Abstract
Cancer-associated fibroblasts (CAFs) are key stromal cells in the tumor microenvironment (TME) that critically contribute to cancer initiation and progression. In bladder cancer (BCa), there is emerging evidence that BCa CAFs are actively involved in cancer cell proliferation, invasion, metastasis, and chemotherapy resistance. This review outlines the present knowledge of BCa CAFs, with a particular emphasis on their origin and function in BCa progression, and provides further insights into their clinical application.
Keywords: Bladder cancer, Cancer-associated fibroblasts, Proliferation, Invasion and metastasis, Chemotherapy resistance
1. Introduction
Bladder cancer (BCa) is one of the most prevalent urinary cancers worldwide, with more than 430,000 new cases per year [1]. BCa can be divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). Approximately 75% of BCa patients present with NIMIBC, which commonly has a favorable outcome. While, 20% of BCa patients develop MIBC, with a 5-year survival rate decreasing to 40% [2]. Therefore, an in-depth understanding of the molecular mechanisms underlying BCa development is important for developing effective therapies for BCa, especially MIBC. It has recently been suggested that BCa progression occurs due to crosstalk between different cell types in the tumor microenvironment (TME) [3]. TME composition varies according to tumor type, but common features include immune cells, stromal cells, blood vessels, and extracellular matrix [4]. Cancer-associated fibroblasts (CAFs) are one of the most important components of the stromal cells [5] and contribute to tumorigenesis by secreting growth factors, supporting angiogenesis, modifying the extracellular matrix (ECM), and suppressing antitumor immune responses [6]. In this review, we summarize the role of CAFs in BCa and their potential applications in therapeutic interventions.
2. Origins and characteristics of CAFs
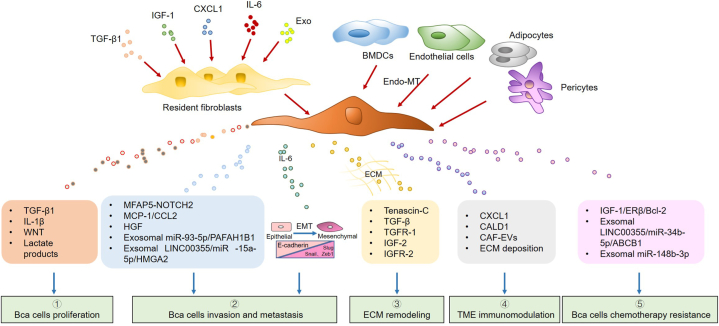
The origin of BCa CAFs remains inconclusive, although they are the most prevalent cell type in the TME. Various studies support CAFs originating from resident fibroblasts, bone marrow-derived mesenchymal stem cells (BMDCs), or endothelial cells that undergo endothelial-mesenchymal transition (Endo-MT).
2.1. The origins of CAFs
CAFs are composed of heterogeneous subtypes that arise from different cellular origins. Kalluri [5] reported that CAFs mainly originate from resident fibroblasts that are activated and reprogrammed in response to paracrine factors and cytokines produced and released by tumor cells. In BCa, Shi et al. reconstructed an in vivo-like TME using a 3-dimensional microfluidic coculture device to investigate the interactions between CAFs and BCa cells and verified that the cytokines secreted by the BCa cell line T24 (a cell line established from a urinary BCa patient) effectively transformed fibroblasts into CAFs [7]. Transforming growth factor-β1 (TGF-β1) [8], chemokine ligand 1 (CXCL1) [9], insulin-like growth factor 1 (IGF-1) [10], interleukin-6 (IL-6) [9], and other factors have been recognized as important biochemical activators of CAFs in BCa. In addition, Long et al. confirmed that blocking IGF-1 in a co-culture system reversed the ability of BCa cells to induce the transformation of normal fibroblasts into CAFs [10]. Recent studies showed that tumor cell-derived exosomes are involved in CAF activation. Goulet et al. [8] reported that exosomes released by BCa cells can be internalized by fibroblasts to promote the expression of CAF markers. Additionally, cancer cell-derived exosomes contain TGF-β and SMAD-dependent signaling is activated in exosome-induced CAFs [8]. TGF-β inhibitors abrogate CAF marker expression in normal fibroblasts [8]. These data indicate that BCa cells induce the transformation of fibroblasts into CAFs via exosome-mediated TGF-β transfer and SMAD pathway activation.
Within the lamina propria there was a layer of cells with the cytological characteristics of both fibroblasts and smooth muscle cells [11]. These cells transform into smooth muscle cells after being stimulated by urothelium-derived Sonic hedgehog (Shh) [12,13], and can be differentiated to fibroblasts after losing the Shh stimulation during BCa development [14]. Despite the fact that their functional roles have not been proven, these cells may be additional sources of BCa CAFs.
In other cancers, endothelial cells [15], BMDCs [6,[16], [17], [18], [19]], pericytes, epithelial cells, and adipocytes [20,21] have been proven to be CAF resources, and physical changes in the ECM are also capable of inducing CAF activation [22]. However, the related mechanisms remain unclear in BCa and require further investigation (Fig. 1).
Fig. 1.
The origins and functions of cancer-associated fibroblasts (CAFs). CAFs can be derived from a variety of cells, including resident fibroblasts, bone marrow-derived mesenchymal stem cells (BMDCs), endothelial cells, pericytes, and adipocytes. CAFs facilitate the development of bladder cancer (BCa) by regulating various cytokines and chemokines.
2.2. CAF characteristics
Compared to quiescent fibroblasts, CAFs are generally larger, with jagged nuclei, more cytoplasmic branches, and thicker endoplasmic reticulum with free ribosomes, Golgi apparatus, and stress fibers [23]. These features promote CAF proliferation, migration, synthesis, and secretion. It is now clear that many different markers can be used to identify CAFs. These markers include fibroblast-specific protein 1 (FSP1), vimentin, podoplanin (PDPN), α-smooth muscle actin (α-SMA), fibroblast-activated protein (FAP), platelet-derived growth factor receptor-α (PDGFRα), PDGFRβ, discoidin domain-containing receptor 2 (DDR2), and desmin [24]. However, none of these markers are specific to CAF, suggesting that other cell types can express them [25]; for instance, α-SMA can be found in vascular muscle cells [26], desmin also serves as a perivascular marker [27], PDPN can also be expressed on lymphatic endothelial cells [28] and vimentin can be observed in some normal stromal cells [29]. The lack of specific markers is one of the main challenges in studying CAFs, which are manipulated in the host tissues.
