Abstract
Objective
As an important factor tumor regulator,long non-coding RNAs (lncRNAs) have aroused extensive attention via the diverse functional mechanisms that were associated with the pathological and physiological processes of HCC. Here, the main purpose of this study was to provide a clear understanding about the expression, functions and potential mechanism of lncRNA CECR7 (Cat Eye Syndrome Chromosome Region, Candidate 7) in HCC.
Methods
RT-qPCR analysis and TCGA database analysis were applied to investigate the expression of CECR7 in HCC cell lines and tissues. Chi-squared Test was employed to explore the correlation between CECR7 expression and HCC clinicopathological features. Besides, Kaplan-Meier curves were constructed to test the effects of CECR7 expression on the prognosis of HCC patients. Transwell assays, MTT assay EdU assay and animal experiments were applied to explore the effects of CECR7 expression on HCC cells migration, invasion, and growth. Furthermore, RNA-seq analysis, luciferase reporter assay and mRNA decay rates assessment were utilized to investigate the mechanism whereby CECR7 regulated EXO1 mRNA. And, rescue experiments were used to determine whether EXO1 was an essential mediator for CECR7 to accelerate HCC cells migration, invasion, and growth.
Results
CECR7 was determined to be significantly overexpressed in HCC cell lines and tissues. CECR7 expression was closely correlated with the tumor size, venous infiltration, TNM stage, 5-year overall survival and disease-free survival of HCC. And, CECR7 played a catalytic role in HCC cells migration, invasion, and growth. Furthermore, CECR7 enhanced the stability of EXO1 mRNA by recruiting RNA binding protein U2AF2. And, EXO1 was determined to be an essential mediator for CECR7 to accelerate HCC cells migration, invasion, and growth.
Conclusion
In a word, our findings demonstrates that the cancer-promoting gene lncRNA CECR7 motivates HCC metastasis and growth through enhanced mRNA stability of EXO1 mediated by U2AF2, proposing a new insight for targeted therapy of HCC.
Keywords: Hepatocellular carcinoma, LncRNA, CECR7, EXO1, U2AF2
1. Introduction
The high morbidity and mortality caused by hepatocellular carcinoma (HCC) remain an urgent issue to be addressed at present [[1], [2], [3]]. Due to the highly complex pathogenesis, researchers still face plenty of challenges regarding HCC therapy [[4], [5], [6]]. Though the great advancement of comprehensive therapy in HCC which is thriving presently deserves affirmation, the full understanding of molecular mechanisms of HCC still has a long way to go. In consequence, it is urgent for us to further elucidate the mechanisms related to HCC development and progression.
As a kind of non-coding RNAs with a length of more than 200 nucleotides, long non-coding RNAs (lncRNAs) have aroused much concern of researchers due to the essential roles and diverse mechanism in cancer progression, though without the ability to encode proteins [[7], [8], [9]]. Like any other type of gene, lncRNAs involved in HCC include both tumor promoters and tumor suppressors [[10], [11], [12], [13], [14]]. Especially, the expression of downstream target gene can be regulated by lncRNAs at transcriptional or post-transcriptional level [11,[15], [16], [17], [18]]. A growing body of evidence indicate that mRNA stability is able to be regulated by lncRNA, during which RNA-binding protein plays a critical role [[19], [20], [21], [22]]. For instance, lncRNA KB-1980E6.3 recruits insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) to strengthen c-Myc mRNA stability in breast cancer cells [23]. LncRNA DUXAP10 facilitates glioma cells stemness by recruiting RNA-binding protein HuR to increase Sox12 mRNA stability [24]. However, the expression and functions of CECR7 (Cat Eye Syndrome Chromosome Region, Candidate 7) in HCC, and whether CECR7 could enhance mRNA stability of target genes remain to be elucidated.
Hereon, this research attempted to figure out the role and mechanism of CECR7 in the malignant process of HCC Data from cell biology experiments, public database, clinical data analysis and animal experiments demonstrate that CECR7 is highly expressed and CECR7 stabilize the downstream target exonuclease 1 (EXO1) mRNA under the mediation of RNA binding protein U2AF2.
2. Materials and methods
2.1. Tissue sample
HCC tissue samples and corresponding adjacent non-tumor tissue samples, which were histopathologically confirmed, were collected from 85 patients who underwent surgery in the Gansu Gem Flower Hospital. All of the samples were stored at −80 °C. No patient received any adjuvant therapy before surgery. This study was approved by the Ethics Committees of the Gansu Gem Flower Hospital and written informed consent was obtained from all patients.
2.2. Cell culture
HCC cell lines (Hep3B, HepG2, HCCLM3, Huh7 and MHCC97H) and human normal liver cell line (LO2) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM (Gibco, Grand Island, NY, USA) containing 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin-streptomycin (Invitrogen, CA, USA). All of the cells were maintained in an incubator (37 °C, 5% CO2).
2.3. Cell transfection
Lentivirus particles containing specific shRNAs against CECR7 (shCECR7#1, shCECR7#2), U2AF2 (shU2AF2) or EXO1 (shEXO1) and their corresponding control sequences (NC), and plasmids containing CECR7, U2AF2 or EXO1 sequence and the empty vector were established and acquired from GeneChem Corporation. Polybrene reagent was used for the transfection of lentivirus particles, while lipofectamine™ 3000 was used for the transfection of overexpression plasmids into HCC cells. Then, 48h–72h after transfection, the collected cells were subjected to further experiments. All the operations were performed according to the manufacturer's instructions.
