Abstract
Conventional methods for improving the hydrophobicity of polypropylene (PP) membranes to prevent wetting phenomena require complex pretreatment procedures in order to activate the surface for enabling the reaction with fluorosilane (FS)-based materials. This study successfully prepared PP membrane contactors with enhanced hydrophobicity through a simple single-step dip-coating method using perfluoroether-grafted silanes for CO2 capture. The FS coating layer on the PP membrane surface was confirmed through ATR-FTIR spectroscopy, XPS, FE-SEM, and EDS. Furthermore, the evaluation of the CO2 absorption performance and long-term stability of the FS-coated PP membrane according to the variation of the gas flow rate (50, 100, 200, 400, and 800 mL/min) confirmed the superior chemical stability and durability of our membranes to those of previously reported hydrophobic membranes. The as-prepared FS-coated PP membrane expands the application scope of gas-liquid membrane contactors for CO2 capture from the flue gas of coal-fired power plants.
Keywords: CO2 capture, Polypropylene, Membrane contactor, Hydrophobic, Surface modification, Fluorosilane
Highlights
-
•
Single-step fluorosilane (FS) dip-coating method was used to modify the PP membrane.
-
•
Abundant C–F moieties in FS enhance hydrophobicity and prevent membrane wetting.
-
•
The long-term stability of the PP membrane increased by 55% after FS coating.
-
•
FS-coated PP is promising for CO2 capture from flue gas of coal-fired power plants.
Abbreviations
- PP
polypropylene
- FS
fluorosilane
- HFMC
hollow fiber membrane contactor
- ATR-FTIR
attenuated total reflection-Fourier transform infrared
- XPS
X-ray photoelectron spectroscopy
- FE-SEM
field-emission scanning electron microscopy
- EDS
energy-dispersive X-ray spectroscopy
1. Introduction
The amount of anthropogenic CO2 emitted by fossil fuel combustion for energy production has increased by 6% annually [1,2]. Anthropogenic CO2 accounts for 80% of the total greenhouse gas emissions and is a significant cause of climate change and global warming [3]. Therefore, considerable efforts have been devoted to the development of CO2 capture technologies to reduce CO2 emissions. In general, strategies designed to reduce CO2 emissions from fossil fuel combustion through CO2 capture can be divided into oxy-combustion, pre-combustion, and post-combustion separation processes. Post-combustion separation is practical because it can easily be applied to all combustion processes in existing power and industrial plants [4,5].
In conventional post-combustion separation processes, packed columns are typically used as reactors. However, industrial-scale reactors occupy a large area and are difficult to operate owing to several issues, including liquid channeling, flooding, and bubble formation [6]. In contrast, a hollow fiber membrane contactor (HFMC) is a hybrid technology that combines membrane separation and absorption and overcomes the above mentioned limitations of packed columns because the role of the membrane as a physical boundary enables sufficient contact between the gas and liquid phases for mass transfer without direct contact and complete mixing [4,7]. HFMCs have recently attracted appreciable attention because they can effectively reduce absorber size. Remarkably, the gas-liquid interface area of an HFMC is 30 times larger than that of a conventional packed column, although using a membrane inevitably leads to additional mass-transfer resistance [8,9]. In addition, HFMCs have the advantage of being easily scalable owing to their modularity [10]. However, HFMCs are susceptible to wetting. The wetting of the membrane with solvents can induce mass-transfer resistance, which increases as the solvent gradually penetrates the membrane's pores during long-term operation, thereby reducing the absorption performance of the membrane [11,12]. Therefore, the ability of the membrane to resist wetting is a critical factor in the selection of the polymer material because it affects the lifetime of the HFMC system [13].
Increasing the hydrophobicity of a membrane is an invaluable method for reducing its wetting. Compared with other polymers, polypropylene (PP) has the advantages of improved hydrophobicity, high chemical stability, and good mechanical strength. In addition, PP has been widely used as a membrane material for HFMC particularly because of its low cost [[14], [15], [16], [17]]. PP costs 36 times lower than polyvinylidene fluoride (PVDF), which possesses superior physical properties, thus making PP relatively advantageous for commercialization [18]. However, the inherent hydrophobicity of the PP membrane is insufficient to prevent its wetting during long-term operation [19]. Therefore, improving the hydrophobicity of the PP membrane to minimize or avoid its wetting with solvents is of utmost priority for the practical application of PP-based HFMCs in CO2 absorption.
Although some attempts have been made to improve the hydrophobicity of PP membranes via surface treatment with a fluorosilane (FS)-based material [20,21] reported studies on improving the hydrophobicity of PP membranes through surface modification for CO2 separation have been scarce. For the surface modification of PP, which contains abundant C–H bonds, it is essential first to replace the inactive C–H bonds with C–OH bonds to facilitate a reaction with an FS molecule [22]. Hydroxyl groups are appropriate reactive sites for immobilizing an FS on the membrane surface through a crosslinking reaction. Currently, the functionalization of the PP membrane surface with hydroxyl groups is mostly accomplished through physical (plasma or UV/ozone treatment) or chemical pretreatment processes (wet acid or alkali treatment) [[22], [23], [24]]. However, physical pretreatment requires special equipment, which incurs high initial installation and operating costs [25]. In contrast, chemical pretreatment could alter the physical properties of the membrane if the treatment conditions are inappropriate [26]. In addition, the chemical pretreatment process is complicated and time-consuming and generally requires two steps: 1) hydroxylation of the PP membrane surface and 2) subsequent reaction with an FS. Therefore, it would be advantageous to simplify the two-step process into a single one. In general, the surface coating process with FS-based materials, such as 1H,1H,2H,2H-perfluorodecyltriethoxysilane, 1H,1H,2H,2H-perfluorodecyltrimethoxysilane, and 1H,1H,2H, 2H-perfluorododecyltrichlorosilane, requires a high-temperature curing process at 353.15 K or higher [22,27,28]. Therefore, the coating processes at high temperatures are unsuitable for PP membranes because of the decomposition issue (melting point of PP: 403.15 K) [29].
