Abstract
In this study, the effects of ultrasonic and steam-cooking treatments on the physicochemical and emulsifying properties of bamboo shoots protein (BSP) were investigated. The particle size and the polydispersity index (PDI) of U-BSP (ultrasonic-BSP) both decreased. Fourier transform infrared spectroscopy (FTIR) showed that the secondary structure of U-BSP was more loose. Furthermore, X-ray diffraction (XRD) and thermogravimetric (TGA) analysis suggested that crystallinity amd thermal stability of U-BSP both deceased. The water and oil holding capacity (WHC/OHC) of U-BSP increased, while steam-cooking treatment had the reverse effect. We also investigated the effects of ultrasonic and steam-cooking treatments on BSP-stabilized emulsions. The viscosity of emulsion stabilized by U-BSP increased and the distribution of emulsion droplets was more uniform and smaller. The results showed that ultrasonic treatment significantly improved the stability of BSP-stabilized emulsions, while steam-cooking treatment had a significant negative impact on the stability of BSP-stabilized emulsions. The work indicated ultrasonication is an effective treatment to improve the emulsifying properties of BSP.
Keywords: Bamboo shoots protein 1, Ultrasonic 2, Steam-cooking 3, Pickering emulsions 4
1. Introduction
The oil droplets and water phase in oil-in-water (O/W) emulsion are immiscible. Hence, O/W emulsion generally exhibit thermodynamic instability and phase separation occurs over time [1]. Based on the above case, amphiphilic surfactants are commonly used as emulsifiers to reduce the interfacial tension. The disadvantages of traditional surfactants are high consumption and poor biocompatibility. Therefore, the utilization of traditional emulsifiers has been greatly limited [2]. Pickering emulsions stabilized by solid particles are more stable and requires lower amount of additives than emulsions stabilized by traditional surfactants [3]. Solid particles are irreversibly adsorbed at the oil-water interface to form a steric barrier, which prevents coalescence and Ostwald ripening in Pickering emulsions [4]. Currently, there is a research hot spot on the preparation of particle-stable Pickering emulsions using food-grade biomacromolecules such as proteins [5,6], gliadin [7,8], cellulose [9,10] and starch [11]. Plant proteins are natural biodegradable, harmless to health, less allergenic, and cheaper than the most commonly used dairy proteins [12,13]. Hence, the plant proteins are used in designing food emulsions has become the first choice of emulsifiers to reduce the environmental footprint of food production [14]. A series of plant proteins, such as soybean [15], sugar beet [15], faba bean [16], pea [17,18], mung bean [19], chickpea [20] and tomato seed [21], have been studied for stabilizing O/W emulsion. Consequently, the food industry urgently needs new and effective plant protein-based Pickering stabilizer [22].
Bamboo shoots are a sustainable food source [23]. Bamboo shoots are also one of the high-quality sources of plant proteins. In order to find natural and cheap plant proteins as emulsions stabilizer, the bamboo shoots protein (BSP) has garnered attention in the food industry [24]. Nevertheless, protein-stabilized emulsions can be quite sensitive to environmental factors, such as temperature, pH and ionic strength [25]. Therefore, it is crucial to implement various physical modifications to enhance the functionality of BSP. These treatments can include homogenization treatment [26], ultrasonic treatment [27] and heat treatment [28]. Ultrasonic technology is frequently employed in protein modification due to its low cost and environmentally friendly advantages. Ultrasonic waves alter the protein's structure and spatial conformation primarily by creating local extreme physical force through acoustic cavitation effects, improving the functional properties of protein, such as solubility, foamability and emulsibility [27]. Furthermore, the extensive protein denaturation resulting from severe heat treatments during the manufacturing process results in poor functional properties of the protein. These may limit the application of protein in food products [29]. The aggregation state of the protein is the key factor affecting the performance of protein-based emulsions [30]. However, a certain degree of thermal denaturation and aggregation is inevitable during the commercial protein production process [31].
This paper aims to provide insight into the physicochemical characterization changes of BSP induced by ultrasonic and steam-cooking treatments. And the influence of ultrasonic and steam-cooking treatments on BSP-stabilized emulsions was also investigated. The treatments effects of BSP were first assessed by examining their particles sizes, polydispersity index (PDI) and ζ-potential. Then fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and thermogravimetric (TGA) analysis were carried out for physicochemical characterization. Furthermore, the solubility and water/oil holding capacity (WHC/OHC) were tested to assess the impact of the treatments on the functional properties of BSP. The rheological properties, morphology, droplet size and stability of O/W emulsion were tested to assess the impacts of the treatments on the emulsifying performance of BSP.
2. Materials and methods
2.1. Materials and chemicals
Bamboo shoots (Phyllostachys heterocycla cv pubescens) were obtained from Jingshi Agricultural Co., Ltd. (Guangde, China). The dried bamboo shoots comprised of 27.93% protein, 29.17% crude fiber, 33.61% total sugar, 6.12% water, 2.37% ash, and 0.41% fat. Soybean oil was purchased from Carrefour supermarket in Hefei. All reagents were of analytical grade and purchased from Macklin Instrument Co., Ltd. (Shanghai, China).
2.2. Preparation and treatment of BSP
BSP: This method was modified according to Yang et al., 2019 [32]. The dried bamboo shoots were first crushed into powder and through an 80 mesh sieve. Bamboo shoots powder (10.0 g) was mixed with NaOH (pH 9.0, 100.0 mL) at 60 °C for 120 min. The bamboo shoot slurry was centrifuged (8500 rpm, 4 °C, 20 min) to collect supernatant. The supernatant was adjusted to pH 3.8 by using 1 mol/L of HCl (4 °C). The precipitate collected by centrifugation. The precipitate was re-dissolved in deionized water, and adjusted to pH 7.0 by using 1 mol/L of NaOH. The solution was transferred into dialysis bag and changed the water every other 12 h within 48 h. The obtained protein was lyophilized for further analysis and named BSP.
U-BSP: The BSP was mixed with distilled water and stirred for 1 h and sonicated at 750 W and 25 kHz for 20 min. The temperature had been always kept at 55 °C. The slurry was freeze-dried to obtain U-BSP.
SC-BSP: BSP was mixed with distilled water and stirred for 1 h at 55 °C. Then the slurry was treated at 120 °C for 20 min by using autoclave sterilizers and freeze-dried to obtain SC-BSP.
SC/U-BSP: SC-BSP was stirred for 1 h and sonicated at 750 W for 20 min. The temperature had been always kept at 55 °C. The slurry was freeze-dried to obtain SC/U-BSP.
