Abstract
Rationale
Clinical care guidelines advise that lung volume recruitment (LVR) be performed routinely by people with neuromuscular disease (NMD) to maintain lung and chest wall flexibility and slow lung function decline. However, the evidence base is limited, and no randomized controlled trials of regular LVR in adults have been published.
Objectives
To evaluate the effect of regular LVR on respiratory function and quality of life in adults with NMD.
Methods
A randomized controlled trial with assessor blinding was conducted between September 2015 and May 2019. People (>14 years old) with NMD and vital capacity <80% predicted were eligible, stratified by disease subgroup (amyotrophic lateral sclerosis/motor neuron disease or other NMDs), and randomized to 3 months of twice-daily LVR or breathing exercises. The primary outcome was change in maximum insufflation capacity (MIC) from baseline to 3 months, analyzed using a linear mixed model approach.
Results
Seventy-six participants (47% woman; median age, 57 [31–68] years; mean baseline vital capacity, 40 ± 18% predicted) were randomized (LVR, n = 37). Seventy-three participants completed the study. There was a statistically significant difference in MIC between groups (linear model interaction effect P = 0.002, observed mean difference, 0.19 [0.00–0.39] L). MIC increased by 0.13 (0.01–0.25) L in the LVR group, predominantly within the first month. No interaction or treatment effects were observed in secondary outcomes of lung volumes, respiratory system compliance, and quality of life. No adverse events were reported.
Conclusions
Regular LVR increased MIC in a sample of LVR-naive participants with NMD. We found no direct evidence that regular LVR modifies respiratory mechanics or slows the rate of lung volume decline. The implications of increasing MIC are unclear, and the change in MIC may represent practice. Prospective long-term clinical cohorts with comprehensive follow-up, objective LVR use, and clinically meaningful outcome data are needed.
Clinical trial registered with anzctr.org.au (ACTRN12615000565549).
Keywords: neuromuscular diseases, amyotrophic lateral sclerosis, lung volume measurements, respiratory therapy, insufflation
Respiratory muscle weakness in neuromuscular disease (NMD), coupled with lung and chest wall stiffness, results in lung volumes that decline over time (1). Respiratory system compromise causes dyspnea, disability, and death of chronic ventilatory failure (2). To minimize the impact of respiratory weakness and stiffness, NMD care guidelines typically recommend airway clearance and volume recruitment techniques (3, 4). However, the underlying evidence base is poor, and recommendations are largely consensus based.
Lung volume recruitment (LVR) is a technique that raises absolute lung volume above the volitional total lung capacity. A resuscitation bag with a mouthpiece or mask is one method of delivering consecutive positive-pressure insufflations until the maximum tolerable inflation is reached, “stacking” a greater volume than can be inspired spontaneously (3, 5). Full exhalation from this upper limit is the maximum insufflation capacity (MIC) or lung insufflation capacity (LIC) (3, 5, 6).
Inflating beyond spontaneous inspiratory capacity (IC) to MIC is hypothesized to stretch the lungs and chest wall, ameliorating respiratory system stiffness and vital capacity (VC) decline. Although short-term improvement in total respiratory system compliance (Crs) has been observed with a single session of LVR, no improvement in VC has been shown (6, 7). Only one randomized controlled trial (RCT) has compared longer term regular LVR with a no-LVR arm, in Duchenne muscular dystrophy (DMD) (8). Katz and colleagues found no differences in respiratory function, but objectively measured adherence was suboptimal, respiratory compromise was mild, and results in children may not generalize to adults (8). In contrast, prior cohort studies of DMD and other slowly progressive NMDs in both children and adults have suggested that regular LVR can increase the MIC over time, even in the face of declining VC (9–12).
To understand the effect of regular LVR on respiratory function and quality of life in adults with NMD, we undertook a 3-month trial of twice-daily LVR compared with an active control. The primary outcome was change from baseline to 3 months in MIC. Secondary outcomes were change from baseline to 3 months in respiratory function, respiratory tract infection (RTI) rate, health-related quality of life (HRQoL), and symptoms. Some of the results of this study have been previously reported in the form of abstracts (13, 14).
Methods
Study Design
Recruitment was via the pediatric NMD, adult domiciliary home ventilation, and progressive neurological disease services in Victoria, Australia. The trial was ethically approved (HREC/15/Austin/117) and prospectively registered at anzctr.org.au (ACTRN12615000565549).
Participants
Patients >14 years old with NMD or restrictive chest wall disease (>3 months postdiagnosis) and forced VC (FVC) <80% of predicted normal (15) were identified at outpatient clinics or through the ventilation service’s database. Exclusion criteria were daily LVR for >6 weeks within the past 6 months, recent (<6 wk) acute respiratory inpatient admission, contraindications to positive-pressure therapy, medical instability, invasive ventilation, recent initiation of noninvasive ventilation (NIV; <3 mo), or nonproficiency in English. All participants provided written informed consent (witnessed verbally if unable to write).
Randomization and Blinding
Participants who effectively performed LVR (as detailed below) were randomized (1:1) to the intervention (LVR) or an active control (breathing exercises [16]) using a computer-generated block scheme with stratification for disease subgroup (rapidly progressive amyotrophic lateral sclerosis [ALS]/motor neuron disease [MND] or other, more slowly progressive NMDs). The randomization schedule was transferred to individually numbered, sealed, opaque envelopes by an independent person and opened sequentially by the physiotherapist administering the intervention. Participants and treating clinicians were unblinded to group allocation. Staff members blinded to group allocation measured respiratory function and HRQoL and performed statistical analyses.
Procedures
Consented participants underwent baseline and final (3-mo) assessment at the trial’s primary hospital. One and 2-month assessments were performed in participants’ homes.
Baseline demographics included diagnosis, age, age at NMD symptom onset, sex, height, weight, current NIV, and gastrostomy use.
Respiratory function testing was performed while seated according to American Thoracic Society/European Respiratory Society standards (17–20) in the following order (for details, see Figures E1–E6 in the data supplement): VC, unassisted peak cough flow (PCF) (3), static lung volumes (functional residual capacity [FRC], total lung capacity, residual volume, IC, and expiratory reserve volume), maximum inspiratory pressure and maximum expiratory pressure (MEP), sniff nasal inspiratory pressure, Crs using the pulse inflation method (7), MIC (3), and PCF from MIC (PCFMIC). The MIC-minus-VC difference (MIC − VC) was calculated from the largest MIC and VC values. Outcomes were expressed as absolute values, percentage of predicted normal (%pn), and z-scores (15, 21, 22).
After baseline testing, a single session of LVR therapy (two sets of five maximal inflations) was performed, and respiratory function tests were repeated (baseline post-LVR assessment). Participants who failed to demonstrate MIC at least 10% greater than VC at the baseline or baseline post-LVR assessment were considered unable to effectively perform LVR and did not proceed to randomization. The single session of LVR and repeat respiratory function testing were also conducted at the final assessment (final post-LVR assessment).
Generic and disease-specific HRQoL was assessed using the Assessment of Quality of Life (AQoL-8D) (23) and the Severe Respiratory Insufficiency Questionnaire (SRI) (24). The Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) was measured in subjects with ALS/MND (25).
An unblinded clinician collected use diaries, adverse events or side effects, and self-reported RTI history (primary care diagnosis with antibiotics or in-hospital admission and diagnosis). If subjects were randomized to LVR, LVR use data were downloaded and inflation pressures checked.
Interventions
Participants received training and written instructions from a respiratory physiotherapist. All participants were instructed to perform at least two treatment sessions daily and provided with use diaries.
Participants in the LVR arm were prescribed five sets of five maximal inflations per session. The number of compressions per maximal inflation was individualized (26), and peak inflation pressure was maintained at <50 cm H2O. A previously validated counter fitted to the LVR kit recorded use objectively (1.6-L manual resuscitation bag, one-way in-line valve, mouthpiece, and nose clip or oronasal mask; LVR kit item number 1034502; Mercury Medical) (26).
Participants in the active, attentional control arm were prescribed 10 minutes of breathing exercises, involving a minimum of five sets of five “diaphragm breaths”(16) (see the data supplement).
Sample Size Estimate and Statistical Analysis
Sample size calculations indicated that 72 participants were required to detect a between-group difference of 150 ml (standard deviation, 310 ml) (27) in mean MIC change over 3 months, with 80% power and α of 0.05. To allow for a 15% withdrawal rate, 83 participants were sought.
Statistical analyses were conducted on an intention-to-treat basis (Stata/IC 15.1 for Mac; StataCorp LLC). Descriptive statistics are presented as mean ± standard deviation, median (interquartile range), or frequencies and percentages as appropriate. Change- over-time data are presented as group mean difference (95% confidence interval).