In this study, to improve the wetting resistance of the PP membrane-based HFMC in the CO2 capture process, the surface of the PP membrane was hydrophobized using perfluoroether-grafted silane in a single step without physical or chemical pretreatment. The selected FS was expected to be suitable for enhancing the hydrophobicity of the PP membrane surface because it could be cured at a relatively low temperature (298.15 K). After surface modification, the chemical structure of the FS-coated PP membrane surface was investigated by attenuated total reflection-Fourier transform infrared (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS) to confirm the presence of the FS coating layer, which contains abundant C–F bonds, using a pristine PP membrane as the control. In addition, the surface morphology and chemical composition of the FS-coated PP membrane were evaluated using field-emission scanning electron microscopy (FE-SEM) and energy-dispersive X-ray spectroscopy (EDS). Furthermore, the CO2-absorption performances (CO2 removal efficiency and CO2 absorption flux) of pristine PP and FS-coated PP membranes were evaluated comparatively by applying them in a gas-liquid membrane contactor. Finally, the long-term stability of the FS-coated PP membrane was investigated and compared with those of other reported hydrophobic membranes. This study aimed to examine the characteristics of FS-coated PP HFMCs and the possibility of improving the wetting resistance of the membrane through membrane surface modification for CO2 capture from coal-fired power plants.
2. Experimental section
2.1. Materials
PP HFMCs were purchased from SepraTeck Co., Korea. Perfluoroether-grafted silane (KY-164, 20.0 wt%) used as the FS was obtained from Shin-Etsu Co., Japan. Hydrofluoroethers (HFE-7200, 99.0 wt%) were supplied by 3 M Deutschland GmbH, Germany. Monoethanolamine (MEA; 99.0 wt%) and ethanol (94.5%) were purchased from DAEJUNG Chemical and Metals Co., Ltd., Korea. CO2 gas (15 vol%, N2 balance) was purchased from Korea Gas & Electric Technology Co., Ltd., Korea.
2.2. Preparation of the FS-coated PP membrane
2.2.1. Coating
The preparation process of the hollow fiber FS-coated PP membrane is illustrated in Fig. 1. A dip-coating method was used for coating the membrane. The cleaned PP membrane was immersed for 1 h in 2 wt% KY-164 diluted with HFE-7200 at 298.15 K. In the case of the membrane contactor, the coating liquid was filled on the shell side, and the absorbent flowed. During immersion, PP and KY-164 adsorbed to the surface of the PP membrane because of hydrophobic interactions between their nonpolar alkyl groups [30].
Fig. 1.
Schematic illustration of the preparation of FS-coated PP membranes.
2.2.2. Curing
Next, the immersed membrane was removed and exposed to 80% relative humidity (RH) for 24 h at 298.15 K in an oven to allow the reaction shown in Fig. 1 to proceed. KY-164 was adsorbed on the membrane surface via hydrophobic interactions under sufficient moisture conditions. The R–OH molecules generated by the hydrolysis of KY-164 facilitated the binding of KY-164 to the PP membrane surface through hydrophobic interactions and hydrogen bonding. Subsequently, a crosslinking reaction occurred through dehydration and condensation reactions. Eventually, the KY-164 on the membrane cured into a stable form on the surface of the PP membrane.
2.2.3. Washing and drying
After the curing process, the modified surface of the PP membrane was washed twice with ethanol to remove unreacted materials and residual solvent. Finally, the modified PP membrane was dried in a vacuum oven at 328.15 K for 24 h.
2.3. CO2 absorption mechanism of MEA used as the absorbent
The absorption of CO2 by primary amines based on the zwitterion mechanism was first proposed by Caplow and reintroduced by Danckwerts [31,32]. When MEA, a primary amine, is reacted with CO2 to form a zwitterion, (Eq. (1)), the zwitterion is instantaneously deprotonated by a basic species (unreacted MEA, OH−, or H2O) present in the system to form a carbamate molecule as shown in Eq. (2).
| (1) |
| (2) |
The resulting carbamate can further react with water to form bicarbonate ( as shown in Eq. (3). Many factors, including the carbamate's chemical stability, influence the reaction kinetics. In particular, the lower the basicity of the amine is, the faster the decomposition of the carbamate is.
| (3) |
Further, CO2 reacts with water to form bicarbonate (Eqs. (4), (5), (6))). Finally, the bicarbonate is converted to carbonate in the presence of a base capable of extracting H+ from it (Eq. (7)). At this time, the base may act as an amine molecule or a hydroxide ion; however, in the case of an amine, the reaction proceeds quickly.
| (4) |
| (5) |
| (6) |
| (7) |
2.4. Experimental apparatus and procedure of CO2 absorption
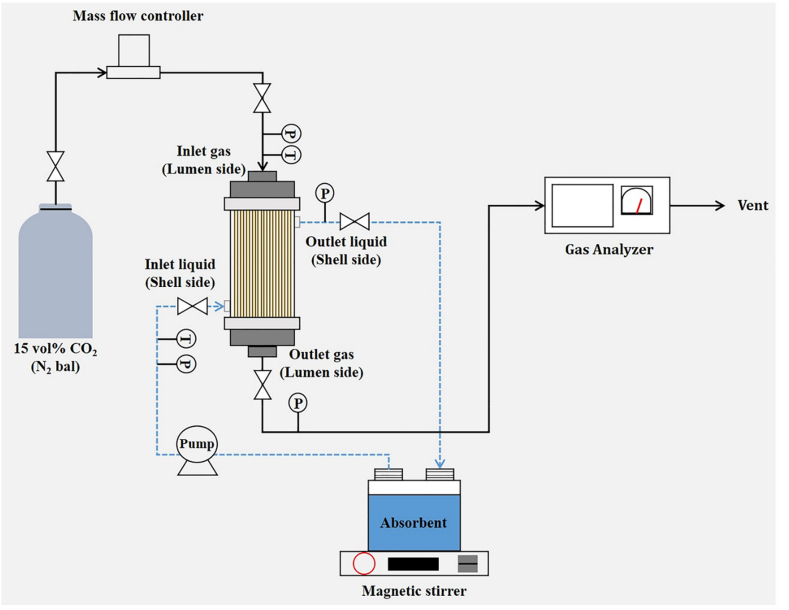
Fig. 2 Shows a schematic of the CO2 absorption apparatus using the FS-coated PP HFMC. In absorption experiments, a 15% CO2/N2 mixture was used as the feed gas to simulate the CO2 concentration in coal-fired power plant emissions [33]. The feed gas was introduced through the lumen side of the fibers of the membrane contactor, and the gas flow rate was adjusted using a mass-flow controller (GMC 1200, Atovac, Korea). The operating conditions of the CO2 absorption are given in Table 1. The CO2 concentrations of the inlet and outlet gases were measured at 1 min intervals using a CO2 gas analyzer (Multi Master, Sensoronic Co., Ltd., Korea).