3. The heterogeneity of CAFs in BCa
Recently, several reports suggest that depending on CAFs’ different origins, their function and phenotype could be diverse and unique. With the advent of single cell sequencing technologies, the understanding of the CAF populations in BCa and their respective contributions to BCa progression has substantially developed. Chen et al. [1] demonstrated the diversity of CAFs in BCa using single cell RNA-sequencing. They discovered that the expression of PDGFRa and RGS5 can separate the CAF population in BCa into inflammatory CAFs (iCAFs) and myofibroblast CAFs (myCAFs). iCAFs secrete a variety of cytokines, including CXCL1, CXCL2, CXCL12, IL-6, and CXCL14, which are thought to be more pro-tumorigenic due to their roles in migration, proliferation, and angiogenesis. Wang et al. confirmed this work by isolating RGS5+myCAFs and PDGFRa + iCAFs in tissue from healthy, NMIBC, MIBC, and metastatic BCa patients. Du et al. also discussed the role of myCAFs subtypes in BCa. They indicated that myCAFs regulated ECM remodeling, tumor metabolism, cancer stemness, and oncological mutations, ultimately influencing treatment responsiveness and prognosis in BCa patients. Another single-cell analysis revealed that COL11A1+fibroblasts are cancer-specific fibroblasts (CSFs) and specifically exist in the tumor tissues of BCa [30]. CSFs exhibit more active CAF subtypes, specifically expressing LRRC15, ITGA11, SPHK1, and FAP, and may contribute to cancer progression by modifying the ECM and stimulating anti-tumor immune responses. Clinical analysis revealed that a high proportion of CSFs is positively correlated with a poor outcome for BCa. The solute carrier family 14 member 1 (SLC14A1) gene, which has been related to BCa, encodes the type-B urea transporter (UT-B), which facilitates the passive movement of urea across the cell membrane [31]. Recently, Ma et al. [32] reported a CAF subpopulation characterized by overexpression of SLC14A1, which is activated by interferon signalling and confers stemness to BCa cells via the paracrine WNT5A pathway. BCa patients with elevated levels of SLC14A1+CAFs were positively associated with a worse outcome and response rate to neoadjuvant chemotherapy or immunotherapy. Targeting STAT1 or STING to inhibit SLC14A1+CAF transformation makes BCa cells more sensitive to chemotherapy.
4. The role of fibroblasts in BCa
CAFs are a key component of the TME, performing a variety of functions such as matrix deposition and remodeling, extensive reciprocal signaling interactions with cancer cells, and crosstalk with infiltrating leukocytes [22,33]. Kobayashi et al. reported that the abundance of CAFs is associated with poor outcomes in different cancers [17]. Du et al. used bioinformatic analysis combined with immunohistochemistry to confirm the cancer-promoting role of CAFs in BCa [34]. In this section, we summarize how CAFs functionally regulate the progression of BCa.
4.1. CAFs and BCa cell proliferation
The proliferation of tumor cells is a critical step in the subsequent invasion. To establish metastatic sites and maintain subsequent growth, tumor cells must recruit supportive stromal cells. Compared with conditioned medium taken from normal fibroblasts, CAF conditioned medium isolated from patients with BCa contained abundant growth-promoting factors. CAFs secrete diverse growth factors, such as TGF-β1, stimulating adjacent BCa cells [35]. Xenograft experiments have shown that CAFs promote BCa tumorigenesis, whereas inhibition of TGF-β1 suppresses the progression of CAF-related cancers [36].
CAF-derived inflammatory factors are also involved in BCa proliferation. Chen et al. confirmed that iCAFs were key factors in tumor progression in BCa by single-cell RNA sequencing of eight BCa samples and three paratumor samples [1]. They further co-cultured iCAFs with BCa cell lines in vitro and validated the pro-proliferative effect of iCAFs in BCa [1]. Yang et al. found that IL-1β is overexpressed in CAFs isolated from BCa tissues [37]. CAF-derived IL-1β activates Wnt signaling in the BCa cell line T24, which upregulates the expression of IL-1β, thereby forming a paracrine Wnt/IL-1β signaling feedback loop to promote BCa cell growth and aggressiveness. Furthermore, inhibition of Wnt signaling could sufficiently suppress the oncogenic effect of CAFs, which supports that CAFs play a role in BCa tumorigenesis through Wnt/IL-1β signaling (Fig. 1, Table 1).
Table 1.
In vitro or in vivo models studying the role of CAFs in BCa.
| Year | Author | Fibroblasts used/source | Animal model | Finding/comments | Ref. |
|---|---|---|---|---|---|
| 2022 | Lu et al. | Tissues from BCa patients | Xenograft mouse model | Exosomal miR-93-5p derived from CAFs confers oncogenicity on BCa cells via sponging PAFAH1B1. | 47 |
| 2022 | Shan et al. | Tissues from BCa patients | Subcutaneous tumor model | CAF-exos and exosomal miR-148b-3p can reduce apoptosis and promote EMT, metastasis and drug resistance in BCa cells and that these effects can be reversed by PTEN overexpression via downregulation of the Wnt/β-catenin pathway. | 70 |
| 2021 | Camargo et al. | Tissues from BCa patients | – | 3D model based on acellular scaffolds provide a novel platform for elucidating the paracrine signaling of BCa and how this molecular signaling can alter the phenotypes of fibroblasts. | 76 |
| 2021 | Yang et al. | Tissues from BCa patients | – | CAFs promote cell proliferation and invasion of human BC cells through Wnt/IL1β signaling feedback. | 37 |
| 2021 | Dong et al. | human Foreskin Fibroblast cells were induced into CAFs by T24 cells | – | CAFs affected T24 cell growth, invasion, and metabolic phenotype through autophagy. | 39 |
| 2021 | Caston et al. | CAF19 | – | Tumors grown in the presence of CAFs were sensitized to the combination of STAT3 and Ref-1 inhibition. | 80 |
| 2020 | Luo et al. | Tissues from BCa patients | – | 1. The exosomes released from CAFs promote BCa cell proliferation and invasion. 2. Regulation of LINC00355 expression in exosomes released from CAFs might be a putative therapeutic strategy against the pathogenesis of BCa. |
69 |
| 2020 | Zhou et al. | Tissues from BCa patients | Xenograft mouse model | 1. CAFs-derived MFAP5 promotes the bladder cancer proliferation and metastasis. 2. MFAP5-mediated PI3K-AKT signaling activated the DLL4/NOTCH2 pathway axis in bladder cancer. |
44 |
| 2019 | Long et al. | Tissues from BCa patients | Xenograft mouse model | CAFs could increase BCa cell resistance to cisplatin by increasing ERβ/Bcl-2 signalling. | 10 |
| 2019 | Mullenders et al. | Tissues from BCa patients | – | Human BCa organoids are successfully derived from resected tumors and biopsies and biopsies and cultured and passaged for prolonged periods. | 77 |
| 2019 | Goulet et al. | Healthy primary bladder fibroblasts (HFs) were induced into CAFs (iCAFs) by bladder cancer-derived exosomes. | – | 1. The IL-6 cytokine was highly expressed by CAFs, and its receptor IL-6R was found on RT4 BCa cells. 2. Inhibition of CAFs-secreted IL-6 by neutralizing antibody significantly reversed the IL-6-induced EMT phenotype. |
50 |
| 2015 | Miao et al. | NIH3T3 fibroblasts | Stroma-rich bladder cancer model (SRBC) | 1. Off targeted nanoparticles (NP) damaged CAF initially inhibited tumor growth, chronic exposure of CAF to cisplatin NP led to elevated secretion of Wnt16 in a paracrine manner that supported tumor cell resistance and stroma reconstruction. 2. Knockdown of Wnt16 in the damaged CAF could be a promising combinatory strategy to improve efficacy of NP delivered cisplatin in a SRBC. |
73 |
| 2015 | Zhuang et al. | Tissues from BCa patients | – | CAFs induces EMT and invasion of human UBC cells through the TGFβ1-ZEB2NAT-ZEB2 axis. | 35 |
| 2015 | Grimm et al. | Tissues from BCa patients | – | Fibroblasts enhance migration and invasion of bladder cancer cells with the involvement of MCP-1 and HGF as stimulatory factors | 45 |
| 2015 | Shi et al. | human Foreskin Fibroblast cells were induced into CAFs by T24 cells | – | Overexpression of monocarboxylate anion transporter 1 and 4 in T24-induced CAFs regulates the progression of bladder cancer cells in a 3D microfluidic device | 7 |
| 2014 | Zhang et al. | NIH 3T3 | SRBC | Antineoplastic effect of Combo NP works by first targeting CAFs and is more effective as an anti-tumor therapy than Combo Free, GMP NP or Cisplatin NP alone. | 81 |
In addition to their ability to secrete factors that promote tumor cell proliferation, CAFs can construct a nutrient-rich microenvironment to metabolically support tumor growth [38]. Autophagy in CAFs can regulate aerobic glycolysis in BCa and further promote BCa cell growth ability [39]. Moreover, CAFs produce lactate, which provides energy for BCa cell proliferation and invasion [7]. This effect was related to the increased expression of monocarboxylate anion transporter 1 (MCT1) and MCT4 in CAFs, suggesting that during disease progression, CAFs depend on glycolysis to transport energy metabolites to cancer cells by means of monocarboxylate transporters [38] (Fig. 1).