The primary analysis used a linear mixed model to examine the fixed effects of treatment group, time, and the interaction between treatment and time, with participant as a random effect. A planned secondary analysis added disease type (ALS/MND or other NMD) into this model. If models were significant, post hoc comparisons of effects were conducted using paired Student’s t tests. Models were repeated for secondary outcomes.
Self-reported diary and LVR counter data were summarized as sessions per day, and their agreement was evaluated using Lin’s concordance correlation coefficient (28) and Bland and Altman’s limits of agreement (29). Dose response was explored by adding use (average sessions/day) as a covariate to the primary model of MIC. Potential mechanisms of action were explored by incorporating change in pressure at MIC, Crs, VC, and FRC into the MIC model as a covariate.
Results
Between September 2, 2015, and May 21, 2019, 80 consecutive participants were recruited (diagnoses are shown in Figure 1). Four participants were unable to perform LVR. The randomized cohort (n = 76) was 47% female, with a median age of 57.0 (30.6–67.6) years and mean baseline VC of 40.3 ± 18.4%pn (Table 1).
Figure 1.
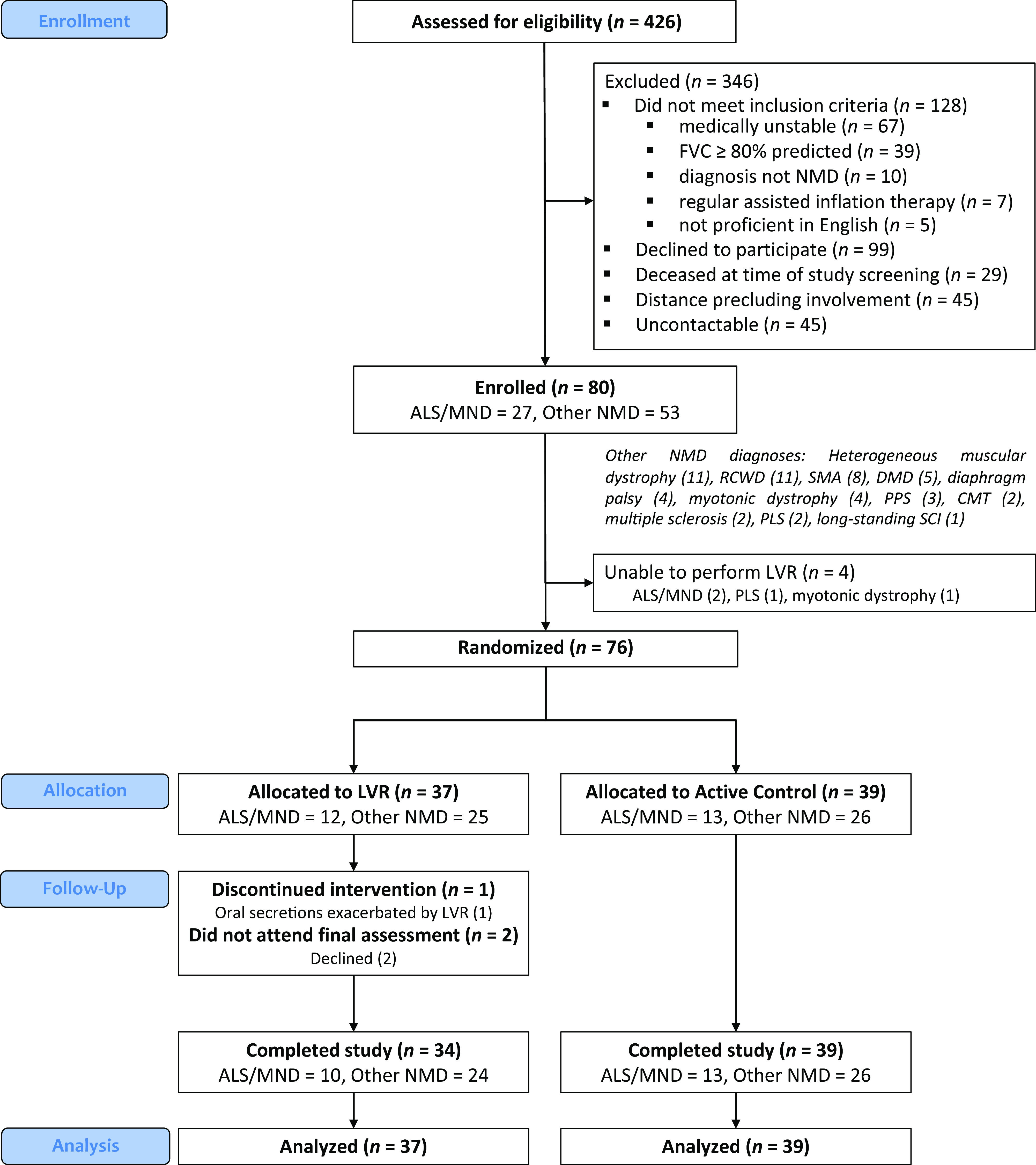
Consolidated Standards of Reporting Trials flow diagram of participants in the LVR in NMD trial. ALS = amyotrophic lateral sclerosis; CMT = Charcot-Marie-Tooth disease; DMD = Duchenne muscular dystrophy; FVC = forced vital capacity; LVR = lung volume recruitment; MND = motor neuron disease; NMD = neuromuscular disease; PLS = primary lateral sclerosis; PPS = postpolio syndrome; RCWD = restrictive chest wall disease; SCI = spinal cord injury; SMA = spinal muscular atrophy.
Table 1.
Baseline demographic and clinical characteristics of randomized participants (n = 76)
| Variable | LVR (n = 37) | Active Control (n = 39) |
|---|---|---|
| Age, yr | 59.3 (27.8 to 68.3) | 56.8 (35.6 to 67.6) |
| Sex, female | 16 (43) | 20 (51) |
| BMI, kg/m2 | ||
| All | 24.7 ± 7.2 | 24.7 ± 7.5 |
| NIV user, yes | ||
| All | 29 (78) | 31 (79) |
| Gastrostomy, yes:no | ||
| All | 12:25 | 8:31 |
| Age at symptom onset, yr | ||
| All | 28.0 (4.8 to 64.2) | 21.7 (3.8 to 62.0) |
| ALS/MND | 63.2 (48.7 to 66.5) | 62.8 (56.6 to 75.0) |
| Other NMD | 9.6 (3.4 to 28.0) | 7.8 (3.1 to 21.7) |
| Time since symptom onset, yr | ||
| All | 12.7 (2.0 to 24.6) | 17.8 (2.2 to 37.0) |
| ALS/MND | 1.9 (1.5 to 2.7) | 2.1 (1.1 to 2.5) |
| Other NMD | 20.9 (12.7 to 34.2) | 24.5 (17.8 to 49.4) |
| VC, % predicted | ||
| All | 40.3 ± 18.2 | 40.3 ± 18.8 |
| ALS/MND | 49.0 ± 10.1 | 56.2 ± 17.7 |
| Other NMD | 36.0 ± 19.8 | 32.4 ± 13.7 |
| VC, z-score | ||
| All | −4.7 ± 1.9 | −4.6 ± 1.9 |
| ALS/MND | −3.6 ± 0.9 | −3.0 ± 1.6 |
| Other NMD | −5.2 ± 2.1 | −5.5 ± 1.6 |
| ALSFRS-R score | 21.3 ± 6.6 | 26.7 ± 7.1 |
| ALSFRS-R bulbar subscore ⩽ 9 (yes) | 6 (50) | 5 (38) |
| ALSFRS-R slope, units/mo | 1.2 [0.8 to 1.6] | 1.0 [0.6 to 1.3] |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; BMI = body mass index; LVR = lung volume recruitment; MND = motor neuron disease; NIV = noninvasive ventilation; NMD = neuromuscular disease; VC = vital capacity.
Data are presented as median (interquartile range), frequency (percentage), mean ± standard deviation, or mean [95% confidence interval]. A bulbar subscore ⩽9 indicates moderate bulbar symptoms.
Seventy-three participants completed the study (Figure 1). No serious adverse events were reported (6,809 participant-days; for details, see the data supplement). One participant with bulbar-onset ALS/MND ceased LVR and withdrew from the study at Month 1, citing exacerbation of upper airway secretions.