Fig. 2.
Schematic of the experimental apparatus used for CO2 absorption.
Table 1.
Operating conditions of the polypropylene-based HFMC.
| Parameters | Value | Unit |
|---|---|---|
| Absorbent | 30 wt% MEA | – |
| CO2 concentration | 15 | % |
| N2 concentration | 85 | % |
| Gas flow rate (QG) | 50, 100, 200, 400, and 800 | mL/min |
| Liquid flow rate (QL) | 5 | mL/min |
| Temperature | 313.15 | K |
Details of the commercial membrane contactor used in the present study are provided in Table 2; 30 wt% MEA was used as the chemical absorbent. The absorbent was flown counter-currently through the shell side of the membrane contactor because the CO2 absorption flux is higher in the counter-current flow than in the co-current flow [34]. To avoid channeling and dead zones, the absorbent was flown from the bottom to the top [35]. The absorption experiments were conducted at 313.15 K and were monitored using a temperature gauge installed at the inlet of the absorbent. A hotplate equipped with a magnetic stirrer was used to maintain the homogeneity and temperature of the absorbent. The absorbent was pumped through the shell side of the membrane contactor using a digital gear pump (5970-Optos, Eldex Laboratories, Inc., USA) to maintain a flow rate of 5 mL/min. It was recirculated back to the absorbent tank. The liquid-side pressure was set to be slightly higher than that of the gas side to avoid gas permeation to the liquid side. The membrane could be wet if the pressure on the liquid side exceeds the liquid entry pressure [36]. The CO2 removal efficiency and CO2 absorption flux were calculated using Eqs. (8), (9)), respectively [37,38]:
| (8) |
| (9) |
where Qin and Qout represent the inlet and outlet gas flow rates (m3/h), respectively; Cin and Cout are the concentrations of the inlet and outlet gases, respectively; Tg is the gas temperature (K); and S represents the gas-liquid mass-transfer area, which is equal to the effective membrane area (m2).
Table 2.
Specifications of the HFMC.
| Parameters | Value | Unit |
|---|---|---|
| Fiber material | Polypropylene | – |
| Housing material | Acryl | – |
| Packing material | Epoxy resin | – |
| Module length | 190 | mm |
| Fiber: outer diameter | 690 | μm |
| Fiber: inner diameter | 500 | μm |
| Number of fibers | 440 | – |
| Effective pore size | 0.225 | μm |
| Surface area | 0.2 | m2 |
2.5. Characterization methods
The amounts of fluorine, carbon, and silicon in the coated hollow fiber membranes were quantitatively determined through XPS analysis (K-alpha, ThermoFisher, USA). The morphology and constituent elements of the surface and cross-section of the coated hollow fiber membrane were investigated using FE-SEM and EDS (S-4800, Hitachi High-Technologies, Japan). ATR-FTIR spectroscopy (FT/IR-4600, Jasco, Japan) was performed to evaluate the surface chemical composition of the coating layer. The FTIR spectrum recorded in the 450–4000 cm−1 with a resolution of 4 cm−1 was averaged over 16 scans.
3. Results and discussion
3.1. Characterization of the FS-coated PP membrane
The surface chemical groups of the pristine PP and modified PP membranes were analyzed by ATR-FTIR spectroscopy. The ATR-FTIR spectra of the samples are shown in Fig. 3(A). The spectra of both samples exhibited the characteristic C–H stretching vibrations of PP at 2835–2950, 1455, and 1375 cm−1. In particular, the peaks observed at 1455 and 1375 cm−1 are attributable to the C–H vibrations of the –CH2 and –CH3 groups, respectively [23]. For the FS-coated PP membrane, the Si–O–Si asymmetric stretching vibrations peak was observed at 1100 cm−1, indicating that KY-164 was crosslinked through dehydration and condensation reactions during the curing process. The peak corresponding to C–F stretching vibrations from the perfluoropolyether unit of KY-164 appeared at 1210 cm−1. In addition, the stretching vibrations of –OH and C–O bonds were observed at 3290 and 1065 cm−1, respectively. These peaks indicate that R–OH was generated during the curing process.
Fig. 3.
(A) ATR-FTIR spectra and (B) XPS profiles of pristine PP and surface-modified PP membranes.
XPS provides qualitative information on the chemical composition of the membrane surface before and after modification [39]. Therefore, the chemical compositions of the pristine PP and FS-coated PP membranes were compared through XPS analysis. As shown in Fig. 3(B), increased Si, F, and O signals were detected for the FS-coated PP membrane. The detailed contents of the different elements in the pristine PP and FS-coated PP membranes are listed in Table 3. Thus, the ATR-FTIR and XPS spectra confirmed the successful formation of the FS-coated PP membrane, as illustrated in Fig. 1. The hydrophobicity of the PP membrane surface was enhanced by the fluorine groups in the FS coating layer, which are highly hydrophobic; this is expected to prevent the wetting of the membrane.
Table 3.
