Abstract
Problem -
Immune cell trafficking and surveillance within the ovary and fallopian tube are thought to impact fertility and also tumorigenesis in those organs. However, little is known of how native cells of the ovary and fallopian tube interact with resident immune cells. Interaction of the Programmed Cell Death Protein-1 (PD-1/PDCD-1/CD279) checkpoint with PD-L1 is associated with downregulated immune response. We have begun to address the question of whether PD-1 ligand or its receptors (PD-L1/-L2) can regulate immune cell function in these tissues of the female reproductive tract.
Method of Study -
PD-1 and ligand protein expression was evaluated in human ovary and fallopian tube specimens, the latter of which included stages of tubal cell transformation and early tumorigenesis. Ovarian expression analysis included the determination of the proteins in human follicular fluid (HFF) specimens collected during in vitro fertilization procedures. Finally, checkpoint bioactivity of HFF was determined by treatment of separately-isolated human T cells and the measurement of interferon gamma (IFNγ).
Results -
We show that membrane bound and soluble variants of PD-1 and ligands are expressed by permanent constituent cell types of the human ovary and fallopian tube, including granulosa cells and oocytes. PD-1 and soluble ligands were present in HFF at bioactive levels that control T cell PD-1 activation and IFNγ production; full-length checkpoint proteins were found to be highly enriched in HFF exosome fractions.
Conclusion -
The detection of PD-1 checkpoint proteins in the human ovary and fallopian tube suggests that the pathway is involved in immunomodulation during folliculogenesis, the window of ovulation, and subsequent egg and embryo immune-privilege. Immunomodulatory action of receptor and ligands in HFF exosomes is suggestive of an acute checkpoint role during ovulation. This is the first study in the role of PD-1 checkpoint proteins in human tubo-ovarian specimens and the first examination of its potential regulatory action in the contexts of normal and assisted reproduction.
1 |. Introduction
There is a critical need to minimize autoimmune responses to oocytes or to other cells that might compromise fertility1,2. The clinical condition termed autoimmune oophoritis is associated with poorer fertility outcomes. The condition manifests in immune cell infiltration of the ovaries, which can take on a cystic appearance3,4. Autoimmune oophoritis can include the presence of anti-ovarian antibodies, and often occurs when another definitive autoimmune condition (e.g., lupus, autoimmune polyglandular syndrome, etc.) has been diagnosed. Recently, a group has shown that there are decreased numbers of effector T regulatory (Treg) cells and increased CD4+ CD69+ T cells in the peripheral blood of women afflicted with primary ovarian insufficiency5, a condition characterized by accelerated ovarian aging early loss of ovarian function.
The PD-1 immune checkpoint6–8 controls immune cell (T9, B10, and dendritic11 cell) activation, favoring identification of interacting cells as “self,” and thus immunosuppression, when induced. With rare exceptions12, the PD-1 receptor has only been detected in myeloid and lymphoid cells, and in tumor cells that co-opt the pathway to evade surveillance and elimination8,13. Transformed cells can downregulate anticancer immune responses by expressing PD-114, ligands PD-L115–17 or PD-L218, or soluble variants of these proteins19–22. We hypothesized that if present, local PD-1 signaling in the ovary and fallopian tube could suppress autoimmunity to native cells including oocytes (within the ovary) and embryos (trafficking within the tubal lumen). PD-1 signaling in the ovary and fallopian tube might also then be involved in the development of early stages of epithelial ovarian cancer (EOC).
PD-1 pathway expression in EOC cells has been shown to correspond to disease stage and patient survival in this manner23–26. Notably, EOC is considered an immunogenic disease27, but the response rate to checkpoint inhibitor immunotherapies has been unexpectedly low28. Information is now available about expression and function of the pathway in normal ovarian cell types, and in a mouse model, blockade of PD-1 signaling in the absence of cancer was shown to compromise the reserve of ovarian follicles29.
The most common histotype of EOC is high grade serous ovarian cancer (HGSOC). The originating cells of HGSOC are increasingly thought to be transformed fallopian tube epithelial (FTE) cells30–36. Early transformation events can result in serous tubal intraepithelial lesions (STILs), which are characterized by a “p53 signature” in morphologically unremarkable FTE cells, visible on immunohistochemical staining as overexpression in 12 or more consecutive non-ciliated cells. Mutations in the tumor suppressor can result in either nuclear stabilization of the mutant p53 variant, or, in loss of p53 expression36. STILs can then progress to another stereotypical stage, the serous tubal intraepithelial carcinoma (STIC)31,38,39. STIC cells detach from the tubal lumen and engraft at distant sites within the peritoneum, including the surface of the ovary at ovulation site(s)40,41.
During a preliminary evaluation of Pd-1 pathway protein expression in the mouse ovary, we detected broad expression of the receptor and ligands in the organ, in somatic immune and non-immune cells and oocytes (consistent with29). There was also an existing report in a publicly-available large-scale gene expression screen (LifeMap Discovery) that showed that PD-1 pathway transcripts are detectable in human cumulus granulosa cells (HCGC). Because of potential relevance of these initial data to ovarian function and ovarian cancer development, a more extensive analysis of the PD-1 checkpoint in the human ovary and fallopian tube was warranted. We tested whether the PD-1 checkpoint acts within the human ovary and fallopian tube in ways suggestive of physiological function, including testing whether soluble PD-1 or ligand proteins are present in human follicular fluid (HFF) at levels capable of regulating immune function.
2 |. Methods
2.1 |. Tissue, cell, and follicular fluid collection and processing
All methods were carried out in accordance with appropriate guidelines and regulations. Specifically, approval for human sample provision and collection was granted by the Colorado Multiple Institutional Review Board as de-identified, discarded tissue (Johnson COMIRB Protocol #17–1428). Paraffin-embedded human ovary specimens were generated using a standard protocol by the University of Colorado Department of Pathology. Mouse tissues were collected under the auspices of protocols 347 and 569 (approved by CU-Anschutz IACUC, Bitler).