Therapy was performed twice daily for a median of 45% (LVR) versus 75% (control) of total study days, with significantly fewer sessions per day in the LVR arm compared with the control arm (1.2 ± 0.7 vs. 1.5 ± 0.5 sessions/day; mean difference, −0.35 [−0.62 to −0.07]). Although a strong relationship existed between the number of LVR sessions per day reported by diary and recorded by the LVR counter (rho = 0.88 [95% confidence interval, 0.80 to 0.95]), more sessions were reported in the diaries than recorded objectively (mean bias [95% limits of agreement], −0.20 [−0.78 to 0.38] sessions/day; see Figure E8). LVR sessions lasted a mean of 10:01 min:sec (median, 7:18 [4:28 to 15:18] min:sec).
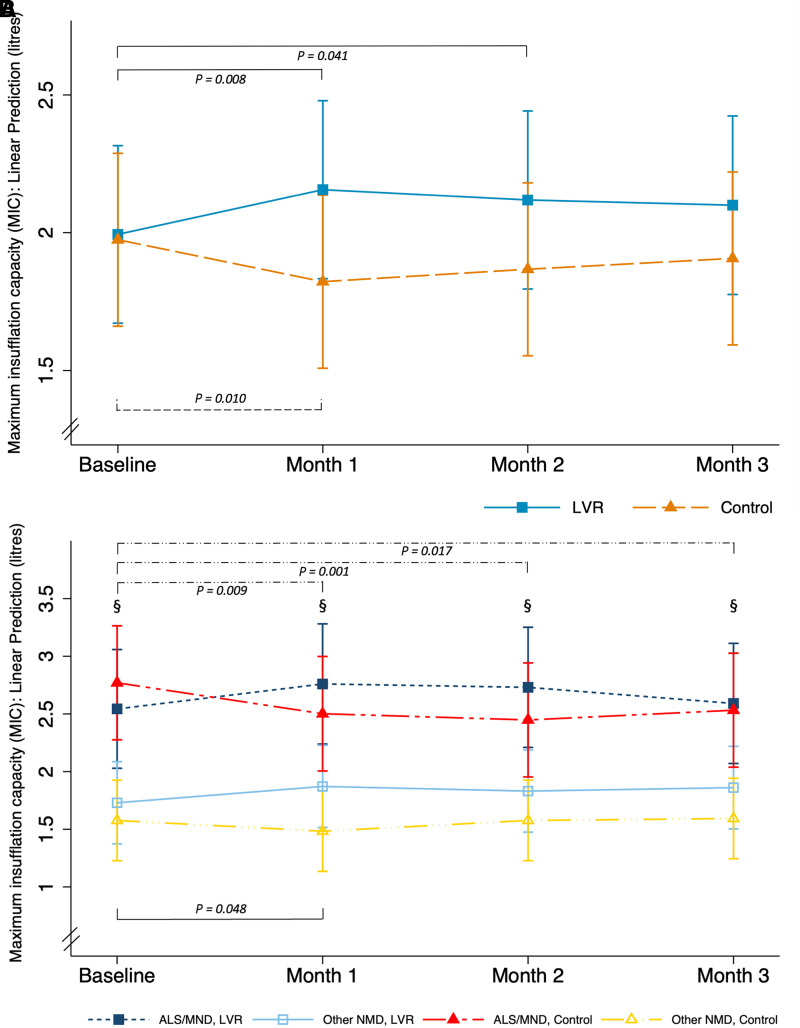
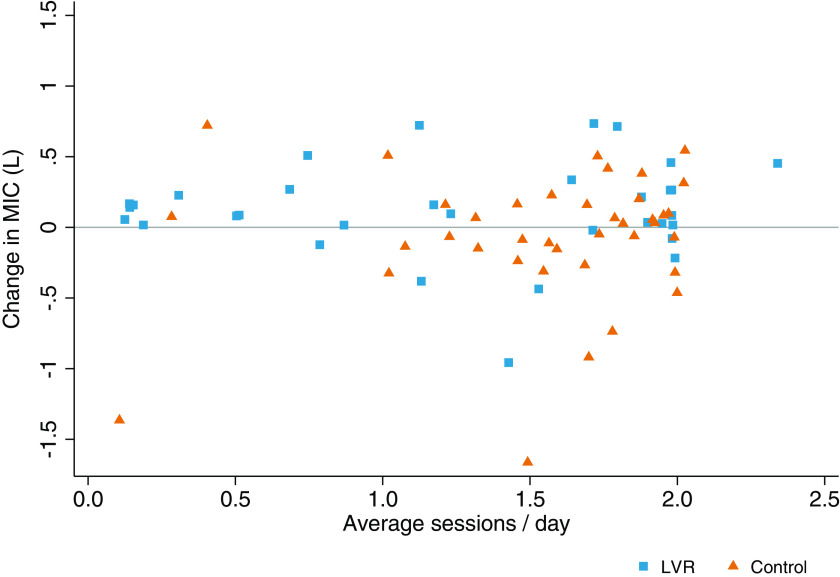
There was a statistically significant difference in the change in MIC between the treatment groups (linear model P = 0.026, interaction effect P = 0.002; Figure 2A). Post hoc testing demonstrated a mean between-group difference of 0.19 (0.00 to 0.39) L over 3 months. MIC increased in the LVR group (estimated effect = 0.11 [−0.02 to 0.23] L), predominantly during the first month of therapy (Figure 2A; observed changes in Table 2). Expressed as a percentage of baseline, MIC increased by 12.2% (3.7% to 20.7%) in the LVR group compared with 0.4% (−6.4% to 7.3%) in the control arm. No dose–response relationship was observed after incorporating use as a covariate (linear model P = 0.17, interaction effect P = 0.07, estimated effect = 0.11 [−0.03 to 0.23] L) (Figure 3). When disease type was introduced in the model, the treatment-by-time interaction effect remained (linear model P < 0.001, interaction effect P < 0.001, disease effect P < 0.001; Figure 2B). Participants with other NMDs had significantly lower MIC values at all time points compared with those with ALS/MND, regardless of treatment allocation (Table 2). Participants with other NMD assigned to LVR demonstrated an increase in MIC (observed mean change, 0.17 [0.07 to 0.27] L), whereas the other subgroups suggested no change (Table 2 and Figure 2B).
Figure 2.
(A and B) Change in the primary outcome, MIC, over time by treatment in the cohort as a whole (primary analysis) (A) and including disease type in the model (exploratory analysis) (B). The linear mixed model illustrates the estimated mean (95% confidence interval) marginal effects. The primary model (A) was significant (P = 0.026), with a significant interaction effect between treatment and time (P = 0.002). The exploratory model (B) was significant (P < 0.001), with a significant main effect of disease (P < 0.001) and interaction effect between treatment and time (P < 0.001). P values refer to statistically significant comparisons, where line patterns represent statistically significant differences over time by treatment group, and § represents differences between disease type (ALS/MND vs. other NMDs, P < 0.001 at baseline, Month 1, Month 2, and Month 3). ALS = amyotrophic lateral sclerosis; Control = active control; LVR = lung volume recruitment; MIC = maximum insufflation capacity; MND = motor neuron disease; NMD = neuromuscular disease.
Table 2.