Surface composition (atom.%) of pristine PP and surface-modified PP membranes.
| C 1s | O 1s | Si 2p | F 1s | |
|---|---|---|---|---|
| Pristine PP | 97.14% | 1.57% | 0.30% | – |
| FS-coated PP | 52.59% | 11.92% | 1.51% | 33.23% |
The surface morphology of the modified PP membrane was observed using SEM. After modification, the shape of the hollow fibers of the PP membrane was maintained. However, the comparison of the structural features of the membrane before and after modification (Fig. 4(A) and (B)) confirmed that the inner diameter of the hollow fibers increased by approximately 71 μm. In comparison, the thickness of the hollow fiber wall increased by about 44 μm. Fig. 4(C) and (D) confirm that the size of the hollow fiber increased significantly after coating treatment. These results indicate that the hollow fiber PP membrane swelled during modification. Membrane swelling occurred because the FS-based coating liquid at the shell side permeated into the lumen side through the pores of the membrane, and the coating liquid was adsorbed and then cured on the surface of the PP membrane to form an FS layer. Consequently, the pores in the membrane increased, more because of the FS layer than the pristine PP (Fig. 4(E)), as shown in Fig. 4(F). In addition, EDS analysis (Fig. 4(G) and (H)) confirmed the formation of a thick fluorine-based coating on the shell side and the presence of a thin fluorinated layer on the lumen side. This result indicates that the coating liquid penetrated the membrane's pores and was adsorbed onto the inner surface. This phenomenon occurs when the membrane substrate is hydrophobic and the viscosity of the coating liquid is low. According to the supplier, the kinematic viscosity of KY-164 is 1.2 mm2/s at 298.15 K, and the kinematic viscosity of HFE-7200 is 0.33 mm2/s at 293.15 K [40]. Tai et al. reported that a coating liquid with low viscosity easily penetrates the pores and surface of the membrane, and the coating extends from the surface to the pores. In contrast, a high-viscosity coating solution does not penetrate the pores and is coated only on the surface [41]. Thus, overall, the shell side of the modified PP hollow fiber membrane and the pore and lumen sides were coated with the FS layer. The effect of swelling caused by surface modification on the CO2-absorption performance of the FS-coated PP hollow fiber membrane is discussed in Section 3.2.
Fig. 4.
Cross-sectional SEM images of pristine PP (A) and surface-modified PP (B) membranes. Plane-view SEM images of pristine PP (C, E) and modified PP membranes (D, F). EDS mapping images for the fluorine component (shown in yellow) on modified membranes (G, H).
3.2. CO2 absorption performance
3.2.1. Effect of gas flow rate on the CO2 removal efficiency and CO2 absorption flux
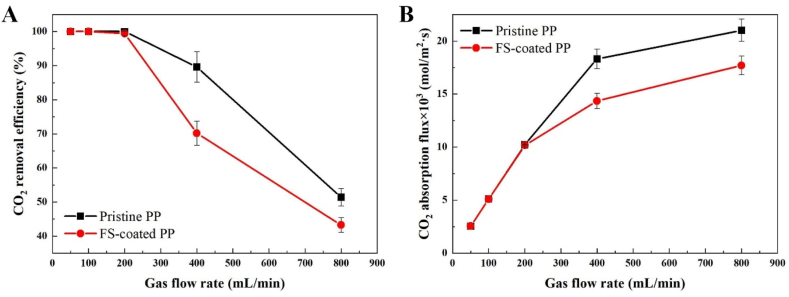
Fig. 5 shows the effect of the gas flow rate on the CO2 removal efficiency and CO2 absorption flux for the pristine and FS-coated PP membranes. The operating conditions of the CO2 absorption experiments are provided in Table 1. As shown in Fig. 5(A), the CO2 removal efficiencies of the systems using pristine PP and FS-coated PP membranes with 30 wt% MEA decreased from 100% to 51.4 and 43.3% as the gas flow rate increased from 50 to 800 mL/min, respectively. In comparison, the CO2 absorption flux of the membranes increased from 2.55 to 21.01 and 17.70 × 10−3 (mol/m2·s), respectively. As the gas flow rate increases, the CO2 absorption flux increases due to decreased gas-phase resistance [42].
Fig. 5.
(A) CO2 removal efficiency and (B) CO2 absorption flux of the systems with pristine PP and modified PP membranes (absorbent: 30 wt% MEA; liquid flow rate: 5 mL/min).
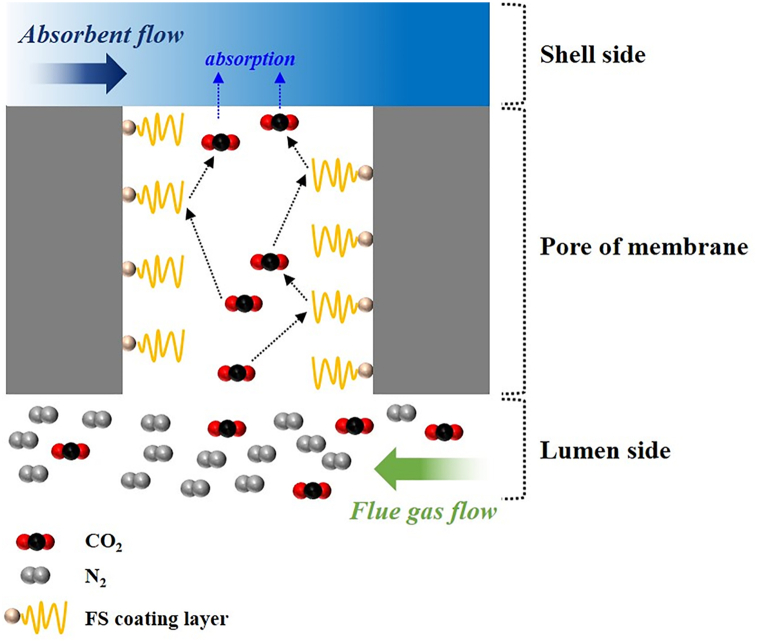
However, the CO2 removal efficiency decreased because the residence time of the inlet gas in the membrane contactor was shortened, and the diffusivity of CO2 was reduced [38]. Interestingly, both the CO2 removal efficiency and CO2 absorption flux of the FS-coated PP membrane with enhanced hydrophobicity decreased in relation to those of the pristine PP membrane. During the CO2 removal process, the CO2-containing gas diffuses from the gas phase to the membrane surface. Then it passes through the membrane's pores to form a uniform and fine bubbles, resulting in dissolution, diffusion, and chemical reactions in the absorbent (MEA in this study). However, the CO2 removal efficiency and CO2 absorption flux of the FS-coated PP were lower than those of pristine PP because the pore pathway was complicated owing to the hydrophobic FS coating layer, leading to an increase in mass-transfer resistance, as illustrated in Fig. 6. Lee and Park reported that the formation of an optimal hydrophobic coating layer is essential for avoiding interference with CO2 transport [42]. Although the fact that the resistance of the membrane phase increases with an increase in membrane thickness, in this study, the effect of increasing the membrane thickness was insignificant because the CO2 removal efficiency and absorption flux of the pristine PP and modified PP membranes were similar at gas flow rates of 50, 100, and 200 mL/min at a slow liquid flow rate of 5 mL/min, which is adequate to evaluate the effect of changing the membrane thickness. Table 4 summarizes the CO2 removal efficiency and absorption flux of the pristine PP and FS-coated PP membranes.