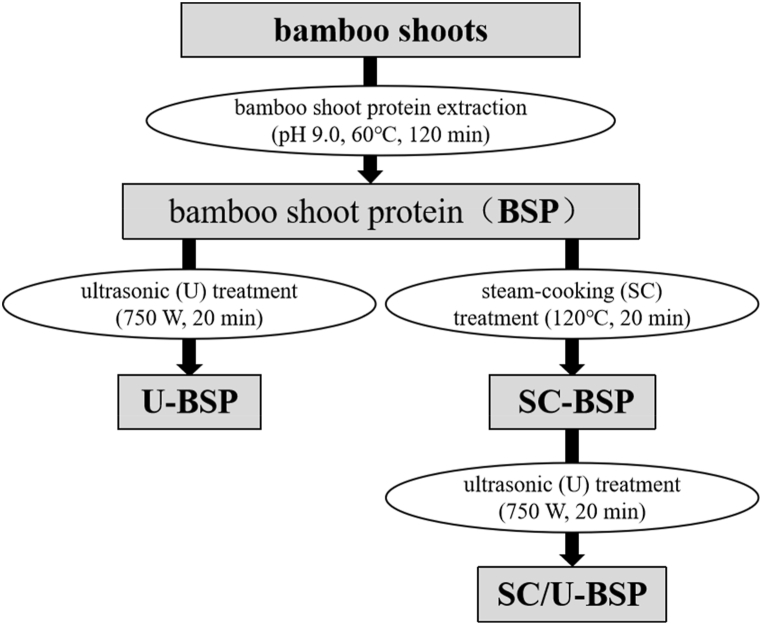
Flow diagram of the preparation process of BSP samples with different treatments is shown in Fig. 1. The Kjeldahl method was used to determine the protein contents of the dried samples. The protein contents (dry basis) of BSP, U-BSP, SC-BSP and U/SC-BSP were 67.90%, 68.29%, 66.19% and 65.56%, respectively.
Fig. 1.
Flow diagram of the preparation process of BSP samples with different treatments.
2.3. Preparation of O/W emulsions
The O/W emulsions were prepared with a solution containing 0.75 wt% protein and soybean oil, and the volume ratio of protein solutions to oil was 7:3. The emulsions were homogenized at 10,000 rpm for 5 min and analyzed within 2 h. Then the emulsions were stored at 4 °C for 28 days to determine their stability.
2.4. Particles size, PDI and ζ-potential measurements
The average particle size, PDI and ζ-potential of the sample were tested using a particle size and ζ-potential analyzer (Zetasizer Nano ZS, Malvern Co., UK). Instrument parameters as follows: fixed angle 90°, scattering angle 173°, relative refractive index 1.590, absorption index 0.001, equilibrium time 120 s, measurement temperature 25 °C [33]. The sample was diluted 100 times with deionized water before measurement. All measurements were repeated triplicate times and the average values were calculated.
2.5. Structure measurement
2.5.1. Fourier transform infrared (FTIR) spectroscopy measurements
1.0 mg sample powder mixed with 100.0 mg of KBr. FTIR spectra scanning range: 4000-400 cm−1 (number of scans: 64, resolution: 4 cm−1) [34]. The changes in the 1600–1700 cm−1 (overlapping amide I band) were interpreted by deconvoluting the bands using Peakfit version 4.12.
2.5.2. X-ray diffraction (XRD) analysis
XRD (PANalytical, Holand) using a Panalytical X'Pert Pro powder. A cobalt anode X-ray tube (Co-Kα radiation) was used to record the patterns. The sample powders were exposed to the X-ray beam at 45 kV and 30 mA. The scan time and step size were 0.5 s/step and 0.02°, respectively. The scanning region of the diffraction angle (2θ) ranged from 5 to 70° [35].
2.5.3. Thermogravimetric analysis (TGA)
The thermal properties of samples were investigated using the TGA technique. Each sample (4 mg) was tested on a TA 2050 instrument (TA Inc, USA) with a heating rate of 20 °C/min under an atmosphere of flowing N2.
2.6. Functional property measurement
2.6.1. Protein solubility measurement
The sample (10.0 mg) was mixed with distilled water (2.0 mL) and centrifuged (10,000 rpm, 4 °C) for 20 min. The protein content in the obtained solution was analyzed following a reported method [36] and bovine serum albumin (BSA) was used as a control.
2.6.2. Water and oil holding capacities (WHC/OHC)
The WHC and OHC of samples were tested according to a reported method [37]. The sample (4.0 g) was mixed with distilled water or soybean oil (20.0 mL) in a centrifuge tube (50.0 mL) and stirring for 30 min. The solution was centrifuged at 3500 rpm/min for 15 min and discarded the supernatant. The sediment was weighed and recorded.
2.7. Emulsion characterization
2.7.1. Flow behavior
The flow properties of emulsions were measured by rheometer (Thermo Haake Ltd., Germany), and followed the method reported by Cao [38]. The tested shear-rate ramp was in the range of 1–100 1/s and a parallel-plate sensor system with 1 mm gap between plates. All the experiments were done at 25 °C.
2.7.2. Optical microscopy analysis
An Olympus optical microscope equipped with a 40-fold objective lens was used to study the microstructure of emulsions (0 h and 24 h).
2.7.3. Droplet size distribution
The droplet size is called as volume-weighted average diameter (d4, 3). The d4, 3 of emulsions stored for 0, 1, 7, 14, 21 and 28 d at 4 °C was determined and calculated [39]. Samples (1.0 g)were diluted with distilled water(50.0 mL). The droplet size was measured by Master Sizer 2000 (Malvern Instruments Co., Ltd., UK) in triplicate. The d4, 3 was calculated according to the following equation [34]:
| (1) |
where ni presents the number of particles with the same diameter, and di stands for particle size.
2.8. Statistical analysis
The data were analyzed by using SPSS 19.0(SPSS Inc., USA) and Origin 9.0(OriginLab, USA). The data were analyzed by following the analysis of variance (ANOVA) method. The statistical significance differences (p < 0.05) were determined by the least significant difference (LSD) test.
3. Results
3.1. Particles size, PDI and ζ-potential distribution
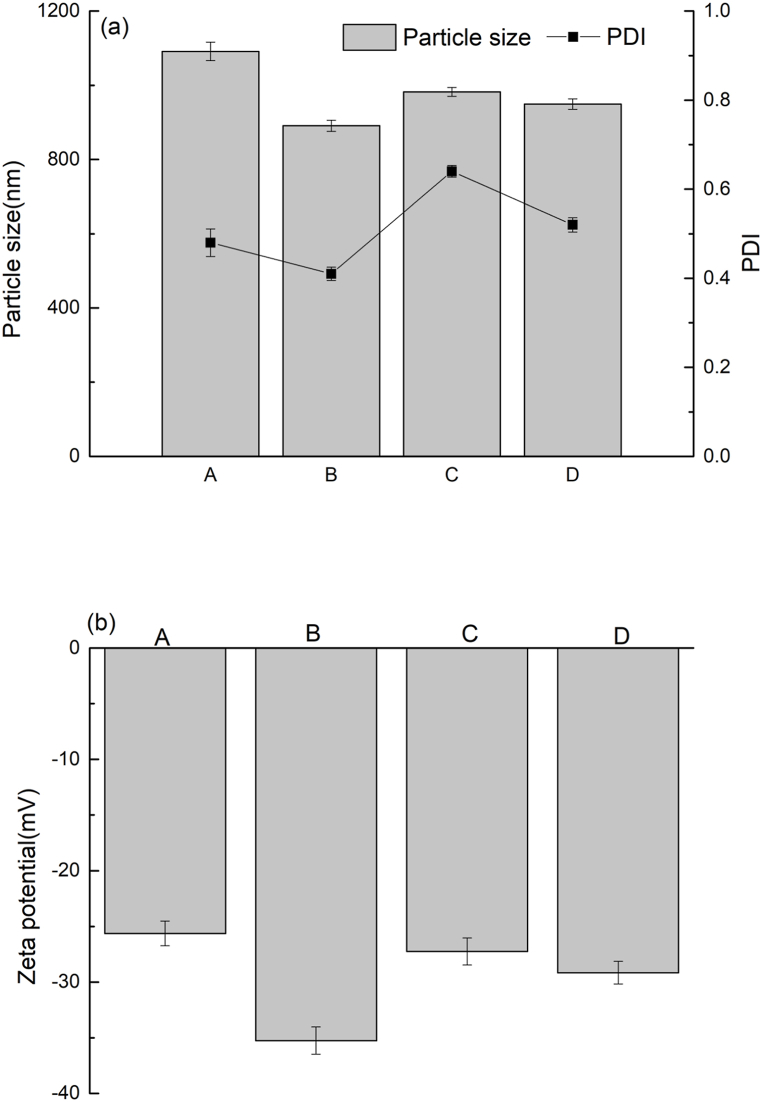
Fig. 2(a) presents the changes in the particle size and polymer dispersity index (PDI) of BSP subjected to different treatment conditions. Fig. 2(b) presents the ζ-potential data for BSP subjected to different treatment conditions. The particle size of BSP, U-BSP, SC-BSP and SC/U-BSP were 1091 ± 25 nm, 891 ± 15 nm, 982 ± 12 nm and 949 ± 14 nm, respectively. The PDI value of BSP, U-BSP, SC-BSP and SC/U-BSP were 0.48 ± 0.031, 0.41 ± 0.015, 0.64 ± 0.013 and 0.52 ± 0.016, respectively. The Zeta Potential of BSP, U-BSP, SC-BSP and SC/U-BSP were −25.63 ± 1.12 mV, −35.26 ± 1.23 mV, −27.25 ± 1.19 mV and −29.16 ± 1.02 mV, respectively. Both ultrasonic and steam-cooking treatments led to a decrease in particle size and an increase in ζ-potential. However, the PDI values of SC/BSP and SC/U-BSP were higher than that of BSP. The findings suggested that partial aggregation of protein occurred after the steam-cooking treatment, which resulted in a decrease in particle homogeneity [40]. The U-BSP had the smallest size, the lowest PDI and the highest ζ-potential. The average particle size of U-BSP decreased with ultrasonic treatment and the particle uniformity was improved.