Pairwise comparisons of observed values: change in respiratory function over time
| Variable | Baseline* |
Baseline to Month 1 |
Month 1 to Month 2 |
Month 2 to Month 3 |
Baseline to Month 3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| LVR | Active Control | LVR | Active Control | LVR | Active Control | LVR | Active Control | LVR | Active Control | |
| MIC, L | ||||||||||
| All | 1.99 ± 1.05 (37) | 1.97 ± 1.03 (39) | 0.18 (0.05 to 0.31) | −0.14 (−0.27 to −0.02) | −0.04 (−0.12 to 0.04) | 0.05 (−0.04 to 0.15) | −0.03 (−0.14 to 0.07) | 0.04 (−0.07 to 0.15) | 0.13 (0.01 to 0.25) | −0.07 (−0.22 to 0.09) |
| ALS/MND | 2.54 ± 0.94 (12) | 2.77 ± 1.22 (13) | 0.28 (−0.08 to 0.65) | −0.26 (−0.64 to 0.13) | −0.04 (−0.25 to 0.17) | −0.03 (−0.28 to 0.21) | −0.16 (−0.51 to 0.18) | 0.08 (−0.19 to 0.36) | 0.02 (−0.34 to 0.38) | −0.24 (−0.64 to 0.16) |
| Other NMD | 1.73 ± 1.02 (25) | 1.58 ± 0.64 (26) | 0.14 (0.01 to 0.27) | −0.09 (−0.19 to 0.01) | −0.04 (−0.14 to 0.06) | 0.09 (0.00 to 0.18) | 0.02 (−0.07 to 0.10) | 0.02 (−0.09 to 0.12) | 0.17 (0.07 to 0.27) | 0.02 (−0.12 to 0.15) |
| VC, L | ||||||||||
| All | 1.58 ± 0.88 (37) | 1.50 ± 0.83 (39) | 0.01 (−0.03 to 0.05) | −0.02 (−0.09 to 0.06) | −0.01 (−0.06 to 0.04) | −0.05 (−0.11 to 0.02) | −0.05 (−0.13 to 0.02) | −0.02 (−0.07 to 0.03) | −0.06 (−0.13 to 0.01) | −0.08 (−0.16 to 0.00) |
| ALS/MND | 2.00 ± 0.62 (12) | 2.20 ± 0.85 (13) | −0.04 (−0.12 to 0.03) | −0.10 (−0.30 to 0.10) | 0.05 (−0.10 to 0.20) | −0.11 (−0.29 to 0.08) | −0.22 (−0.42 to −0.03) | −0.06 (−0.21 to 0.09) | −0.23 (−0.36 to −0.09) | −0.27 (−0.45 to −0.08) |
| Other NMD | 1.38 ± 0.93 (25) | 1.16 ± 0.57 (26) | 0.03 (−0.02 to 0.08) | 0.02 (−0.05 to 0.09) | −0.03 (−0.08 to 0.02) | −0.02 (−0.07 to 0.03) | 0.01 (−0.05 to 0.07) | 0.00 (−0.04 to 0.04) | 0.01 (−0.05 to 0.07) | 0.01 (−0.05 to 0.06) |
| MIC − VC, L | ||||||||||
| All | 0.41 ± 0.54 (37) | 0.47 ± 0.43 (39) | 0.17 (0.03 to 0.32) | −0.13 (−0.25 to −0.01) | −0.03 (−0.11 to 0.05) | 0.10 (0.02 to 0.18) | 0.03 (−0.06 to 0.12) | 0.06 (−0.03 to 0.15) | 0.19 (0.09 to 0.30) | 0.02 (−0.12 to 0.15) |
| ALS/MND | 0.55 ± 0.73 (12) | 0.57 ± 0.56 (13) | 0.32 (−0.07 to 0.72) | −0.16 (−0.51 to 0.20) | −0.09 (−0.30 to 0.13) | 0.07 (−0.13 to 0.27) | 0.06 (−0.20 to 0.33) | 0.15 (−0.06 to 0.35) | 0.25 (−0.04 to 0.54) | 0.03 (−0.33 to 0.39) |
| Other NMD | 0.41 ± 0.42 (25) | 0.42 ± 0.35 (26) | 0.11 (−0.03 to 0.26) | −0.12 (−0.23 to −0.01) | −0.01 (−0.10 to 0.08) | 0.11 (0.03 to 0.20) | 0.02 (−0.07 to 0.11) | 0.01 (−0.08 to 0.11) | 0.17 (0.06 to 0.28) | 0.01 (−0.12 to 0.14) |
| Crs, ml/cm H2O | ||||||||||
| All | 35 ± 20 (35) | 40 ± 30 (34) | 3.7 (0.7 to 6.7) | 3.5 (−0.9 to 7.8) | −0.1 (−4.3 to 4.0) | −1.6 (−6.0 to 2.7) | 0.6 (−6.5 to 7.7) | 1.9 (−2.0 to 5.7) | 6.3 (0.4 to 12.1) | 4.8 (−3.8 to 13.3) |
| ALS/MND | 41 ± 15 (11) | 55 ± 33 (10) | 5.9 (0.7 to 11.1) | 3.8 (−7.4 to 15.1) | 1.4 (−11.3 to 14.1) | −3.7 (−12.8 to 5.5) | 5.4 (−21.6 to 32.4) | 1.5 (−8.4 to 11.3) | 14.4 (−7.7 to 36.5) | 4.4 (−18.0 to 26.8) |
| Other NMD | 32 ± 22 (24) | 33 ± 28 (24) | 2.9 (−0.9 to 6.7) | 3.3 (−1.5 to 8.2) | −0.7 (−4.8 to 3.4) | −0.6 (−5.8 to 4.6) | −1.1 (−6.3 to 4.1) | 2.0 (−1.7 to 5.8) | 3.3 (−3.0 to 6.9) | 4.9 (−5.5 to 23.2) |
| FRC, L | ||||||||||
| All | 1.31 ± 0.76 (30) | 1.45 ± 1.07 (32) | 0.06 (−0.05 to 0.18) | 0.09 (−0.12 to 0.30) | −0.02 (−0.13 to 0.10) | 0.12 (0.02 to 0.21) | −0.08 (−0.18 to 0.02) | −0.10 (−0.21 to 0.01) | −0.02 (−0.15 to 0.10) | 0.06 (−0.09 to 0.21) |
| ALS/MND | 1.80 ± 0.98 (8) | 2.35 ± 1.23 (10) | 0.19 (−0.08 to 0.46) | 0.00 (−0.67 to 0.68) | −0.08 (−0.42 to 0.26) | 0.10 (−0.15 to 0.35) | −0.09 (−0.33 to 0.15) | 0.07 (−0.17 to 0.31) | 0.05 (−0.34 to 0.44) | 0.10 (−0.44 to 0.64) |
| Other NMD | 1.13 ± 0.59 (22) | 1.05 ± 0.70 (22) | 0.02 (−0.11 to 0.14) | 0.12 (−0.08 to 0.33) | 0.01 (−0.11 to 0.13) | 0.12 (0.01 to 0.23) | −0.08 (−0.20 to 0.05) | −0.17 (−0.29 to −0.06) | −0.05 (−0.19 to 0.10) | 0.05 (−0.08 to 0.18) |
| RV, L | ||||||||||
| All | 0.92 ± 0.53 (30) | 1.08 ± 0.79 (32) | 0.02 (−0.08 to 0.11) | 0.09 (−0.11 to 0.30) | 0.01 (−0.09 to 0.11) | 0.11 (0.03 to 0.19) | −0.02 (−0.11 to 0.07) | −0.06 (−0.17 to 0.04) | −0.00 (−0.11 to 0.10) | 0.11 (−0.04 to 0.25) |
| ALS/MND | 1.30 ± 0.65 (8) | 1.64 ± 0.79 (10) | 0.17 (−0.07 to 0.41) | 0.05 (−0.57 to 0.67) | −0.01 (−0.25 to 0.24) | 0.15 (−0.03 to 0.34) | −0.11 (−0.38 to 0.16) | 0.06 (−0.17 to 0.29) | 0.07 (−0.36 to 0.49) | 0.19 (−0.37 to 0.75) |
| Other NMD | 0.79 ± 0.43 (22) | 0.82 ± 0.66 (22) | −0.04 (−0.13 to 0.05) | 0.11 (−0.09 to 0.31) | 0.02 (−0.10 to 0.14) | 0.10 (−0.01 to 0.19) | 0.01 (−0.08 to 0.11) | −0.11 (−0.23 to −0.00) | −0.02 (−0.12 to 0.08) | 0.07 (−0.04 to 0.19) |
| TLC, L | ||||||||||
| All | 2.57 ± 1.23 (30) | 2.65 ± 1.47 (32) | 0.02 (−0.08 to 0.13) | 0.06 (−0.14 to 0.26) | −0.00 (−0.12 to 0.12) | 0.07 (−0.03 to 0.18) | −0.13 (−0.23 to −0.02) | −0.09 (−0.20 to 0.01) | −0.09 (−0.21 to 0.03) | 0.01 (−0.14 to 0.15) |
| ALS/MND | 3.27 ± 1.20 (8) | 3.93 ± 1.71 (10) | 0.16 (−0.09 to 0.40) | −0.01 (−0.61 to 0.58) | −0.04 (−0.41 to 0.33) | −0.05 (−0.31 to 0.21) | −0.27 (−0.57 to 0.04) | −0.01 (−0.22 to 0.21) | −0.10 (−0.57 to 0.37) | −0.13 (−0.64 to 0.39) |
| Other NMD | 2.32 ± 1.17 (22) | 2.06 ± 0.90 (22) | −0.02 (−0.14 to 0.09) | 0.09 (−0.11 to 0.29) | 0.01 (−0.11 to 0.14) | 0.12 (0.00 to 0.24) | −0.08 (−0.18 to 0.02) | −0.13 (−0.26 to −0.00) | −0.09 (−0.21 to 0.03) | 0.05 (−0.06 to 0.17) |