Fig. 6.
Schematic of gas permeation through the pores of the FS-coated PP membrane during absorption experiments.
Table 4.
Summary of the CO2 removal efficiency and absorption flux of the pristine PP and FS-coated PP membranes.
| Membrane | Gas flow rate (mL/min) | CO2 removal efficiency (%) | CO2 absorption flux 103 (mol/m2·s) |
|---|---|---|---|
| Pristine PP |
50 | 100 | 2.55 |
| 100 | 100 | 5.11 | |
| 200 | 100 | 10.22 | |
| 400 | 89.6 | 18.32 | |
| 800 |
51.4 |
21.01 |
|
| FS-coated PP | 50 | 100 | 2.55 |
| 100 | 100 | 5.11 | |
| 200 | 99.4 | 10.17 | |
| 400 | 70.2 | 14.35 | |
| 800 | 43.3 | 17.70 |
3.2.2. Long-term CO2 absorption performance of the surface-modified PP membrane
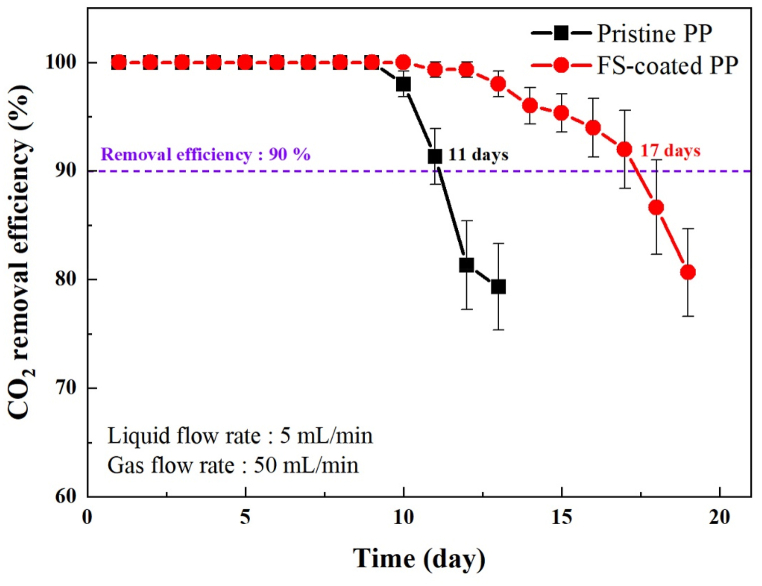
CO2 absorption experiments were conducted at the same CO2 absorption flux to investigate the long-term stability and wetting resistance of the pristine PP and FS-coated PP membranes using 30 wt% MEA as the absorbent (Fig. 7). In the case of pristine PP, the CO2 removal efficiency decreased rapidly after 10 days of the CO2 absorption experiment. The period over which a 90% removal rate was maintained for 11 days. Note that most CO2 capture processes are aimed at 90% CO2 removal efficiency from coal-fired power plants [35]. In contrast, in the case of the FS-coated PP, the CO2 removal efficiency gradually decreased from the 11th day onward, and 90% removal efficiency was maintained for 17 days. These results indicate that the CO2 removal efficiency of the modified membrane system decreased gradually. The period with 90% CO2 removal efficiency was also extended because the FS coating layer increased the wetting resistance of the membrane. Therefore, these results confirm that the FS-coated PP possesses high chemical stability and durability, even under extreme operating conditions in contact with an MEA solution for a long time.
Fig. 7.
Long-term CO2-absorption performances of pristine PP and surface-modified PP membranes.
3.2.3. Comparison of the long-term stabilities of the FS-coated PP membrane and other reported membranes
The long-term stability of the FS-coated PP membrane was compared with those of recently reported hydrophobic membranes. As shown in Table 5, the membrane developed in this study exhibited long-term competitive stability even though these experiments were conducted under more severe conditions than those in the literature (concentration of absorbent: 30 wt% MEA, absorption temperature: 313.15 K, and liquid-to-gas ratio: 0.1 in this study). It is well known that the absorbent concentration or absorption temperature increases the wettability of the membrane [13]; therefore, the FS-coated PP membrane can effectively resist wetting in harsher conditions. Considering this, the results of this study suggest that FS-coated PP membranes can be directly applied in CO2 capture from the flue gas of coal-fired power plants.
Table 5.