HFF and HCGC samples were isolated at the University of Colorado Center for Advanced Reproductive Medicine during egg retrieval procedures. Aspirates from the largest follicle within each ovary were collected, and removal of the oocyte and proximal-most HCGC was performed by mechanical cutting. Remaining cumulus cells within HFF were then stored at room temperature (RT) until batch processing to separate GC from HFF by centrifugation (5 minutes, 2000 × g). Cell-free HFF was removed from the cell pellet, and was either aliquoted, and frozen, or processed further for exosome preparations (below). Resuspended HCGC were either flash-frozen or placed into culture media.
Cell-free HFF was optionally sub-fractionated into exosome-rich or exosome-depleted (soluble protein) fractions as follows. HFF was processed using an exosome isolation kit (Total Exosome Isolation Kit, Invitrogen, #4484450 and Total Exosome Isolation Reagent, Invitrogen #4484453) per the manufacturer’s instructions.
Ovaries from 8-week-old C57BL/6 mice were isolated, rinsed in PBS, and placed into 4% paraformaldehyde prior to further processing (Immunostaining, below).
2.2 |. PD-1 pathway gene expression analyses (immunostaining, western blots, and ELISA assays)
Immunostaining
Ovary, fallopian tube or control tonsil samples were embedded in paraffin and sectioned. Clinical specimens provided for research purposes were sectioned by the University of Colorado Pathology Research Core service. Briefly, after deparaffinization and rehydration, antigen retrieval was performed by incubating the sections in 10 mM citrate buffer pH 6.0 at 120°C for 30 min. Endogenous peroxidase blocking was performed by using 3% hydrogen peroxide (Labchem, Cat # LC154301). Sections were then washed in water, then in PBS containing 0.05% Tween 20 (0.05% TBST). Tissue sections were blocked in 1% (wt/vol) bovine serum albumin plus 10% normal goat serum at room temperature (RT) for 90 min. After blocking, the tissues were incubated overnight at 4°C with first antibody in a humid chamber. After three washes 0.05% TBST, sections were incubated for 30 min goat anti-rabbit (abcam, #6721). Signal amplification was performed using an ABC Kit (Vectastain, #PK6100) for 30 min at RT followed by DAB (Vector Labs, #SK-4100) substrate. Nuclei were counterstained with Harris hematoxylin for 2 min. Following rinsing, slides were dehydrated and mounted in Permafluor (Fisher Scientific, # SP15–500). Negative control sections were submitted to the same procedures, except that the first antibody was replaced by blocking solution. Pictures were taken using an Olympus microscope (Model: CKX41SF, SN:2B77130).
Western Blot Analyses
Protein isolation from whole ovaries was performed by using TPER Tissue Protein Extraction (Thermo Scientific, #78510). Protease Inhibitor Cocktail (Sigma, #P8340) and Phosphatase Inhibitor Cocktail (Calbiochem, #524625) were each added at a 1:100 dilution. Where applicable, immunoprecipitation was performed using lysis buffer (Cell Signaling Technologies, #9803) and Protein A (#9863) according to the manufacturer’s protocol. Tissue or cell digestion was performed by agitation with stainless steel beads (NextAdvance, #SSB14B-RNA) for 5 min at speed 12 at 4°C in a bullet blender. Supernatant was recovered and the Bradford assay was performed for protein quantification. Supernatants were electrophoresed on a 4–12% polyacrylamide gel (Invitrogen), and proteins were transferred to a HyBond PVDF membrane (Amersham). Membranes were blotted with antibodies against the protein targets in the summary table below. HRP-conjugated second antibody was used followed by chemi-luminescence imaging using X-ray film. Optionally, a near-IR second antibody (LI-COR) was used and blots were imaged using the LI-COR Odyssey imaging system and densitometric analysis was performed using ImageStudio (LI-COR). For experiments comparing exosome and soluble proteins in fractions of the same HFF specimen (HFF processing, above), equal concentrations of exosome and soluble protein fractions from each sample were loaded onto SDS-page gels so that the relative amount of protein in each fraction could be estimated.
ANTIBODIES / SUPPLIERS
| Target | Supplier | Catalog # |
|---|---|---|
| HUMAN | ||
| PD-1 | Cell Signaling Technology | #86163 |
| PD-1† | R&D Systems | #AF1086 |
| PD1 (phospho Y248) | abcam | #ab206378 |
| PD-L1 | ThermoFisher | #PA5–20343 |
| PD-L2 | BIO-RAD | AHP1704 |
| MOUSE | ||
| Pd-1 | ProSci, Inc. | #4065 |
| Pd-L1† | ThermoFisher | #PA5–20343 |
| Pd-L2‡ | BIO-RAD | #AHP1704 |
Blocking antibody for T cell assays
Same antibody cross-reacts with human and mouse.
ELISA Assays
ELISA assays were performed according to manufacturer’s instructions. Each sample was run in triplicate, and each assay included positive and negative controls in addition to standard curve and “unknown” HFF samples.
| Target | Supplier | Catalog # |
|---|---|---|
| sPD-1 | Aviscera Bioscience, Inc. | #SK00808–01 |
| sPD-L1 | Abcam | #ab214565 |
| sPD-L2 | Abcam | #ab231928 |
2.3 |. T cell isolation, handling, and activation assay
T cell provision and analyses were performed by the Human Immune Monitoring Shared Resource at CU|AMC. The source of T cells was from a Bonfils Leukopak (LRS chamber), first sorted with a Miltenyi pan T cell isolation kit, and then sorted on the AutoMacs instrument. Media for cell culture and activation or control treatment were RPMI + 10% FBS + 2 mM L-glut + 200U/ml penicillin/streptomycin. T cell activation was performed by anti-CD3/anti-CD28 treatment (ThermoFisher, #11132D). Where specified, PD-1 blocking antibody (R&D Systems/Techne, #AF1086) was included at the 10 μg/ml. IFNγ levels were measured using the MSD V-PLEX assay (Mesoscale, Inc., #K151QOD) according to manufacturer’s instructions. HFF samples were diluted 50-fold in assay kit Diluent 2 for the assay.
