Abstract
Ferroptosis is a form of regulated cell death that was first formally proposed a decade ago. While its role in cancer cell death was initially understudied, it has recently gained considerable interest from researchers. In recent years, a growing number of studies have focused on the role of ferroptosis in cancer progression, with the goal of developing novel ferroptosis-inducing cancer therapies. This study aims to present the developmental trend and hotspots of research on ferroptosis-inducing cancer therapy using bibliometric analysis. A literature search was conducted using the Web of Science Core Collection on October 1st, 2022, to retrieve articles and reviews pertaining to ferroptosis and cancer published from 2012 to 2022. Microsoft Excel 2016, VOSviewer 1.6.18 and CiteSpace (version 6.1. R6) were utilized to conduct the bibliometric analysis of publication trends, authorship, and citation networks, with a focus on identifying countries, institutions, journals, and authors contributing to the field. These analyses were used to predict future trends in this area. A total of 2839 articles were identified and extracted for analysis. The number of publications has increased almost every year, with a sharp increase after 2018. China produced the most publications in this area, followed by the United States. Central South University was the institution that published the most papers. Frontiers in Oncology was the journal with the highest number of publications, while Cell had the greatest impact factor. Daolin Tang was the most productive author and Dixon SJ was the most influential author. Co-occurrence and burst analyses of keywords and references were conducted to identify the developmental trends and hotspots in ferroptosis-inducing cancer therapy research. Main research directions have shifted from investigating the mechanism of ferroptosis to developing novel ferroptosis-targeting cancer therapies. Emerging topicsfocus on the role of ferroptosis in solid tumor therapy. Based on our bibliometric analysis, we predict that research on ferroptosis in cancer therapy will continue to be a hot topic in the future, with a growing number of treatment modalities related to ferroptosis being developed. Our study provides valuable insights into the current state and future trends of research in this field, serving as a useful guide for researchers seeking to make important contributions in this area.
Keywords: Ferroptosis, Cancer therapy, Bibliometric analysis, CiteSpace, VOSviewer
1. Introduction
Ferroptosis is a form of regulated cell death that was first proposed by Dixon et al., in 2012 [1]. Unlike other types of cell death, ferroptosis is triggered by the accumulation of lipid peroxides and the production of reactive oxygen species (ROS) on cellular membranes, both of which are driven by active ferrous ions [2,3]. Ferroptosis occurs when the accumulation of lipid peroxide exceeds the upper limit of cell tolerance [4,5]. Evidence of ferroptosis has been observed in various diseases, including cancers [6], neurodegenerative diseases [7], cardiovascular diseases [8,9], stroke [10] and so on. As a novel form of regulated cell death, ferroptosis has garnered significant attention from researchers seeking new therapeutic strategies for cancer.
Over the years, researchers have achieved significant progress in understanding the relationship between ferroptosis and cancer. Polyunsaturated fatty acid-containing membrane-localized lipids is the key factor in the occurrence of ferroptosis [11]. In the past few years, researchers have identified four currently recognized ferroptosis suppressing pathways. The latest advances suggested that GSH-GPX4 (glutathione peroxidase-4) pathway is one of the most common pathways that resists ferroptosis. The glutathione-dependent lipid hydroperoxidase GPX4 can reduce the accumulation of lipid hydroperoxides by converting them into non-toxic lipid alcohols on cellular membrane, thereby preventing ferroptosis in cancer cells [5]. Ferroptosis suppressor protein 1 (FSP1)/CoQ10 is another ferroptosis suppressing pathway. In plasma membrane, FSP1 could mediate the reduction of CoQ, which is an antioxidant, thus protecting cancer cells from ferroptosis [12]. In 2020, a novel system was found to suppress ferroptosis by James et al. In their study, they found tetrahydrobiopterin (BH4), a radical-trapping antioxidant, which restrains ferroptosis by protecting lipid membranes from autoxidation [13]. Moreover, dihydroorotate dehydrogenase (DHODH) was identified to function parallelly to GPX4 and FSP1 [14]. DHODH could reduce ubiquinone to ubiquinol and suppress ferroptosis in mitochondria, inactivation of DHODH in GPX4lowcancer cells could induce ferroptosis. These findings suggest that ferroptosis plays a suppressor role in the development of cancer, indicating that targeting ferroptosis in cancer could be a novel therapeutic therapy.
Bibliometric analysis is a valuable tool for quantitative analysis of literature and statistical analysis of developmental trends and hotspots in a given field [15]. It is also using for describing the contributions of countries, institutions, journals and authors. Through the intuitive cooperation network, bibliometric analysis can effectively present the collaboration relationship between countries, institutions and authors [16,17]. As previously mentioned, considerable progress has been made in the study of ferroptosis targeted cancer treatments in recent years. Herein, we conducted a bibliometric analysis to evaluate the research focus, emerging trends and future research priorities in the field of targeted therapy of ferroptosis in cancer.
2. Method
2.1. Data source and search strategy
Web of science is a widely recognized academic database that contains a plethora of valuable global academic information in the world. The Science Citation Index Expanded (SCIE) database of the Web of Science Core Collection (WoSCC), which is a central database with mainstream journals in natural science, was employed for literature retrieval in this study. To avoid citation duplication and database updating, all source publication searches and data download were performed on October 1st, 2022. The following search formulas: TS= (ferroptosis OR ferroptotic) AND TS= (cancer OR tumor OR neoplasm OR carcinoma OR
Adenocarcinoma OR leukemia OR sarcoma OR lymphoma OR oncology OR melanoma OR osteosarcoma OR maligancy). We limited the publication time range from January 1st, 2010 to October 1st, 2022, the document types to articles or reviews and the document language to English. The search yielded a total of 3269 papers. Two independent researchers filtered the title, abstract of these articles to determine that these articles focus on the relationship between tumor and ferroptosis. Ultimately, 2839 studies were included and applied in this study. We extracted some important information of these articles such as title, keywords, authors, institutions, countries and regions, publication year, publishing journals and saved them in plain files for further analysis.
2.2. Bibliometric analyzing
All data was imported to Microsoft Excel 2016, VOSviewer 1.6.18 and CiteSpace (version 6.1. R6) for further analysis.
Microsoft Excel 2016 was used to analyze trend of annual publications of countries/institutions, number of citations of top countries, top publication and citations of journals, most productive authors, maximum occurrences keywords and most cited references.