2.4. Transwell assays
Transwell assays were applied for detecting cells migration and invasion abilities. For invasion ability test, the Matrigel (BD Biosciences, CA, USA) Matrigel matrix (BD Bioscience, USA) was precoated on polycarbonate membranes at a concentration of 1:30 in DMEM without FBS. Cells (2 × 105) were resuspended with 200 μl serum-free DMEM and seeded into the top chamber. Then, 24 h later, the cells in the upper chamber were carefully removed with a cotton swab, and the cells in the lower surface of the membrane were fixed with 4% paraformaldehyde and then stained with 0.5% crystal violet.
2.5. Quantitative Real-time RT-PCR (RT-qPCR)
Total RNA from tissue samples and cell lines was isolated by TRIzol reagent (Invitrogen Carlsbad, CA) according to the product manual. Then, 1 μg RNA was reversely transcribed into cDNA using PrimeScript RT Reagent Kit (Takara, Dalian, China). The SYBR Green MasterMix (Takara) was used for RT-qPCR analysis. Gene expression analysis was performed using the 2−ΔΔCT method. GAPDH was used as the internal reference. The primer sequences were provided as follow. CECR7-Forward: 5′-GAACACAGCCGAAGTGGAAT-3′,
CECR7-Reverse: 5′-ACAGAAACTGTGGGGTCAGG-3’.
U2AF2-Forward: 5′-CGGCAGCTCAACGAGAATAAA-3′,
U2AF2-Reverse: 5′-GGGAACGAATCAGTCCACCG-3’.
EXO1-Forward: 5′-TGAGGAAGTATAAAGGGCAGGT-3′,
EXO1-Reverse: 5′-AGTTTTTCAGCACAAGCAATAGC-3’.
GAPDH-Forward: 5′-GGAGCGAGATCCCTCCAAAAT-3′,
GAPDH-Reverse: 5′-GGCTGTTGTCATACTTCTCATGG-3’.
2.6. MTT assay
The transfected cells were seeded in 96-well plates (2000 cells/well). MTT (10μL/well, Sigma, USA) was added to each well at 0, 24, 48, and 72h after seeding and incubated for 4h at 37 °C. Then, DMSO (100μL/well, Sigma, USA) was applied for the removal of formazan crystals. A microplate reader (Bio-Rad, Richmond, CA) was utilized to assess the absorption at 490 nm.
2.7. EdU (5-ethynyl-2′-deoxyuridine) assay
The proliferative ability was determined using Cell-Light™ EdU Apollo®567 In Vitro Imaging Kit (RiboBio Co., Ltd. Guangzhou, China) according to the manufacturer's instructions. About 1 × 105 HCC cells in 96-well plates were cultured for 24 h, and then maintained in the medium containing 50 mmol/L EdU for 2 h at 37 °C. Cells were washed and fixed with 4% paraformaldehyde, glycine, and 0.5% TritonX-100 in PBS. Next, cells were stained with 100 μL Apollo dye solution for 30min at room temperature. Cells were subsequently stained using Hoechst and incubated for 30min and were observed with an Olympus IX53 microscope (Tokyo, Japan).
2.8. Dual-luciferase reporter assay
The 3′-UTR fragment of EXO1 with CECR7 binding site was established by Guangzhou RiboBio Co., Ltd. (Guangzhou, China) to form the pmiR-RB-ReportTM vector EXO1-3′-UTR-wild-type (EXO1-3′-UTR- Wt) (RiboBio). To mutate the putative binding site of CECR7 in the 3′-UTR-containing vector, the sequence of the putative binding site was mutated and formed the EXO1-3′-UTR-mutated-type (EXO1-3′-UTR- Mut). Wild-type vector or mutated-type vector was transfected into HCC cells using Lipofectamine 3000 (Invitrogen, USA). Relative luciferase activities were measured 48h after transfection and renilla luciferase activity was normalized by firefly luciferase activity.
2.9. Subcellular localization of CECR7
The PARIS Kit (Life Technologies, Carlsbad, CA) was applied to conduct the separation of nuclear and cytosolic fractions based on the manufacturer's instructions. Then, the nuclear or cytosolic fractions were subjected to RT-qPCR analysis. The GAPDH acted as the internal reference of cytoplasmic, while U6 acted as the internal reference of nuclear RNA.
2.10. RNA immunoprecipitation (RIP) assay
RIP assay was conducted by using the EZ-Magna RIP kit (Millipore, Billerica, MA) based on the manufacturer's instructions. Lysates of HCC cells were cultured in RIP containing magnetic beads conjugated with antibodies against U2AF2 (NBP2-33397, Novus, USA) or control IgG (NBP2-34250, Novus, USA) for 6h at 4 °C. Finally, RNAs after purification were analyzed by RT-qPCR.
2.11. Western blot
Cells were lysed by RIPA buffer to obtain total protein lysates and then separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After being transferred to PVDF membranes (Millipore, Billerica, MA, USA), the blots were blocked by 5% nonfat milk for 1h. The protein lysates were incubated with primary antibody against U2AF2 (1:1000, NBP2-33397, Novus, USA), EXO1 (1:1000, NBP2-16391, Novus, USA) or β-actin (1:1000, ab8226, Abcam, USA). The blots were detected using an enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA).