3 |. Results
3.1 |. Ovarian and tubal PD-1 pathway protein expression
In an immunohistochemical study of both pre- and postmenopausal human ovarian tissue (Fig. 1), we found that PD-1, PD-L1, and PD-L2 are widely expressed, and this was conserved in the mouse ovary (Fig. S1; human immune cell colorimetric positive controls are shown in Fig. S1`, and human tonsil positive are controls shown in Fig. S2). In human premenopausal specimens (n=8 unique patient samples evaluated), PD-1 was detectable in the oocytes of non-growing (including primordial stage) and growing follicles (Figs. 1a, b), but unlike the mouse (Fig. S1), some oocytes appeared negative (examples in 1b). Like the mouse, PD-1 was present at low relative levels in granulosa cells of growing follicles (red arrow, 1b, c; see also 4e), and cells of the ovarian cortex (red arrows 1d). The receptor was also detected in what appeared to be immune cells subjacent to the fallopian tube lumen (1e, denoted by *), with epithelial cells of the tubal lumen faintly positive (arrow, 1e’). In addition, cells positive for PD-1 with the appearance of immune cells were detectable throughout the stromal/extrafollicular tissue of each ovary specimen (arrowheads, 1b, d), and their identities were probed by double immunofluorescence staining and multispectral imaging (Vectra). Macrophages double-positive for PD-1 and CD68 were detected, and T cells double-positive for PD-1 and CD3 were detected in ovary specimens (Fig. S3).
Figure 1. Premenopausal expression of PD-1 receptor and ligands in human ovarian follicles and fallopian tube.
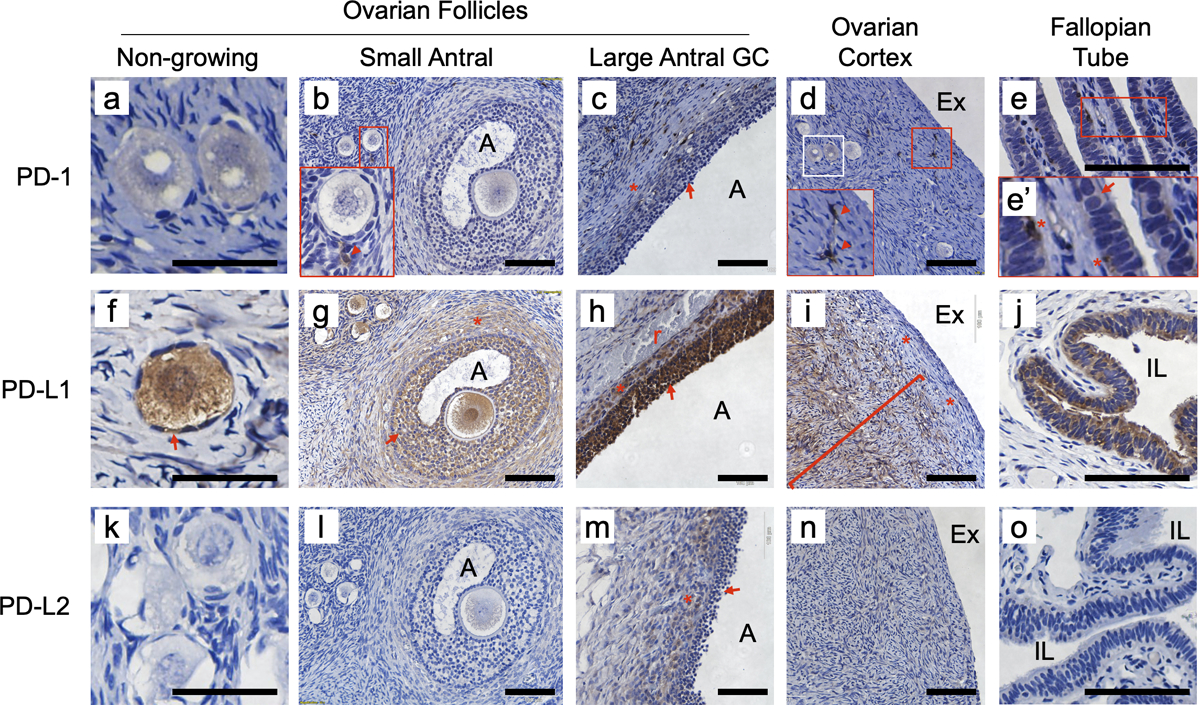
Brown stain indicates expression. PD-1 was detected in some oocytes (compare faintly positive examples in panel a with negative example in b; region from white box in panel d is shown in panel a), and granulosa cells of large antral follicles (c, arrow; theca interna denoted by *). PD-1 was present at high levels in relatively rare cells throughout the ovary (red arrowheads in insets, b and d) and the stromal compartment of the fallopian tube (inset e’, *); some of these are likely to be T cells and macrophages (see Figure S2). PD-1 was also detectable at low levels in the luminal epithelium of the fallopian tube (e and e’, arrow). PD-L1 was consistently expressed in oocytes (f, g) of all sizes, pregranulosa cells of non-growing follicles (f, arrow) and granulosa cells (f-h, arrows) and the theca interna (g, h *) of growing follicles. PD-L1 was also broadly expressed in stromal cells of the inner ovarian cortex (i, l - left), but was not detectable in cells of the OSE or in stromal layers immediately below the surface (i, red bracket and *’s; compare to Figs. 2e–g). PD-L1 was also expressed the luminal epithelial cells of the fallopian tube (j). PD-L2 was detected only in the granulosa cells (arrow) and theca interna (*) of large antral follicles (m, compare to panels c and h). Control circulating immune cell expression of PD-1 and ligands is shown in Supplemental Fig. S1’a–c, representative no first antibody control shown in Fig. S1’d.. A - follicular antrum, Ex - extra-ovarian space, IL - intraluminal space of tube, r - red blood cells. Scale bars in panels a, e, g, k, m and q = 100 μm, bars in other panels are 50 μm
The PD-1 activating ligand PD-L115–17 was expressed broadly in the premenopausal ovary and fallopian tube. PD-L1 was detected in all oocytes in all specimens (examples in 1fg, gh and 4e), in granulosa cells of follicles of all sizes (1f-h, arrows, also 4e), cells of the theca interna of growing follicles (1g, h – indicated by *), cells of the ovarian cortex (1i, compare to 1d and n), and FTE cells (1j). PD-L1 expression in the stromal cells of the premenopausal ovarian cortex was consistently found not to extend to the ovary surface; instead, PD-L1 was absent from the layers of the ovary closest to surface. PD-L2 was less widely expressed in these cell types compared to PD-L1, with all oocytes and granulosa cells of follicles up to the small antral stages negative (1k, l), and only granulosa cells of larger follicles (1m, arrow; Fig. 4f) and cells of the theca interna (1m, *) were consistently positive.