CiteSpace is a citation visualization software developed by Chaomei Chen[18], which was used to analyze the basic article information like authors, keywords and so on. It provides an experimental platform to preform co-occurrence visual network, observe the research development process, detect topic change trends in a specific field and predict the future research direction[19]. We visualized collaboration relationship between countries, institutions, authors and co-citation relationship of authors. Moreover, we employed CiteSpace to conduct a burst analysis and a timeline view of keywords to demonstrate research hotpots and cited references with strong burst was also done to present the high influential references. The high-frequency keywords were clustered by CiteSpace. Furthermore, in order to explore the scientific distribution, we made a dual-map overlay of journals.
VOSviewer was developed by Nees Jan van Eck and Ludo Waltman in 2009 and used to analyze bibliographic coupling indicators[20]. VOSviewer is also a powerful tool which can display large bibliometric network maps. In this study, we used it to analysis the co-occurrence of keywords.
3. Results
3.1. Global publications trend analysis
The search yielded a total of 2839 articles on the relation between tumor and ferroptosis from WoSCC with 2144 papers (75.52%) classified as articles and 694 papers (24.48%) as reviews. The distribution of publications and citations over time can reflect the evolution of a research field. Fig. 1A and B presents the cumulative and annual number of papers published from 2012 to 2022. The first article was published in 2012 [1], which was a pioneering work in this field. However, the number of annual publications remained low in the following four years. Since 2016, the number of papers has gradually increased. From 2018 to the present, there has been an apparent and qualitative leap, and the cumulative number of papers is increasing dramatically. In 2022, the annual number of publications has already exceeded 1000, indicating that research on ferroptosis and oncology has become a hotspot and that scholars are enthusiastic about it.
Fig. 1.
The annual number (A) and cumulative number(B) of publications on ferroptosis and cancer from 2012 to 2022.
3.2. Country and institution analysis
Over the past decade, numerous institutions from 76 countries have contributed papers to this field. As illustrated in Table 1, among these 76 countries, only the top 8 countries have produced more than 50 publications, primarily from Asia, America, and Europe. The People's Republic of China leads the way with the largest output of publications (2008, 70.73%), followed by the United States (506, 25.20%), Germany (139, 4.90%) and Japan (122, 4.30%). While the first fundamental paper was published in USA, China has far surpassed other countries in terms of the number of documents issued. But there are different results when concerning the citation time, the frequency of citations of China (41,965 times) is lower than that of American (50,809 times). This discrepancy may be attributed to the large number of papers published in China were in recent years and needed to be verified. As depicted in Fig. 2A, a network visualization map of international research collaboration among the leading countries, China has the biggest node. The width of the connection indicates the degree of cooperation between countries. Strong connections between countries denote dense cooperations between countries. For example, the People's Republic of China has a close collaboration with the USA, Germany cooperates tightly with USA. Additionally, countries such as USA (0.43), Germany (0.36) and People's Republic of China (0.21) has high centrality, implying that these countries played a crucial role as a bridge in international cooperation between countries.
Table 1.
List of top 10 countries publishing research on ferroptosis in cancer therapy.
| Ranking | Country | Number of publications | Citation | Average citation |
|---|---|---|---|---|
| 1st | CHINA | 2008 | 41,965 | 20.90 |
| 2nd | USA | 506 | 50,809 | 100.41 |
| 3rd | GERMANY | 139 | 12,651 | 91.01 |
| 4th | JAPAN | 122 | 8185 | 67.09 |
| 5th | ITALY | 69 | 1739 | 27.60 |
| 6th | FRANCE | 61 | 4356 | 71.41 |
| 7th | SOUTH KOREA | 50 | 2234 | 44.68 |
| 8th | AUSTRALIA | 46 | 3992 | 86.78 |
| 9th | CANADA | 38 | 2843 | 74.82 |
| 10th | RASSIA | 38 | 2054 | 54.05 |
Fig. 2.
Analysis of countries (A) and institutions (B) engaged in ferroptosis in cancer therapy.
All these papers were published in 424 institutions, of which the leading top 10 productive were listed in Table 2. Among them, the top five institutions are Cent South Univ (113, 3.98%), Zhejiang Univ (106, 3.73%), Shanghai Jiao Tong Univ (99, 3.49%), Guangzhou Med Univ (90, 3.17%) and Fudan Univ (85, 2.99%). The top 15 institutions are all from People's Republic of China, with Columbia University from the United States ranking 16th, reflecting the popularity of this field in China. As shown in Fig. 2B, collaboration between institutions is more prevalent than between countries. Columbia Univ has a broad collaboration with other institutions. The top institutions for centrality are Chinese Acad Sci (0.28), followed by Columbia Univ (0.24) and Harvard Med Sch (0.21), highlighting their critical role in facilitating cooperation between institutions. Furthermore, Chinese institutions' articles appear to be younger than those of other countries, with Columbia University being the earliest institution to publish research in this field.
Table 2.
List of top 10 productive institutions related to ferroptosis in cancer therapy.
| Ranking | Institution | Country | Count | % |
|---|---|---|---|---|
| 1st | Cent South Univ | China | 113 | 3.98% |
| 2nd | Zhejiang Univ | China | 106 | 3.73% |
| 3rd | Shanghai Jiao Tong Univ | China | 99 | 3.49% |
| 4th | Guangzhou Med Univ | China | 90 | 3.17% |
| 5th | Fudan Univ | China | 85 | 2.99% |
| 6th | Sun Yat Sen Univ | China | 84 | 2.96% |
| 7th | Chinese Acad Univ | China | 82 | 2.89% |
| 8th | Shandong Univ | China | 58 | 2.04% |
| 9th | Sichuan Univ | China | 57 | 2.01% |
| 10th | Wuhan Univ | China | 57 | 2.01% |
3.3. Journal and co-cited journal analysis
A total of 2839 papers were published in 634 journals. Table 3 presents the top 10 journals that contribute to this field. Frontiers in oncology published the most articles as the leading journal with 126 publications representing 4.44% of all publications. It was followed by frontiers in cell and developmental biology (102, 3.59%), cell death & disease (64, 2.25%), biochemical and biophysical research communications (55, 1.94%), frontiers in genetics (54, 1.94%). These journals accounted for 20.57% of all publications in this field.
Table 3.