2.12. Microarray mRNA expression analysis
Global mRNA expression was analyzed by the PrimeView Human Gene Expression Array (Affymetrix). Total RNA was converted into cRNA. The products were purified and quantified for chip hybridization. At the end of hybridization, the chips were removed and washed in a Boo Slide Washer8 chip washer, and the washed chips were scanned using an Agilent chip scanner (G2565CA) to obtain hybridization images. The fluorescent signal was scanned by GeneChip Scanner 3000 (Affymetrix). The fold change (FC) of gene expression in shCECR7#1 cells was calculated relative to NC cells. A gene was defined as differentially expressed if its log2|FC| > 0.5.
2.13. Animal experiments
The protocol for the mice experiments in this study was approved by the Institutional Animal Ethical Committee of the Gansu Gem Flower Hospital (#GGFH-202201056). The 4-weeks-old female BALB/c nude mice aged four weeks old were housed in a specific pathogen-free (SPF) condition. For in vivo metastasis evaluation, HCC cells at a density of 105 cells/100 μL were injected into the mice tail vein. The mice were sacrificed 6 weeks after the injection. Hematoxylin-eosin staining was applied to observe the formation of metastatic lung nodes. For in vivo growth evaluation, HCC cells at a density of 2 × 106/200 μL were subcutaneously injected into the mice flank. Then, the tumor size was measured every week. The mice were sacrificed 5 weeks after cell injection. Then, the weight of resected tumor nodules was measured. And part of the tumor nodule was stored at −80 °C and the rest was fixed in 4% formaldehyde solution for the subsequent experiments.
2.14. Statistical analysis
Statistical analyses were performed by SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) and Graphpad Prism 8.0 (San Diago, CA, USA). Quantification values were presented as Mean ± S.D. Continuous data were analyzed by one-way ANOVA, and data that were normally distributed were analyzed using the two-tailed Student's t-test. Categorical variables were analyzed by the chi-square tests. Relationships between clinicopathological characteristics were investigated by the chi-square test or Fisher's exact test. Kaplan–Meier curve analysis and the log-rank test were used for the survival analysis. And Pearson's correlation coefficient analysis was used for the genes expression relationship. P<0.05 was considered statistically significant.
3. Results
3.1. Highly expressed lncRNA CECR7 indicates poor prognosis of HCC
CECR7 expressions in HCC cell lines (HCCLM3, Hep3B, Huh7,HepG2, and MHCC97H) were significantly upregulated compared to the human normal liver cell line (LO2) as shown by RT-qPCR analysis (P < 0.05, respectively, Fig. 1A). And, HCC tissues had higher CECR7 expression in comparison to the adjacent non-tumor tissues (P < 0.05, Fig. 1B). Additionally, the analysis of TCGA data from UALCAN (http://ualcan.path.uab.edu) consistently showed an upregulated expression of CECR7 in HCC (P < 0.001, Fig. 1C). To evaluate whether CECR7 expression was associated with HCC clinicopathologic features, 82 patients were categorized into two subgroups (low/high CECR7 group) in accordance with the median expression of CECR7 in HCC tissues. The analysis conducted with Chi-square test revealed that CECR7 expression was closely related to tumor size (P = 0.014), venous infiltration (P = 0.018), and TNM stage (P = 0.015) (Table 1). Besides, the 5-year overall survival (OS) and disease-free survival (DFS) of HCC patients with higher CECR7 expression were much worse than the patients with lower CECR7 expression (P = 0.0110, Fig. 1D; P = 0.0038, Fig. 1E). Consistently, the above finding was obtained from the data of StarBase (http://starbase.sysu.edu.cn) (Fig. 1F). In brief, our data demonstrate that CECR7 is significantly increased in HCC and CECR7 expression is positively associated with HCC progression.
Fig. 1.
Highly expressed lncRNA CECR7 indicates poor prognosis of HCC. (A) RT-qPCR analysis was applied to explore the expression of CECR7 in HCC cell lines (Hep3B, HepG2, HCCLM3, Huh7 and MHCC97H) and a human normal liver cell line (LO2) (Mean ± SD; n = 3). ***P < 0.001 vs. LO2; two-way ANOVA. (B) RT-qPCR analysis was applied to explore the expression of CECR7 in 82 pairs of HCC tissues and adjacent non-tumor (NT) tissues. ***P < 0.001; Student's t-test. (C) TCGA data from public dataset UALCAN (http://ualcan.path.uab.edu) for CECR7 expression in HCC. (D, E) Kaplan-Meier curves were established to investigate the effects of CECR7 expression on 5-year overall survival (OS) and disease-free survival (DFS) of HCC. Log-rank test. (f) Survival data from StarBase (http://starbase.sysu.edu.cn).
Table 1.
Correlation between CECR7 expression and the clinicopathologic characteristics in HCC.
| Characteristics | Cases (n = 82) | CECR7 Expression |
P | ||
|---|---|---|---|---|---|
| High (n = 41) | Low (n = 41) | ||||
| Age (year) | <50 | 25 | 10 | 15 | 0.230 |
| ≥50 | 57 | 31 | 26 | ||
| Gender | Male | 68 | 33 | 35 | 0.557 |
| Female | 14 | 8 | 6 | ||
| HBV | Absent | 10 | 3 | 7 | 0.177 |
| Present | 72 | 38 | 34 | ||
| Serum AFP level (ng/mL) | <400 | 18 | 12 | 6 | 0.109 |
| ≥400 | 64 | 29 | 35 | ||
| Tumor size (cm) | <5 | 35 | 12 | 23 | 0.014 |
| ≥5 | 47 | 29 | 18 | ||
| Number of tumor nodules | 1 | 66 | 30 | 36 | 0.095 |
| ≥2 | 16 | 11 | 5 | ||
| Cirrhosis | Absent | 21 | 14 | 7 | 0.077 |
| Present | 61 | 27 | 34 | ||
| Venous infiltration | Absent | 56 | 23 | 33 | 0.018 |
| Present | 26 | 18 | 8 | ||
| Edmondson-Steiner grading | I + II | 53 | 23 | 30 | 0.106 |
| III + IV | 29 | 18 | 11 | ||
| TNM stage | I + II | 58 | 24 | 34 | 0.015 |
| III + IV | 24 | 17 | 7 | ||
HCC, hepatocellular carcinoma; HBV, hepatitis B virus.
