: 10.1097/CCE.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Academies of Sciences, Engineering, and Medicine. 2020. https://www.nap.edu/catalog/25765/rapid-expert-consultation-on-crisis-standards-of-care-for-the-covid-19-pandemic-march-28-2020
- 50. Papadimos TJ, Marcolini EG, Hadian M, Hardart GE, Ward N, Levy MM, et al. Ethics of outbreaks position statement. Part 1: therapies, treatment limitations, and duty to treat. Crit Care Med . 2018;46:1842–1855. doi: 10.1097/CCM.0000000000003416. [DOI] [PubMed] [Google Scholar]
- 51. Shively NR, Moffa MA, Paul KT, Wodusky EJ, Schipani BA, Cuccaro SL, et al. Impact of a telehealth-based antimicrobial stewardship program in a community hospital health system. Clin Infect Dis . 2020;71:539–545. doi: 10.1093/cid/ciz878. [DOI] [PubMed] [Google Scholar]