Abstract
Massive amounts of wastewater are produced by the textile industry, and this waste needs to be appropriately managed. Agricultural waste wheat straw (WS), a biosorbent that is both economically available and environmentally acceptable, was used in this work to treat textile effluent. Microbial treated modification approaches were utilized for WS to study the dye removal from textile wastewater. Total 15 different isolates were screened for the dye degradation ability from Surat textile industrial effluent. The most significant deterioration was seen in PPSUHB3 when compared to other isolates. The amount of methylene blue dye removal was examined using the isolate PPSUHB3 due to its high efficiency. Based on 16s rDNA sequencing, it was predicted that the isolate PPSUHB3 was Bacillus licheniformis, having great capacity to degrade dye & wheat straw by producing efficient enzyme. The isolate showed the highest decolorization % of MB dye during optimization with WS absorbent which was verified using FTIR and SEM. The dye removal process parameters were statistically optimized using a central composite design (CCD). Wheat straw with particle sizes of 180–250 mm was discovered to be a possible adsorbent for the removal of colour. The maximum removal of MB (55.89%) was obtained using a statistical experimental design at pH 6.36, Temperature 44.6 °C, and Bacteria Concentration 3.04%. The created model is highly significant, according to the ANOVA, which found an R2 value of 0.9812 for it. The validation experiment revealed that the experimental and projected results were strikingly similar. The study found that using bacterial treated wheat straw as an adsorbent may remove wastewater that contains colours at a low cost.
Keywords: Bacterial treated biosorbent, Agro-waste, Wastewater, SEM analysis, FTIR, Plackett–Burman design
1. Introduction
Over the past few decades, there has been a sharp increase in the amount of hazardous chemicals released into surface water [1]. One of the primary sources of pollution is the creation of wastewater. All sorts of industrial effluent, including wastewater that contains colours, must be successfully handled. About 7 lakh metric tonnes of dyes are used annually. Textile, leather, cosmetics, and food industries are just a few that require dyeing for casting and finishing the wrapping of finished items. A wide range of wastewater discharges from industrial products need to be treated since they are polluted with dyes. The use of synthetic dyes in the textile sector is about 30%. This sector today employs 35 million people worldwide, has risen by $1 trillion, and accounts for 7% of all exports [2]. Additionally, it is predicted that 5%–10% of these colours will be squandered and dumped right into water bodies. Because dyes usually have more intricate and durable structures, they can be more challenging to completely remove from wastewater [3]. Most of the time, dye concentrations in the water can be found to be more than 1 mg L−1. Around 10,000 distinct pigments and dyes are produced globally on an annual basis, and significant amounts of dissolved dyes are released into diverse bodies of water [1,4].
The release of untreated effluents containing coloured dyes into water bodies harms the ecosystem. It blocks light from reaching the water, which lowers the rates of photosynthesis in phytoplankton-coloured algae and has an impact on the aquatic biota. Additionally, known for being poisonous, mutagenic, and carcinogenic are the dyes. Acid, basic, direct, and reactive dyes are examples of dyes having aromatic molecular structures that are challenging to appropriately treat dye effluent for. The very intricate aromatic structure aids biological breakdown while improving molecular stability [2]. Contrarily, as dyes are naturally toxic and poisonous and can have a variety of negative impacts on human health, they can offer serious health hazards when combined with drinking water [3]. For instance, vomiting, cyanosis, an accelerated heartbeat, tissue necrosis, shock, jaundice, cancer, and mammalian cell mutation. It is therefore essential to clean wastewater that has been contaminated with these colours as soon as possible. The present technologies for dye removal from wastewater have a number of serious limitations, including high prices, the creation of harmful byproducts, and high energy requirements [1].