List of top 10 journals and co-cited journal related to ferroptosis in cancer therapy.
| Ranking | Journal | Count | IF (2022) | Country | JCR (2022) | Co-cited Journal | Citation | IF (2022) | Country | JCR (2022) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st | Frontiers in oncology | 126 | 5.738 | Switzerland | Q2 | Cell | 7238 | 66.85 | USA | Q1 |
| 2nd | Frontiers in cell and developmental biology | 102 | 6.081 | Switzerland | Q1 | Nature | 6632 | 69.504 | England | Q1 |
| 3rd | Cell Death & Disease | 64 | 9.685 | England | Q1 | Proceedings Of the National Academy of Sciences of The United States of America | 3720 | 12.779 | USA | Q1 |
| 4th | Biochemical and Biophysical Research Communications | 55 | 3.322 | USA | Q3 | Journal Of Biological Chemistry | 3247 | 4.7 | USA | Q2 |
| 5th | Frontiers in genetics | 54 | 4.772 | Switzerland | Q1 | Cell Death and Differentiation | 3100 | 12.067 | England | Q1 |
| 6th | Frontiers in pharmacology | 49 | 5.988 | Switzerland | Q1 | Cancer Research | 3090 | 13.312 | USA | Q1 |
| 7th | Oxidative Medicine and Cellular Longevity | 49 | 7.21 | USA | Q1 | Free Radical Biology and Medicine | 2726 | 8.101 | USA | Q1 |
| 8th | International Journal of Molecular Sciences | 46 | 6.208 | Switzerland | Q1 | Cell Death & Disease | 2571 | 9.685 | England | Q1 |
| 9th | Redox Biology | 39 | 10.787 | Netherlands | Q1 | Nature Communications | 2514 | 17.694 | England | Q1 |
| 10th | Frontiers in immunology | 39 | 8.786 | Switzerland | Q1 | Biochemical And Biophysical Research Communications | 2311 | 3.322 | USA | Q1 |
The impact factor (IF) is a widely used metric to judge the authority of a journal in particular fields which is related to its co-citation frequency [21]. Higher IF means publications in that journal have more citations and journals is more important in its field. Journals with higher IFs are considered to be more important in their respective fields. As can be seen in Table 3, Cell is the most frequently cited journal with its cited frequency exceeding 7000. Nature was the second with cited frequency of 6632, followed by P Natl Acad Sci USA (3720), J Biol Chem (3247) and Cell Death Differ (3100).
To further explore the relationships between citing and cited journals, we used CiteSpace to create a dual-map overlay of journals. The left side represents citing journals, while the right side represents cited journals. The color path that is from left side to right side represents the relationship of their citation relationship. In Fig. 3, there are mainly one orange path, which indicates that papers published in “Molecular, Biology, Genetics” journals were mostly frequently cited in papers published in “Molecular, Biology, Immunology” journals.
Fig. 3.
A dual-map overlay of journals related to ferroptosis in cancer therapy.
The analysis of the top contributing journals and their impact factors, as well as the relationship between citing and cited journals, provides valuable insights into the publishing trends and the most influential sources in this field.
3.4. Author and co-cited author analysis
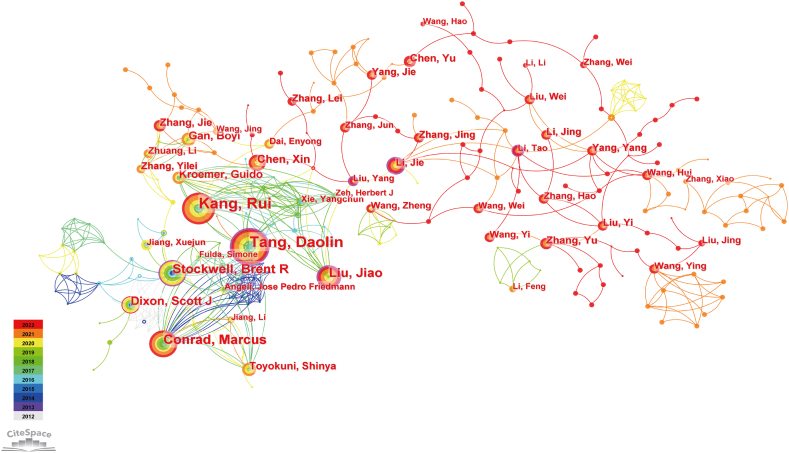
More than 1,5000 researchers co-authored these publications related to ferroptosis and cancer. Among them, Daolin Tang headed the list with 58 documents, followed by Rui Kang (n = 54), Conrad Marcus (n = 29), Jiao Liu (n = 28), and Stockwell Brent R (n = 26) (Table 4). We set the minimum number of documents of an author to 5, and 363 authors met the threshold. The collaboration map in Fig. 4 show authors with high centrality having a purple ring around the node. As we can see, Daolin Tang (0.20), Jiao Liu (0.20) and Stockwell Brent R (0.14) not only have the highest productivity but also high centrality, indicating their close cooperative relationship with other authors in this field. This suggests enhanced cooperation may lead to more productive outcomes.
Table 4.
List of top 10 productive and co-cited authors.
| Ranking | Author | Count | Country | Co-cited Author | Count | Country |
|---|---|---|---|---|---|---|
| 1st | Tang DL | 58 57 | China | Dixon SJ | 2903 | USA |
| 2nd | Kang R | 54 54 | China | Yang WS | 2291 | USA |
| 3rd | Conrad M | 29 26 | Germany | Stockwell Brent R | 1227 | USA |
| 4th | Liu J | 28 28 | China | Angeli JPF | 1007 | Germany |
| 5th | Stockwell Brent R | 26 26 | USA | Doll S | 978 | Germany |
| 6th | Dixon SJ | 19 21 | USA | Gao MH | 978 | USA |
| 7th | Chen X | 18 19 | China | Jiang L | 707 | USA |
| 8th | Kroemer G | 16 17 | France | Hassannia B | 646 | Belgium |
| 9th | Gan BY | 15 17 | USA | Chen X | 633 | China |
| 10th | Gu W | 15 16 | USA | Sun XF | 611 | China |
Fig. 4.
Collaboration map of author of the articles related to ferroptosis in cancer therapy.
When two or more authors are cited by one paper simultaneously, they are considered to be co-cited authors [22]. As shown in Table 4, the numbers of citation of the top 10 co-cited authors over 600 times. The most frequently co-cited author is Dixon Scott J (2903 citations), far ahead of the authors behind, who also has a high article output, which indicates that he has achieved a lot of convincing and impactful results.
Keywordanalysis; Keyword co-occurrence analysis can well illustrate the research frontiers and hotspots in research on ferroptosis and cancer. This analysis was performed on the 2839 articles mentioned above. We merged similar keywords (immune therapy and immunotherapy, cell-death and cell death) and generated a keyword co-occurrence map using VosViewer to analyze the relationship between different subjects (Fig. 5A). Fig. 5B shows a density map, which can intuitively display frequency of keywords. Table 5 shows the frequency of keywords. The frequency of keyword “ferroptosis” is far ahead (n = 1872), followed by cell-death (n = 636), cancer (n = 631), apoptosis (n = 461) and iron (n = 448). In Fig. 5A, the node represents keywords, and the size of node corresponds to the frequency of the keywords. The colors of the nodes represent particular year: color from purple to yellow indicates time from far to near. In early years, keywords “cell death, cancer, apoptosis” appear more frequently, while keyword “hepatocellular cancer, gastric cancer, prognosis” appear more in recent years. This trend shows that the research focus in this field is shifted to specific cancer diseases.