Comparison of the long-term stability of the FS-coated PP membrane with those of previously reported membranes.
| Membrane | Inlet gas (gas flow rate) | Liquid absorbent (gas flow rate) | Liquid-to-gas ratio | Absorption temperature (K) | Long-term stability | Ref. |
|---|---|---|---|---|---|---|
| FS-coated PP | 15% CO2 in N2 (50 mL/min) | 30 wt% MEA (5 mL/min) | 0.1 | 313.15 | Decline 10% after 17 d | This study |
| PVDF-TDMS-1.5 | 19% CO2 in N2 (20 mL/min) | 1 M DEA (50 mL/min) | 2.5 | Not reported | Decline 17% after 17 d | [43] |
| Al2O3-FAS | 15% CO2 in N2 (50 mL/min) | 20 wt% MEA (10 mL/min) | 0.2 | 293.15 | Decline 15% after 3 d | [42] |
| PVDF + LDPE | 20% CO2 in N2 (150 mL/min) | 1 M MEA (50 mL/min) | 0.33 | Not reported | Decline 14% after 1 d | [44] |
| PVDF + DL-4% SiO2-80 | 19% CO2 in N2 (100 mL/min) | 1 M DEA (50 mL/min) | 0.5 | 293.15 | Decline 18% after 6 d | [45] |
| PP | 20% CO2 in N2 (200 mL/min) | 1 M MEA (17 mL/min) | 0.08 | 293.15 | Decline 14% after 7 d | [14] |
| PVDF-PA | 19% CO2 in N2 (130 mL/min) | Water (70 mL/min) | 0.54 | 297.15 | Decline 17% after 2 d | [46] |
4. Conclusion
This study reports a simple single-step dip-coating method to prepare a hollow PP fiber membrane contactor with enhanced hydrophobicity using perfluoroether-grafted silane as the surface modifier for CO2 capture. The FS coating layer contained abundant C–F bonds, as revealed by the surface analysis (ATR-FTIR, XPS, and SEM/EDS). The enhanced hydrophobicity of the FS-coated PP membrane was confirmed by evaluating the CO2 absorption performance in a gas-liquid membrane contactor. The FS-coated PP membrane's CO2 removal efficiency and CO2 absorption flux were comparable to those of pristine PP at gas flow rates of 50, 100, and 200 mL/min. In terms of long-term stability, the FS-coated PP membrane maintained 90% CO2 removal efficiency for a significantly longer duration (55% increase in time) than the pristine PP membrane, which confirms its higher chemical stability and durability, even under extreme operating conditions in contact with a 30 wt% MEA solution for a long time. In addition, the FS-coated PP membrane exhibited greater long-term stability than previously reported hydrophobic membranes. The results of this study suggest that FS-coated PP membranes can be used directly to capture CO2 in the flue gas emitted from coal-fired power plants. Our future study will explore the effects of various operating conditions (such as liquid flow rate, liquid concentration, liquid temperature, feed gas composition) on CO2 absorption performance and membrane wetting using mathematical modeling.
Author contribution statement
Kwanghwi Kim, Suhan Kim: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Heejun Lee: Performed the experiments. Hyun Sic Park, Hojun Song: Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Ulsan Metropolitan City and Korea Institute of Industrial Technology (“Development of Carbon Dioxide Capture and Utilization Technology for Greenhouse Gas Reduction,” Project No. IZ-22-0078).
References
- 1.Chabanon E., Roizard D., Favre E. Modeling strategies of membrane contactors for post-combustion carbon capture: a critical comparative study. Chem. Eng. Sci. 2013;87:393–407. doi: 10.1016/j.ces.2012.09.011. [DOI] [Google Scholar]
- 2.Dai Z., Noble R.D., Gin D.L., Zhang X., Deng L. Combination of ionic liquids with membrane technology: a new approach for CO2 separation. J. Membr. Sci. 2016;497:1–20. doi: 10.1016/j.memsci.2015.08.060. [DOI] [Google Scholar]
- 3.WMO . vol. 13. World Meteorological Organization; 2017. The state of greenhouse gases in the atmosphere based on global observations through 2016. (WMO Greenhouse Gas Bulletin (GHG Bulletin)). [Google Scholar]
- 4.Qazi S., Gómez-Coma L., Albo J., Druon-Bocquet S., Irabien A., Sanchez-Marcano J. CO2 capture in a hollow fiber membrane contactor coupled with ionic liquid: influence of membrane wetting and process parameters. Sep. Purif. Technol. 2020;233 doi: 10.1016/j.seppur.2019.115986. [DOI] [Google Scholar]
- 5.Zhao S., Feron P.H.M., Deng L., Favre E., Chabanon E., Yan S., Hou J., Chen V., Qi H. Status and progress of membrane contactors in post-combustion carbon capture: a state-of-the-art review of new developments. J. Membr. Sci. 2016;511:180–206. doi: 10.1016/j.memsci.2016.03.051. [DOI] [Google Scholar]
- 6.Wang M., Lawal A., Stephenson P., Sidders J., Ramshaw C. Post-combustion CO2 capture with chemical absorption: a state-of-the-art review. Chem. Eng. Res. Des. 2011;89:1609–1624. doi: 10.1016/j.cherd.2010.11.005. [DOI] [Google Scholar]
- 7.Mansourizadeh A., Ismail A.F., Matsuura T. Effect of operating conditions on the physical and chemical CO2 absorption through the PVDF hollow fiber membrane contactor. J. Membr. Sci. 2010;353:192–200. doi: 10.1016/j.memsci.2010.02.054. [DOI] [Google Scholar]