The removal of dyes can be accomplished through physical, chemical, or biological processes [3]. When treating wastewater that contains reactive colours, such as through biodegradation, many techniques have been considered [5]. Biosorption [6] coagulation and flocculation [7], electrocoagulation [8], adsorption [9], ozone treatment [10], membrane separation [11], chemical oxidation [12], photocatalysis [13], sonochemical [14], nanotechnology [15], and photo-Fenton processes [16], in the developing countries; In terms of the environment and the cost of lessening the environmental impact of these dyes through biodegradation, these technologies are prohibitively expensive to use. The biodegradation process can be thought of as a feasible candidate approach for removing undesirable hazardous components from contaminated wastewater because it is a cheap and easily accessible way of treatment.
The most used dye, methylene blue (MB), has caused a substantial amount of environmental pollution because of its toxicity [17]. MB may disrupt photosynthesis and result in eye burns, which may result in both human and animal eyes becoming permanently damaged [18]. Moreover, MB can cause tissue necrosis, cyanosis, jaundice, vomiting, an increase in heart rate, and more in people [19]. Because of this, there are significant ecological concerns with the removal of MB from industrial effluents.
Microbial decolorization method enables thorough cleaning of coloured effluent in a natural manner since it predominantly converts the dyes to much simpler components of CO2, NH3, & H2O by beginning the deterioration of the dyes' bonds [20]. Numerous bacterial species have been discovered to easily decolorize azo dyes when grown in anaerobic environments. Streptococcus faecalis, Eubacterium sp., Proteus vulgaris, Clostridium sp., and Bacteroides sp. Azo dyes cannot be the only carbon or energy source employed since the aerobic decolorization of azo dyes can also occur in the presence of an external carbon source. Bacillus sp., Pseudomonas sp., Sphingomonas sp., and Xanthomonas sp. have all been seen to reductively oxidise polymeric azo dyes in the presence of additional carbon sources in an aerobic environment [21].
However, the application of activated carbon on a wider scale is constrained due to the price, high running expenses, and challenges with regeneration. A better dye removal adsorbent that is made of readily available, inexpensive, and renewable materials is therefore constantly needed. The removal of dyes has been recommended for a wide range of cheap adsorbents. Agricultural waste (sawdust, bark, fruit peel) [22], industrial waste (fly ash, red mud, metal hydroxide sludge) [23], clay materials (zeolites, diatomite, dolomite) [24], bio-adsorbents (biomass, peat) [25], and others are among them (cotton, starch). Agricultural wastes are considered to be a key precursor because of environmental issues. This is due to the fact that they are affordable, renewable, secure, readily available, and easily verifiable sources. These contain a significant amount of carbon and few ash [26]. These readily available materials are regarded as wastes because so much of them are produced annually. As a result, using waste biomass to remove toxic pollutants will result in the formation of energy from waste [3].
Wheat straw from agricultural waste was used in this project as the solid support. Every year, 300 million tonnes of straw are abandoned or burned in China, causing resource waste in addition to soil and air environment degradation [27]. The advantages of this technology over the traditional solid fermentation system are as follows:
-
(1)
Wheat straw is a particularly plentiful sort of reusable agricultural residues in nature, making the process more affordable and ecologically friendly [28,29].
-
(2)
When microorganisms degraded wheat straw, they continuously produced a significant number of ligninolytic enzymes [30,31].
-
(3)
Due to P. chrysosporium ability to disrupt the surface structures of the wheat straw and produce new adsorption sites, it can be employed again as effective bioadsorbents for eliminating colours from contaminated wastewater following fermentation [32,33].
The microbial treated agriculture waste absorbent has great impact on the decolorizing of dye and textile effulent due to hybrid effect of microorganisms and absorbant. This hybrid method has novel aspect for effective treatment of textile effluent.