Fig. 5.
(A) CiteSpace visualization map of the keyword analysis. (B) A density map of keywords. (C) Network map of keyword clusters analysis. (D) Timeline view of keyword related to ferroptosis in cancer therapy.
Table 5.
List of top 20 keywords.
| Ranking | Keyword | Count | Ranking | Keyword | Count |
|---|---|---|---|---|---|
| 1st | ferroptosis | 1872 | 11th | activation | 290 |
| 2nd | cell death | 636 | 12th | autophagy | 244 |
| 3rd | cancer | 631 | 13th | cancer cell | 243 |
| 4th | apoptosis | 461 | 14th | cell | 241 |
| 5th | iron | 448 | 15th | resistance | 231 |
| 6th | expression | 437 | 16th | inhibition | 219 |
| 7th | death | 411 | 17th | lipid-peroxidation | 202 |
| 8th | metabolism | 402 | 18th | prognosis | 181 |
| 9th | oxidative stress | 390 | 19th | pathway | 157 |
| 10th | mechanism | 345 | 20th | gpx4 | 151 |
By clustering common keywords, a total of 10 clusters were obtained, each cluster represents a research direction (Fig. 5C). For instance, cluster 0 is the largest cluster with 86 keywords, the most common keywords are gastric cancer, pancreatic cancer, invasion, immune infiltration and receptor, indicating that tumor of digestive tract was a popular research topic and attracted more attention of researchers in last decade. Cluster 1 was the earliest cluster based on the time whose keyword first appeared, the most used keywords are cell death, cancer, expression, oxidative stress and metabolism. In the cluster 2, the most used keywords are lipid peroxidation, peroxidation, homeostasis, polyunsaturated fatty acid and hydrogen peroxide. Cell, sensitivity, target, nrf2 and gpx4 are the most frequent keywords in cluster 3. In cluster 4, keywords with the most repetition are colorectal cancer, biology, molecular mechanism, gene expression and metastasis. There are 62 keywords in cluster 5, including immunotherapy, immune microenvironment, long noncoding RNA, endoplasmic reticulum, stress and prognostic signature. Cluster 6 contains 56 keywords, including breast cancer, therapy, proliferation, survival and lung cancer. In the cluster 7, tumor microenvironment, photodynamic therapy, delivery, drug delivery are the most frequent keywords in all 55 keywords. There are 47 keywords in cluster 8, including promotes ferroptosis, cystine/glutamate, antiporter, gene signature, cisplatin resistance and inhibits ferroptosis. Cluster 9 contains the minimal keywords. There are 37 keywords in cluster 9, including promote, artemisinin, complex, temozolomide and cycle arrest. Moreover, a time line viewer of keywords based on the keywords clustering was built using CiteSpace, which could explain the trend of research topics in this field over time (Fig. 5D). It is intuitive to see the research focus at each stage and explore the evolution track.
“Burst” means that the frequency of keywords suddenly rises sharply and lasts for a period of time. Keyword burst analysis can reflect the evolution of frontier hotspots in a field and predict the future direction to a certain extent. The top 30 keywords with the strongest citation bursts were presented by CiteSpace (Fig. 6). In Fig. 6, the blue line represents the time span, while the red line represents the time period of keyword burst. Among the top 20 keywords with citation bursts, “tumor suppression” has the strongest burst, suggesting ferroptosis in tumor suppression is a hotspot from 2016 to 2018. This indicated that ferroptosis may play an important role in anti-tumor. “Cancer cell” and “hydrogen peroxide” is the keyword with the longest burst duration (6 years), meaning researchers have focused on biochemical mechanism of cancer cell metabolism for a long time. Moreover, in recent years, keyword “tumor immunity” and “immunity status” began to explode, showing that attention had shifted to finding immune therapy of cancer through ferroptosis pathway.
Fig. 6.
Time trend of keyword burst related to ferroptosis in cancer.
3.5. References analysis and co-cited reference analysis
Co-cited references refer to a group of articles that are frequently cited together in other articles. The frequency of co-citations can present the academic value of articles and the evolutionary process in a research field [23,24]. Highly cited articles mean they play a pivotal role in the cooperation with other articles. Table 6 summarizes the top ten most cited references. These articles were mainly published between 2012 and 2019, which may be because the articles published in the past 3 years need more time and more experiments to be verified. Among these ten articles, most articles were cited more than 500 times. All these articles are published in journals with high impact factors, the top 3 references were all from cell. Dixon SJ is the only author who has two papers in the top ten most cited references. The titles of these two references are “Ferroptosis: an iron-independent form of nonapoptotic cell death” [1] and “Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis” [25]. The former was groundbreaking work, who first proposed the concept of ferroptosis. In their statement, ferroptosis is an iron-dependent form of nonapoptotic cell death that is morphologically, biochemically, and genetically distinct from known forms of cell death, which can be triggered by erastin through inhibiting system Xc−-mediated cystine-glutamate exchange. And activation of ferroptosis results in the destruction of certain cancer cells, and could protect organisms against neurodegeneration when inhibiting this process. The study further identified that ferroptosis is regulated by a distinct set of genes including acyl-CoA synthetase family member 2 (ACSF2), citrate synthase (CS) and so on,. Moreover, they found a compound named ferrostatin-1 that could effectively inhibit ferroptosis in cancer cell. These findings provided a novel strategy for cancer therapy. Furthermore, their studies discovered sorafenib facilitated ferroptosis in cancer cells through inhibiting system Xc− function. This may open up new applications for sorafenib in the treatment of cancer in future. The second most cited paper was published in 2014, titled “regulation of ferroptotic cancer cell death by GPX4” [5]. In their mouse models experiment, they discovered the role of GPX4 in reducing lipid peroxide and the importance in protecting cells from lipid peroxides. Moreover, they first proved that GPX4 could mitigate ferroptosis. Results of mouse xenografts model experiments showed inactivation of GPX4 could induce ferroptosis and inhibit tumor growth, which bring hope for the applications of ferroptosis-induced compounds. An increasing number of studies on GPX4 have further confirmed its crucial role in the ferroptosis induced cancer therapy. The third most cited article was a review published in cell by Stockwell Brent R [26]. This article reviewed the mechanisms underlying ferroptosis and strengthened the connection between ferroptosis and other diseases. The article titled “Ferroptosis as a p53-mediated activity during tumor suppression” ranked fifth [27]. It concluded that p53 and p533KR sensitized cells to ferroptosis by repressing the expression of SLC7A11 (a key component of the cystine/glutamate antiporter), which is highly expressed in certain tumors. According their conclusions, p53 could suppress cystine uptake in vivo and ROS stress jointly lead to ferroptosis. They called it “a new mode based on p53 regulation of cystine metabolism, ROS responses and ferroptosis”, which discovered a novel mechanism of tumor suppression.