AFP, alpha-fetoprotein; TNM, tumor-node-metastasis.
3.2. CECR7 promotes metastasis of HCC cells both in vitro and in vivo
Next, we respectively altered CECR7 expression of MHCC97H and Hep3B with pcDNA/CECR7 and CECR7 shRNAs and RT-qPCR analysis was performed to test the efficiency (P < 0.001, respectively,Fig. 2A and B). Then, Transwell assays were employed to investigate the effect of CECR7 expression on migration and invasion ability in MHCC97H and Hep3B subclones. Results indicated that the number of MHCC97H cells passing through the chamber membrane was significantly suppressed by CECR7 knockdown (P < 0.05, Fig. 2C), while ectopic expression of CECR7 significantly enhanced Hep3B cell migration and invasion (P < 0.05, Fig. 2D). Furthermore, the intravenous transplantation tumor model was established and lung tissues obtained from the models were subjected to HE staining. The data indicated that the formation rate of lung metastasis was significantly decreased in CECR7 knockdown group (P < 0.01, Fig. 2E). Thus, the above findings revealed that CECR7 promoted metastasis of HCC cells both in vitro and in vivo.
Fig. 2.
CECR7 promotes HCC cells metastasis both in vitro and in vivo. (A) Lentivirus encoding shRNAs targeting CECR7 (shCECR7#1, shCECR7#2) and control were used to knockdown CECR7 expression in MHCC97H cells. RT-qPCR analysis was applied to confirm the efficiency (Mean ± SD; n = 3). ***P < 0.001 vs. NC; two-way ANOVA. (B) The pcDNA plasmids encoding CECR7 or control were used to overexpress CECR7 expression in Hep3B cells. RT-qPCR analysis was applied to confirm the efficiency. (Mean ± SD; n = 3). ***P < 0.001, Student's t-test. (C, D) Transwell assays were applied to detect migration and invasion ability changes in MHCC97H and Hep3B cells (Mean ± SD; n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA, Student's t-test. (E) The intravenous transplantation tumor model was established. Paired lung tissues were isolated and subjected for H&E staining (Mean ± SD; n = 3). **P < 0.01, Student's t-test.
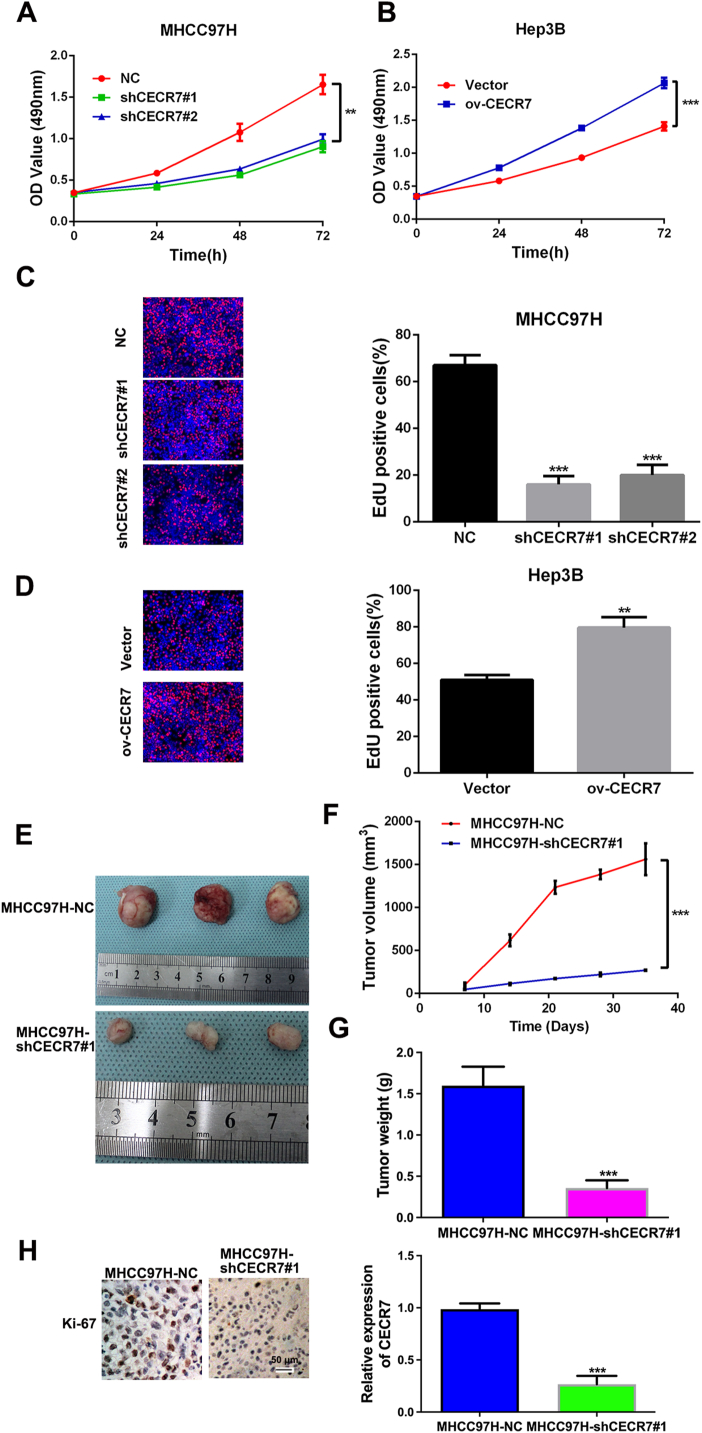
3.3. CECR7 promotes HCC cells growth both in vitro and in vivo
Next, we tried to investigate whether CECR7 had any influence on HCC cells growth. MTT assay showed a suppressed cell viability in CECR7-knockdown subclones of MHCC97H (P < 0.01, Fig. 3A). In contrast, overexpressed CECR7 promoted the cell viability of Hep3B cells (P < 0.001, Fig. 3B). Additionally, the suppressed proliferation ability was also found in EdU assay which was conducted in CECR7-knockdown subclones of MHCC97H (P < 0.001, Fig. 3C), while CECR7 overexpression had a promoting effect on Hep3B cell proliferation (P < 0.01, Fig. 3D). Furthermore, the subcutaneous xenograft tumor model was constructed, and the tumor growth was observed. The growth curves and the final tumor weight strongly indicated that tumor growth was significantly inhibited in CECR7 knockdown group (Fig. 3E–G). In addition, a weaker Ki-67 staining was observed in HCC nodules tissues of CECR7 knockdown group. In brief, the above findings suggest that CECR7 not only promotes HCC cells growth both in vitro and in vivo.