Fig. 2.
Particle size (a), PDI (a) and Zeta (b) of BSP (A), U-BSP (B), SC-BSP (C) and SC/U-BSP (D).
3.2. Effects of treatments on the structure of BSP
3.2.1. Analysis of FTIR spectra
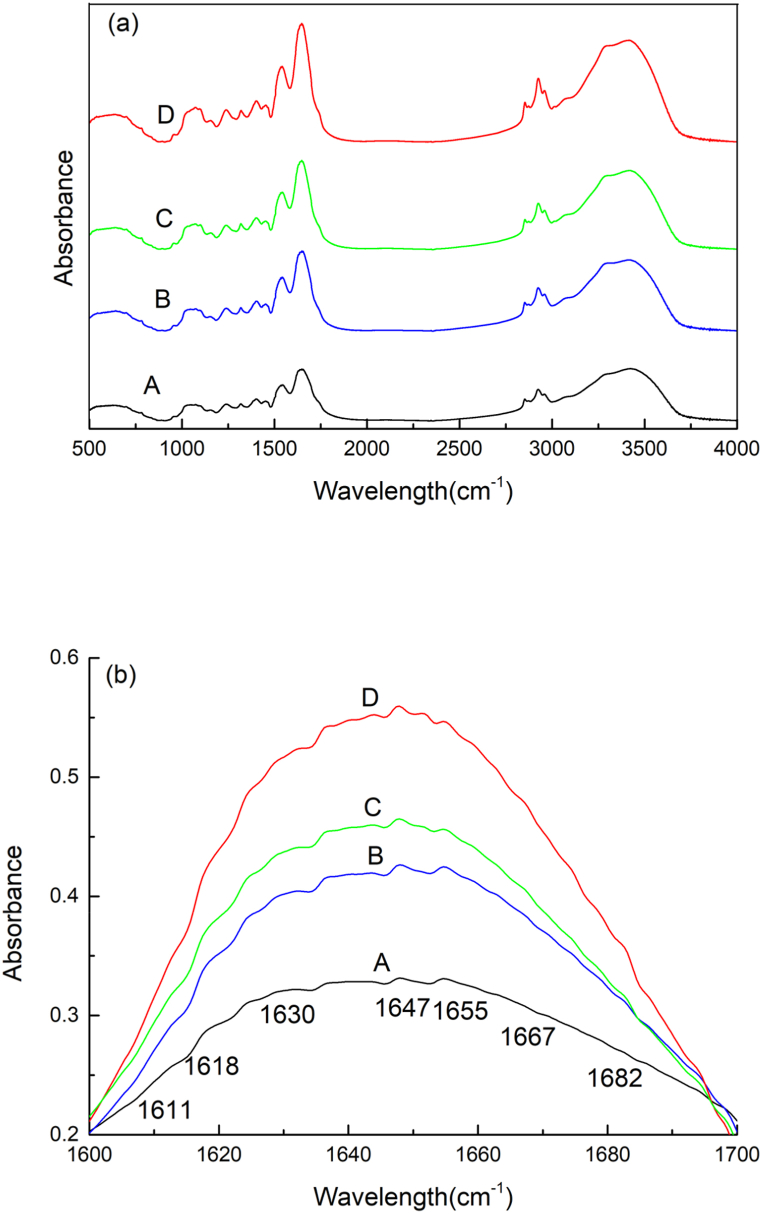
The secondary structure of the protein was commonly required by FTIR (Fig. 3(a)). The secondary structure of the protein was often based on the amide I band analysis (1700–1600 cm−1, Fig. 3(b)) [41]. Gaussian peak analysis of the amide I band was performed to analyze the secondary structure of the samples according to Chen Wang et al., 2011 [42]. These secondary structure of protein includes α-helix (1650–1660 cm−1), β-sheet (1610–1640 cm−1, 1670–1690 cm−1), β-turn (1660–1670 cm−1), and random coil (1640–1650 cm−1) [43]. Table 1 shows the influence of different treatments on the secondary structure of BSP. The secondary structural components of U-BSP, SC-BSP and SC/U-BSP showed difference from BSP. Compared to BSP, the α-helix content of U-BSP decreased by 5.92%, while the contents of β-sheet, β-turn and random coil increased by 1.49%, 0.76% and 3.67%, respectively. The α-helix structure is the most tightly connected structure in protein molecules. Hence, the α-helix content is low indicated the protein molecular structure is relatively loosen and the hydrophobic sites in the protein are exposed largely [42]. The α-helix and random coil contents of SC-BSP and SC/U-BSP both increased compared to BSP. This might be due to the fracture of some secondary bonds of β-sheet and β-turns, which rearranged into a random coil and an α-helix. These results indicated that steam-cooking induced protein dissociation and reaggregation, thus affecting the secondary structure of BSP.
Fig. 3.
FTIR spectral profiles (a:500-4000 cm−1, b:1600-1700 cm−1) recorded for BSP(A), U-BSP(B), SC-BSP(C) and SC/U-BSP(D).
Table 1.
Effects of treatment conditions on the secondary structure of BSP.
| BSP (%) | U-BSP (%) | SC-BSP (%) | SC/U-BSP (%) | |
|---|---|---|---|---|
| α-helix | 19.28 ± 0.39c | 13.36 ± 0.11a | 23.61 ± 0.38a | 20.32 ± 0.34d |
| β-sheet | 35.03 ± 0.92b | 30.52 ± 0.14b | 20.52 ± 0.95d | 21.56 ± 0.23a |
| β-turn | 22.88 ± 0.66a | 29.64 ± 0.17c | 20.20 ± 0.17bc | 20.49 ± 0.36c |
| random coil | 22.81 ± 0.87c | 33.48 ± 1.04b | 35.67 ± 1.13a | 37.63 ± 0.96b |
(Values followed by different letters in the same line have significant differences at p < 0.05.)