Table 6.
List of top 10 cited references related to ferroptosis in cancer therapy.
| Ranking | Year | Author | Journal | Title | Citation |
|---|---|---|---|---|---|
| 1st | 2012 | Dixon SJ | Cell | Ferroptosis: an iron-dependent form of nonapoptotic cell death | 1819 |
| 2nd | 2014 | Yang WS | Cell | Regulation of ferroptotic cancer cell death by GPX4 | 1151 |
| 3rd | 2017 | Stockwell Brent R | Cell | Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease | 975 |
| 4th | 2015 | Jiang L | Nature | Ferroptosis as a p53-mediated activity during tumor suppression | 599 |
| 5th | 2016 | Xie Y | Cell Death Differentiation | Ferroptosis: process and function | 585 |
| 6th | 2014 | Angeli JPF | Nature Cell Biology | Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice | 527 |
| 7th | 2019 | Wang WM | Nature | CD8+ T cells regulate tumor ferroptosis during cancer immunotherapy | 505 |
| 8th | 2019 | Hassannia B | Cancer Cell | Targeting Ferroptosis to Iron Out Cancer | 494 |
| 9th | 2017 | Doll S | Nature Chemistry Biology | ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition | 490 |
| 10th | 2014 | Dixon SJ | Elife | Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis | 464 |
The top 25 references according to the timeline of citation bursts are shown in Fig. 7. The top-ranked article according to citation burst strength was “regulation of ferroptotic cancer cell death by GPX4”, which ranked second in terms of citation time. Article “Ferroptosis as a p53-mediated activity during tumor suppression” ranked second in terms of citation burst strength. The following article is “Inactivation of the ferroptosis regulator GPX4 triggers acute renal failure in mice [28]”. In this study, researchers demonstrated GPX4 as a protective factor in ferroptosis and they also discovered that Liproxstatin-1 was able to suppress ferroptosis in cells. According to the year of citation burst, 12 references (48%) were from 2016 to 2020, 9 of which were about regulation of ferroptosis in tumor cells. These studies found many novel molecular which could induce or protect cancer cells against ferroptosis. This means that regulation pathway in ferroptosis continue to receive attention in recent years. For example, Sun XF et al. [29] have discovered that NRF2 could prevent hepatocellular carcinoma (HCC) cells against ferroptosis. Inhibition of NRF2 expression sensitized HCC cell to erastin and sorafenib in tumor xenograft models. NRF2 provide insight into the ferroptosis-targeted treatment of HCC. In another study, a study in 2015 found the iron-carrier protein transferrin and amino acid glutamine can be the ferroptosis inducers [30]. And studies have already confirmed that p53 tumor suppressor could regulates ferroptosis by the cystine/glutamate antiporter SLC7A11 [31]. Therefore, ferroptosis could be a therapeutic option. Moreover, Christophe L et al. [32] emphasized the role of retinoblastoma (Rb) protein in modulation of ferroptosis in HCC cells. In their experiment, Rb-negative HCC cells were more likely to occur ferroptosis when exposure to sorafenib.
Fig. 7.
Citation bursts of references related to ferroptosis in cancer.
4. Discussion
The developmental stage of research in the field of ferroptosis and cancer can be understood through an analysis of published papers. Fig. 1 presents the growth trend of publication of papers related to ferroptosis and cancer. The annual growth curve can be divided into two periods, including steady development stage (2012–2017) and rapid development stage (2018–2022). In the first 6 years, this field began to attract people's attention and became popular since 2019. Several articles may well explain this development. The initial publication by Dixon et al., in 2012 introduced the concept of ferroptosis and highlighted its unique morphology and mechanism of action, generating interest and leading to further research in the field [1]. This aroused the interest of researchers, and the researchers began to grope about the mechanism of ferroptosis. During this period, many milestone discoveries have emerged and leaded to the rapid growth of the field. We have made some breakthrough discoveries about the mechanism of ferroptosis. Several authoritative studies have identified the specific lipids that drive to ferroptosis in 2017, which provide key markers for detecting ferroptosis and lay a solid foundation for subsequent research [33,34]. Moreover, in the past few year researchers have discovered many new key ferroptosis inducers or suppressors. Researchers discovered FIN56 and FINO2 acted as ferroptosis inducers in 2016 and phospholipids promoted ferroptosis in 2017 [34,35]. Second, the development of some important technologies has further promoted the development of the field. In 2018, Gaschler et al. firstly used stimulated Raman scattering microscopy to illustrate the role of mitochondria and endoplasmic reticulum in contributing to ferroptosis [36]. After that, many studies began to explore the role of various subcellular organelles in the ferroptosis [14,37,38]. Third, researchers have realized the universality and importance of ferroptosis. Ferroptosis has been proven to be involved in the progress of many types of cancers [26]. One of the most important functions of ferroptosis is tumor suppression. BAP1 can promote ferroptosis and suppress tumor tumorigenesis [39]. These authoritative and important discoveries have driven progress in the field of ferroptosis. With continued efforts by researchers, additional targets were discovered, and studies identified several drugs that could induce ferroptosis in cancer cells [35,40,41], providing a novel approach to treating tumors. This greatly aroused the enthusiasm of researchers. Thus, a considerable number of studies were carried out and many high-quality articles were published in recent years. Although there are still three months left in 2022, the publication has already exceeded that in 2021. As time going by, the research system is also constantly improving, and the quality of articles also increased in general. First, only 80 articles published before 2018 were cited more than 100 times, while 211 articles published after 2018 were cited more than 100 times. Of the articles published before 2018, only 92 were in Journal Citation Reports Quartile 1, far less than those published after 2018 (Table 3). Secondly, due to breakthroughs in research technology and the emergence of more fundamental and heavyweight research results, our current research is moving closer to clinical practice.