Figure 4. Detection of PD-1 receptor and ligands in human follicular fluid.


ELISA assays were used to determine whether sPD and/or PD-1 ligands are present in HFF. PD-1 was detected in 20 of 32 samples measured (a, inset plot shows same data points between 0 and 1750 pg/ml expanded vertically, note single sample with greater than 15,000 pg/ml PD-1), while PD-L1 (b) and PD-L2 (c) were detected in all samples. Subsequent analysis detected full-length and shorter variants of each protein as enriched in HFF exosomal fractions (see Fig. S6). Representative examples of granulosa cells (gc) in large antral follicles, including HCGC are shown in panels d-f for each protein; this expression pattern may represent membrane and soluble forms of the proteins. PD-L2 was also detected consistently in cell-free contents (*) of ovarian (g) and fallopian tube (h) lymphvascular channels. Note that in the example ovarian lymphvascular channel (g), white blood cells are negative for PD-L2 (arrow), while the cell-free vessel contents in the fixed tissue are positive. Also note that the contents of the fallopian tube channel (h, *) are positive for PD-L2 while the contents of the vein at top right are negative; tubal luminal epithelium is at bottom right. (i) Western blot analysis of full-length and soluble PD-1 in HFF and media conditioned by HCGC, soluble form(s) enhanced by immunoprecipitation (IP) indicated by asterisks. Contents of lanes as follows: Lane 1: MW Marker, 2: media conditioned by HCGC (“HCGC media”) for 24 hours, 3: HFF, 4: HCGC media, anti-PD-1 IP, 5: HCGC media, IP no-first antibody control, 6: HCGC media, unrelated first antibody control, 7: HFF, anti-PD-1 IP, 8: HFF, IP no-first antibody control, 9: HFF, unrelated first antibody control, 10: HFF, IP no-first antibody control. (j) Western blot detection of ligands PD-L1 and -L2 in HFF. A first immunoprecipitation (IP) step was required to detect the ligands (equal amounts of protein are loaded with and without IP).
Because the risk of HGSOC increases after menopause41, we also evaluated ovarian specimens from postmenopausal patients (Fig. 2, representative data from n=5 unique patient samples shown). In the postmenopausal ovary, PD-1 expression was limited to sparse cells with the appearance of tissue resident immune cells within the ovarian cortex (2a, inset), and was faintly detectable in cells of the ovarian surface epithelium (OSE) (2b) or in sub-surface epithelial inclusions (2c, d). PD-L1 was expressed throughout the ovarian cortex (2e, f), including stromal cells near the organ surface and within cells of the OSE (2f). PD-L2 was not detected in any of the same cell types or structures evaluated in postmenopausal specimens (2i-l).
Figure 2. Postmenopausal human ovarian PD-1 pathway expression.

As seen with premenopausal specimens, PD-1 expression was highest in sparse cells with the appearance of surveilling immune cells in the ovarian cortex (a, inset), but was low or absent in cells of the OSE (b) and sub-surface epithelial inclusions (c, *). PD-L1 was widely expressed throughout postmenopausal ovarian cortex (d-f), and unlike the premenopausal ovary, was detectable at comparable levels in cell layers at the surface of the ovary (red brackets, panels d, e; compare to Fig. 1j). PD-L1 was also detected in cells of the OSE (d,e) and in cells of epithelial inclusions (example in f, note lack of surface OSE in this area). PD-L2 was mostly absent from postmenopausal specimens (g-i). No first antibody control shown in panel j. Ex - extra-ovarian space.
Despite their having been removed from patients for reasons unrelated to ovarian pathology, 4 of the 5 postmenopausal human ovary specimens harbored surface epithelial inclusions (2c, f and i) thought to develop via invagination of cells normally at the ovarian surface42. These structures were only positive for PD-L1 (2f).
3.2 |. Tubal expression during tumorigenesis
Given the consistent expression of PD-L1 in normal FTE cells, we also asked whether its expression might change when tubal cells become transformed and acquire a “p53 signature36,37.” We limited our analysis to lesions exhibiting a p53 stabilization signature, and photomicrographs of hematoxylin-eosin staining as well as PD-L1 immunostaining in serial sections of the same lesion were prepared. Representative images of tubal lesions, including a serial section stained with hematoxylin and eosin are shown in Figure 3. Cells overexpressing p53 (3e) in p53 Signature/STIL lesions were found to maintain expression of PD-L1 (3i). FTE cells within STIC lesions were also PD-L1 positive (panels b, f, j and c, g, k), as were all other cases of more advanced tubal lesions (example more advanced tubal lesion exhibiting mucosal involvement by invasive serous carcinoma, d, h, l). Thus, expression of PD-L1 ligand was maintained in the tubal epithelium throughout all stages of transformation evaluated.
Figure 3. PD-L1 expression in p53-variant tubal epithelial lesions.

As seen in the normal fallopian tube (Fig. 1k), PD-L1 is expressed in tubal lesions that have been implicated as precursors of HGSOC. Hematoxylin and eosin stained sections used for clinical/pathological staging are shown in panels a-d, with adjacent serial sections stained for p53 (e-h) and PD-L1 (i-l) below. Both normal (p53 low/absent, arrowheads e, f, h) and p53-overexpressing (brown staining, p53 row of images) FTE cells were positive for PD-L1, in lesions of all types evaluated.