3.2.2. Analysis of XRD patterns
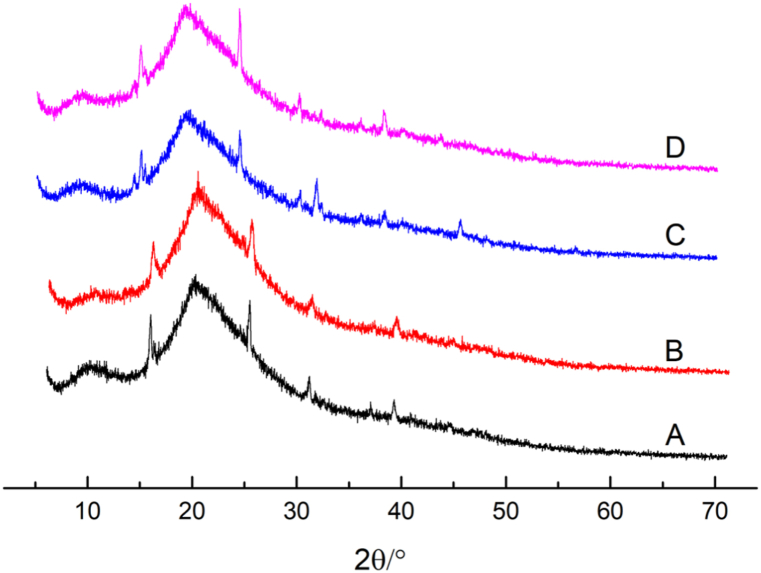
The crystal structure and compatibility of BSP with different components are observed using the XRD technique. The diffraction peaks at 9.09° and 19.60° are characteristic peaks of the protein [44]. Fig. 4 shows that ultrasonic and steam-cooking treatments do not change the characteristic peaks of BSP. The crystallinity of samples was calculated according to the reported method [45]. The percent crystallinity of BSP, U-BSP, SC-BSP and SC/U-BSP was 67.55% 62.3%, 60.75% and 59.87%, respectively. The results indicated that ultrasonic and steam-cooking treatments both destroyed the regular arrangement of protein molecules and increased the disorder of molecular structure.
Fig. 4.
XRD patterns recorded for BSP (A), U-BSP (B), SC-BSP (C) and SC/U-BSP (D).
3.3. Thermal properties
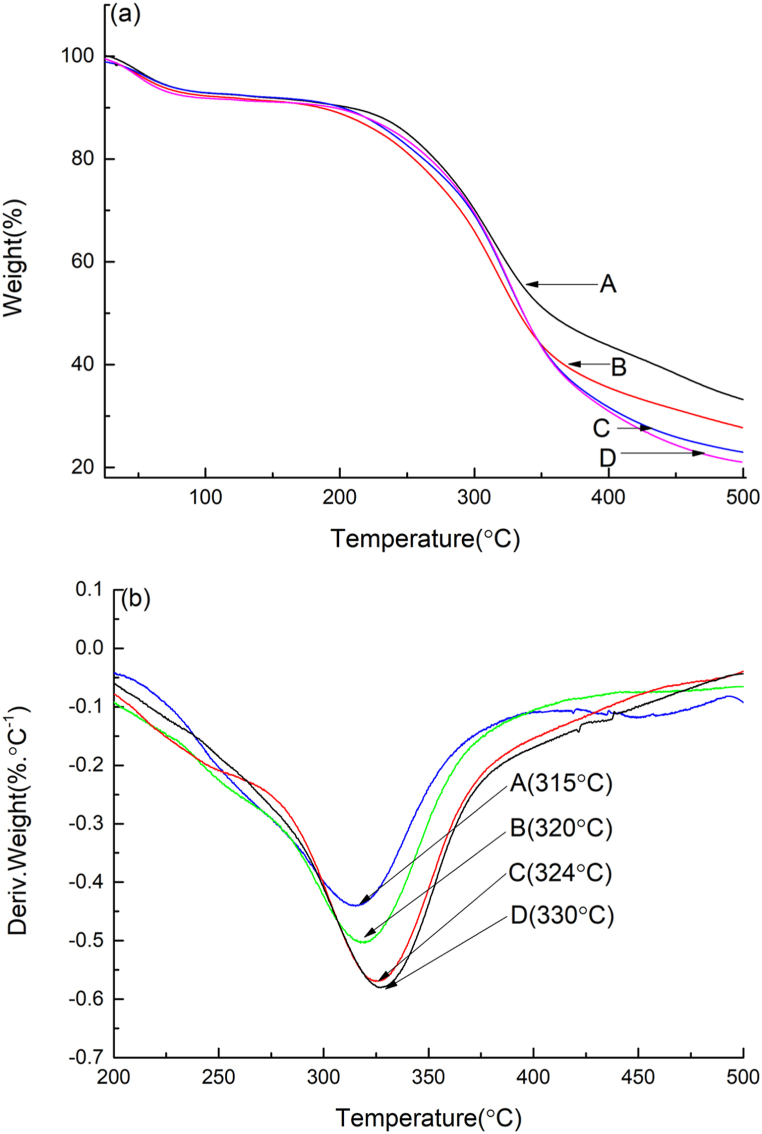
The thermal properties of BSP, U-BSP, SC-BSP and SC/U-BSP were investigated using the TGA technique (Fig. 5). Samples were heated from 25 to 500 °C, and the heating rate was 20 °C/min. Typical TGA curves of BSP, U-BSP, SC-BSP and SC/U-BSP are presented in Fig. 5(a). BSP begins to lose weight at approximately 213 °C. While U-BSP begins to lose weight at approximately 192 °C, earlier than BSP. However, SC-BSP and SC/U-BSP begin to lose weight at 205 °C and 203 °C, respectively. This indicates that ultrasonic and steam-cooking treatments reduced the initial decomposition temperature of BSP. On the contrary, plenty of particles were gathered together after steam-cooking treatment, resulting in the initial decomposition temperature of SC-BSP and SC/U-BSP slightly increased. The major weight loss region for BSP, U-BSP, SC-BSP and SC/U-BSP is similar (230–400 °C) [46]. The residues at 500 °C for BSP, U-BSP, SC-BSP and SC/U-BSP are approximately 33.28%, 27.78%, 23.33% and 21.04%, respectively. Derivative thermogravimetry (DTG) was shown in Fig. 5(b). The maximum thermal weight loss rates (Rmax, %/°C) for BSP, U-BSP, SC-BSP and SC/U-BSP were recorded to be 0.43, 0.50, 0.56 and 0.58, respectively. These results indicated that the steam-cooking treatment destroyed the structure of BSP more severely than ultrasonic treatment. These findings are consistent with the results of XRD.
Fig. 5.
TGA (a) curves and DTG (b) curves recorded for BSP (A), U-BSP (B), SC-BSP (C) and SC/U-BSP (D).