4.2. CAFs and BCa invasion and metastasis
Invasion and metastasis are the most common causes of cancer-related death. Immunohistochemical analysis of primary tumors in patients with BCa showed that the presence of CAFs was increased compared to that in normal bladder tissue [40] and that the expression of CAF markers CD90, FAP, and PDGFRβ positively correlated with BCa aggressiveness [41]. Furthermore, hierarchical clustering analysis of 344 BCa patients revealed that a patient cluster with dominant FAP expression had poorer 5-year survival than other groups [41]. A study further confirmed these findings, and found that FAP expression was positively correlated with muscle invasion and negatively correlated with survival in patients with BCa of the basal phenotype (aggressive phenotype) [42] and demonstrated that CAFs play an important role in the progression of BCa and lead to aggressive disease.
CAFs found around cancer regions can not only promote the proliferation of cancer cells but also enhance their invasiveness through different proinvasive molecules and cellular interactions, including cytokines, chemokines, and various inflammatory mediators [43]. Microfibrillar-associated protein 5 (MFAP5), secreted by CAFs, is an oncogenic protein and a component of elastic microfibers in several types of tumors. MFAP5 has been reported to be upregulated in BCa and is positively correlated with poor prognosis in patients with BCa. CAF-derived MFAP5 promotes BC malignancy by activating the NOTCH2 signaling pathway with the direct MFAP5/NOTCH2 complex and indirect PI3K/AKT/DLL4 (Table 1) [44]. Moreover, the downregulation of NOTCH2 by short hairpin RNA or treatment with the inactivating antibody NRR2Mab reversed the adverse effects of MFAP5 stimulation in vitro and in vivo. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is an invasion-promoting factor in BCa. This finding was supported by Grimm et al. [45], who revealed that BCa cell invasion was induced by stimulation with CAF-derived MCP-1/CCL2 (Table 1). Hepatocyte growth factor (HGF) plays a crucial role in cancer progression, is a ligand for the c-Met receptor, and promotes tumor cell migration and invasion in various cancer types [46]. Patients with muscle infiltration or high-grade disease show higher tissue expression of HGF than patients with NMIBC and low-grade disease [45]. HGF is secreted by fibroblasts under the influence of BCa cells. However, CAF-derived HGF immediately promoted the migratory and invasive properties of the BCa cells. Therefore, a complex cytokine-based signaling loop is the basis for mutual and malignant crosstalk between BCa cells and fibroblasts (Fig. 1). In addition to soluble factors, CAFs-secreted exosomes are also reported to have a vital role in the invasiveness of BCa cells. The CAFs-secreted exosomes contained a high level of miR-93-5p, which increased BCa cell mobility by targeting PAFAH1B1 [47]. PAFAH1B1 encodes the noncatalytic alpha subunit of the intracellular Ib isoform of platelet-activating factor aceteylhydrolase. The overexpression of PAFAH1B1 inhibited the promotive function of miR-93-5p on cell viability, migration, and invasion [47]. LINC00355 is also found in the CAFs-derived exosomal of BCa. By competitively binding to miR-15a-5p, exosomal LINC00355 increases the expression of HMGA2, thus enhancing BC cell invasion and cell proliferation [48].
Epithelial-mesenchymal transition (EMT) is a process in which epithelial tumor cells lose polarity and intercellular adhesion properties and become mesenchymal-like to gain a propensity for migration and invasion. Expression analysis of FAP, α-SMA, SDF1, S100A4, PDGFRβ, and EMT markers (Zeb1, Slug, Snail, and E-cadherin) was performed in 49 BCa cases at different stages [49]. The results showed that invasiveness was significantly related to decreased E-cadherin (epithelial marker) and increased expression of Zeb1, Slug, and Snail (mesenchymal markers) in tumor cells and increased expression of S100A4, α-SMA, and PDGFRβ in stromal cells [49]. In addition, the loss of E-cadherin and increase in S100A4 and PDGFRβ are closely related to lymph node metastasis [49]. These findings demonstrate that different subtypes of fibroblasts are associated with EMT and tumor progression in BCa to varying extents [49]. Regarding BCa cell invasiveness related to EMT, Goulet et al. [50] demonstrated that CAF-derived IL-6 enhanced the motility and invasiveness of BCa cells via EMT (Fig. 1). When incubated in a medium containing IL-6, BCa cells showed downregulated epithelial marker expression, upregulated mesenchymal marker expression, and enhanced capacity to invade through the ECM, while inhibition of IL-6 could significantly reverse the CAF-induced EMT and invasiveness. Additionally, Runt-Related Transcription Factor 2 (RUNX2) is related to EMT. Immunohistochemical images revealed that tissues with higher RUNX2 expression also had deeper staining of CAFs markers. Liu et al. demonstrated that RUNX2 is a transcription factor related to CAFs, which is overexpressed in BCa and affects the prognosis of patients [51].
The pre-metastatic niche (PMN) is a supportive tissue microenvironment undergoing a series of molecular and cellular changes to form the fertile ‘‘soil’’ in preparation for metastatic tumor cell “seed’’ colonization [52]. CAFs could potentially have a substantial impact on the development of PMN [53]. Silvers et al. reported that BCa-derived EVs induced tenascin-C expression in CAFs in an NF-κB-dependent manner. The increased secreting of tenascin-C from CAFs in regional lymph nodes induced PMN formation and promote BCa cell metastasis [54]. In breast and lung cancer, tumor stroma reprogramming is mediated by the heat shock factor 1-driving transcriptional program in CAFs and tumor cells by activating the TGF-b and SDF-1 pathways, which enables tumor metastasis [52].