3.3 |. Soluble PD-1 pathway bioactivity in human follicular fluid
The expression of PD-1 pathway proteins pre- and post-menopause were suggestive of ovarian and tubal immunomodulation involving PD-1 checkpoint signaling (Discussion, below). How the pathway might act acutely to regulate immune function remained unclear, and we thus moved to the analysis of potential PD-1 pathway bioactivity in live human cells and fluid isolated from human ovaries in the clinic.
HCGC and (human) follicular fluid (HFF) are readily available as discarded material after clinical egg retrieval procedures. Using the collection technique of Jungheim et al.43,44 that allows the collection of i) HCGC and ii) undiluted HFF free of cells, we collected a series of cell samples and fluid aliquots from 60 patients. Where possible, samples were collected from the largest peri-ovulatory follicle in the left ovary, and separately, in the right ovary. Analysis of soluble receptor and ligand levels was first performed by ELISA assay. Soluble PD-1 was detectable in only a subset of HFF samples by ELISA. 12 of 32 samples contained zero detectable PD-1, and samples that did contain PD-1 ranged from approximately 200 pg/ml to greater than 16500 pg/ml (Fig. 4a). In contrast, soluble PD-L1 (4b) and PD-L2 (4c) were detected in all HFF samples, and their approximate ranges were between 20 and 160 pg/ml and 2800 and 11000 pg/ml, respectively. Detection of soluble PD-1 receptor and ligands in HFF samples corresponded to their detection in the granulosa cells (including HCGC) seen in immunostained specimens that contained large follicles (4d-f, see also Fig. 1c, i, o). Strikingly, the contents of lymphvascular channels also demonstrated high levels of immunoreactivity for PD-L2 (4g, h). Because the PD-L2 signal was uniform throughout acellular lymphvascular contents, extracellular/secreted PD-L2 is further supported.
Full-length PD-1, along with PD-L1 and PD-L2 protein, as well as shorter splice variants were also detected in each of 4 patient HCGC samples and HFF samples by western blot with optional immunoprecipitation (IP; representative data in 4i, j). In the case of sPD-1 IP, we also assessed conditioned media used to culture HCGC cells for 24 hours (4i). Low molecular weight bands corresponding to the correct sizes of sPD-1 splice variants were detected when anti-PD-1 antibody was used for IP, but these bands were not present when no first antibody, or, an unrelated antibody were used (not shown). IP was required for the detection of sPD-L1 and sPD-L2 on western blots (4j).
Hypothesizing that patients might show a consistent HFF soluble PD-1 pathway “signature,” we first tested whether any correlation between protein concentrations occured i) for the targets between samples collected from the left and right ovary (Fig. S4), ii) for levels of the soluble ligands with each other (Fig. S5a), or iii) if any correlation existed between levels and key patient demographic information (Fig. S5b–d). While PD-1 levels were not correlated between samples collected from the largest follicle in the left versus right ovary (S4a) highly significant correlations for both soluble ligands were detected (S4b, c). This suggests that the production of the soluble ligands is a tightly regulated process in individuals. In addition, significant positive correlations were noted between patient age and both PD-L1 and -L2, and patient BMI and PD-L1 (but not PD-L2; Fig. S5). No correlation was noted between sPD-1 or the ligands and the fertilization rate per patient per mature metaphase II egg, nor the percentage of embryos that developed to blastocyst per patient in the limited number of specimens assessed during this study (not shown, see Discussion, below). These data suggest that at least in the context of in vitro fertilization (IVF) treatment, the soluble PD-1 checkpoint immunomodulatory milieu can vary between patients and is subject to modification by aging and body composition.
The detection of bands corresponding to the sizes of full-length PD-1 and ligands in cell-free HFF (Fig. 4) suggested that an alternative mode of production and delivery of full-length proteins to HFF might be occurring. We hypothesized that full-length proteins might be loaded into exosomes as has been reported for PD-L146–49 and secreted into HFF in that fashion. After processing HFF from 7 unique patients into exosome-enriched and exosome-depleted (soluble protein) fractions, we found that full-length receptor and both ligands, as well as shorter species suggestive of splicing were highly enriched in the exosome fractions of each specimen (Fig. S6).
We next addressed the question of whether HFF can exhibit physiological immunomodulatory activity, and whether this relates to soluble PD-1 pathway factor concentration(s). For these experiments, we added different volumes of HFF or PBS vehicle to human T cells (isolated separately, in 50 μl culture media) that were optionally activated using a standard anti-CD3/anti-CD28 regime (CD3/CD28 activated)50. Interferon gamma (IFNγ) production and tyrosine 248 phosphorylation of PD-1 (P-Y248 PD-1) were monitored as measures of relative T cell activation51,52 and ligand interaction with T cell PD-1 receptors, respectively (Fig. 5).
Figure 5. Bioactivity of follicular fluid upon T cell activation.

T cells were incubated with hFF without (a) or with (b) concurrent CD3/CD28 activation and IFNγ was measured 24 hours later. Critically, IFNγ was not detectable in any hFF sample prior to addition to T cells (not shown). In non-activated T cells, some hFF samples stimulated IFNγ production above baseline (blue points within oval; mean values for 3 replicates shown). When the same hFF samples were applied to CD3/CD28-activated T cells, net IFNγ production was calculated by subtracting the mean amount produced by vehicle-treated, activated controls (set to zero, dashed line). Compared to vehicle, IFNγ production was either diminished (“Inhibitory”) or enhanced (“Stimulatory”) by hFF treatment. All 5 samples shown to stimulate IFNγ production in non-activated T cells (a) were found to have “Stimulatory” action upon activated T cells (b). Panel c shows western blots of T cell lysates after the described treatments, probed for “active” P-Y248 PD-1 and β-actin housekeeping protein. hFF treatment corresponded to increased P-Y248 PD-1 in both Unactivated and Activated T cells, with a volume-dependent dose-response apparent in Activated T cells.