- 8.Favre E., Svendsen H.F. Membrane contactors for intensified post-combustion carbon dioxide capture by gas-liquid absorption processes. J. Membr. Sci. 2012:407–408. doi: 10.1016/j.memsci.2012.03.019. 1–7. [DOI] [Google Scholar]
- 9.Villeneuve K., Albarracin Zaidiza D.A., Roizard D., Rode S. Modeling and simulation of CO2 capture in aqueous ammonia with hollow fiber composite membrane contactors using a selective dense layer. Chem. Eng. Sci. 2018;190:345–360. doi: 10.1016/j.ces.2018.06.016. [DOI] [Google Scholar]
- 10.Li J.-L., Chen B.-H. Review of CO2 absorption using chemical solvents in hollow fiber membrane contactors. Sep. Purif. Technol. 2005;41:109–122. doi: 10.1016/j.seppur.2004.09.008. [DOI] [Google Scholar]
- 11.Mosadegh-Sedghi S., Rodrigue D., Brisson J., Iliuta M.C. Wetting phenomenon in membrane contactors – causes and prevention. J. Membr. Sci. 2014;452:332–353. doi: 10.1016/j.memsci.2013.09.055. [DOI] [Google Scholar]
- 12.Zhang H.-Y., Wang R., Liang D.T., Tay J.H. Theoretical and experimental studies of membrane wetting in the membrane gas-liquid contacting process for CO2 absorption. J. Membr. Sci. 2008;308:162–170. doi: 10.1016/j.memsci.2007.09.050. [DOI] [Google Scholar]
- 13.Ibrahim M.H., El-Naas M.H., Zhang Z., Van der Bruggen B. CO2 capture using hollow fiber membranes: a review of membrane wetting. Energy Fuel. 2018;32:963–978. doi: 10.1021/acs.energyfuels.7b03493. [DOI] [Google Scholar]
- 14.Lv Y., Yu X., Jia J., Tu S.-T., Yan J., Dahlquist E. Fabrication and characterization of superhydrophobic polypropylene hollow fiber membranes for carbon dioxide absorption. Appl. Energy. 2012;90:167–174. doi: 10.1016/j.apenergy.2010.12.038. [DOI] [Google Scholar]
- 15.Islam S.Z., Arifuzzaman M., Rother G., Bocharova V., Sacci R.L., Jakowski J., Huang J., Ivanov I.N., Bhave R.R., Saito T., Sholl D.S. A membrane contactor enabling energy-efficient CO2 capture from point sources with deep eutectic solvents. Ind. Eng. Chem. Res. 2023;62:4455–4465. doi: 10.1021/acs.iecr.3c00080. [DOI] [Google Scholar]
- 16.Amirabedi P., Akbari A., Yegani R., Raveshiyan S. CO2 stripping from monoethanolamine through a polypropylene/CH3SiO2 composite hollow-fiber membrane contactor. Chem. Eng. Technol. 2022;45:1512–1521. doi: 10.1002/ceat.202100630. [DOI] [Google Scholar]
- 17.Imtiaz A., Othman M.H.D., Jilani A., Khan I.U., Kamaludin R., Ayub M., Samuel O., Kurniawan T.A., Hashim N., Puteh M.H. A critical review in recent progress of hollow fiber membrane contactors for efficient CO2 separations. Chemosphere. 2023 doi: 10.1016/j.chemosphere.2023.138300. [DOI] [PubMed] [Google Scholar]
- 18.Khisri S., deMontigny D., Tontiwachwuthikul P. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009;65:290–297. doi: 10.1016/j.seppur.2008.10.035. [DOI] [Google Scholar]
- 19.Lv Y., Yu X., Tu S.-T., Yan J., Dahlquist E. Wetting of polypropylene hollow fiber membrane contactors. J. Membr. Sci. 2010;362:444–452. doi: 10.1016/j.memsci.2010.06.067. [DOI] [Google Scholar]
- 20.Chen Z., Xie H.-Y., Li Y.-J., Chen G.-E., Xu S.-J., Xu Z.-L. Smart light responsive polypropylene membrane switching reversibly between hydrophobicity and hydrophilicity for oily water separation. J. Membr. Sci. 2021;638 doi: 10.1016/j.memsci.2021.119704. [DOI] [Google Scholar]
- 21.Raveshiyan S., Amirabedi P., Yegani R., Pourabbas B., Tavakoli A. CO2 absorption through PP/fSiO2 nanocomposite hollow fiber membrane contactor. Polyolefins J. 2022;9:61–71. [Google Scholar]
- 22.Wang Y., He G., Shao Y., Zhang D., Ruan X., Xiao W., Li X., Wu X., Jiang X. Enhanced performance of superhydrophobic polypropylene membrane with modified antifouling surface for high salinity water treatment. Sep. Purif. Technol. 2019;214:11–20. doi: 10.1016/j.seppur.2018.02.011. [DOI] [Google Scholar]
- 23.Amirabedi P., Akbari A., Yegani R. Fabrication of hydrophobic PP/CH3SiO2 composite hollow fiber membrane for membrane contactor application. Sep. Purif. Technol. 2019;228 doi: 10.1016/j.seppur.2019.115689. [DOI] [Google Scholar]
- 24.Jaleh B., Etivand E.S., Mohazzab B.F., Nasrollahzadeh M., Varma R.S. Improving wettability: deposition of TiO2 nanoparticles on the O2 plasma activated polypropylene membrane. Int. J. Mol. Sci. 2019;20:3309. doi: 10.3390/ijms20133309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardani A.K., Ariono D., Yespin Y., Sihotang D.R., Wenten I.G. Preparation of hydrophilic polypropylene membrane by acid dipping technique. Mater. Res. Express. 2019;6 doi: 10.1088/2053-1591/ab10cf. [DOI] [Google Scholar]
- 26.Liu Q.-F., Lee C.-H., Kim H. Performance evaluation of alkaline treated poly(vinylidene fluoride) membranes. Separ. Sci. Technol. 2010;45:1209–1215. doi: 10.1080/01496391003775808. [DOI] [Google Scholar]
- 27.Huang Y.-X., Liang D.-Q., Luo C.-H., Zhang Y., Meng F. Liquid-like surface modification for effective anti-scaling membrane distillation with uncompromised flux. J. Membr. Sci. 2021;637 doi: 10.1016/j.memsci.2021.119673. [DOI] [Google Scholar]