4.3. CAFs and extracellular matrix production and remodeling
Maintenance of the ECM is essential for normal tissue homeostasis [55]. The generation, recruitment and activation of CAFs results in ECM remodeling and stiffening, which could disrupt normal tissue architecture and promote tumorigenesis [[56], [57], [58]]. The COL11A1+fibroblasts have been identified in BCa and showed the overexpression level of ECM-associated genes, TGF-β, TGFR-1, IGF-2 and IGFR-2. Such characteristics suggested that COL11A1+fibroblasts could regulate ECM remodeling [30]. Another CAF subpopulation, myCAFs, is frequently found near tumour margins and responsible for ECM deposition and potential stromal stiffening [59]. Millet et al. employed a tissue-engineered tumor model with stiffness levels comparable to those of a bladder tumor and demonstrated that increasing stiffness can impact oncogenic signaling within the urothelial cells [60]. Notably, increased stiffness can promote CAF activation even further, resulting in a self-perpetuating positive feedback loop [57]. This loop is considered to arise following the activation of transcription factor Yes-associated protein (YAP) which subsequently regulates the downstream expression of genes involved in cytoskeletal and matrix remodeling. Tenascin-C (Tn-C) plays a critical role in ECM reorganization [61] and is abundantly expressed in CAFs [43]. In the early 1990s, Tiitta et al. [61,62] reported that Tn-C increased during EMT transition in the urinary bladder wall and was further deposited to induce BCa aggressiveness (Fig. 1). Immunohistochemistry confirmed the relationship between the expression of Tn-C and TGF-β1 in BCa and supported that aggressiveness-associated Tn-C deposition is associated with tumor-stroma crosstalk and EMT [61].
4.4. CAFs and TME immunomodulation
The tumor immune microenvironment (TIME) has been linked to the clinical prognosis of cancer patients [63]. Recently, a growing number of researchers have begun to focus on CAFs' immunosuppressive effect, which is achieved through interactions with TIME components, particularly immune cells. FAP is abundant in the stroma which is related to tumor staging and aggressiveness in BCa patients. According to Gil et al. [64], FAP+CAFs have been associated with immune-cold TMEs characterized by inadequate infiltration of CD8+T-cells and a significant loss of human leukocyte antigen expression on tumor cells. The deposition of a highly crosslinked ECM, which serves as a physical barrier to exclude lymphocytes in peritumour regions, is another factor that FAP+CAFs contribute to poor CD8+T cell infiltration [65]. This barrier prevents lymphocytes from reaching cancer cells and releasing their full cytotoxic potential [65]. In addition, Feng et al. [66]elucidated a complex crosstalk between CAFss, BCa cells, and CD8+T cells mediated by extracellular vesicles (EVs). CAF-EVs reduced apoptosis and increased invasion of T24 cells, decreased proliferation of CD8+T cells, and decreased levels of IFN-, IL-2, and TNF- secreted by CD8+T cells. Using mouse models of BCa, they demonstrated that CAF-EVs increased tumor volume and weight, upregulated the expression of programmed death-ligand 1 (PD-L1), and attenuated the infiltration of CD8+T cells in vivo [66].
Tumor-associated macrophages (TAMs) are the important component of the TIME, and the dominant immune cells in the vicinity of CAF-populated areas, indicating that these two cell types interact closely [67]. A 3D co-culture study of BCa cells and TAMs/CAFs revealed that CXCL1 secretion in these stromal cells plays a crucial role in cellular adhesion and interaction between cancer cells and these stromal cells [9]. Furthermore, in vivo model revealed that CXCL1-expressing TAMs/CAFs promoted tumor growth [9]. Caldesmon 1 (CALD1) is one of the main genes associated with CAFs and outcomes in BCa [34]. Du et al. [34] demonstrated that CALD1 was associated with the infiltration level of numerous Tumor-Infiltrating Immune Cells (TIICs) in the TME, particularly macrophages M2 and CD8+T cells. It was also demonstrated that a high level of CALD1 expression may result in an increase in immune checkpoint-related transcripts, including PD-L1 [34].
Collectively, CAFs promote immunosuppression through direct or indirect cross-talk with immune cells and by teaching immune cells to express tolerant characteristics.
4.5. CAFs and chemoresistance and recurrence
Cancer chemotherapy efficacy is gradually improving; however, achieving a complete cure with chemotherapy is still difficult and has been the main goal for the treatment of advanced cancer [68]. Emerging evidence suggests that CAFs confer substantial resistance to cancer therapeutics by impairing drug delivery and biochemical signaling. When BCa cells are co-cultured with CAFs, their apoptotic rates decrease, whereas their viability, colony-forming ability, and chemoresistance increase [10]. The mechanism indicated that CAFs activated IGF-1/ERβ signaling in BCa cells, which further led to upregulation of the anti-apoptotic gene Bcl-2 [10]. Blocking IGF-1/ERβ/Bcl-2 signaling partially abrogated the ability of CAFs to increase BCa chemoresistance, which was also confirmed in animal experiments [10]. Exosomes derived from CAFs can also promote chemotherapy resistance in various human tumors by delivering bioactive molecules. Luo et al. [69] found that CAF-derived exosomal LINC00355 promotes BCa cell resistance to cisplatin by regulating the miR-34b-5p/ABCB1 axis (Fig. 1, Table 1). Shan et al. [70] indicated that CAF-derived exosomal miR-148b-3p can promote drug resistance to doxorubicin and paclitaxel in BCa cells, and these effects were reversed by PTEN overexpression via the downregulation of the Wnt/β-catenin pathway. CAFs-derived miR-146a-5p generates a niche that promotes BCa stemness and chemoresistance. Clinical evidence indicated that the elevated levels of exosomal miR-146a-5p in the serum of BCa patients were correlated with both tumor stage and relapse risk [71] (Fig. 1). Recent studies have shown that CAFs communicate with BCa cells, altering their metabolism and sensitivity to drugs. Yu and colleagues demonstrated that the CD10 positive CAFs induce chemoresistance via regulating lipid metabolism of cancer stem cells. Additionally, the exposure of CAFs to environmental pollutant could promote BCa progression through an altered metabolism. Bisphenol A (BPA) is an endocrine-disrupting molecule used in plastics [72]. BPA molecules can be released in food and the environment, which causes humans and animals to be continuously exposed, which is defined as an environmental pollutant [72]. Pellerin and colleagues found that BPA could exacerbate the metabolic switch in CAFs via an increased glycolytic metabolism, leading to greater acidification of the extracellular environment and promoting BCa progression and recurrence [72]. Taken together, these results illustrate the critical roles for CAFs within the bladder TME and indicate that they promote BCa chemoresistance and recurrence.