| ERV, L | ||||||||||
| All | 0.39 ± 0.28 (30) | 0.38 ± 0.37 (32) | 0.05 (−0.00 to 0.10) | −0.00 (−0.05 to 0.04) | −0.03 (−0.07 to 0.02) | 0.00 (−0.05 to 0.05) | −0.06 (−0.13 to 0.00) | −0.04 (−0.08 to −0.00) | −0.02 (−0.08 to 0.04) | −0.04 (−0.09 to 0.00) |
| ALS/MND | 0.51 ± 0.41 (8) | 0.71 ± 0.49 (10) | 0.02 (−0.06 to 0.10) | −0.05 (−0.21 to 0.11) | −0.07 (−0.17 to 0.03) | −0.05 (−0.23 to 0.13) | 0.02 (−0.08 to 0.12) | 0.01 (−0.07 to 0.09) | −0.01 (−0.15 to 0.13) | −0.09 (−0.23 to 0.05) |
| Other NMD | 0.35 ± 0.22 (22) | 0.23 ± 0.17 (22) | 0.06 (−0.01 to 0.12) | 0.01 (−0.03 to 0.05) | −0.01 (−0.06 to 0.04) | 0.03 (−0.01 to 0.06) | −0.09 (−0.17 to −0.01) | −0.06 (−0.10 to −0.02) | −0.02 (−0.10 to 0.05) | −0.03 (−0.08 to 0.02) |
| IC, L | ||||||||||
| All | 1.25 ± 0.70 (30) | 1.19 ± 0.56 (32) | 0.02 (−0.07 to 0.10) | −0.03 (−0.10 to 0.04) | −0.04 (−0.14 to 0.06) | −0.04 (−0.09 to 0.02) | −0.04 (−0.10 to 0.01) | −0.00 (−0.06 to 0.06) | −0.05 (−0.11 to 0.00) | −0.06 (−0.14 to 0.01) |
| ALS/MND | 1.47 ± 0.47 (8) | 1.58 ± 0.62 (10) | −0.04 (−0.18 to 0.11) | −0.01 (−0.28 to 0.25) | 0.04 (−0.02 to 0.10) | −0.14 (−0.31 to 0.03) | −0.17 (−0.26 to −0.08) | −0.11 (−0.26 to 0.04) | −0.16 (−0.30 to −0.01) | −0.27 (−0.48 to −0.05) |
| Other NMD | 1.17 ± 0.76 (22) | 1.01 ± 0.43 (22) | 0.04 (−0.07 to 0.15) | −0.04 (−0.09 to 0.01) | −0.07 (−0.21 to 0.07) | 0.00 (−0.04 to 0.05) | −0.00 (−0.06 to 0.05) | 0.04 (−0.01 to 0.09) | −0.02 (−0.08 to 0.04) | 0.01 (−0.04 to 0.06) |
| MIP, cm H2O | ||||||||||
| All | 35.1 ± 18.3 (36) | 43.1 ± 22.1 (37) | N/A | N/A | N/A | N/A | N/A | N/A | −1.8 (−4.2 to 0.6) | −2.0 (−4.9 to 0.8) |
| ALS/MND | 32.9 ± 18.9 (11) | 41.5 ± 18.5 (12) | N/A | N/A | N/A | N/A | N/A | N/A | −5.1 (−11.9 to 1.7) | −7.2 (−12.3 to −2.1) |
| Other NMD | 36.0 ± 18.3 (25) | 43.8 ± 23.9 (25) | N/A | N/A | N/A | N/A | N/A | N/A | −0.8 (−3.4 to 1.8) | 0.4 (−2.8 to 3.6) |
| MEP, cm H2O | ||||||||||
| All | 48.0 ± 26.2 (34) | 49.0 ± 27.4 (32) | N/A | N/A | N/A | N/A | N/A | N/A | −2.0 (−4.6 to 0.7) | −0.5 (−4.6 to 3.6) |
| ALS/MND | 44.8 ± 24.4 (12) | 55.0 ± 29.7 (8) | N/A | N/A | N/A | N/A | N/A | N/A | −4.0 (−7.2 to −0.8) | −12.0 (−16.7 to −7.4) |
| Other NMD | 49.8 ± 27.6 (22) | 47.0 ± 26.9 (24) | N/A | N/A | N/A | N/A | N/A | N/A | −1.1 (−4.7 to 2.4) | 3.4 (−1.0 to 7.7) |
| SNIP, cm H2O | ||||||||||
| All | 23.1 ± 12.7 (37) | 28.7 ± 14.4 (37) | N/A | N/A | N/A | N/A | N/A | N/A | 0.5 (−3.0 to 3.9) | −0.8 (−3.6 to 2.1) |
| ALS/MND | 20.1 ± 8.4 (12) | 25.6 ± 9.1 (12) | N/A | N/A | N/A | N/A | N/A | N/A | −2.9 (−8.3 to 2.4) | −6.0 (−8.9 to −3.1) |
| Other NMD | 24.5 ± 14.2 (25) | 30.1 ± 16.5 (25) | N/A | N/A | N/A | N/A | N/A | N/A | 1.6 (−2.7 to 6.0) | 1.7 (−2.0 to 5.4) |
| PCF, L/min | ||||||||||
| All | 173 ± 59 (37) | 177 ± 80 (39) | −5.6 (−14.8 to 3.6) | −10.9 (−27.9 to 6.2) | −2.7 (−11.3 to 6.0) | −3.3 (−13.0 to 6.3) | −5.2 (−15.2 to 4.8) | 4.3 (−5.2 to 13.9) | −15.4 (−25.7 to −5.2) | −8.0 (−23.4 to 7.5) |
| ALS/MND | 180 ± 66 (12) | 192 ± 62 (13) | 8.0 (−13.4 to 29.5) | −13.8 (−44.1 to 16.6) | −9.7 (−35.7 to 16.3) | −13.5 (−34.4 to 7.5) | −20.7 (−50.5 to 9.1) | 4.5 (−14.5 to 23.5) | −28.0 (−47.1 to −8.9) | −15.9 (−38.1 to 6.2) |
| Other NMD | 169 ± 57 (25) | 170 ± 87 (26) | −11.1 (−21.0 to −1.2) | −9.5 (−31.6 to 12.5) | −0.1 (−8.7 to 8.5) | 1.4 (−9.5 to 12.2) | 0.6 (−8.4 to 9.6) | 4.2 (−7.5 to 15.9) | −10.2 (−22.6 to 2.2) | −4.0 (−25.1 to 17.2) |
| PCFMIC, L/min | ||||||||||
| All | 165 ± 49 (37) | 183 ± 62 (39) | 18.4 (6.1 to 30.8) | 3.2 (−9.1 to 15.6) | −4.4 (−17.0 to 8.3) | 3.1 (−6.5 to 12.7) | 3.9 (−6.1 to 13.9) | −2.8 (−13.0 to 7.3) | 19.6 (6.4 to 32.8) | 2.3 (−10.7 to 15.3) |
| ALS/MND | 168 ± 53 (12) | 215 ± 68 (13) | 11.6 (−8.3 to 31.6) | −5.1 (−22.9 to 12.8) | −3.6 (−25.1 to 17.9) | −3.6 (−14.8 to 7.5) | −8.9 (−22.2 to 4.5) | −3.6 (−28.0 to 20.9) | 3.8 (−8.6 to 16.2) | −14.7 (−39.1 to 9.7) |
| Other NMD | 164 ± 47 (25) | 166 ± 53 (26) | 21.1 (5.1 to 37.2) | 7.1 (−9.6 to 23.7) | −4.6 (−20.9 to 11.6) | 6.2 (−7.1 to 19.6) | 8.8 (−4.0 to 21.7) | −2.5 (−13.2 to 8.2) | 26.5 (8.5 to 44.4) | 10.8 (−4.5 to 26.0) |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; Crs = total respiratory system compliance; ERV = expiratory reserve volume; FRC = functional residual capacity; IC = inspiratory capacity; LVR = lung volume recruitment; MEP = maximal expiratory pressure; MIC = maximum insufflation capacity; MIP = maximal inspiratory pressure; MND = motor neuron disease; N/A = not applicable (outcome not collected at interim time points); NMD = neuromuscular disease; PCF = peak cough flow; PCFMIC = peak cough flow from maximum insufflation capacity; RV = residual volume, SNIP = sniff nasal inspiratory pressure; TLC = total lung capacity; VC = vital capacity.
Data are presented as mean ± standard deviation and mean change (95% confidence interval). Results were not obtainable in all subjects, because of bulbar impairment, technical issues, or fatigue. Values in boldface type indicate statistically significant within-group changes over time using paired Student’s t test (P < 0.05).
Values in parentheses are numbers of participants with technically acceptable measurements at baseline.
Figure 3.