3.4. Functional properties
Protein solubility is an important functional property. It significantly affects other properties of protein such as foaming, emulsifying and rheological properties. Table 2 shows the solubility of BSP, U-BSP, SC-BSP and SC/U-BSP at pH 7.0. The solubility of BSP dispersion was around 21.24%. Compared to BSP, the solubility of U-BSP increased by 5.63%. This increase may be attributed to the degradation of most polymers resulting from ultrasonic treatment, which ultimately leaded to exposure of a large number of hydrophilic groups and decreased the inter-molecular interactions between hydrophobic groups on the surface of BSP molecules. These contributed to the hydration of BSP and increased their solubility. In contrast, the solubility of SC-BSP and SC/U-BSP decreased by 1.72% and 1.21%, respectively. The WAC of SC-BSP decreased to the minimum (9.69 ± 0.73 g/g). This suggested that hydrophilic groups was destroyed by steam-cooking and resulting in a decrease in WAC. The WAC value of SC/U-BSP was slightly higher than that of SC-BSP. The increased WAC of SC/U-BSP can be potentially attributed to the partial dissociation of protein under ultrasonic treatment. The dissociated samples interacted with water molecules to improve the degree of protein hydration. Compared to BSP, U-BSP exhibited a high OHC value (6.03 ± 0.15 g/g). This increase may be potentially attributed to the exposure of hydrophobic groups by ultrasound, which enhanced the interaction between oil and protein. Conversely, a reduction in OHC was also observed in SC-BSP and SC/U-BSP (compared to BSP), demonstrating that the hydrophilic and hydrophobic groups of BSP were both destroyed through steam-cooking treatment.
Table 2.
Effects of treatment conditions on the Solubility, WHC and OHC of BSP.
| BSP | U-BSP | SC-BSP | SC/U-BSP | |
|---|---|---|---|---|
| Solubility (%) | 23.24 ± 1.32a | 26.87 ± 2.84d | 21.52 ± 1.12 bc | 22.03 ± 1.17d |
| WHC (g/g) | 10.31 ± 0.86d | 13.78 ± 1.41a | 9.69 ± 0.73b | 9.84 ± 0.65c |
| OHC (g/g) | 4.83 ± 0.32c | 6.03 ± 0.15b | 4.61 ± 0.14a | 4.75 ± 0.37b |
(Values followed by different letters in the same line have significant differences at p < 0.05.).
3.5. Properties of O/W emulsions
3.5.1. Flow behavior of O/W emulsions
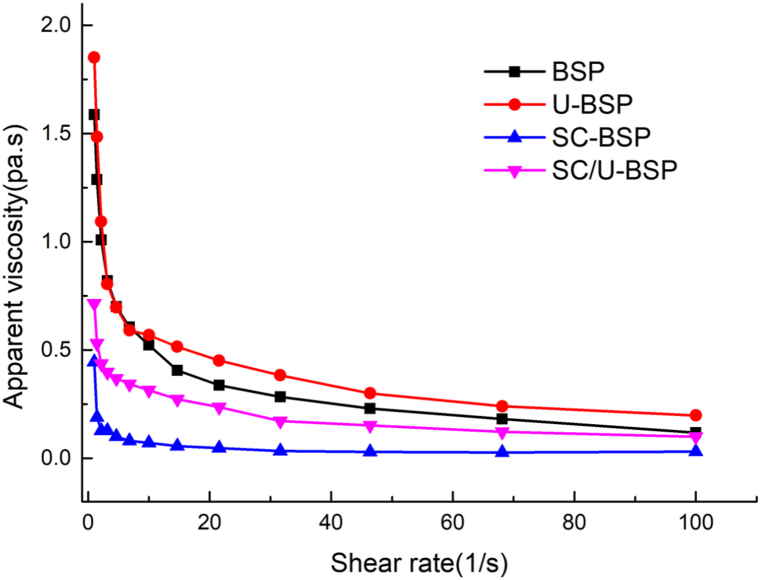
The rheological properties are one of the most important properties of food. The particle size of emulsion, viscosity and composition of continuous phase will affect the rheological properties of emulsion. The shear force and viscosity of the emulsions stabilized by BSP, U-BSP, SC-BSP and SC/U-BSP particles are presented in Fig. 6. All of the investigated emulsions showed pseudoplastic behavior. The shear thinning behavior of emulsions stabilized with U-BSP particles was more obvious than that of lotion stabilized with BSP, SC-BSP or SC/U-BSP particles. Upon the addition of SC-BSP and SC/U-BSP, there was a decrease in the viscosity of the stabilized emulsions, compared to BSP-stabilized emulsions. In contrast, the viscosity of U-BSP-stabilized emulsions increased due to the droplet size of emulsions decreased and a strong 3D network in the continuous phase was formed by U-BSP particles.
Fig. 6.
Viscosity versus shear rate for O/W emulsions stabilized upon the addition of BSP, U-BSP, SC-BSP and SC/U-BSP
3.5.2. Microphotographs of O/W emulsions
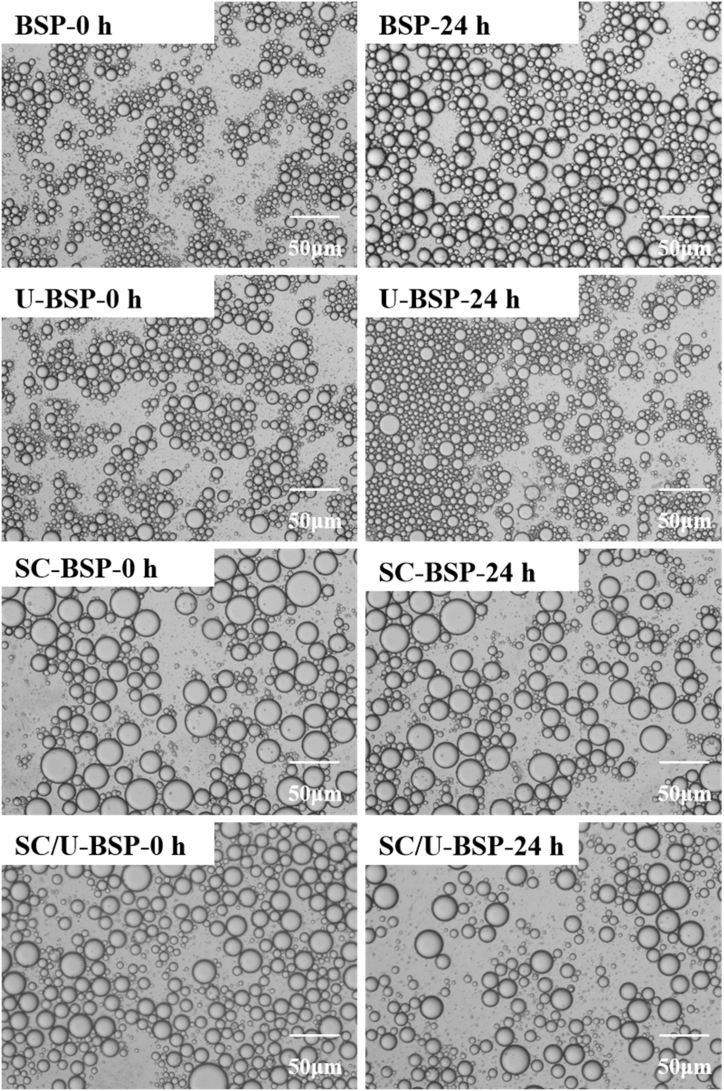
Fig. 7 shows the optical microphotographs of O/W emulsions stabilized with BSP, U-BSP, SC-BSP and SC/U-BSP particles at 0 and 24 h. The droplets became finer upon the addition of U-BSP to the emulsions. Furthermore, the emulsions stabilized by SC-BSP or SC/U-BSP had a larger droplet size than those stabilized by BSP particles. Steam-cooking treatment leaded to a decrease in the uniformity of BPS particle, which was not conducive to the stability of emulsion, and the size of the formed emulsion droplets increased.