Previous studies have shown that therapeutic nanoparticles (NPs) can distribute and deplete CAFs to improve therapeutic efficacy. However, resistance developed after repeated exposure to chemotherapeutic NPs. Miao et al. [73] revealed that the increased secretion of Wnt16 induced by chronic exposure of CAFs to cisplatin NPs could support tumor cell resistance and TME reconstruction (Table 1). Thus, Wnt16 knockdown in impaired CAFs could be a potential combination strategy to enhance the effect of NP-delivered cisplatin in a stroma-enriched model of BCa.
4.6. Negative role of CAFs in BCa
Despite numerous results indicating CAFs’ pro-tumorigenic effects, they can be tumour suppressive in some contexts [74]. In BCa, the activation of stroma-specific Hedgehog (Hh) in CAFs inhibits the growth of tumors that are mediated by bone morphogenetic protein (BMP) signaling in cancer cells. This finding suggests the existence of CAF subtypes that have tumor-suppressing functions [14]. Consequently, it is crucial to have a better understanding of the diversity of the stromal-cell populations as well as the specific functional roles that each of these populations plays in BCa tissue.
5. Models to investigate the biology of CAFs in BCa progression
5.1. In vitro models
In vitro research is important for the exploration of CAFs biology. The co-culture system is the most common method for studying CAFs' interactions with other cells [9]. Recently, advances in tissue culture techniques have led to the use of three-dimensional (3D) membrane matrices with a variety of compositions [75]. Shi et al. presented a 3D microfluidic co-culture device to reconstruct an in vivo-like TME for the investigation of CAFs and BCa cells interactions [7]. Camargo et al. [76] developed a 3D model based on acellular scaffolds to recreate BCa in vitro that closely describes the in vivo behavior of tumour cells. This 3D BCa model may provide a novel platform for elucidating the paracrine signaling of BCa and how this molecular signaling can alter the phenotypes of fibroblasts. Human organoids have been used to investigate BCa progression. Unlike the previously 3D culture methods, organoids can be passaged for prolonged periods and thus massively expanded [77], allowing researchers to conduct functional studies on TME components [77,78]. Although in vitro studies provide valuable information and are still important in cancer research, they have some limitations, including the inability to account for complex tumor properties like the change of tumore size, the lack of immune and vascular contributions, and th absence of genetic drift in cancer cells.
5.2. In vivo models
Mouse models of cancer allow researchers to study tumor biology in a complex and dynamic physiological system [79]. Conventional method for studying CAF of BCa in vivo is co-injection of CAFs with tumor cells subcutaneously and orthotopically in mice [10,44,47,70]. However, as the tumor grows, it may affect the stromal architecture, thereby altering the composition of the CAF population in favor of particular subpopulations. Genetically engineered mouse models (GEMMs) are another method for studying CAFs in vivo [75]. In cancer research, GEMMs offer a distinct advantage for observing the phenotypes and transcriptomic landscape of CAFs. However, a significant limitation of these models is the broad range of cell types that are labeled due to the lack of fibroblast-specificity in these markers. While they have improved our general understanding of the origins and functions of CAFs, their inability to target all or specific CAF subpopulations has left unanswered questions. Despite these noteworthy efforts to generate in vivo models for fibroblast research, additional efforts are required to develop models that, provided a marker specific to the population of interest, can be used to clarify the cell of origin upon loss of expression of that marker and identify markers that are specific to CAFs regardless of their cell of origin in order to capture the full heterogeneity of the CAF population.
6. Future clinical application of CAFs in BCa treatment
Chemotherapy and radiation therapy are conventional cancer treatments that target actively growing cancer cells. However, the efficacy of these therapies varies greatly. Formidable challenges, such as tumor resistance, recurrence, and metastasis, remain in clinical practice, demonstrating the complexity of the mechanisms underlying tumor heterogeneity and evolution. The TME is a key factor influencing tumor evolution and heterogeneity. Targeting the TME may provide a new strategy for tumor treatment. The modulation of the TME by CAFs during cancer progression makes them promising targets for cancer treatment [3]. In a study by Goulet et al. [50], the proliferation, migration, and invasion of BCa cells increased after incubation with CAF supernatants. This effect could be impaired by the IL-6 antibody. Caston et al. [80] hindered crosstalk between tumors and CAFs by deactivating Ref-1 and STAT3, leading to an improved tumor response (Table 1). Moreover, Zhang et al. reported that gemcitabine monophosphate and cisplatin nanoparticles (Combo NPs) have synergistic antitumor effects because of the combined effects of increased uptake of chemotherapeutic drugs by the tumor, apoptosis of cancer cells, and CAF depletion with alterations in collagen deposition [81]. Compared to free Combo, Combo NPs showed enhanced inhibition of tumor growth with no obvious toxicity [81].
Considering the promising results of CAF-targeted therapies, attempts have been made to directly deplete CAF in other cancers. For example, the FAP-specific antibody sibrotuzumab has been used to treat FAP-positive colorectal cancer. In Phase I trials, sibrotuzumab was well tolerated and halted tumor progression [82], it was unsuccessful in improving survival in patients with metastatic colorectal cancer in a phase II trial [83]. In addition, some treatments target the interactions between CAFs and their surrounding microenvironment. For instance, it has been found that components targeting CAF-derived ECM deprive cancer cells of their protective niche, whereas the hedgehog (Hh) pathway promotes crosstalk between CAFs and cancer cells and drives ECM remodeling [84]. To date, several Hh inhibitors such as vismodegib, sonidegib, and IPI-926, have been tested in combination with chemotherapy for the treatment of pancreatic ductal adenocarcinoma (NCT01383538vi and EDALINE trial iii), with few patients responding to treatment [85]. However, studies on CAF-targeted interventions in BCa are limited to preclinical in vitro and in vivo models. Therefore, therapeutically effective drugs for CAF need to be further developed in the clinical treatment of BCa.
Although the TME is rich in fibroblasts, this has been neglected in the past few decades. The role of CAFs is becoming increasingly important in cancer biology. Targeting CAFs may be an effective way to inhibit cancer progression and metastasis through TME remodeling. However, the plasticity and heterogeneity of CAFs present a major challenge for the exploration of effective clinical treatments [5,6]. To eliminate tumor-promoting CAFs or manipulate their interconvertibility to convert these CAFs into anti-tumorigenic subtypes, future research must be focused on CAF subtypes [86], identify more specific targets [87], and further clarify the mechanisms associated with each subtype [17]. Alternatively, downstream signaling of CAFs could be targeted with the aim of reverting cells back to a resting phenotype.
7. Conclusion
BCa CAFs are the most prevalent cellular component of the bladder TME and have a wide range of functions in BCa progression, including cancer initiation, invasion, metastasis, and therapy resistance [2,50]. Recently, a preliminary understanding of BCa CAFs has been gained, including key characteristics such as their origin, specific markers, biological heterogeneity, and mechanisms of treatment resistance. With deeper understanding of the interaction between CAFs and the TME, an increasing amount of evidence demonstrates that BCa CAFs will become targets for anticancer therapy [81]. Researchers are combining drugs targeting CAF-secreted cytokines with immunotherapies to improve the antitumor effect [6,23]. However, CAF-targeted strategies for cancer therapy remain in the fledging period. To promote the integration of CAF findings into BCa clinical management, further research is required to precisely identify the CAF population through careful characterization, and to target the identified characteristics with appropriate treatments.