In terms of distribution of countries, China and USA conducted the most publication output, followed by European countries and Japan, among which, China is the only one developing country. China and USA have contributed almost 90% of the articles, which is attributed to the emphasis and amount of financial support of these countries on the ferroptosis in cancer. However, despite the large number of publications from China, their average citations were among the lowest of the top ten productive countries. There may be several reasons for this. First, this is likely due to the fact that the research in this field in China started relatively late. More authoritative results require more time and longer sustained research. Second, most articles from China were published in recent years and require further verification. And due to the short publication time of the article, the citation records still need to be improved. Third, the research scale of many studies is relatively small and lack sufficient original discoveries and significant achievements. Fig. 2 shows the collaborations between countries, and the centrality illustrates USA dominated the countries’ cooperation. The average citation and centrality imply USA is still the most influential country in this field. Moreover, as it shows, the green connection lines are the most, and the strength of orange lines are thicker than those of green and purple, indicating there is more international cooperation in recent years. Distribution of institutions was generally consistent with the country distribution. In the early period, foreign institutions have ushered this field, especially Columbia University. The earliest article about ferroptosis and the top three most cited references were from Columbia, indicating its basic groundbreaking work and major achievements. Although China was a latecomer, the top ten productive institutions are all Chinese universities, showing that there is a research upsurge in Chinese universities. And the collaboration network also shows most papers in Chinese agencies were published in recent years. The collaboration of institutions was stronger than countries, suggesting most cooperation is domestic rather than international. Lack of international cooperation may hinder the development of the field. First, tumor is a highly heterogeneous disease, the same type of tumor varies greatly in different countries and regions. Lack of international cooperation will make research results less convincing and universal. Moreover, the lack of international cooperation reduces opportunities for mutual learning and the sharing of research resources, slowing down the pace of development. International cooperation can provide a larger platform for exchange of ideas and learning, where new ideas can collide and emerge. Researchers can deeper understand the research progress in this field. In recent years, the cooperation between countries and institutions is becoming more and more frequent, which is also one of the reasons why the quantity and quality of articles has been improving in recent years. Therefore, international cooperation should be encouraged to generate more ideas and promote the development of this field.
Daolin Tang is the most productive author, who is well-known for his contribution in ferroptosis field. His major contribution was the discovery that NRF2 is a suppressing factor in regulation of ferroptosis, which could activate p62-Keap1-NRF2 pathway and protect HCC cells against ferroptosis [29]. In another study, Tang revealed a new mechanism of acquired sorafenib resistant in HCC patients and discovered metallothionein (MT)-1G as a ferroptosis suppressor factor [42]. Inhibiting MT‐1G enhances the sensitivity of HCC cells to sorafenib in vivo. Tang specifically focused on the ferroptosis regulation mechanism of HCC and ferroptosis-inducing HCC therapy, whose findings positively compensate for the limitations of traditional cancer therapy. Dixon Scott J although did not publish many articles, he is still recognized as the most influential pioneer in ferroptosis field. He first proposed the concept of ferroptosis, which is different from apoptosis, necrosis and other known type of cell death [1]. Dixon further illustrated the mechanism of ferroptosis and the overwhelming, iron-dependent accumulation of lethal lipid ROS is the characterize of ferroptosis, which is considered as the foundation and source of subsequent ferroptosis research. Moreover, he firstly discovered ferroptosis activator erastin and ferroptosis inhibitor ferrostatin-1. This provided a research direction for later researchers, most of whom focused on finding new molecules that regulate the ferroptosis. More and more studies have discovered novel ferroptosis inhibitors and inducers, as well as new mechanisms of ferroptosis [5,27]. In November 2019, Dixon et al. found a novel ferroptosis suppressing pathway called ferroptosis suppressor protein 1 (FSP1)/CoQ10 [12], which is the second discovered ferroptosis resistance mechanism that could suppress ferroptosis Wan Seok Yang is also an influential professor from Columbia University. He also participated in the proposal of the concept of ferroptosis, whose article found a key regulator GPX4 and played an important role in the following search on ferroptosis mechanism [5]. These researchers are considered as the pioneers in this field and still have a profound and lasting impact on future research.
Keyword analyses have presented the evaluation of hot research topics in the field of ferroptosis targeted therapy. The most frequent keywords are ferroptosis, cell-death, cancer, apoptosis and iron, indicating that the research mainly focus on the ferroptosis mechanism in cancer cell. Fig. 5A shows that keyword “cell death, cancer, apoptosis” appear more actively in early stage, which meant researchers were interested in the biochemical mechanism of cancer cell metabolism at the beginning. And cluster 1 in Fig. 5D also confirms this conclusion. The clustering analysis of keywords can show direction and scope in this field. Cluster 0 is mainly about research of the role of ferroptosis in progression and therapy of gastric cancer and pancreatic cancer. Cluster 1 is mainly about the mechanism of cell death induced by ferroptosis. The keywords in cluster 2 are mainly related to the research of peroxidation of ferroptosis in cells. Cluster 3 may be the study of some specific drug targets that is favorable for tumor treatment. In cluster 4, most keywords are about the research of the role of ferroptosis in progression and therapy of colorectal cancer. The keywords of cluster 5 are mainly about the study of immune therapy targeting for ferroptosis to treat cancer. The keywords in cluster 6 is mainly about research of the role of ferroptosis in breast cancer therapy. Cluster 7 represents the research direction on radiotherapy targeting for ferroptosis. Cluster 8 may focus more on the ferroptosis-inducing/suppressing molecules. The keywords in cluster 9 are more related to ferroptosis induction drugs like artemisinin. Moreover, keywords time line viewer and citation burst could reflect the evolutionary trajectory of research on ferroptosis targeting cancer therapy. Combining the above figures, we can draw a development path for ferroptosis in the field of cancer therapy. Early researches focused more on the mechanism of ferroptosis in cells and its role in the process of cell death. Later, more and more ferroptosis-related pathways were discovered, researchers gradually shift research direction to specific cancers like gastric cancer, breast cancer, colon cancer and so on. According to the reference citation burst analysis, we can find most recent articles are about the novel regulation molecular or pathway that could promote or suppress ferroptosis in cancer cells, which means that ferroptosis regulation continue to receive attention in recent years. Through further understanding of the mechanism of ferroptosis, more means will be found to regulate ferroptosis of various tumor cells in the future. And we further analyzed the changes in methodology of ferroptosis in the literature and found that in addition to developing new compounds that could regulate ferroptosis in cancer cells, current research also focuses on developing methods that can reliably detect ferroptosis [38,43]. The detection of markers is very important for the determination of ferroptosis, which is of great significance for subsequent clinical research. Based on the results of co-occurrence and burst analyses of keywords and references, it can be found that in the future the mechanism research of ferroptosis is still a hot spot in the future, but as time goes on, the mechanism research becomes more and more sophisticated, and the clinical research of ferroptosis inducing cancer therapy will gradually increase. More methods will be developed to treat tumors based on known mechanisms. Furthermore, researchers discovered many novel ferroptosis inducers that may trigger ferroptosis in cancer cells. In recent years, researchers found that some classic anti-tumor drugs like sorafenib were related to ferroptosis, which provides a new way to treat cancer and many novel cancer treatments have been explored. Studies related to ferroptosis targeting cancer therapy strategies may be the hotspots in future study [41,[44], [45], [46], [47]].