Dose–response relationship: change in maximum insufflation capacity (MIC; liters) and self-reported average number of treatment sessions per day. The average number of treatment sessions per day for both groups was determined from participant self-report diaries (mean, 1.2 ± 0.7 [LVR] vs. 1.5 ± 0.5 [control] sessions/day; mean difference, −0.35 [−0.62 to −0.07]; P = 0.015). The light gray line represents no change in MIC over the 3-month study duration. Control = active control; LVR = lung volume recruitment.
Analysis of secondary outcomes showed an interaction effect only for MIC − VC difference (linear model P = 0.003, interaction effect P = 0.004; see Figure E9), with post hoc testing demonstrating a mean between-group difference of 0.18 (0.00 to 0.35) L. The model for VC suggested decline over time (linear model P = 0.03) but no treatment or interaction effects (P = 0.65; see Figure E10A). Adding disease to this model suggested that the decline occurred predominantly in participants with ALS/MND, regardless of treatment (mean difference, Month 3 minus baseline, −0.25 [−0.36 to −0.14] L; see Figure E10B). PCFMIC improved over time (linear model P = 0.03, no treatment or interaction effects [P = 0.14]; see Figure E11). Models of Crs, FRC, residual volume, total lung capacity, expiratory reserve volume, IC, PCF, maximum inspiratory pressure, and sniff nasal inspiratory pressure showed no effects of treatment. Adding disease to the models demonstrated a larger decline in MEP among participants with ALS/MND in the control group (linear model P = 0.002, interaction effect P = 0.009; see Figure E12; mean difference, −8.1 [−2.9 to −13.2] cm H2O; Table 2).
The peak inflation pressure achieved during the measurement of MIC was unchanged over time in both treatment groups (LVR mean change, 3.5 [−0.7 to 7.7] cm H2O; control mean change, 1.7 [−2.2 to 5.6] cm H2O). No model or interaction effects were found when pressure at MIC was added to the MIC model as a covariate (linear model P = 0.16, interaction effect P = 0.053; estimated effect = 0.12 [−0.02 to 0.26] L). There was no relationship between change in MIC and Crs (linear model P = 0.27, interaction effect P = 0.09), VC (linear model P = 0.17, interaction effect P = 0.055), or FRC (linear model P = 0.07, interaction effect P = 0.02).
The immediate response to a single LVR session was larger at baseline than at the final assessment. MIC, MIC − VC, Crs, PCFMIC, and the PCFMIC-minus-PCF difference all increased after a single session of LVR when participants were naive, but no similar single-session change was apparent at Month 3 (Table 3).
Table 3.
Comparison between the immediate effect of lung volume recruitment at study commencement (baseline) and conclusion (Month 3) for respiratory function variables
| Variable | Δ at Baseline | n B | Δ at Month 3 | n F | Difference in Immediate Effect* | n | |
|---|---|---|---|---|---|---|---|
| Mean Difference (95% CI) | Mean Difference (95% CI) | Mean Difference (95% CI) | P Value | ||||
| MIC, L | 0.13 (0.04 to 0.21)† | 76 | −0.01 (−0.07 to 0.05) | 66 | −0.11 (−0.21 to −0.02) | 66 | 0.02 |
| VC, L | −0.02 (−0.06 to 0.02) | 76 | −0.01 (−0.04 to 0.03) | 66 | 0.02 (−0.02 to 0.07) | 66 | 0.25 |
| MIC − VC, L | 0.15 (0.06 to 0.23)† | 76 | −0.01 (−0.07 to 0.05) | 66 | −0.14 (−0.23 to −0.04) | 66 | 0.005 |
| Crs, ml/cm H2O | 4.6 (0.9 to 8.3)† | 63 | 0.8 (−1.4 to 3.0) | 60 | −2.2 (−6.8 to 2.3) | 54 | 0.33 |
| FRC, L | −0.02 (−0.09 to 0.04) | 48 | −0.05 (−0.09 to −0.01)† | 48 | 0.01 (−0.06 to 0.07) | 41 | 0.83 |
| RV, L | −0.01 (−0.07 to 0.05) | 48 | −0.03 (−0.06 to 0.01) | 48 | 0.01 (−0.05 to 0.08) | 41 | 0.68 |
| TLC, L | −0.02 (−0.08 to 0.04) | 48 | −0.04 (−0.09 to 0.00)† | 48 | 0.01 (−0.05 to 0.06) | 41 | 0.82 |
| ERV, L | −0.01 (−0.05 to 0.02) | 48 | −0.02 (−0.05 to 0.01) | 48 | −0.01 (−0.06 to 0.04) | 41 | 0.79 |
| IC, L | 0.01 (−0.03 to 0.05) | 48 | 0.01 (−0.02 to 0.05) | 48 | −0.00 (−0.05 to 0.05) | 41 | 0.99 |
| PCF, L/min | −7.1 (−16.5 to 2.4) | 76 | −9.2 (−14.6 to −3.7)† | 66 | −0.8 (−10.4 to 8.9) | 66 | 0.87 |
| PCFMIC, L/min | 12.3 (4.5 to 20.2)† | 75 | −2.8 (−9.6 to 4.0) | 65 | −14.1 (−25.9 to −2.3) | 64 | 0.02 |
| PCFMIC−PCF | 19.5 (6.6 to 32.3)† | 75 | 6.1 (−1.6 to 13.8) | 65 | −13.6 (−29.8 to 2.7) | 64 | 0.10 |
Definition of abbreviations: Crs = total respiratory system compliance; Δ at baseline = baseline post-LVR minus baseline; Δ at Month 3 = final post-LVR minus Month 3; ERV = expiratory reserve volume; FRC = functional residual capacity; IC = inspiratory capacity; LVR = lung volume recruitment; MIC = maximum insufflation capacity; MIC − VC = maximum insufflation capacity minus vital capacity difference; n = number of randomized participants with immediate effect data at both time points; nB = number of randomized participants with paired data at the baseline assessment; nF = number of participants with paired data at the final, 3-month assessment; PCF = peak cough flow; PCFMIC = peak cough flow from maximum insufflation capacity; PCFMIC−PCF = peak cough flow from maximum insufflation capacity minus peak cough flow difference; RV = residual volume; TLC = total lung capacity; VC = vital capacity.
Data are presented as between-group mean difference (95% confidence interval). Results were not obtainable in all subjects, because of bulbar impairment, technical issues, or fatigue. P values represent paired t tests between baseline and Month 3 assessments (boldface type indicates statistically significant difference [P < 0.05]).
Difference in immediate effect = Δ at Month 3 minus Δ at baseline.
Statistically significant difference on paired t test within the stated time point (e.g., baseline post-LVR assessment minus baseline; P < 0.05).
No treatment, time, or interaction effects were observed in generic HRQoL or the SRI summary scale (AQoL-8D model P = 0.64, SRI summary scale model P = 0.23). The ALSFRS-R summary score significantly declined over 3 months (ALSFRS-R model P < 0.001, time effect P < 0.001), with no difference between treatment groups (LVR, −4.5 [−6.9 to −2.1] points; control, −6.3 [−9.7 to −2.9] points; mean difference, 1.8 [−2.4 to 6.0] points).
The number of participants reporting RTIs was not significantly different over the 6,809 participant-days between the treatment groups (LVR, n = 4 of 37; control, n = 5 of 39).
Discussion
In adults with NMD, 3 months of regular LVR improved the MIC and MIC − VC difference compared with control breathing exercises, but there were no observable differences in respiratory function, HRQoL, symptoms, and RTI rate. Exploratory analyses suggested that MEP declined more in the control arm than the LVR arm among participants with ALS/MND, but the importance of this single finding is debatable.
We observed a mean MIC increase in the LVR arm of 130 (10 to 250) ml or 12% (4% to 21%), attributable largely to participants with slowly progressive NMD. This improvement is similar to the gain of 154 (−13 to 322) ml in LIC reported in a 3-month, prospective, uncontrolled study of 16 adults with ALS/MND, postpolio syndrome, or myotonic dystrophy (mean FVC, 60%pn) (27). In contrast, an uncontrolled study of 18 children and young adults with slowly progressive NMD demonstrated no change in MIC after 4 to 6 months of regular LVR (30). Participants had milder disease (not using NIV with better lung function; mean FVC, 1.78 ± 0.60 L) than the comparable subgroup in our study, suggesting that LVR may demonstrably improve MIC only once a critical, although as yet unclear, degree of VC impairment is apparent.