Funding sources
This work was supported by the National Natural Science Foundation of China (grant numbers: 82102685); Science and Technology Program of Guangzhou (grant number: 202102020936); Medical Science and Technology Program of Guangdong (grant number: A2023203).
Author contributions
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jian Deng, Email: zhdengjian2008@163.com.
Wen-Fei Wei, Email: weiwenfei.good@163.com.
Abbreviations
- BCa
bladder cancer
- NMIBC
non-muscle-invasive BCa
- MIBC
muscle-invasive BCa
- TME
tumor microenvironment
- CAFs
cancer-associated fibroblasts
- BMDCs
bone-marrow-derived mesenchymal stem cells
- Endo-MT
endothelial-mesenchymal transition
- TGF-β1
transforming growth factor-β 1
- PDGFR
platelet derived growth factor receptor
- SHH
sonic hedgehog
- BMP
bone morphogenetic protein
- FSP1
fibroblast-specific protein 1
- α-SMA
α-smooth muscle actin
- FAP
fibroblast activation protein
- DDR2
discoidin domain-containing receptor 2
- MCT1
monocarboxylate anion transporter 1
- MCT4
monocarboxylate anion transporter 4
- MFAP5
microfibrillar-associated protein 5
- EMT
epithelial-mesenchymal transition: EMT
- Tn-C
Tenascin-C
- ECM
extracellular matrix
- GMP
gemcitabine monophosphate
- NP
nanoparticles
References
- 1.Chen Z., Zhou L., Liu L., Hou Y., Xiong M., Yang Y., Hu J., Chen K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020;11:5077. doi: 10.1038/s41467-020-18916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li F., Teng H., Liu M., Liu B., Zhang D., Xu Z., Wang Y., Zhou H. Prognostic value of immune-related genes in the tumor microenvironment of bladder cancer. Front. Oncol. 2020;10:1302. doi: 10.3389/fonc.2020.01302. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran L., Xiao J.F., Agarwal N., Duex J.E., Theodorescu D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer. 2021;21:104–121. doi: 10.1038/s41568-020-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. : CB. 2020;30:R921–r925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H., Enomoto A., Woods S.L., Burt A.D., Takahashi M., Worthley D.L. Cancer-associated fibroblasts in gastrointestinal cancer, Nature reviews. Gastroenterol. Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 7.Shi H., Jiang H., Wang L., Cao Y., Liu P., Xu X., Wang Y., Sun L., Niu H. Overexpression of monocarboxylate anion transporter 1 and 4 in T24-induced cancer-associated fibroblasts regulates the progression of bladder cancer cells in a 3D microfluidic device. Cell Cycle. 2015;14:3058–3065. doi: 10.1080/15384101.2015.1053666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringuette Goulet C., Bernard G., Tremblay S., Chabaud S., Bolduc S., Pouliot F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFbeta signaling. Mol. Cancer Res. : MCR. 2018;16:1196–1204. doi: 10.1158/1541-7786.MCR-17-0784. [DOI] [PubMed] [Google Scholar]
- 9.Miyake M., Hori S., Morizawa Y., Tatsumi Y., Nakai Y., Anai S., Torimoto K., Aoki K., Tanaka N., Shimada K., Konishi N., Toritsuka M., Kishimoto T., Rosser C.J., Fujimoto K. CXCL1-Mediated interaction of cancer cells with tumor-associated macrophages and cancer-associated fibroblasts promotes tumor progression in human bladder cancer. Neoplasia. 2016;18:636–646. doi: 10.1016/j.neo.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long X., Xiong W., Zeng X., Qi L., Cai Y., Mo M., Jiang H., Zhu B., Chen Z., Li Y. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERβ/Bcl-2 signalling. Cell Death Dis. 2019;10:375. doi: 10.1038/s41419-019-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiseman O.J., Fowler C.J., Landon D.N. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 12.Cao M., Tasian G., Wang M.H., Liu B., Cunha G., Baskin L. Urothelium-derived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation. 2010;79:244–250. doi: 10.1016/j.diff.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelsohn C. Going in circles: conserved mechanisms control radial patterning in the urinary and digestive tracts. J. Clin. Invest. 2006;116:635–637. doi: 10.1172/JCI27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin K., Lim A., Zhao C., Sahoo D., Pan Y., Spiekerkoetter E., Liao J.C., Beachy P.A. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell. 2014;26:521–533. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin F., Wang N., Zhang T.C. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life. 2012;64:717–723. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez M.E., Martin E.E., Anwar T., Arellano-Garcia C., Medhora N., Lama A., Chen Y.C., Tanager K.S., Yoon E., Kidwell K.M., Ge C., Franceschi R.T., Kleer C.G. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep. 2017;18:1215–1228. doi: 10.1016/j.celrep.2016.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D., Sahai E., Rullan A. SnapShot: cancer-associated fibroblasts. Cell. 2020;181:486–486 e481. doi: 10.1016/j.cell.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S., Friedman R., Varro A., Tycko B., Wang T.C. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y., Du L., Lin L., Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets, Nature reviews. Drug discovery. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 20.Bochet L., Lehuédé C., Dauvillier S., Wang Y.Y., Dirat B., Laurent V., Dray C., Guiet R., Maridonneau-Parini I., Le Gonidec S., Couderc B., Escourrou G., Valet P., Muller C. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 21.Bartoschek M., Oskolkov N., Bocci M., Lövrot J., Larsson C., Sommarin M., Madsen C.D., Lindgren D., Pekar G., Karlsson G., Ringnér M., Bergh J., Björklund Å., Pietras K. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., Hynes R.O., Jain R.K., Janowitz T., Jorgensen C., Kimmelman A.C., Kolonin M.G., Maki R.G., Powers R.S., Puré E., Ramirez D.C., Scherz-Shouval R., Sherman M.H., Stewart S., Tlsty T.D., Tuveson D.A., Watt F.M., Weaver V., Weeraratna A.T., Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T., Han C., Wang S., Fang P., Ma Z., Xu L., Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeltz C., Primac I., Erusappan P., Alam J., Noel A., Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin. Cancer Biol. 2020;62:166–181. doi: 10.1016/j.semcancer.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Ziani L., Chouaib S., Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front. Immunol. 2018;9:414. doi: 10.3389/fimmu.2018.00414. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podkalicka P., Mucha O., Kaziród K., Bronisz-Budzyńska I., Ostrowska-Paton S., Tomczyk M., Andrysiak K., Stępniewski J., Dulak J., Łoboda A. Age-dependent dysregulation of muscle vasculature and blood flow recovery after hindlimb ischemia in the mdx model of duchenne muscular dystrophy. Biomedicines. 2021;9 doi: 10.3390/biomedicines9050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang M.L., Kim H.S., You J., Choi Y.S., Kwon B.J., Park C.H., Baek W., Kim M.S., Lee Y.J., Im G.I., Yoon J.K., Lee J.B., Sung H.J. Hydrogel cross-linking-programmed release of nitric oxide regulates source-dependent angiogenic behaviors of human mesenchymal stem cell. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay5413. eCollection 2020 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astarita J.L., Acton S.E., Turley S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front. Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurmik M., Ullmann P., Rodriguez F., Haan S., Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int. J. Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. Epub 2019 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J., Lu S., Lu T., Han D., Zhang K., Gan L., Wu X., Li Y., Zhao X., Li Z., Shen Y., Hu S., Yang F., Wen W., Qin W. Single-cell analysis reveals the COL11A1(+) fibroblasts are cancer-specific fibroblasts that promote tumor progression. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1121586. eCollection 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou R., Kong X., Yang B., Xie Y., Chen G. SLC14A1: a novel target for human urothelial cancer. Clin. Transl. Oncol. 2017;19:1438–1446. doi: 10.1007/s12094-017-1693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z., Li X., Mao Y., Wei C., Huang Z., Li G., Yin J., Liang X., Liu Z. Interferon-dependent SLC14A1(+) cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell. 2022;40:1550–1565.e1557. doi: 10.1016/j.ccell.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Farhood B., Najafi M., Mortezaee K. Cancer-associated fibroblasts: secretions, interactions, and therapy. J. Cell. Biochem. 2018 doi: 10.1002/jcb.27703. [DOI] [PubMed] [Google Scholar]
- 34.Du Y., Jiang X., Wang B., Cao J., Wang Y., Yu J., Wang X., Liu H. The cancer-associated fibroblasts related gene CALD1 is a prognostic biomarker and correlated with immune infiltration in bladder cancer. Cancer Cell Int. 2021;21:283. doi: 10.1186/s12935-021-01896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang J., Lu Q., Shen B., Huang X., Shen L., Zheng X., Huang R., Yan J., Guo H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015;5 doi: 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calon A., Tauriello D.V., Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 2014;25:15–22. doi: 10.1016/j.semcancer.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Yang F., Guo Z., He C., Qing L., Wang H., Wu J., Lu X. Cancer-associated fibroblasts promote cell proliferation and invasion via paracrine Wnt/IL1β signaling pathway in human bladder cancer. Neoplasma. 2021;68:79–86. doi: 10.4149/neo_2020_200202N101. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Outschoorn U.E., Lisanti M.P., Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Dong D., Yao Y., Song J., Sun L., Zhang G. Cancer-associated fibroblasts regulate bladder cancer invasion and metabolic phenotypes through autophagy. Dis. Markers. 2021;2021 doi: 10.1155/2021/6645220. eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexa A., Baderca F., Lighezan R., Izvernariu D. Myofibroblasts reaction in urothelial carcinomas. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2009;50:639–643. [PubMed] [Google Scholar]
- 41.Mezheyeuski A., Segersten U., Leiss L.W., Malmström P.U., Hatina J., Östman A., Strell C. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci. Rep. 2020;10:281. doi: 10.1038/s41598-019-55013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvete J., Larrinaga G., Errarte P., Martín A.M., Dotor A., Esquinas C., Nunes-Xavier C.E., Pulido R., López J.I., Angulo J.C. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum. Pathol. 2019;91:61–68. doi: 10.1016/j.humpath.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Xing F., Saidou J., Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z., Cui D., Sun M.H., Huang J.L., Deng Z., Han B.M., Sun X.W., Xia S.J., Sun F., Shi F. CAFs-derived MFAP5 promotes bladder cancer malignant behavior through NOTCH2/HEY1 signaling. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2020;34:7970–7988. doi: 10.1096/fj.201902659R. [DOI] [PubMed] [Google Scholar]
- 45.Grimm S., Jennek S., Singh R., Enkelmann A., Junker K., Rippaus N., Berndt A., Friedrich K. Malignancy of bladder cancer cells is enhanced by tumor-associated fibroblasts through a multifaceted cytokine-chemokine loop. Exp. Cell Res. 2015;335:1–11. doi: 10.1016/j.yexcr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Xia M., Jin K., Wang S., Wei H., Fan C., Wu Y., Li X., Li X., Li G., Zeng Z., Xiong W. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X., Wang J., Dong B., Wang L., Liu Y. Exosomal miR-93-5p from cancer-associated fibroblasts confers malignant phenotypes on bladder cancer cells by targeting PAFAH1B1. Anti Cancer Drugs. 2023;34:439–450. doi: 10.1097/CAD.0000000000001453. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Luo G., You S., Zhang L., Liang C., Chen X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell proliferation and invasion by regulating miR-15a-5p/HMGA2 axis. Acta Biochim. Biophys. Sin. 2021;53:673–682. doi: 10.1093/abbs/gmab041. [DOI] [PubMed] [Google Scholar]
- 49.Schulte J., Weidig M., Balzer P., Richter P., Franz M., Junker K., Gajda M., Friedrich K., Wunderlich H., Östman A., Petersen I., Berndt A. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem. Cell Biol. 2012;138:847–860. doi: 10.1007/s00418-012-0998-0. [DOI] [PubMed] [Google Scholar]
- 50.Goulet C.R., Champagne A., Bernard G., Vandal D., Chabaud S., Pouliot F., Bolduc S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer. 2019;19:137. doi: 10.1186/s12885-019-5353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B., Pan S., Liu J., Kong C. Cancer-associated fibroblasts and the related Runt-related transcription factor 2 (RUNX2) promote bladder cancer progression. Gene. 2021;775 doi: 10.1016/j.gene.2021.145451. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Sceneay J., Smyth M.J., Möller A. The pre-metastatic niche finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 54.Silvers C.R., Messing E.M., Miyamoto H., Lee Y.F. Tenascin-C expression in the lymph node pre-metastatic niche in muscle-invasive bladder cancer. Br. J. Cancer. 2021;125:1399–1407. doi: 10.1038/s41416-021-01554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen J.S., Clayton A., Pearson H.B. Cancer-associated fibroblast heterogeneity, activation and function: implications for prostate cancer. Biomolecules. 2022:13. doi: 10.3390/biom13010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohan V., Das A., Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 2020;62:192–200. doi: 10.1016/j.semcancer.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Burley A., Rullan A., Wilkins A. A review of the biology and therapeutic implications of cancer-associated fibroblasts (CAFs) in muscle-invasive bladder cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar M., Nguyen T., Gundre E., Ogunlusi O., El-Sobky M., Giri B., Sarkar T.R. Cancer-associated fibroblasts: the chief architect in the tumor microenvironment. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1089068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bordeleau F., Brownell D., Chabaud S., Huot M.E., Bolduc S. Recreating heterogeneity of bladder cancer microenvironment to study its recurrences and progression. Stem Cell Invest. 2023;10:5. doi: 10.21037/sci-2023-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millet M., Bollmann E., Ringuette Goulet C., Bernard G., Chabaud S., Huot M.E., Pouliot F., Bolduc S., Bordeleau F. Cancer-associated fibroblasts in a 3D engineered tissue model induce tumor-like matrix stiffening and EMT transition. Cancers. 2022:14. doi: 10.3390/cancers14153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berndt A., Richter P., Kosmehl H., Franz M. Tenascin-C and carcinoma cell invasion in oral and urinary bladder cancer. Cell Adhes. Migrat. 2015;9:105–111. doi: 10.1080/19336918.2015.1005463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiitta O., Wahlström T., Virtanen I., Gould V.E. Tenascin in inflammatory conditions and neoplasms of the urinary bladder. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;63:283–287. doi: 10.1007/BF02899274. [DOI] [PubMed] [Google Scholar]