Fig. 3.
CECR7 promotes HCC cells growth both in vitro and in vivo. (A, B) MTT assay was used to explore the effects of CECR7 expression on MHCC97H and Hep3B cells viability (Mean ± SD; n = 3). **P < 0.01, ***P < 0.001, two-way ANOVA. (C, D) EdU assay was used to explore the effects of CECR7 expression on MHCC97H and Hep3B cells viability proliferation (Mean ± SD; n = 3). **P < 0.01, ***P < 0.001, two-way ANOVA. Student's t-test. (E-G) Subcutaneous xenograft tumor model was established, and the tumor growth was measured every week. Five weeks later, the mice were euthanasiaed, then the tumor nodules were resected, and the tumor weight was measured (Mean ± SD; n = 3). ***P < 0.001, two-way ANOVA. Student's t-test. (H) Immunohistochemical staining for Ki-67 in the tumor sections, and the result was evaluated by Image J (Mean ± SD; n = 3). ***P < 0.001, Student's t-test.
3.4. CECR7 increases EXO1 expression by enhancing the stability of EXO1 mRNA
In order to probe into the underline mechanism of CECR7 involved in growth and metastasis of HCC cells, RNA-sequencing was applied to identify the targeted mRNA of CECR7 in CECR7-knockdown subclones of MHCC97H. Especially, EXO1 was focused due to its most remarkable decrease when CECR7 was lost (Fig. 4A). Then, we verified that knockdown of CECR7 inhibited mRNA level of EXO1 (P < 0.001, Fig. 4B). In contrast, overexpression of CECR7 increased mRNA level of EXO1 (P < 0.001, Fig. 4C). EXO1 expression was determined to be upregulated in HCC tissues by RT-qPCR analysis (P < 0.001, Fig. 4D), which was consistently found in TCGA data analysis from StarBase (http://starbase.sysu.edu.cn) (P < 0.001, Fig. 4E). Besides, the analyses both in our cohort (Fig. 4F) and StarBase (http://starbase.sysu.edu.cn) (Fig. 4G) revealed that there existed a positive correlation between CECR7 and EXO1 expression in HCC. Next, we attempted to figure out the underline mechanism by which CECR7 increased EXO1 expression. CECR7 was determined to be mainly located in in the cytoplasm by separation of nuclear and cytosolic fractions assay (Fig. 4H), suggesting that CECR7 might increase the expression of EXO1 mRNA through strengthening the stability of EXO1 mRNA. Additionally, the luciferase reporter which contained EXO1 promoter was established to test whether CECR7 had any effect on the EXO1 transcription. It was unfortunate that neither overexpression nor silencing of CECR7 influenced the luciferase activity of EXO1 promoter (Fig. 4I and J). Subsequently, stable HCC subclones of CECR7 overexpression or silencing were constructed, then mRNA decay rates were assessed. Actinomycin D was used to block the RNA synthesis of these subclones, then RT-qPCR analysis was applied to measure the degradation of EXO1 mRNA at different time point. The results revealed that EXO1 mRNA in CECR7 knockdown group had a shorter half-life (Fig. 4K), while overexpression of CECR7 extended the half-life of EXO1 mRNA (Fig. 4L). Thus, the above findings suggest that CECR7 increases EXO1 expression through strengthening the stability of EXO1 mRNA.
Fig. 4.