- 28.Meng S., Ye Y., Mansouri J., Chen V. Fouling and crystallisation behaviour of superhydrophobic nano-composite PVDF membranes in direct contact membrane distillation. J. Membr. Sci. 2014;463:102–112. doi: 10.1016/j.memsci.2014.03.027. [DOI] [Google Scholar]
- 29.Zhang R., Xiong Y., Liu Q., Hu S. Improved cell morphology and thermal properties of expanded polypropylene beads by the addition of PP with a high melting point. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.45121. [DOI] [Google Scholar]
- 30.Ben-Naim A.Y. Springer Science and Business Media; 2012. Hydrophobic Interactions. [Google Scholar]
- 31.Caplow M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968;90:6795–6803. doi: 10.1021/ja01026a041. [DOI] [Google Scholar]
- 32.Danckwerts P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979;34:443–446. doi: 10.1016/0009-2509(79)85087-3. [DOI] [Google Scholar]
- 33.Lee J.H., Lee D.W., Kwak C., Kang K., Lee J.H. Technoeconomic and environmental evaluation of sodium bicarbonate production using CO2 from flue gas of a coal-fired power plant. Ind. Eng. Chem. Res. 2019;58:15533–15541. doi: 10.1021/acs.iecr.9b02253. [DOI] [Google Scholar]
- 34.Kim S., Scholes C.A., Heath D.E., Kentish S.E. Gas-liquid membrane contactors for carbon dioxide separation: a review. Chem. Eng. J. 2021;411 doi: 10.1016/j.cej.2021.128468. [DOI] [Google Scholar]
- 35.Lee H.J., Park Y.G., Kim M.K., Lee S.H., Park J.H. Study on CO2 absorption performance of lab-scale ceramic hollow fiber membrane contactor by gas/liquid flow direction and module design. Sep. Purif. Technol. 2019;220:189–196. doi: 10.1016/j.seppur.2019.03.011. [DOI] [Google Scholar]
- 36.Sohaib Q., Vadillo J.M., Gómez-Coma L., Albo J., Druon-Bocquet S., Irabien A., Sanchez-Marcano J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020;223 doi: 10.1016/j.ces.2020.115719. [DOI] [Google Scholar]
- 37.Ghaee A., Ghadimi A., Sadatnia B., Ismail A.F., Mansourpour Z., Khosravi M. Synthesis and characterization of poly(vinylidene fluoride) membrane containing hydrophobic silica nanoparticles for CO2 absorption from CO2/N2 using membrane contactor. Chem. Eng. Res. Des. 2017;120:47–57. doi: 10.1016/j.cherd.2017.01.032. [DOI] [Google Scholar]
- 38.Lv Y., Yu X., Tu S.-T., Yan J., Dahlquist E. Experimental studies on simultaneous removal of CO2 and SO2 in a polypropylene hollow fiber membrane contactor. Appl. Energy. 2012;97:283–288. doi: 10.1016/j.apenergy.2012.01.034. [DOI] [Google Scholar]
- 39.Kontturi E., Thüne P.C., Niemantsverdriet J. Cellulose model surfaces simplified preparation by spin coating and characterization by X-ray photoelectron spectroscopy, infrared spectroscopy, and atomic force microscopy. Langmuir. 2003;19:5735–5741. doi: 10.1021/la0340394. [DOI] [Google Scholar]
- 40.Rausch M.H., Kretschmer L., Will S., Leipertz A., Fröba A.P. Density, surface tension, and kinematic viscosity of hydrofluoroethers HFE-7000, HFE-7100, HFE-7200, HFE-7300, and HFE-7500. J. Chem. Eng. Data. 2015;60:3759–3765. doi: 10.1021/acs.jced.5b00691. [DOI] [Google Scholar]
- 41.Tai Z.S., Othman M.H.D., Mustafa A., Ravi J., Wong K.C., Koo K.N., Hubadillah S.K., Azali M.A., Alias N.H., Ng B.C., Mohamed Dzahir M.I.H., Ismail A.F., Rahman M.A., Jaafar J. Development of hydrophobic polymethylhydrosiloxane/tetraethylorthosilicate (PMHS/TEOS) hybrid coating on ceramic membrane for desalination via membrane distillation. J. Membr. Sci. 2021;637 doi: 10.1016/j.memsci.2021.119609. [DOI] [Google Scholar]
- 42.Lee H.J., Park J.H. Effect of hydrophobic modification on carbon dioxide absorption using porous alumina (Al2O3) hollow fiber membrane contactor. J. Membr. Sci. 2016;518:79–87. doi: 10.1016/j.memsci.2016.06.038. [DOI] [Google Scholar]
- 43.Pang H., Chen Z., Gong H., Du M. Fabrication of a super hydrophobic polyvinylidene fluoride-hexadecyltrimethoxysilane hybrid membrane for carbon dioxide absorption in a membrane contactor. J. Membr. Sci. 2020;595 doi: 10.1016/j.memsci.2019.117536. [DOI] [Google Scholar]
- 44.Ahmad A.L., Mohammed H.N., Ooi B.S., Leo C.P. Deposition of a polymeric porous superhydrophobic thin layer on the surface of poly(vinylidenefluoride) hollow fiber membrane. Pol. J. Chem. Technol. 2013;15:1–6. doi: 10.2478/pjct-2013-0036. [DOI] [Google Scholar]
- 45.Chen Z., Shen Q., Gong H., Du M. Preparation of a novel dual-layer polyvinylidene fluoride hollow fiber composite membrane with hydrophobic inner layer for carbon dioxide absorption in a membrane contactor. Sep. Purif. Technol. 2020;248 doi: 10.1016/j.seppur.2020.117045. [DOI] [Google Scholar]
- 46.Pang H., Gong H., Du M., Shen Q., Chen Z. Effect of non-solvent additive concentration on CO2 absorption performance of polyvinylidenefluoride hollow fiber membrane contactor. Sep. Purif. Technol. 2018;191:38–47. doi: 10.1016/j.seppur.2017.09.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.