In this study, the improvement in MIC in the LVR group was evident 1 month after randomization, with no additional increase over time (Figure 2). Furthermore, participants who performed LVR more frequently did not have greater gains in MIC. Given that this was a sample of naive participants, it is plausible that the improvement attributable to LVR reflects a practice effect. Like other authors (27), we speculate that LVR may acclimatize participants to tolerate a higher tolerable inflation capacity rather than causing a physiological increase in absolute lung volume.
The hypothesis that the effects of LVR reflect learning how to breath-stack is supported by the immediate effects results. We observed improvements in MIC, MIC − VC, PCFMIC, PCFMIC-minus-PCF difference, and Crs immediately after a single session of LVR at baseline, a result that may be interpreted as indicating that LVR improves lung and/or chest wall compliance (6). However, this acute response was not replicated at Month 3, despite participants’ being more familiar with LVR and able to stack more volume during the technique (greater MIC − VC difference). We speculate that the immediate effects of LVR seen at baseline in naive participants were attributable to a learning effect as opposed to a treatment effect and that once the technique is learned, this immediate, measurable benefit fades.
In this 3-month trial, longer lasting higher absolute MIC did not translate into improvement in VC, Crs, or PCF. Participants had moderately to severely compromised lung function, and given the age at symptom onset and duration of weakness in the other NMD subgroup, chest wall restriction may have been “fixed” and less modifiable with LVR. Domiciliary NIV may also have confounded the effects of LVR by modifying lung and chest wall distensibility, although comparable NIV use in both groups mitigates this factor.
Two recent pediatric RCTs have similarly failed to demonstrate an effect of regular volume recruitment therapy on respiratory function. Katz and colleagues’ 2-year trial of usual care versus usual care plus LVR in 70 participants with DMD revealed no differences in the rate of FVC %pn decline (mean difference, 1.9% [−6.9% to 10.7%]), MIC − VC trajectory, or any secondary outcomes between groups (8). Median FVC at baseline was 85%pn, and only 6% of participants were NIV users, indicating that LVR was initiated when respiratory compromise was mild. In a study of 34 children with muscular dystrophy and worse respiratory function (median FVC, 56%pn), volume recruitment using a mechanical insufflation–exsufflation device improved FVC more than a no-treatment control at interim visits but not at 12 months (31). Taken together, the pediatric and adult RCT data suggest that prophylactic LVR does not modify respiratory mechanics within 2 years. It is plausible that regular LVR needs to be performed earlier and for longer than in our study for a benefit to be conferred, but initiating LVR too early may also be fruitless (8, 31). To our knowledge, there are no published data detailing the onset or trajectory of decline in Crs in people with NMD, and the optimal time or “sweet spot” for commencing regular LVR is currently unknown and may differ among specific diagnoses.
Consistent with other studies (27, 31, 32), we found that patient-reported outcomes of symptoms (SRI) or generic HRQoL (AQoL-8D) did not change over time or between groups, even in the context of deteriorating physical function in participants with ALS/MND. Moreover, although regular LVR increased MIC, the RTI rate was similar between treatments. This result corroborates data from longer trials showing low RTI rates regardless of treatment (8, 32), indicating that acute respiratory complications are thankfully rare. Studies that test whether regular LVR improves clinical outcomes such as RTI rate, symptoms, or the timing of NIV are needed, although Rafiq and colleagues estimated that more than 200 participants would be required to detect a relative risk reduction of 0.5 in RTI (32).
We objectively measured LVR use and showed that LVR was performed as prescribed for 45% of study days, similar to other clinical trials (between 41% and 50% [8, 27, 31], with one trial reporting 71% [32]). A cohort of 181 participants prescribed daily prophylactic mechanical insufflation– exsufflation suggested that real-world use may be even lower (31% adherent) (33). We believe that this choice by patients to undertake regular respiratory therapy or not is an important consideration for research translation. Competing activities, limited time, forgetting, oppositional behaviors, no perceived benefit, discomfort, and difficulty performing LVR have been identified as factors contributing to nonadherence with recommended therapy (27, 31).
Self-report methods of adherence overestimate LVR use (26); hence, it is critical that future studies collect objective measures of LVR. Participant-reported outcomes and qualitative data evaluating participant-perceived benefits and barriers to treatment are also necessary. With limited level 1 evidence to support care recommendations, the decision to prescribe regular prophylactic therapy should incorporate individual patient views on the perceived benefit and burden of adding another task to daily care routines.
Strengths and Limitations
The delivered dose of LVR in the intervention arm may not have been enough to produce a clinically meaningful change. It is plausible that 3 months of therapy may be too short to produce physiological changes in people with long-standing disease, but previous uncontrolled studies have demonstrated improvements in LIC, PCF, VC, and static lung volume over similar durations (27, 30, 34, 35).
Although the prescribed dose was consistent with that reported in the literature (9, 10, 27, 30, 31), actual use was reduced (average, 1.2 sessions/day). Although this more closely reflects real-world clinical practice, reduced adherence may have affected the study’s ability to detect a change. Exploratory analyses were undertaken and suggested that use did not confound the results, but the study was not powered for this analysis.
An active control was chosen to match participant-to-therapist interaction and daily treatment duration, as sham LVR is impossible. It is unlikely that our active control minimized any opportunity to observe benefits from LVR, because breathing exercises as used in our control arm do not deliver lasting physiological change in healthy participants (36) nor in those with NMD (37).
We have used the label “MIC” for the primary outcome herein, as at the time of trial registration, the terms “MIC” and “LIC” were used interchangeably to refer to the exhaled volume from the maximum tolerable insufflation capacity, regardless of the method to assist inflation. In a recent review, MIC and LIC were separated according to whether the glottis is actively controlled during insufflation (3). Our measurement using an LVR kit with a one-way valve is thus more consistent with the contemporary definition of LIC.
The noninvasive and portable Crs method we used obtained measurements in 91% of participants at baseline and allowed serial follow-up at participants’ homes each month. However, the absence of invasive esophageal pressure measurement and the resultant inability to differentiate between lung and chest wall compliance is a limitation of this technique.
Retrospective case series have suggested the rate of FVC decline slows after LVR initiation (9–12), but these cohorts are frequently methodologically compromised, especially by case ascertainment biases and incomplete follow-up. We found no relationship between the change in MIC and the change in VC or Crs that was attributable to LVR. Our results are therefore inconsistent with previously hypothesized mechanisms: that LVR maintains or improves lung and chest wall distensibility over time and slows the decline in VC.
Conclusions
LVR increases MIC when prescribed twice daily for 3 months in adults with NMD. We did not observe differences in other respiratory variables (HRQoL, symptoms, and RTI rate), and we found no direct evidence to support the hypotheses that regular LVR modifies respiratory mechanics or slows the rate of lung volume decline. Moreover, given that participants were naive to LVR, the time course of improvement, the lack of a dose–response relationship between LVR and change in Crs, and the attenuation of immediate response to LVR at 3 months, we speculate that the MIC improvement we found primarily reflects a practice effect.
Our data suggest that LVR may need to be commenced within a specific and as yet unknown window of restrictive impairment for it to have a prophylactic effect, if at all. The longer term clinical impact of increasing this measurable volume remains to be determined, and prospective cohorts with objective LVR use and clinically meaningful outcome data are now needed.
Acknowledgments
Acknowledgment
The authors acknowledge the assistance of the following research assistants and physiotherapists who helped conduct participant assessments: Carmel Nicholls, Sandra Henderson, Alyssa Rigoni, Krisha Saravanan, Rebecca Dirago, Phoebe Naughton, Marlena Ahrens, Megan Hawkins, Luke McDonald, and Sarah Retica. The authors also thank Associate Professor Graham Hepworth for statistical consulting (Statistical Consulting Centre, University of Melbourne).
Footnotes
Supported by National Health and Medical Research Council/Motor Neurone Disease Research Institute of Australia cofunded Postgraduate Scholarship 2014/GNT1093831, Mavis Gallienne MND Victoria research grant GIA 1703, Institute for Breathing and Sleep research grants 2014 and 2018, and Physiotherapy Research Foundation seeding grant S14-013. In-kind support was provided by the Institute for Breathing and Sleep, Victorian Respiratory Support Service, and Department of Respiratory and Sleep Medicine, Austin Health. The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions: All authors were involved in the trial design, contributed to drafting the manuscript, revised it critically for important intellectual content, and approved the final version for publication. N.L.S., D.J.B., and M.E.H. were responsible for oversight and governance of the trial. D.A.M. provided substantial contributions to the conception and design of the study and assistance with the lung volume recruitment counter. P.D.R. provided respiratory physiology and measurement expertise. N.L.S., L.R., and C.C. standardized the interventions, and L.R. and C.C. conducted all participant training. N.L.S. was the trial manager, recruited participants, and performed all blinded data collection. N.L.S., D.J.B., and M.E.H. had full access to all the data in the study, performed the statistical analyses, and verified the data reported in the manuscript.