Fig. 7.
Microphotographs of O/W emulsions stabilized using BSP, U-BSP, SC-BSP and SC/U -BSP recorded at 0 h (BSP-0 h, U-BSP-0 h, SC-BSP-0 h, SC/U -BSP-0 h)and 24 h (BSP-24 h, U-BSP-24 h, SC-BSP-24 h, SC/U -BSP-24 h).
3.5.3. Particle size analysis of O/W emulsions
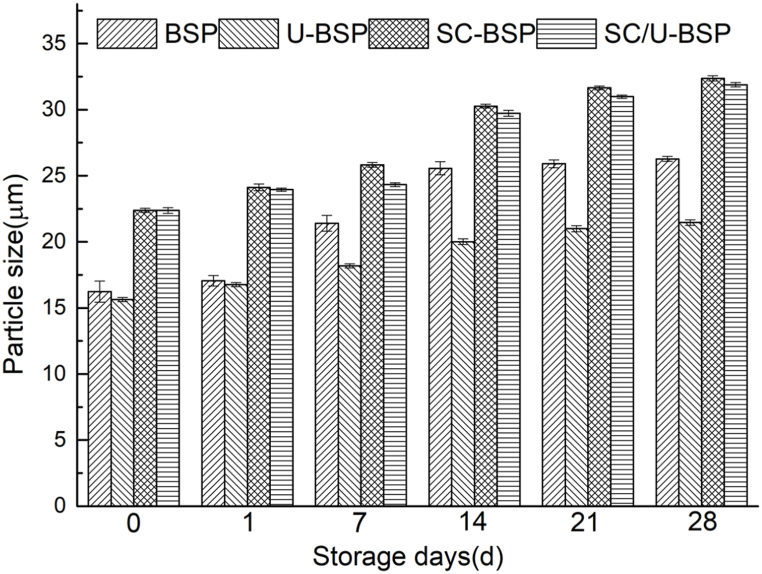
The parameter “d4, 3″ presents the volume-weighted average diameter, which is highly susceptible to changes in disaggregation, aggregation, or flocculation properties. Fig. 8 shows the changes in d4, 3 of BSP, U-BSP, SC-BSP and SC/U-BSP particles were used as stabilizers of O/W emulsions over storage times (0, 1, 7, 14, 21 and 28 d). At 28 d post-preparation, coarse emulsions were formed in the samples. The d4, 3 of all emulsions increased with time. The droplet aggregation may be attributed to the interaction among oil droplets and the rearrangement process occurring at the interface[47].
Fig. 8.
Emulsion size of BSP, U-BSP, SC-BSP and SC/U-BSP at different storage times (0, 1, 7, 14, 21 and 28 d, respectively).
There was no significant difference in the d4, 3 of fresh emulsions (0 d) prepared with BSP and U-BSP. However, the d4, 3 of BSP-stabilized emulsions increased significantly after two weeks of storage, owing to flocculation and coalescence. The droplet size of BSP reached 26.27 μm after 28 d of storage, which was 1.22 times larger than the size recorded for U-BSP. It was evident that U-BSP-stabilized emulsions were the most stable within 28 ds? This could be due to the ultrasonic treatment improved the interfacial packing and conformation of the samples. In summary, the shelf life of U-BSP-stabilized emulsions was longer than BSP-stabilized emulsions. In contrast, emulsions prepared with SC-BSP and SC/U-BSP exhibited a sharp increase in d4, 3 over time and the droplet size reached 32.37 μm and 31.89 μm after 28 d, respectively. The results showed that the emulsification performance of BSP particles decreased after steam-cooking treatment.
4. Conclusions
Ultrasonic and steam-cooking treatments both altered the structure of BSP to varying degrees, and then significantly influenced its functional properties. Ultrasonic treatment decreased the crystal structure, thermal stability and particle size of BSP. While, the particle homogeneity and flexibility of U-BSP were promoted, resulting in improved the solubility and water-holding capacity of the particles. The viscosity of emulsion stabilized by U-BSP increased and the distribution of emulsion droplets was more uniform and smaller. This was due to ultrasonic treatment increased the electrostatic repulsion and spatial repulsion among emulsion droplets, and the aggregation of emulsion droplets slowed down. Therefore, ultrasonic treatment enhanced the emulsifying ability of BSP. On contrast, the PDI of SC/BSP and SC/U-BSP both increased indicated a decrease in the uniformity of particle distribution. The α-helical content and random coil content of SC/BSP and SC/U-BSP increased, indicating that the secondary structure of BSP reaggregated after steam-cooking treatment. Hence, the solubility and water-holding capacity of the BSP particles after steam-cooking treatment were both decreased. The viscosity and uniformity of emulsion stabilized by SC/BSP or SC/U-BSP both decreased and the droplet size increased. These results indicated that steam-cooking treatment had a significant negative impact on the emulsifying ability of BSP. These findings provide a useful theoretical basis for understanding the effect of ultrasonic and steam-cooking treatments on the BSP-stabilized emulsions.
Funding
This research was funded by Anhui Science & Technology Important Special Project (No.202003a06020007).
Author contribution statement
Jingjing Du, Jian Jiang: Conceived and designed the experiments; Wrote the paper. Qian Zhu, Jiagang Guo: Performed the experiments; Analyzed and interpreted the data. Yuhan Wu, Zhangqing Hu, Song Yang: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Song Yang, Email: 178815275@qq.com.
Jian Jiang, Email: 591760009@qq.com.