At present, research on ferroptosis has made great achievements. Many mechanisms and pathways have been elucidated, providing a foundation for researchers to develop corresponding cancer therapy strategies targeting these targets in future. Currently, based on the achievements of previous predecessors, several therapies targeting ferroptosis have been applicated to treat cancer, including chemotherapy, immunotherapy and radiotherapy. In chemotherapy, a diverse range of cancers have gradually become resistant to multiple chemotherapeutics drugs, leading to poor chemotherapeutic response [48]. However, studies have suggested that ferroptosis-inducting drugs such as sorafenib, artemisinins may be promising treatment that can reversing chemotherapy resistance to improve chemotherapeutic response [25,32,49,50]. Studies in lung cancer and HCC have shown sorafenib inhibits the activity of system Xc- and induces ferroptosis in cancer cells. Similarly, erlotinib, selumetinib, gefitinib, and other kinase inhibitors have also been shown to induce ferroptosis [29,32]. Artemisinins generate free radicals against cancer and promote ferritinophagy to trigger ferroptosis [51]. In head and neck squamous cell carcinoma, artemisinins are effective in inducing cell death and ferroptosis by cell cycle arrest. In addition to chemotherapy, immunotherapy can also treat cancers through ferroptosis. Immunotherapy can activate the innate immune system to enhance our anti-cancer capacity. PD-L1 antibodies promote ferroptosis by facilitating accumulation of lipid peroxidation. Besides, the combination of anti-PD-L1 antibodies and ferroptosis activators could suppress tumor growth more significantly in vitro and in vivo [41]. TGFβ1 inhibits SLC7A11 transcription and activate ZEB1 to result in ferroptosis [52,53], enabling immunotherapy in cancers possible. As for radiotherapy, which used to be considered directly inducing DNA damage or other adverse events in cancer cells and causing cell death [54,55], was also been reported that it could directly promote ferroptosis in cancer cells [44,56]. When receiving radiotherapy, the level of SLC7A11 decreases, which is regulated by Ataxia telangiectasia-mutated (a key protein kinase in DNA damage repair) [57]. Radiotherapy also upregulates ACSL4, leading the occurrence of ferroptosis [58]. In addition, the anti-cancer effect is enhanced when radiotherapy is combined with ferroptosis drugs [44,58,59]. These studies have revealed the regulatory mechanism of ferroptosis in cancers and have significant and groundbreaking implications for the application of ferroptosis-targeted cancer therapy. However, the vast majority researches focus on precision medicine of tumors. The application of ferroptosis in early screening and prognosis prediction of solid tumor unsatisfactory.
4.1. Limitation
This study utilized a variety of bibliometric software to provide insight into the developmental trends and research hotspots in the field of ferroptosis-inducing cancer therapy. However, there are some limitations to this study that should be acknowledged. First, the literature included in this study is not exhaustive as all data was downloaded from WOSCC exclusively. Excluding data from other search engines such as Embase and PubMed may limit the comprehensiveness of the study. Additionally, the type of publications was limited to article and review, other types of publication like books, letter, and conference abstracts were excluded from the screening process. Second, only the English article were searched. Because it is difficult to mix different language papers in CiteSpace and VOSviewer. Third, in some analysis process, the software was stuck due to the large amount of data, so we reduced some data, which may cause a bias for results. Finally, this study focused primarily on high-citation references, potentially excluding high-quality papers published more recently. The future database update may solve this problem.
5. Conclusion
In this study, we use CiteSpace and VOSviewer to visualize the developmental trend and hotspots in the field of targeted therapy of ferroptosis in cancer, providing a landscape for the future researchers. The results demonstrate that the attention has dramatically increased and developed rapidly in recent 5 years, thus a great many of high-quality papers publishing. The study also suggests that the focus on ferroptosis in cancer therapy will continue in the foreseeable future and that research in the field will lead to a climax of development. Basic study on mechanisms of ferroptosis in cancer cells will also expand and researches will focus more on the role of ferroptosis in therapy strategy for cancer. Additionally, new treatment modalities related to ferroptosis, such as chemotherapy, radiotherapy, or combined therapies, will continue to be explored and may provide new insights into cancer treatment. Nonetheless, this study provides a clearer understanding of the current state of research in ferroptosis-inducing cancer therapy and can serve as a foundation for future research in the field.
Authors’ contributions
Chen Huang: Conceived and designed the experiments; Wrote the paper.
Jvdan Zeng: Conceived and designed the experiments.
Zai Luo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Jun Zhang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yang Zheng; Qianqian Cai: Performed the experiments; Analyzed and interpreted the data.
Haoliang Zhang; Jie Jiang; Mingyu Duan; Yanmin Chen; Jiayang Xia; Zhengjun Qiu: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. Material/referenced in article.
Additional information
No additional information is available for this paper.
Fundings
This article is supported by the National Natural Science Foundation of China (No.82072662), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (2019142), Shanghai three-year action plan to promote clinical skills and clinical innovation in municipal hospitals (SHDC2020CR4022), and the 2021 Shanghai ‘Rising Stars of Medical Talent’ Youth Development Program: Outstanding Youth Medical Talents.
Availability of data and materials
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jvdan Zeng, Email: zjd2013210881@163.com.
Chen Huang, Email: richard-hc@sohu.com.