CECR7 increases EXO1 expression by stabilizing EXO1 mRNA. (A) Heat-map for RNA-seq data in CECR7-knockdown of MHCC97H cells. Among the downstream genes of CECR7, EXO1 is the most downregulated gene upon CECR7 was knockdown. (B, C) RT-qPCR analysis was used to detect EXO1 mRNA expression in CECR7-knockdown or CECR7-overexpressing cells (Mean ± SD; n = 3). ***P < 0.001, two-way ANOVA. Student's t-test. (D) RT-qPCR analysis was applied to detect EXO1 expression in 82 paired HCC tissues and NT tissues. ***P < 0.001, Student's t-test. (E) TCGA data analysis from StarBase (http://starbase.sysu.edu.cn). (F) Pearson correlation analysis was used to explore the correlation between CECR7 expression and EXO1 expression in HCC tissues. (G) Correlation analysis from StarBase (http://starbase.sysu.edu.cn). (H) RNA separation of nuclear and cytosolic fractions was conducted to determine the subcellular localization of CECR7. (I, J) Luciferase reporter containing EXO1 promoter was used to detect whether CECR7 affected the EXO1 transcription. (K, L) Actinomycin D was applied to block RNA synthesis. RT-qPCR analysis was used to detect the remaining EXO1 mRNA (Mean ± SD; n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA.
3.5. CECR7 stabilize EXO1 mRNA under the mediation of U2AF2 protein
To explore the RNA binding protein, by which CECR7 increased the stability of EXO1 mRNA, we applied starBase (http://starbase.sysu.edu.cn) to predict the protein associated with both CECR7 and EXO1 mRNA. U2AF2 was predicted to be the potential RNA binding protein for both CECR7 and EXO1 mRNA (Fig. 5A). Furthermore, the interaction was verified by RIP assay in HCC cells (Fig. 5B and C). Besides, neither U2AF2 mRNA nor protein expression was affected by U2AF2 knockdown (Fig. 5D) or CECR7 overexpressing (Fig. 5E). Furthermore, U2AF2 overexpressing reversed the repression of EXO1 mRNA by CECR7 shRNA#1 (Fig. 5F), and the EXO1 mRNA induction induced by overexpressed CECR7 was abrogated by U2AF2 shRNA (Fig. 5G). Collectively, the above data suggested that CECR7 stabilize EXO1 mRNA under the mediation of U2AF2 protein in HCC.
Fig. 5.
CECR7 stabilize EXO1 mRNA under the mediation of U2AF2 protein. (A) The platform starBase (http://starbase.sysu.edu.cn) was applied to predict the potential RNA binding protein for both CECR7 and EXO1 mRNA. (B, C) RIP assay using antibody against U2AF2 to determine the enrichment of CECR7 and EXO1 mRNA in HCC cells (Mean ± SD; n = 3). **P < 0.01, ***P < 0.001, Student's t-test. (D, E) RT-qPCR analysis and Western blot were used to detect the effect of CECR7 on U2AF2 expression. (F, G) U2AF2-overexpressing reversed the repression of EXO1 mRNA by CECR7 shRNA#1, and the EXO1 mRNA induction induced by CECR7-overexpressing was abrogated by U2AF2 shRNA (Mean ± SD; n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA.
3.6. EXO1 mediates the effects of CECR7 on HCC cells metastasis
Then, we tried to explore whether CECR7 promoted metastasis of HCC cells under the mediation of EXO1. The data from RT-qPCR and Western blot revealed that EXO1 expression was repressed by CECR7 knockdown, and EXO1 overexpression rescued the inhibitory effect in MHCC97H cells (Fig. 6A). In contrast, overexpressed CECR7 increased EXO1 expression, while the promotion effect was rescued by EXO1 shRNA in Hep3B cells (Fig. 6B). As expected, rescue experiments of Transwell assays manifested that EXO1 overexpressing rescued the inhibiting effects of CECR7 silencing on the migration and invasion of MHCC97H cells (Fig. 6C). Besides, the motivating effects of CECR7 overexpression on migration and invasion of Hep3B cells were offset by EXO1 silencing (Fig. 6D). Taken together, we demonstrate that EXO1 mediates the effects of CECR7 on HCC cell metastasis.
Fig. 6.
EXO1 mediates the effects of CECR7 on HCC cells metastasis. (A, B) Co-transfections were conducted by using the indicated lentivirus and plasmids, then RT-qPCR analysis and Western blot were used to confirm the efficiency (Mean ± SD; n = 3). ***P < 0.001, two-way ANOVA. (C, D) Transwell assays were applied to detect the migration and invasion ability changes in different HCC subclones (Mean ± SD; n = 3). **P < 0.01, ***P < 0.001, two-way ANOVA.
3.7. EXO1 mediates the effects of CECR7 on HCC cells growth
In order to investigate whether EXO1 mediated the effects of CECR7 on HCC cells growth, the rescue experiments were applied. MTT assay indicated that the suppression effects of CECR7 knockdown on MHCC97H cells viability was rescued by EXO1 overexpression (Fig. 7A). In contrast, the accelerating effect of overexpressed CECR7 on Hep3B cells viability was abolished by EXO1 silencing (Fig. 7B). Moreover, EdU assay showed that the inhibitory proliferation ability of MHCC97H cells led by CECR7 shRNA was rescued by EXO1 restoration (Fig. 7C), while the enhancing effect of upregulated CECR7 on Hep3B cells viability was counteracted by EXO1 knockdown (Fig. 7D). Taken together, the above data demonstrate that EXO1 mediates the effect of CECR7 on HCC cells growth.
Fig. 7.
EXO1 mediates the effects of CECR7 on HCC cells growth. (A, B) MTT assay was applied to detect the viability changes in different HCC subclones (Mean ± SD; n = 3). **P < 0.01, ***P < 0.001, two-way ANOVA. (C, D) Edu assay was applied to detect the proliferation changes in different HCC subclones (Mean ± SD; n = 3). *P < 0.05, **P < 0.01, two-way ANOVA.