References
- 1.Yuan Tianzhong, Zeng Jinsong, Wang Bin, Cheng Zheng, Chen Kefu. Pickering emulsion stabilized by cellulosic fibers: morphological properties-interfacial stabilization-rheological behavior relationships. Carbohydr. Polym. 2021;269(7):118339–1118346. doi: 10.1016/j.carbpol.2021.118339. [DOI] [PubMed] [Google Scholar]
- 2.Bouhoute Meryem, Taarji Noamane, de Oliveira Felipe Lorena, Habibi Youssef, Kobayashi Isao, Zahar Mohammed, Isoda Hiroko, Nakajima Mitsutoshi, Neves Marcos A. Microfibrillated cellulose from Argania spinosa shells as sustainable solid particles for O/W Pickering emulsions. Carbohydr. Polym. 2021;251:116–119. doi: 10.1016/j.carbpol.2020.116990. [DOI] [PubMed] [Google Scholar]
- 3.Papa Mady Sy, Mouhamed Dieng Sidy, Diarra Mounibé. Stability and physicochemical properties of pickering emulsions: an overview. Appl. Phys. Res. 2019;11(1):41. doi: 10.5539/apr.v11n1p41. [DOI] [Google Scholar]
- 4.Feng Xin, Dai Hongjie, Liang Ma, Fu Yu, Yu Yong, Zhou Hongyuan, Guo Ting, Zhu Hankun, Wang Hongxia, Zhang Yuhao. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces. 2020;196:294–303. doi: 10.1016/j.colsurfb.2020.111294. [DOI] [PubMed] [Google Scholar]
- 5.Matsumiya Kentaro, Brent S. Murray. Soybean protein isolate gel particles as foaming and emulsifying agents. Food Hydrocolloids. 2016;3(28):206–215. doi: 10.1016/j.foodhyd.2016.03.028. [DOI] [Google Scholar]
- 6.Zhang Shuning, Holmes Melvin, Ettelaie Rammile, Sarkar Anwesha. Pea protein microgel particles as Pickering stabilisers of oil-in-water emulsions: responsiveness to pH and ionic strength. Food Hydrocolloids. 2020;102:580–589. doi: 10.1016/j.foodhyd.2019.105583. [DOI] [Google Scholar]
- 7.Hu Ya Qiong, Wei Yin Shou, Hua Zhu Jian, Qi Jun Ru, Guo Jian, Wu Lei Yan, Tang Chuan He, Yang Xiao Quan. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocolloids. 2016;5(28):300–310. doi: 10.1016/j.foodhyd.2016.05.028. [DOI] [Google Scholar]
- 8.Zhou Fu Zhen, Yu Xin Hao, Zeng Tao, Yin Shou Wei, Tang Chuan He, Yang Xiaoquan. Fabrication and characterization of novel water-insoluble protein porous materials derived from pickering high internal phase emulsions (HIPEs) stabilized by gliadin/chitosan complex particles. J. Agric. Food Chem. 2019;67:3423–3431. doi: 10.1021/acs.jafc.9b00221. [DOI] [PubMed] [Google Scholar]
- 9.Xie Bing, Zhang Xingzhong, Luo Xiaogang, Wang Yixiang, Li Yan, Li Bin, Liu Shilin. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020;331:100–108. doi: 10.1016/j.foodchem.2020.127108. [DOI] [PubMed] [Google Scholar]
- 10.Deng Zilong, Jung Jooyeoun, John Simonsen, Zhao Yanyun. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocolloids. 2018;6(12):229–237. doi: 10.1016/j.foodhyd.2018.06.012. [DOI] [Google Scholar]
- 11.Marto Joana, Duarte Aida, Simões Sandra, Gonçalves Lídia Maria, Gouveia Luís Filipe, António José Almeida, Ribeiro Helena Margarida. Starch-based pickering emulsions as platforms for topical antibiotic delivery: in vitro and in vivo studies. Polymers. 2019;11 doi: 10.3390/polym11010108. 108-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva Ronivaldo Rodrigues Enzymatic hydrolysis of non-animal proteins for improving nutritional and sensory properties of foods. J. Food Biochem. 2021;45:13891–13901. doi: 10.1111/jfbc.13891. [DOI] [PubMed] [Google Scholar]
- 13.Adriano Profeta, Marie-Christin Baune, Sergiy Smetana, Keshia Broucke, Van Royen Geert, Weiss Jochen, Sophie Hieke, Volker Heinz, Nino Terjung. Consumer preferences for meat blended with plant proteins – empirical findings from Belgium - ScienceDirect. Future Foods. 2021;4:397–406. doi: 10.1016/j.fufo.2021.100088. [DOI] [Google Scholar]
- 14.Yi Hua Xin, Jie Hong Chiang, Jeyakumar Henry Christiani. Application of plant proteins as alternative emulsifiers in double emulsions: using kappa-carrageenan for complex coacervation and microencapsulation of riboflavin. Int. J. Food Sci. Technol. 2022;57:2402–2415. doi: 10.1111/ijfs.15599. [DOI] [Google Scholar]
- 15.Jozef Bernard Marie Delahaije Roy, Alexandra Kiskini, Alexander Wierenga Peter. Towards predicting the emulsion properties of plant protein extracts from sugar beet (Beta vulgaris L.) leaf and soybean (Glycine max) Colloids Surf. A Physicochem. Eng. Asp. 2022;646:1–11. doi: 10.1016/J.COLSURFA.2022.128950. [DOI] [Google Scholar]
- 16.Chang Liu, Pei Ruisong, Marina Heinonen. Faba bean protein: a promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022;369:1–10. doi: 10.1016/j.foodchem.2021.130. [DOI] [PubMed] [Google Scholar]
- 17.Ma Tiantian, Xiong Youling L., Jiang Jiang. Calcium-aided fabrication of pea protein hydrogels with filler emulsion particles coated by pH_(12)-shifting and ultrasound treated protein. Food Hydrocolloids. 2022;125:1–10. doi: 10.1016/j.foodhyd.2021.10739. [DOI] [Google Scholar]
- 18.Li Sisheng, Jiao Bo, Shi Meng, Fu Weiming, Shah Faisal, Li Xiaomin, Liu Hongzhi, Wang Qiang. Edible mayonnaise-like Pickering emulsion stabilized by pea protein isolate microgels: effect of food ingredients in commercial mayonnaise recipe. Food Chem. 2022;376:131866–131872. doi: 10.1016/J.FOODCHEM.2021.13186. [DOI] [PubMed] [Google Scholar]
- 19.Wang Ying, Zhao Jing, Zhang Shucheng, Zhao Xiangzhong, Liu Yuanfa, Jiang Jiang, Xiong Youling L. Structural and rheological properties of mung bean protein emulsion as a liquid egg substitute: the effect of pH shifting and calcium. Food Hydrocolloids. 2022;126:1–13. doi: 10.1016/j.foodhyd.2022.10748. [DOI] [Google Scholar]
- 20.Felix Manuel, Cermeno Maria, Fitzgerald Richard J. Influence of hydrolysis on the bioactive properties and stability of chickpea-protein-based O/W emulsions. J. Agric. Food Chem. 2020;68:10118–10127. doi: 10.1021/acs.jafc.0c0242. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar Anwesha, Kamaruddin Hannah, Bentley Annie, Wang Shikai. Emulsion stabilization by tomato seed protein isolate: influence of pH, ionic strength and thermal treatment. Food Hydrocolloids. 2016;57:160–168. doi: 10.1016/j.foodhyd.2016.01.01. [DOI] [Google Scholar]
- 22.Zembyla MorfoMurray, Sarkar Brent S. Anwesha. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: a review. Trends Food Sci. Technol. 2020;104:49–59. doi: 10.1016/j.tifs.2020.07.02. [DOI] [Google Scholar]
- 23.Wang M.L., Harrison M.L., Tonnis B.D., Pinnow D., Davis J., Irish B.M. Total leaf crude protein, amino acid composition and elemental content in the USDA-ARS bamboo germplasm collections. Plant Genetic Resources. 2018;16:185–187. doi: 10.1017/S147926211700005. [DOI] [Google Scholar]
- 24.Kaur Bajwa Harjit, Santosh Oinam, Koul Ashwani, Bisht M.S., Nirmala Chongtham. Quantitative determination of macroelement and microelement content of fresh and processed bamboo shoots by wavelength dispersive X‐ray fluorescence spectrometry. X Ray Spectrom. 2019;48:637–643. doi: 10.1002/xrs.304. [DOI] [Google Scholar]