References
- 1.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death [J] Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mou Y., Wang J., Wu J., et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer [J] J. Hematol. Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Cheng Y., Mao C., et al. Emerging mechanisms and targeted therapy of ferroptosis in cancer [J] Mol. Ther. 2021;29(7):2185–2208. doi: 10.1016/j.ymthe.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer [J] Nat. Rev. Cancer. 2022;22(7):381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W.S., SriRamaratnam R., Welsch M.E., et al. Regulation of ferroptotic cancer cell death by GPX4 [J] Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan B. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity [J] Signal Transduct. Targeted Ther. 2022;7(1):128. doi: 10.1038/s41392-022-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cong L., Dong X., Wang Y., et al. On the role of synthesized hydroxylated chalcones as dual functional amyloid-β aggregation and ferroptosis inhibitors for potential treatment of Alzheimer's disease [J] Eur. J. Med. Chem. 2019;166:11–21. doi: 10.1016/j.ejmech.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Wang H., An P., Xie E., et al. Characterization of ferroptosis in murine models of hemochromatosis [J] Hepatology. 2017;66(2):449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han C., Liu Y., Dai R., et al. Ferroptosis and its potential role in human diseases [J] Front. Pharmacol. 2020;11:239. doi: 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alim I., Caulfield J.T., Chen Y., et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke [J] Cell. 2019;177(5):1262–1279.e1225. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications [J] Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bersuker K., Hendricks J.M., Li Z., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis [J] Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soula M., Weber R.A., Zilka O., et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers [J] Nat. Chem. Biol. 2020;16(12):1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao C., Liu X., Zhang Y., et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer [J] Nature. 2021;593(7860):586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin B., Wu X., Xu G., et al. Evolutions of the management of colorectal cancer liver metastasis: a bibliometric analysis [J] J. Cancer. 2021;12(12):3660–3670. doi: 10.7150/jca.52842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Lu Y., Qian Y., et al. Emerging trends and research foci in artificial intelligence for retinal diseases: bibliometric and visualization study [J] J. Med. Internet Res. 2022;24(6) doi: 10.2196/37532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guler A.T., Waaijer C.J., Palmblad M. Scientific workflows for bibliometrics [J]. Scientometrics. 2016;107:385–398. doi: 10.1007/s11192-016-1885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C., Hu Z., Liu S., et al. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace [J] Expet Opin. Biol. Ther. 2012;12(5):593–608. doi: 10.1517/14712598.2012.674507. [DOI] [PubMed] [Google Scholar]
- 19.Science Mapping. A systematic review of the literature [J] Journal of Data and Information Science. 2017;2(2):1–40. [Google Scholar]
- 20.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping [J] Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y., Ren X., Chen D. Steroid treatment in macular edema: a bibliometric study and visualization analysis [J] Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.824790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao L., Zhang J., Zhang Z., et al. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021 [J] Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.840956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma D., Yang B., Guan B., et al. A bibliometric analysis of pyroptosis from 2001 to 2021 [J] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.731933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang C., Liu D., Fan Y., et al. Visualization and bibliometric analysis of cAMP signaling system research trends and hotspots in cancer [J] J. Cancer. 2021;12(2):358–370. doi: 10.7150/jca.47158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon S.J., Patel D.N., Welsch M., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis [J] Elife. 2014;3 doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stockwell B.R., Friedmann Angeli J.P., Bayir H., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease [J] Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L., Kon N., Li T., et al. Ferroptosis as a p53-mediated activity during tumour suppression [J] Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedmann Angeli J.P., Schneider M., Proneth B., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice [J] Nat. Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Ou Z., Chen R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells [J] Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao M., Monian P., Quadri N., et al. Glutaminolysis and transferrin regulate ferroptosis [J] Mol. Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagoda N., von Rechenberg M., Zaganjor E., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels [J] Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louandre C., Marcq I., Bouhlal H., et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells [J] Cancer Lett. 2015;356(2 Pt B):971–977. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Doll S., Proneth B., Tyurina Y.Y., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition [J] Nat. Chem. Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagan V.E., Mao G., Qu F., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis [J] Nat. Chem. Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimada K., Skouta R., Kaplan A., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis [J] Nat. Chem. Biol. 2016;12(7):497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaschler M.M., Hu F., Feng H., et al. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis [J] ACS Chem. Biol. 2018;13(4):1013–1020. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou Y., Henry W.S., Ricq E.L., et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion [J] Nature. 2020;585(7826):603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng H., Schorpp K., Jin J., et al. Transferrin receptor is a specific ferroptosis marker [J] Cell Rep. 2020;30(10):3411–3423.e3417. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Shi J., Liu X., et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression [J] Nat. Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer S.L., Saha A., Liu J., et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth [J] Nat. Med. 2017;23(1):120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W., Green M., Choi J.E., et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy [J] Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Niu X., Chen R., et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis [J] Hepatology. 2016;64(2):488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J., Schorpp K., Samaga D., et al. Machine learning classifies ferroptosis and apoptosis cell death modalities with TfR1 immunostaining [J] ACS Chem. Biol. 2022;17(3):654–660. doi: 10.1021/acschembio.1c00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang X., Green M.D., Wang W., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11 [J] Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.Cd-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y., Li L., Lan J., et al. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma [J] Mol. Cancer. 2022;21(1):11. doi: 10.1186/s12943-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang F., Xiao Y., Ding J.H., et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy [J] Cell Metabol. 2023;35(1):84–100.e108. doi: 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Yang M., Wu X., Hu J., et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma [J] J. Hepatol. 2022;76(5):1138–1150. doi: 10.1016/j.jhep.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y., Shen Y., Chen C., et al. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? [J] Cancer Biol Med. 2019;16(4):630–646. doi: 10.20892/j.issn.2095-3941.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G.Q., Benthani F.A., Wu J., et al. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis [J] Cell Death Differ. 2020;27(1):242–254. doi: 10.1038/s41418-019-0352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hangauer M.J., Viswanathan V.S., Ryan M.J., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition [J] Nature. 2017;551(7679):247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ooko E., Saeed M.E., Kadioglu O., et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells [J] Phytomedicine. 2015;22(11):1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Kim D.H., Kim W.D., Kim S.K., et al. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells [J] Cell Death Dis. 2020;11(5):406. doi: 10.1038/s41419-020-2618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viswanathan V.S., Ryan M.J., Dhruv H.D., et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway [J] Nature. 2017;547(7664):453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baidoo K.E., Yong K., Brechbiel M.W. Molecular pathways: targeted α-particle radiation therapy [J] Clin. Cancer Res. 2013;19(3):530–537. doi: 10.1158/1078-0432.Ccr-12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adjemian S., Oltean T., Martens S., et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death [J] Cell Death Dis. 2020;11(11):1003. doi: 10.1038/s41419-020-03209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye L.F., Chaudhary K.R., Zandkarimi F., et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers [J] ACS Chem. Biol. 2020;15(2):469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka S., Ballif B.A., Smogorzewska A., et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage [J] Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 58.Lei G., Zhang Y., Koppula P., et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression [J] Cell Res. 2020;30(2):146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sleire L., Skeie B.S., Netland I.A., et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion [J] Oncogene. 2015;34(49):5951–5959. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.
Not applicable.