4. Discussion
A large body of studies proved the crucial roles of lncRNAs in HCC progression [[25], [26], [27], [28]]. Here, we verified the high expression of the novel lncRNA CECR7 in HCC tissues and cell lines. Clinically, CECR7 was closely associated with tumor size, venous infiltration, and TNM stage. Besides, CECR7 expression was negatively related to the prognosis of HCC patients. The findings strongly demonstrate the potential oncogene role of CECR7 in HCC. Furthermore, cytological experiments and animal experiments were conducted to investigate the effects of CECR7 on HCC metastasis and growth. As expected, our findings collectively demonstrated that CECR7 was promotor for HCC cells metastasis and growth. Thus, these data demonstrate that CECR7 plays the oncogenic role in HCC by enhancing cells metastasis and growth.
Diverse molecular mechanisms of lncRNAs involved in the regulation of targeted mRNA expression have been identified, including transcriptional and post-transcriptional levels [14,29,30]. In particular, lncRNAs located in cytoplasm can modulate the downstream mRNA expression through regulating the mRNA stability with the assistance of some RNA-binding proteins [25]. For example, lncRNA UCID promotes HCC metastasis via stabilizing of Snail mRNA [31]. And, lncRNA LINC01093 inhibits HCC development through the mediation of RNA-binding protein IGF2BP1 to induce the degradation of GLI1 mRNA [32]. In this study, on the basis of Microarray mRNA expression analysis which was conducted in CECR7 knockdown subclone of MHCC97Hand the control subclone, EXO1 was selected to be further studied due to the most remarkable reduction in CECR7 knockdown subclone. Studies have showed that EXO1 has been proved to be an oncogene in HCC, and there exists a close relationship between EXO1 expression and FOXP3 activity. Here, we verified the elevated expression of EXO1 in HCC. Moreover, there existed a positive correlation between CECR7 expression and EXO1 mRNA expression in HCC. Additionally, the stability of EXO1 mRNA rather than the transcription activity was modulated by CECR7.
RNA-binding proteins paly essential roles in the regulation of mRNA stability by lncRNA [33]. Here, Bioinformatics analysis tools were employed to predict the protein candidates which might associate with both CECR7 and EXO1 mRNA. Through a series of experiments, we demonstrate that CECR7 regulated EXO1 expression through recruiting U2AF2 protein to modulate EXO1 mRNA stability. Additionally, rescue experiments showed that the influences of CECR7 on HCC cells migration, invasion and growth were reversed by artificially expression changing of EXO1. Obviously, in our study, CECR7 promoted HCC metastasis and growth at least in part under the mediation of EXO1. However, And the further mechanism about how EXO1 promoted HCC progression is the limitations of our current studym, which is also needed to be further studied in our future researches. EXO1 is a member of the Rad2 family of exonucleases and has an N-terminal catalytic domain that is conserved in other Rad2 family proteins, and the C-terminus is predicted to be largely unstructured but is involved in protein–protein interactions [34]. The overexpression of EXO1 has been reported in several other cancers, which in part is related to increased DNA repair activity [35]. And it's worth noting that EXO1 dysfunction could alter other DNA repair pathways, leading to replication stress followed by genomic instability and the development of cancer [34,35]. Thus, based on the findings in our study, we suspect that CECR7 played its roles in HCC cells metastasis and growth at least in part through EXO1, during which EXO1 might affect the DNA stability of some metastasis-related and growth-related genes, or affect the protein–protein interactions of some related genes and so on.
Taken together, our data identified a novel lncRNA, which was upregulated in HCC. In terms of clinical significance, CECR7 expression was positively related to large tumor size, venous infiltration, advanced TNM stage. And the patients with higher CECR7 expression had worse prognosis. Besides, CECR7 facilitated HCC metastasis and growth, which further indicated its oncogenic role in HCC. Furthermore, CECR7 induced EXO1 expression by enhancing the stability of EXO1 mRNA through the interaction with RNA binding protein U2AF2.
Ethics approval and informed consent
According to the Declaration of Helsinki, the current study was approved by the Institutional Animal Ethical Committee of the Gansu Gem Flower Hospital. Written informed consent was obtained from all patients.
Author contribution statement
Liang Zhao, Xiaobin Yao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qing Zang, Guodong Liang: Contributed reagents, materials, analysis tools; Performed the experiments; Wrote the paper.
Funding statement
This study received no specific funding or grant.
Data availability statement
Data will be made available on request.