Data sharing: Requests for access to deidentified data can be made to the corresponding author. All reasonable requests will be contingent on successful application to the authors’ institutional research ethics committee.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. De Troyer A, Borenstein S, Cordier R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax . 1980;35:603–610. doi: 10.1136/thx.35.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benditt JO, Boitano LJ. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med . 2013;187:1046–1055. doi: 10.1164/rccm.201210-1804CI. [DOI] [PubMed] [Google Scholar]
- 3. Chatwin M, Toussaint M, Gonçalves MR, Sheers N, Mellies U, Gonzales-Bermejo J, et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med . 2018;136:98–110. doi: 10.1016/j.rmed.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 4. Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, et al. DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol . 2018;17:347–361. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheers N, Howard ME, Berlowitz DJ. Respiratory adjuncts to NIV in neuromuscular disease. Respirology . 2019;24:512–520. doi: 10.1111/resp.13431. [DOI] [PubMed] [Google Scholar]
- 6. Sheers NL, Berlowitz DJ, Dirago RK, Naughton P, Henderson S, Rigoni A, et al. Rapidly and slowly progressive neuromuscular disease: differences in pulmonary function, respiratory tract infections and response to lung volume recruitment therapy (LVR) BMJ Open Respir Res . 2022;9:e001241. doi: 10.1136/bmjresp-2022-001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molgat-Seon Y, Hannan LM, Dominelli PB, Peters CM, Fougere RJ, McKim DA, et al. Lung volume recruitment acutely increases respiratory system compliance in individuals with severe respiratory muscle weakness. ERJ Open Res . 2017;3:00135-2016. doi: 10.1183/23120541.00135-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katz SL, Mah JK, McMillan HJ, Campbell C, Bijelić V, Barrowman N, et al. Routine lung volume recruitment in boys with Duchenne muscular dystrophy: a randomised clinical trial. Thorax . 2022;77:805–811. doi: 10.1136/thoraxjnl-2021-218196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang SW, Bach JR. Maximum insufflation capacity. Chest . 2000;118:61–65. doi: 10.1378/chest.118.1.61. [DOI] [PubMed] [Google Scholar]
- 10. Bach JR, Mahajan K, Lipa B, Saporito L, Goncalves M, Komaroff E. Lung insufflation capacity in neuromuscular disease. Am J Phys Med Rehabil . 2008;87:720–725. doi: 10.1097/PHM.0b013e31817fb26f. [DOI] [PubMed] [Google Scholar]
- 11. Katz SL, Barrowman N, Monsour A, Su S, Hoey L, McKim D. Long-term effects of lung volume recruitment on maximal inspiratory capacity and vital capacity in Duchenne muscular dystrophy. Ann Am Thorac Soc . 2016;13:217–222. doi: 10.1513/AnnalsATS.201507-475BC. [DOI] [PubMed] [Google Scholar]
- 12. McKim DA, Katz SL, Barrowman N, Ni A, LeBlanc C. Lung volume recruitment slows pulmonary function decline in Duchenne muscular dystrophy. Arch Phys Med Rehabil . 2012;93:1117–1122. doi: 10.1016/j.apmr.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 13. Sheers N, Howard M, Rautela L, Chao C, Rochford P, Berlowitz D. Lung volume recruitment therapy in people with neuromuscular disease. Respirology . 2021;26:24. [Google Scholar]
- 14. Sheers N, Howard M, Rautela L, Chao C, Rochford P, Berlowitz D. A randomised controlled trial of lung volume recruitment in people with neuromuscular disease. Eur Respir J . 2020;56:1262. [Google Scholar]
- 15. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gosselink RAAM, Wagenaar RC, Rijswijk H, Sargeant AJ, Decramer MLA. Diaphragmatic breathing reduces efficiency of breathing in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 1995;151:1136–1142. doi: 10.1164/ajrccm.151.4.7697243. [DOI] [PubMed] [Google Scholar]
- 17. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41:507–522. doi: 10.1183/09031936.00069712. [DOI] [PubMed] [Google Scholar]
- 19. American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med . 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 20. Kelley A, Garshick E, Gross ER, Lieberman SL, Tun CG, Brown R. Spirometry testing standards in spinal cord injury. Chest . 2003;123:725–730. doi: 10.1378/chest.123.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruschi C, Cerveri I, Zoia MC, Fanfulla F, Fiorentini M, Casali L, et al. Reference values of maximal respiratory mouth pressures: a population-based study. Am Rev Respir Dis . 1992;146:790–793. doi: 10.1164/ajrccm/146.3.790. [DOI] [PubMed] [Google Scholar]
- 22. Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax . 1995;50:371–375. doi: 10.1136/thx.50.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson J, Khan M, Iezzi A, Sinha K, Mihalopoulos C, Herrman H, et al. 2009.
- 24. Ghosh D, Rzehak P, Elliott MW, Windisch W. Validation of the English severe respiratory insufficiency questionnaire. Eur Respir J . 2012;40:408–415. doi: 10.1183/09031936.00152411. [DOI] [PubMed] [Google Scholar]
- 25. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. BDNF ALS Study Group (Phase III) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci . 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 26. Naughton PE, Sheers N, Berlowitz DJ, Howard ME, McKim DA, Katz SL. Objective measurement of lung volume recruitment therapy: laboratory and clinical validation. BMJ Open Respir Res . 2021;8:e000918. doi: 10.1136/bmjresp-2021-000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaminska M, Browman F, Trojan DA, Genge A, Benedetti A, Petrof BJ. Feasibility of lung volume recruitment in early neuromuscular weakness: a comparison between amyotrophic lateral sclerosis, myotonic dystrophy, and post-polio syndrome. PM R . 2015;7:677–684. doi: 10.1016/j.pmrj.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 28. Lin LIK. A concordance correlation coefficient to evaluate reproducibility. Biometrics . 1989;45:255–268. [PubMed] [Google Scholar]
- 29. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet . 1986;1:307–310. [PubMed] [Google Scholar]
- 30. Marques TBC, de Carvalho Neves J, Portes LA, Salge JM, Zanoteli E, Reed UC. Air stacking: effects on pulmonary function in patients with spinal muscular atrophy and in patients with congenital muscular dystrophy. J Bras Pneumol . 2014;40:528–534. doi: 10.1590/S1806-37132014000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawnani H, Mayer OH, Modi AC, Pascoe JE, McConnell K, McDonough JM, et al. Randomized trial of lung hyperinflation therapy in children with congenital muscular dystrophy. Pediatr Pulmonol . 2020;55:2471–2478. doi: 10.1002/ppul.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rafiq MK, Bradburn M, Proctor AR, Billings CG, Bianchi S, McDermott CJ, et al. A preliminary randomized trial of the mechanical insufflator-exsufflator versus breath-stacking technique in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener . 2015;16:448–455. doi: 10.3109/21678421.2015.1051992. [DOI] [PubMed] [Google Scholar]
- 33. Chatwin M, Simonds AK. Long-term mechanical insufflation-exsufflation cough assistance in neuromuscular disease: patterns of use and lessons for application. Respir Care . 2020;65:135–143. doi: 10.4187/respcare.06882. [DOI] [PubMed] [Google Scholar]
- 34. Nygren-Bonnier M, Markström A, Lindholm P, Mattsson E, Klefbeck B. Glossopharyngeal pistoning for lung insufflation in children with spinal muscular atrophy type II. Acta Paediatr . 2009;98:1324–1328. doi: 10.1111/j.1651-2227.2009.01343.x. [DOI] [PubMed] [Google Scholar]
- 35. Nygren-Bonnier M, Wahman K, Lindholm P, Markström A, Westgren N, Klefbeck B. Glossopharyngeal pistoning for lung insufflation in patients with cervical spinal cord injury. Spinal Cord . 2009;47:418–422. doi: 10.1038/sc.2008.138. [DOI] [PubMed] [Google Scholar]
- 36. Vieira DS, Mendes LP, Elmiro NS, Velloso M, Britto RR, Parreira VF. Breathing exercises: influence on breathing patterns and thoracoabdominal motion in healthy subjects. Braz J Phys Ther . 2014;18:544–552. doi: 10.1590/bjpt-rbf.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nardin R, O’Donnell C, Loring SH, Nie R, Hembre K, Walsh J, et al. Diaphragm training in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis . 2008;10:56–60. doi: 10.1097/CND.0b013e31818cf6df. [DOI] [PubMed] [Google Scholar]