- 25.Dickinson Eric. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocolloids. 2019;96:209–223. doi: 10.1016/j.foodhyd.2019.05.02. [DOI] [Google Scholar]
- 26.Li Quanyang, Zhao Zhengtao. Interfacial characteristics, colloidal properties and storage stability of dairy protein-stabilized emulsion as a function of heating and homogenization. RSC Adv. 2020;10:11883–11891. doi: 10.1039/D0RA00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Sullivan Jonathan, Murray Brian, Flynn Cal, Norton Ian. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids. 2016;53:141–154. doi: 10.1016/j.foodhyd.2015.02.00. [DOI] [Google Scholar]
- 28.Chen Ye Bao, Zhu Xue Feng, Tong Xun Liu, Lin Wei Feng, Tang Chuan He, Liu Ruihai. Improving freeze-thaw stability of soy nanoparticle-stabilized emulsions through increasing particle size and surface hydrophobicity. Food Hydrocolloids. 2019;87:404–412. doi: 10.1016/j.foodhyd.2018.08.02. [DOI] [Google Scholar]
- 29.Zhu Peineng, Huang Weijuan, Chen Lingyun. Develop and characterize thermally reversible transparent gels from pea protein isolate and study the gel formation mechanisms. Food Hydrocolloids. 2022;125:1–10. doi: 10.1016/j.foodhyd.2021.10737. [DOI] [Google Scholar]
- 30.Matsuyama Shingo, Maeda Kazuhiro, Nakauma Makoto, Funami Takahiro, Nambu Yuko, Matsumiya Kentaro, Matsumura Yasuki. Stabilization of whey protein isolate-based emulsions via complexation with xanthan gum under acidic conditions. Food Hydrocolloids. 2021;111:1–10. doi: 10.1016/j.foodhyd.2020.10636. [DOI] [Google Scholar]
- 31.Amagliani Luca, Ben Sassi Elyes, Buczkowski Johann, Schmitt Christophe. Influence of protein source on the morphology, physicochemical and flow properties of protein-based emulsion particles to be used as texture modulators. Food Hydrocolloids. 2020;101:1–8. doi: 10.1016/j.foodhyd.2019.10558. [DOI] [Google Scholar]
- 32.Yang Tao, Xun Liu Tong, Ting Li Xiu, He Tang Chuan. Novel nanoparticles from insoluble soybean polysaccharides of Okara as unique Pickering stabilizers for oil-in-water emulsions[J] Food Hydrocolloids. 2019;94(9):255–267. doi: 10.1016/j.foodhyd.2019.03.03. [DOI] [Google Scholar]
- 33.Li Wenjun, Maria Chountoulesi, Lemonia Antoniadi, Angelis Apostolis, Lei Jiandu, Maria Halabalaki, Costas Demetzos, Sofia Mitakou, Skaltsounis Leandros A., Wang Chengzhang. Development and physicochemical characterization of nanoliposomes with incorporated oleocanthal, oleacein, oleuropein and hydroxytyrosol. Food Chem. 2022;8:384–390. doi: 10.1055/s-0041-173690. [DOI] [PubMed] [Google Scholar]
- 34.Xia Tao, Cai Yongjian, Liu Tongxun, Zhao Long, Huang Lihua, Deng Xinlun, Zhao Qiangzhong, Zhao Mouming. Effects of pretreatments on the structure and functional properties of okara protein. Food Hydrocolloids. 2019;90:394–402. doi: 10.1016/j.foodhyd.2018.12.02. [DOI] [Google Scholar]
- 35.Fidele Benimana, Potoroko Irina Y., Pathak Prateek, Sonawane Shirish H., Shriram Sonawane, Bagale Uday D. Ultrasound‐assisted synthesis of nanoemulsion/protein blend for packaging application. Food Sci. Nutr. 2022;10:1537–1547. doi: 10.1002/fsn3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson G.L. A simplification of the protein assay of Lowry et al. Anal. Biochem. 1978;83:346–356. doi: 10.1016/0003-2697(77)90043-. [DOI] [PubMed] [Google Scholar]
- 37.Ge Jiao, Sun Cui-Xia, Mata Analucia, Corke Harold, Gan Ren-You, Fang Yapeng. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocolloids. 2020;112:1–13. doi: 10.1016/j.foodhyd.2020.106288. [DOI] [Google Scholar]
- 38.Cao Mengmeng, Zhang Xingcai, Zhu Yuqing, Liu Yikun, Ma Li, Xing Chen, Zou Liqiang, Liu Wei. Enhancing the physicochemical performance of myofibrillar gels using Pickering emulsion fillers: rheology, microstructure and stability. Food Hydrocolloids. 2022;128:1–11. doi: 10.1016/j.foodhyd.2022.10760. [DOI] [Google Scholar]
- 39.Davood Zaeim, Ana-Isabel Mulet-Cabero, Read Sophia A. Liu Weilin, Han Jianzhong, Wilde Peter J.. Effect of oil droplet size on the gastric digestion of milk protein emulsions using a semi-dynamic gastric model. Food Hydrocolloids. 2022;124:1–12. doi: 10.1016/j.foodhyd.2021.10727. [DOI] [Google Scholar]
- 40.Wang Ruican, Liu Jingyuan, Guo Shuntang. Binding of phytate to soybean protein during the heat treatment of soymilk and its effect on protein aggregation[J] Food Hydrocolloids. 2018;84(10):368–378. doi: 10.1016/j.foodhyd.2018.06.03. [DOI] [Google Scholar]
- 41.Cui Qiang, Wang Guorong, Gao Da, Wang Lin, Zhang Anqi, Wang Xibo, Xu Ning, Jiang Lianzhou. Improving the gel properties of transgenic microbial transglutaminase cross-linked soybean-whey mixed protein by ultrasonic pretreatment - ScienceDirect[J] Process Biochem. 2020;91(4):104–112. doi: 10.1016/j.procbio.2019.12.00. [DOI] [Google Scholar]
- 42.Wang Chen, Jiang Lianzhou, Wei Dongxu, Yang Li, Sui Xiaonan, Wang Zhongjiang, Li Dan. Effect of secondary structure determined by FTIR spectra on surface hydrophobicity of soybean protein isolate[J] Science & Technology of Food Industry. 2011;15(1):4819–4827. doi: 10.1016/j.proeng.2011.08.90. [DOI] [Google Scholar]
- 43.Zhao Fei, Zhang Daofang, Li Xiangyang, Dong Haizhou. High-pressure homogenization pretreatment before enzymolysis of soy protein isolate: the effect of pressure level on aggregation and structural conformations of the protein[J] Molecules. 2018;23(7):17752–177514. doi: 10.3390/molecules2307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Zhongqi, Cheng Huai N., Olanya O. Modesto, Joseph Uknalis, Zhang Xiaodong, Koplitz Brent D., He Jibao. Surface characterization of cottonseed meal products by SEM, SEM-EDS, XRD and XPS analysis[J] J. Mater. Sci. Res. 2017;7(1):28–40. doi: 10.5539/jmsr.v7n1p2. [DOI] [Google Scholar]
- 45.Eka Putri Anita, Winarsih Suci, Kurniawan Budhy, Rezky Dicky, Munazat, Watanabe Isao. Improvement of the crystallinity of La2CuO4 nanoparticles using the vacuum treatment. Mater. Sci. Forum. 2021;1028:44–49. https://10.4028/www.scientific.net/MSF.1028.4 [Google Scholar]
- 46.Yi Feng, Guo Zhao-Xia, Zhang Li-Xia, Yu Jian, Li Qiang. Soluble eggshell membrane protein: preparation, characterization and biocompatibility[J] Biomaterials. 2004;27(19):4591–4599. doi: 10.1016/j.biomaterials.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 47.Du Feifei, Qi Yue, Huang Hongbing, Wang Peng, Xu Xinglian, Yang Zongyun. Stabilization of O/W emulsions via interfacial protein concentrating induced by thermodynamic incompatibility between sarcoplasmic proteins and xanthan gum. Food Hydrocolloids. 2022;3:1–12. doi: 10.1016/j.foodhyd.2021.10724. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.