The effect of HFF upon T cell IFNγ production varied between patients (n=16, Fig. 5). This was first seen in samples where “naïve” T cells were treated with HFF but were not activated by the anti-CD3/anti-CD28 regime (5A). While all of these T cell samples treated with PBS vehicle did not produce detectable IFNγ (not shown), five of sixteen samples treated with HFF (5/16) produced detectable IFNγ above background (2162.9 ± 635.1 pg/ml; mean ± SEM). Next, we assessed T cells that were CD3/CD28-activated. To determine the net effect of HFF treatment upon IFNγ, we subtracted the mean IFNγ produced from activated T cells treated with PBS vehicle from the amount produced from T cells treated with the individual HFF samples. This calculation revealed that HFF could either induce a net increase or decrease in IFNγ relative to that control (Fig. 5B). Of the 16 samples, 9 were found to be “stimulatory” and 7 were found to be “inhibitory” of IFNγ production. It was notable that all 5 of the samples that induced IFNγ in unstimulated T cells (5A) were “stimulatory”, and enhanced the effect of CD3/CD28 activation upon IFNγ production (5B).
Last, we addressed the question of whether T cell PD-1 signaling was modulated by HFF addition. Here we measured P-Y248 PD-1 in T cells treated with varying amounts of HFF. Western blots of T cell lysates (unstimulated or CD3/CD28-stimulated) collected after treatment with PBS vehicle of HFF are shown for three different patient HFF samples. HFF was shown to increase P-Y248 PD-1 in both unstimulated and stimulated T cells. Further, there was a correspondence between increasing HFF volume and P-Y248 PD-1 in CD3/CD28-stimulated T cells. We interpret these data as showing that soluble ligands are indeed present at bioactive levels, at least in this PD-1 phosphorylation assay. Given the consistent T cell P-Y248 PD-1 response to HFF treatment, we expect that the cytokine profile of individual HFF specimens is critical in determining the ultimate T cell and overall immune response in real time, and this is being explored in ongoing studies.
4 |. Discussion
We show herein that membrane bound and soluble PD-1 pathway receptor and ligand proteins are expressed in resident (non-lymphoid/non-myeloid) cells of the human ovary, and can be found in the fluid collected from peri-ovulatory follicles during IVF retrieval procedures. These data are reminiscent of one of our group’s prior findings evaluating PD-1 pathway expression and function in a mouse model where follicle numbers were evaluated after antibody (pembrolizumab) PD-1 blockade29.
Full-length PD-1 and ligands were found to be highly enriched in exosome fractions in HFF. We further show that these factors (particularly PD-L1) are present in variable amounts within human ovarian cortex pre- and postmenopausally, in a pattern that is consistent with the increased incidence of EOC after menopause. Why the distribution of PD-L1 is more extensive within the ovarian cortex (e.g., nearer the surface) after menopause is currently unknown. Finally, we also show that the soluble ligands are present in HFF at “bioactive” levels capable of regulating T cell activation, perhaps impacting immune surveillance at the time of ovulation in vivo.
Our data evaluating net T cell activation in the context of different concentrations of soluble PD-1 pathway members evoke recent findings of Karunarathne et al.53. That group showed that PD-L1 and PD-L2 have opposing actions upon T cell activation in a (mouse) model of malaria (see54 for a review). They showed that as expected, PD-L1:PD-1 interaction suppresses T cell activation, in that case rendering mice susceptible to malarial death. In contrast, PD-L2 and sPD-L2 delivery rescued this lethality, and additional experiments showed that the rescue resulted from the blocking of PD-L1’s interaction with the receptor by PD-L2. sPD-L2 is consistently present at higher concentrations than sPD-L1 in HFF. In combination, these data predict that ovarian immune surveillance is likely to be enhanced by sPD-L2, and that this can be blocked in when sPD-1 production is high. The net impact of HFF upon immune cell action can be appreciated to involve these PD-1 checkpoint factors as well as their cytokine “profiles” (below).
PD-1 pathway regulatory activity in HFF specifically via the T cell-activating soluble ligands, and the effect of concurrent soluble PD-1 expression have important implications. First, it has been reported that sPD-1 variant is not produced in “healthy individuals”.8,55–57 Ovarian granulosa cells can instead be recognized as a contributing, physiological source of soluble receptor, including the full-length protein found to be enriched in exosomes. Second, if pathway members regulate the surveillance and elimination of transformed cells in vivo, individual variability in physiological (e.g., ovarian including HFF) levels may contribute to individual variability in ovarian cancer risk (below). This concept has been evaluated in the context of rheumatoid arthritis risk56, and PD-L1 has been shown to be a prognostic factor in established ovarian cancer patients58. Interestingly, sPD-L1 can be generated by proteolytic cleavage21,22 as well as alternative splicing, and we will need to determine how and under what circumstances spliced variants are produced in ovarian follicles.
4.1 |. Strengths and limitations
Immune cells have been isolated from HFF samples59, and they may contribute soluble PD-1 and ligands to the fluid prior to and at the time of collection. While we confirmed that granulosa cells can express soluble PD-1 pathway proteins (Fig. 4i–k) using multiple approaches, the relative amount of these proteins that originate from immune cells versus non-immune ovarian cells (e.g., theca, granulosa, oocyte) remains unclear. Despite immune cells being found outside of intact, growing ovarian follicles, they may contribute to the protein content of HFF in situ, and are likely to enter the HFF sample when the wall of the follicle and its neighboring blood and lymph vessels are mechanically ruptured during the egg retrieval procedure (see below). In addition, clinical gonadotrophic stimulation has been shown to alter levels of detectable cytokines in follicular fluid and also the complement of immune cells retrieved60. This stimulation may also be impacting the expression and action of the PD-1 checkpoint in immune and non-immune cells of the ovary. We are currently limited as far as conclusions we can draw about how the PD-1 checkpoint signaling pathway impacts reproductive function in the ovary, fallopian tube, and even within oocytes and embryos. A large expansion of the analysis of soluble factors within follicular fluid to a greater number of samples, and a systematic analysis of embryology and pregnancy outcomes will be required to determine how this pathway impacts reproductive performance. Accordingly, the time scale of the development of EOC between the reproductive and post-menopausal periods, makes the monitoring of acute surveillance of immunological response to transformed cells as EOC lesions arise highly challenging and in need of long-term study.