Ethical approval
The study protocol was duly approved by the Ethics Committees of the Gansu Gem Flower Hospital. All experiments were carried out according to the guidelines of Helsinki declaration as well as the International Conference on Harmonization of Good Clinical Practice. All participants signed written informed consent for participation in the study as well as the publication of research data.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19862.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
References
- 1.Sangro B., et al. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18(8):525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet J.M., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021;18(4):223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim E., Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp. Mol. Med. 2020;52(12):1898–1907. doi: 10.1038/s12276-020-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittermeier C., Konopa A., Muehlich S. Molecular mechanisms to target cellular senescence in hepatocellular carcinoma. Cells. 2020;9(12):2540. doi: 10.3390/cells9122540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimassa L., et al. Systemic treatment of HCC in special populations. J. Hepatol. 2021;74(4):931–943. doi: 10.1016/j.jhep.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Malla R.R. Long noncoding RNAs: potential mediators of liver cancer metastasis. Crit. Rev. Oncog. 2021;26(1):21–33. doi: 10.1615/CritRevOncog.2020035666. [DOI] [PubMed] [Google Scholar]
- 8.Yue J., et al. LncRNAs link cancer stemness to therapy resistance. Am. J. Cancer Res. 2021;11(4):1051–1068. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P.S., Wang Z., Yang C. Dysregulations of long non-coding RNAs - the emerging "lnc" in environmental carcinogenesis. Semin. Cancer Biol. 2021;76:163–172. doi: 10.1016/j.semcancer.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie C., et al. Functional long non-coding RNAs in hepatocellular carcinoma. Cancer Lett. 2021;500:281–291. doi: 10.1016/j.canlet.2020.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z., et al. The role of long noncoding RNAs in hepatocellular carcinoma. Mol. Cancer. 2020;19(1):77. doi: 10.1186/s12943-020-01188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanzafame M., et al. The role of long non-coding RNAs in hepatocarcinogenesis. Int. J. Mol. Sci. 2018;19(3):682. doi: 10.3390/ijms19030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong C.M., Tsang F.H., Ng I.O. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat. Rev. Gastroenterol. Hepatol. 2018;15(3):137–151. doi: 10.1038/nrgastro.2017.169. [DOI] [PubMed] [Google Scholar]
- 14.Klingenberg M., et al. Non-coding RNA in hepatocellular carcinoma: mechanisms, biomarkers and therapeutic targets. J. Hepatol. 2017;67(3):603–618. doi: 10.1016/j.jhep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Shi X., et al. The Hippo pathway in hepatocellular carcinoma: non-coding RNAs in action. Cancer Lett. 2017;400:175–182. doi: 10.1016/j.canlet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Qiu L., et al. Long non-coding RNAs in hepatitis B virus-related hepatocellular carcinoma: regulation, functions, and underlying mechanisms. Int. J. Mol. Sci. 2017;18(12):2505. doi: 10.3390/ijms18122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv H., et al. Noncoding RNAs in liver cancer stem cells: the big impact of little things. Cancer Lett. 2018;418:51–63. doi: 10.1016/j.canlet.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y.H., et al. Long non-coding RNAs as mediators of tumor microenvironment and liver cancer cell communication. Int. J. Mol. Sci. 2018;19(12):3742. doi: 10.3390/ijms19123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho J.J.D., et al. Wiley Interdiscip Rev RNA; 2021. Translational Remodeling by RNA-Binding Proteins and Noncoding RNAs; p. e1647. [DOI] [PubMed] [Google Scholar]
- 20.Takayama K.I., et al. Targeting epigenetic and post-transcriptional gene regulation by PSF impairs hormone therapy-refractory cancer growth. Cancer Res. 2021;81(13):3495–3508. doi: 10.1158/0008-5472.CAN-20-3819. [DOI] [PubMed] [Google Scholar]
- 21.Sato M., et al. The lncRNA Caren antagonizes heart failure by inactivating DNA damage response and activating mitochondrial biogenesis. Nat. Commun. 2021;12(1):2529. doi: 10.1038/s41467-021-22735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papoutsoglou P., et al. The noncoding MIR100HG RNA enhances the autocrine function of transforming growth factor beta signaling. Oncogene. 2021;40(21):3748–3765. doi: 10.1038/s41388-021-01803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu P., et al. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene. 2021;40(9):1609–1627. doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu B., et al. Long noncoding RNA DUXAP10 promotes the stemness of glioma cells by recruiting HuR to enhance Sox12 mRNA stability. Environ. Toxicol. 2021;36(5):840–849. doi: 10.1002/tox.23087. [DOI] [PubMed] [Google Scholar]
- 25.Lim L.J., et al. Roles and regulation of long noncoding RNAs in hepatocellular carcinoma. Cancer Res. 2019;79(20):5131–5139. doi: 10.1158/0008-5472.CAN-19-0255. [DOI] [PubMed] [Google Scholar]
- 26.Mai H., et al. Molecular pattern of lncRNAs in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38(1):198. doi: 10.1186/s13046-019-1213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Z.S., et al. Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma. World J. Gastroenterol. 2020;26(29):4240–4260. doi: 10.3748/wjg.v26.i29.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bu F.T., et al. LncRNA NEAT1: shedding light on mechanisms and opportunities in liver diseases. Liver Int. 2020;40(11):2612–2626. doi: 10.1111/liv.14629. [DOI] [PubMed] [Google Scholar]
- 29.Huo X., et al. Dysregulated long noncoding RNAs (lncRNAs) in hepatocellular carcinoma: implications for tumorigenesis, disease progression, and liver cancer stem cells. Mol. Cancer. 2017;16(1):165. doi: 10.1186/s12943-017-0734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S., et al. Ageing Res Rev; 2021. Epigenetic Regulation of Melanogenesis. [DOI] [PubMed] [Google Scholar]
- 31.Yuan S., et al. LncRNA UCID promotes hepatocellular carcinoma metastasis via stabilization of Snail. OncoTargets Ther. 2021;14:725–736. doi: 10.2147/OTT.S277951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J., et al. A novel, liver-specific long noncoding RNA LINC01093 suppresses HCC progression by interaction with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett. 2019;450:98–109. doi: 10.1016/j.canlet.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Li L., et al. Multidimensional crosstalk between RNA-binding proteins and noncoding RNAs in cancer biology. Semin. Cancer Biol. 2021;75:84–96. doi: 10.1016/j.semcancer.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Goellner E.M., Putnam C.D., Kolodner R.D. Exonuclease 1-dependent and independent mismatch repair. DNA Repair. 2015;32:24–32. doi: 10.1016/j.dnarep.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperka T., Wang J., Rudolph K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012;13(9):579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.