- 63.Mao X., Xu J., Wang W., Liang C., Hua J., Liu J., Zhang B., Meng Q., Yu X., Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gil-Julio H., Perea F., Rodriguez-Nicolas A., Cozar J.M., González-Ramirez A.R., Concha A., Garrido F., Aptsiauri N., Ruiz-Cabello F. Tumor escape phenotype in bladder cancer is associated with loss of HLA class I expression, T-cell exclusion and stromal changes. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22147248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–79. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 66.Feng R., Li Z., Ge G., Wang C., Jia Y., Ouyang J. Endocr Metab Immune Disord Drug Targets; 2023. Cancer-associated Fibroblast-Derived Extracellular Vesicles Mediate Immune Escape of Bladder Cancer via PD-L1/pd-1 Expression. [DOI] [PubMed] [Google Scholar]
- 67.Ireland L.V., Mielgo A. Macrophages and fibroblasts, key players in cancer chemoresistance. Front. Cell Dev. Biol. 2018;6:131. doi: 10.3389/fcell.2018.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bu L., Baba H., Yasuda T., Uchihara T., Ishimoto T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020;111:3468–3477. doi: 10.1111/cas.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo G., Zhang Y., Wu Z., Zhang L., Liang C., Chen X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim. Biophys. Sin. 2021 doi: 10.1093/abbs/gmab023. [DOI] [PubMed] [Google Scholar]
- 70.Shan G., Zhou X., Gu J., Zhou D., Cheng W., Wu H., Wang Y., Tang T., Wang X. Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/β-catenin pathway and upregulating PTEN. Cellular oncology (Dordrecht) 2021;44:45–59. doi: 10.1007/s13402-020-00500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuang J., Shen L., Li M., Sun J., Hao J., Li J., Zhu Z., Ge S., Zhang D., Guo H., Huang R., Yan J. Cancer research; 2023. Cancer-associated Fibroblast-Derived miR-146a-5p Generates a Niche that Promotes Bladder Cancer Stemness and Chemoresistance. [DOI] [PubMed] [Google Scholar]
- 72.Pellerin È., Chabaud S., Pouliot F., Pelletier M., Bolduc S. Bisphenol A alters the energy metabolism of stromal cells and could promote bladder cancer progression. Cancers. 2021;13 doi: 10.3390/cancers13215461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miao L., Wang Y., Lin C.M., Xiong Y., Chen N., Zhang L., Kim W.Y., Huang L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J. Contr. Release : official journal of the Controlled Release Society. 2015;217:27–41. doi: 10.1016/j.jconrel.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gieniec K.A., Butler L.M., Worthley D.L., Woods S.L. Cancer-associated fibroblasts-heroes or villains? Br. J. Cancer. 2019;121:293–302. doi: 10.1038/s41416-019-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caligiuri G., Tuveson D.A. Activated fibroblasts in cancer: perspectives and challenges. Cancer Cell. 2023;41:434–449. doi: 10.1016/j.ccell.2023.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camargo S., Gofrit O.N., Assis A., Mitrani E. Paracrine signaling from a three-dimensional model of bladder carcinoma and from normal bladder switch the phenotype of stromal fibroblasts. Cancers. 2021;13 doi: 10.3390/cancers13122972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mullenders J., de Jongh E., Brousali A., Roosen M., Blom J.P.A., Begthel H., Korving J., Jonges T., Kranenburg O., Meijer R., Clevers H.C. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc. Natl. Acad. Sci. U.S.A. 2019;116:4567–4574. doi: 10.1073/pnas.1803595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serex L., Sharma K., Rizov V., Bertsch A., McKinney J.D., Renaud P. Microfluidic-assisted bioprinting of tissues and organoids at high cell concentrations. Biofabrication. 2021;13 doi: 10.1088/1758-5090/abca80. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W., Moore L., Ji P. Mouse models for cancer research. Chin. J. Cancer. 2011;30:149–152. doi: 10.5732/cjc.011.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caston R.A., Shah F., Starcher C.L., Wireman R., Babb O., Grimard M., McGeown J., Armstrong L., Tong Y., Pili R., Rupert J., Zimmers T.A., Elmi A.N., Pollok K.E., Motea E.A., Kelley M.R., Fishel M.L. Combined inhibition of Ref-1 and STAT3 leads to synergistic tumour inhibition in multiple cancers using 3D and in vivo tumour co-culture models. J. Cell Mol. Med. 2021;25:784–800. doi: 10.1111/jcmm.16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J., Miao L., Guo S., Zhang Y., Zhang L., Satterlee A., Kim W.Y., Huang L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J. Contr. Release : official journal of the Controlled Release Society. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott A.M., Wiseman G., Welt S., Adjei A., Lee F.T., Hopkins W., Divgi C.R., Hanson L.H., Mitchell P., Gansen D.N., Larson S.M., Ingle J.N., Hoffman E.W., Tanswell P., Ritter G., Cohen L.S., Bette P., Arvay L., Amelsberg A., Vlock D., Rettig W.J., Old L.J. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 83.Hofheinz R.D., al-Batran S.E., Hartmann F., Hartung G., Jäger D., Renner C., Tanswell P., Kunz U., Amelsberg A., Kuthan H., Stehle G. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 84.Cazet A.S., Hui M.N., Elsworth B.L., Wu S.Z., Roden D., Chan C.L., Skhinas J.N., Collot R., Yang J., Harvey K., Johan M.Z., Cooper C., Nair R., Herrmann D., McFarland A., Deng N., Ruiz-Borrego M., Rojo F., Trigo J.M., Bezares S., Caballero R., Lim E., Timpson P., O'Toole S., Watkins D.N., Cox T.R., Samuel M.S., Martín M., Swarbrick A. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018;9:2897. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira B.A., Vennin C., Papanicolaou M., Chambers C.R., Herrmann D., Morton J.P., Cox T.R., Timpson P. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5:724–741. doi: 10.1016/j.trecan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Wei W.F., Chen X.J., Liang L.J., Yu L., Wu X.G., Zhou C.F., Wang Z.C., Fan L.S., Hu Z., Liang L., Wang W. Periostin(+) cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 2020 doi: 10.1002/1878-0261.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su S., Chen J., Yao H., Liu J., Yu S., Lao L., Wang M., Luo M., Xing Y., Chen F., Huang D., Zhao J., Yang L., Liao D., Su F., Li M., Liu Q., Song E. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856 e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.