4.2 |. Context
Much more mechanistic information about the regulation of immune surveillance within the ovary pre- and post-menopause (and indeed, the immune cell repertoire of the organ) is needed. Our finding that the distribution of somatic ovarian cortex cells that express PD-L1 changes between pre- and postmenopausal life may relate to the increased incidence of EOC after menopause41, and again, different susceptibility in individuals. Currently, the changes noted pre-and post-menopause are only a correlation. It is likely that the global decline in numbers of T cells that results from aging61 and also, the onset of menopause62, contributes as well. Because the application of immunotherapy (checkpoint blockade therapy, or, chimeric antigen receptor, or, “CAR T”) to HGSOC is in its infancy, these data and prior data from the mouse29 will inform treatment design so that basal PD-1 action in native cells of the ovary and fallopian tube is accounted for.
4.3 |. Clinical implications
The broader-than-expected action of the pathway in the ovary may also reflect an immune-privilege mechanism that reduces auto-immunity to cells of the follicle, including developing oocytes63–65. Several groups, including Fahmi et al.66 and Kollman et al.60 have evaluated HFF immune cell populations and cytokine levels, and the potential impact of altered ovarian immune function upon the likelihood of assisted conception. Data from the latter paper showed that gonadotrophic stimulation alters the cytokine profile of isolated HFF compared to HFF collected after a “natural cycle,” albeit where the “natural cycle” included a hormonal (hCG) ovulation trigger. Our direct experimental evaluation of immunomodulation of T cell activity by HFF (Fig. 5) suggests that immune checkpoint signaling may impact ovarian function and reproductive outcomes in combination with cytokine expression and response.
The degree to which EOC arises from transformed FTE cells that then engraft at healing ovulation sites40,41,67 or initiates from ovary-resident cells68 is difficult to determine. Regardless, immune checkpoint regulation of T cell activation by FTE and stroma, or, by cells of the ovary could represent a physiological immune-regulatory mechanism in those tissues. Ovulatory follicular fluid containing soluble PD-1 pathway members may regulate immune surveillance at the ovarian surface and adjacent extra-ovarian peritoneal space. Suppressed PD-1 signaling at this time53 could represent a pro-surveillance, “protective” state. Enhanced PD-1 signaling at the time of ovulation would instead reduce T cell surveillance, potentially enhancing the risk of EOC cell survival. Intriguingly, the significant correlations between soluble PD-1 pathway protein concentrations from the largest follicle of patient left and right ovaries (Fig. S4) suggests that sPD-L1 and -L2 production is tightly regulated within individuals, and may relate to overall HGSOC risk. If so, favoring the pro-surveillance state at the time of ovulation may represent a route towards reducing that risk.
Overall, we interpret these findings as suggestive of a PD-1 pathway-regulated balance between protecting the ovary, including its oocytes and embryos passing through the fallopian tube, from autoimmune reactions, while ensuring that the immune system can function in these organs to protect against infection and malignant transformation. Future large-scale studies of follicular fluid and possibly ovarian cells in the assisted reproduction clinic may reveal relationships between PD-1 pathway action and pregnancy outcomes, and reveal strategies to improve those outcomes. Similar long-term studies may also reveal events that correspond to the increased risk of EOC after menopause.
Supplementary Material
Acknowledgements
Drs. Jill Slansky, Kian Behbahkt, Saketh Guntupalli and Bradley Corr are acknowledged for their suggestions while the manuscript was being developed. Melissa Rosario and David Russell collected human follicular fluid and granulosa-lutein cells discarded after clinical IVF procedures and are gratefully acknowleged for their efforts. Jennifer Ann McWilliams and Angela Minic performed T cell activation assays. Dr. Sunhyo Ryo performed “active” P-Y248 T cell western blots.
Funding
This study was supported by University of Colorado Department of Obstetrics and Gynecology Division of Gynecologic Oncology funds awarded to J.J. and B.G.B. J.J. is also supported by University of Colorado Department of Obstetrics and Gynecology General Research Funds. B.G.B acknowledges additional support by NIHR00CA194318.
Footnotes
Conflict of interest statement
No conflicts of interest are noted or occurred during the preparation of this manuscript.
References
- 1.Ferguson TA, Green DR & Griffith TS Cell death and immune privilege. Int. Rev. Immunol 21, 153–172 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Spanel-Borowski K Footmarks of innate immunity in the ovary and cytokeratin-positive cells as potential dendritic cells. Adv Anat Embryol Cell Biol 209, vii–99 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Sedmak DD, Hart WR & Tubbs RR Autoimmune oophoritis: a histopathologic study of involved ovaries with immunologic characterization of the mononuclear cell infiltrate. Int. J. Gynecol. Pathol 6, 73–81 (1987). [PubMed] [Google Scholar]
- 4.Jacob S & Koc M Autoimmune oophoritis: a rarely encountered ovarian lesion. Indian J Pathol Microbiol 58, 249–251 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M et al. Decreased effector regulatory T cells and increased activated CD4+ T cells in premature ovarian insufficiency. Am. J. Reprod. Immunol 81, e13125 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Maeda A, Nishimura H, Kurosaki T & Honjo T PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U.S.A 98, 13866–13871 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki T & Honjo T PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol 19, 813–824 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Zhu X & Lang J Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget 8, 97671–97682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karyampudi L et al. PD-1 Blunts the Function of Ovarian Tumor-Infiltrating Dendritic Cells by Inactivating NFκB. Cancer Res. 76, 239–250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibult ML et al. PD-1 is a novel regulator of human B-cell activation. Int. Immunol 25, 129–137 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Lee J et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell. Cardiol 46, 169–176 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Wang W et al. Programmed cell death-1 is expressed in large retinal ganglion cells and is upregulated after optic nerve crush. Exp. Eye Res 140, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ & Sharpe AH PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol 26, 677–704 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng SY et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med 193, 839–846 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong H, Zhu G, Tamada K & Chen L B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med 5, 1365–1369 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Freeman GJ et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med 192, 1027–1034 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latchman YE et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U.S.A 101, 10691–10696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latchman Y et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol 2, 261–268 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Cheng S et al. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int. J. Biol. Markers 30, e364–368 (2015). [DOI] [PubMed] [Google Scholar]
- 20.He XH, Liu Y, Xu LH & Zeng YY Cloning and identification of two novel splice variants of human PD-L2. Acta Biochim. Biophys. Sin. (Shanghai) 36, 284–289 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Chen Y et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56, 231–238 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Shi B, Du X, Wang Q, Chen Y & Zhang X Increased PD-1 on CD4(+)CD28(−) T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metab. Clin. Exp 62, 778–785 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Liu YL & Zamarin D Combination Immune Checkpoint Blockade Strategies to Maximize Immune Response in Gynecological Cancers. Curr Oncol Rep 20, 94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia C & Ring KL The Role of PD-1 Checkpoint Inhibition in Gynecologic Malignancies. Curr Treat Options Oncol 19, 70 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Toker A et al. Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from that in Melanoma. Clin. Cancer Res 24, 5685–5696 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Drakes ML et al. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J Ovarian Res 11, 43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittica G, Genta S, Aglietta M & Valabrega G Immune Checkpoint Inhibitors: A New Opportunity in the Treatment of Ovarian Cancer? Int J Mol Sci 17, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Martin A & Sanchez-Lorenzo L Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 125 Suppl 24, 4616–4622 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Xu PC, et al. Effects of PD-1 blockade on ovarian follicles in a prepubertal female mouse. J Endocrinol, 252, 15–30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kindelberger DW et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol 31, 161–169 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Lee Y et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol 211, 26–35 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Medeiros F et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol 30, 230–236 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Piek JM et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol 195, 451–456 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Piek JM et al. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol. Oncol 90, 491 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Cass I et al. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol 106, 1327–1334 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Labidi-Galy SI et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 8, 1093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perets R & Drapkin R It’s Totally Tubular….Riding The New Wave of Ovarian Cancer Research. Cancer Res. 76, 10–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarboe E et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int. J. Gynecol. Pathol 27, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Vang R, Shih I. e. M. & Kurman RJ Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology 62, 44–58 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Yang-Hartwich Y et al. Ovulation and extra-ovarian origin of ovarian cancer. Sci Rep 4, 6116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia D, Nagaoka Y, Katsumata M & Orsulic S Inflammation is a key contributor to ovarian cancer cell seeding. Sci Rep 8, 12394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torre LA et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 68, 284–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar H et al. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J. Natl. Cancer Inst 88, 1810–1820 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Jungheim ES, Frolova AI, Jiang H & Riley JK Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J. Clin. Endocrinol. Metab 98, E1364–1368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jungheim ES et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril 95, 1970–1974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordonnier M et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles 9, 1710899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang Y et al. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens Bioelectron 148, 111800 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Fan Y et al. Exosomal PD-L1 Retains Immunosuppressive Activity and is Associated with Gastric Cancer Prognosis. Ann. Surg. Oncol 26, 3745–3755 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Tucci M et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology 7, e1387706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trickett A & Kwan YL T cell stimulation and expansion using anti-CD3/CD28 beads. J. Immunol. Methods 275, 251–255 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Kemp RA, Backstrom BT & Ronchese F The phenotype of type 1 and type 2 CD8+ T cells activated in vitro is affected by culture conditions and correlates with effector activity. Immunology 115, 315–324 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhoef CM et al. Lymphocyte stimulation by CD3-CD28 enables detection of low T cell interferon-gamma and interleukin-4 production in rheumatoid arthritis. Scand. J. Immunol 50, 427–432 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Karunarathne DS et al. Programmed Death-1 Ligand 2-Mediated Regulation of the PD-L1 to PD-1 Axis Is Essential for Establishing CD4(+) T Cell Immunity. Immunity 45, 333–345 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Crompton PD & Pierce SK PD-L2 Elbows out PD-L1 to Rescue T Cell Immunity to Malaria. Immunity 45, 231–233 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Dai S, Jia R, Zhang X, Fang Q & Huang L The PD-1/PD-Ls pathway and autoimmune diseases. Cell. Immunol 290, 72–79 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Wan B et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol 177, 8844–8850 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Nielsen C, Ohm-Laursen L, Barington T, Husby S & Lillevang ST Alternative splice variants of the human PD-1 gene. Cell. Immunol 235, 109–116 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Hamanishi J et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U.S.A 104, 3360–3365 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Droesch K, Fulgham DL, Liu HC, Rosenwaks Z & Alexander NJ Distribution of T cell subsets in follicular fluid. Fertil. Steril 50, 618–621 (1988). [PubMed] [Google Scholar]
- 60.Kollmann Z, Schneider S, Fux M, Bersinger NA & Wolff M. von. Gonadotrophin stimulation in IVF alters the immune cell profile in follicular fluid and the cytokine concentrations in follicular fluid and serum. Hum. Reprod 32, 820–831 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Palmer S, Albergante L, Blackburn CC & Newman TJ Thymic involution and rising disease incidence with age. Proc. Natl. Acad. Sci. U.S.A 115, 1883–1888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farage MA, Miller KW & Maibach HI Effects of menopause on Autoimmune Diseases. Expert Rev. of Obstet. Gynecol 7, 557–571 (2012). [Google Scholar]
- 63.Kovanci E & Schutt AK Premature ovarian failure: clinical presentation and treatment. Obstet. Gynecol. Clin. North Am 42, 153–161 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Silva CA et al. Autoimmune primary ovarian insufficiency. Autoimmun Rev 13, 427–430 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Forges T, Monnier-Barbarino P, Faure GC & Bene MC Autoimmunity and antigenic targets in ovarian pathology. Hum. Reprod. Update 10, 163–175 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Fahmi HA, Hunter AG, Markham RJ & Seguin BE Immunosuppressive activity of bovine follicular fluid on bovine T lymphocytes in vitro. J. Dairy Sci 68, 3312–3317 (1985). [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Coffey DM, Ma L & Matzuk MM The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice. Endocrinology 156, 1975–1981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J et al. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc. Natl. Acad. Sci. U.S.A 109, 3921–3926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