A collection of mathematical and statistical techniques known as the Response Surface Methodology (RSM) may be used to evaluate the significance of various influencing variables even when there are complex linkages. In order to build a model that explains how these optimal circumstances affect the response, RSM is used to determine a system's ideal operational conditions [34]. The Central Composite Design (CCD) under RSM has been extensively used as the experimental design for fitting a quadratic surface to optimize the effective parameters with a minimum number of experiments and to analyze the interaction between the parameters [[35], [36], [37]]. Using potential native adsorbents that have been selected, the current research aims to identify low-cost substitutes for adsorbents and statistically optimize the dye removal process parameters.
2. Materials and methods
2.1. Isolation and screening of potential bacteria for dye degradation
In a sample jar, samples were taken from decomposing WS. Five grams of material were taken from each location and finely milled into powder. For enriching purpose, it was added in 100 mL BHM medium. Five times, 1 mL of this culture solution was used to test prospective microorganisms for higher laccase activity after being transferred to fresh BHM media after 7 days [38]. Constant streaking procedures were used to create pure cultures, which were kept at 4 °C. Using the plate method, the pure cultures were tested for laccase activities. In brief, the cultures were spotted on nutrient agar plates supplemented with ABTS solution and incubated at 35 ± 2 °C for 24–36 h. Laccase production was suggested by the appearance of a clear hue surrounding the colonies of microbes [39]. Bergey's manual categorization was used to determine biochemical factors such as gram staining, shape, motile bacteria, catalase, voges-proskauer (VP), citrate, ortho-nitrophenyl--galactoside (ONPG), malonate, nitrate reduction, and carbohydrate consumption [40].
2.2. Molecular characterization
The DNA from a single strain of bacteria was extracted using conventional methods. The isolated pure DNA was then amplified using forward and reverse primers (27F - 5′ AGAGTTTGATCMTGGCTCAG 3′ and 1492R - 5′ TACGGYTACCTTGTTACGACTT3′) in PCR (Polymerase Chain Reaction). The following temperatures were used for PCR: denaturation (93–95 °C, 30 s), primer annealing (50–70 °C, 30 s), and elongation (72 °C, 1 min). Purity of the amplified DNA was examined, and using 16S rDNA sequencing, it was molecularly described (sequenced). To determine the functional and evolutionary relationships of that isolate, NCBI blast was used [41].
2.3. Adsorbent selection
BSI standard sieve diameters of 180 and 250 mm were used to separate solid samples (100 g) of each substance. By sieving, each of the adsorbent substances (Wheat straw powder) was divided into two samples based on various particle size distributions. The samples were divided into fine (180 mm) and coarse categories (250 mm). For later use, the produced samples were kept in airtight containers. In order to preserve the manufactured adsorbent materials for future usage, they were dried for 48 h at 60 °C in hot air oven. In contrast to standard laboratory drying processes at 105 °C, a cooler, longer drying cycle was adopted because higher temperature tends to melt or burn the goods [42].
2.4. Preparation of chemical treated wheat straw
It was sieved after washed with distilled water and dried for 12 h at 110 °C. After being pulverized and dried, the wheat straws were immersed for 24 h at room temperature in a solution of 5 N NaOH and 5 N HCL, allowing them to thoroughly adsorb all of the chemicals [43]. The acidic and alkali solution was filtered out and washed with distilled water in order to get rid of any potential residue from the 5 N NaOH and 5 N HCL solution [44]. Near neutral pH was achieved by pH adjustment. The cleaned wheat straw underwent a second 24 hr oven drying [45]. The preparation was finished by placing the dried wheat straw in a zip-top bag with a label that was easy to read and a desiccator so it could be used later.
2.5. Preparation of microbial treated wheat straw
Wheat straw was collected at Surat, India (21.1702° N, 72.8311° E). The isolate was subsequently grown in Luria broth for 24 h while the conditions were held at 37 °C and 150 rpm. The isolates' initial cellular density, which was 106 cells/mL, was obtained by centrifugation. The cells were then suspended in medium that had been autoclaved at 115 °C for 10 min with 20 g of sieved powder and 80 mL of BHM broth. The cells were then rinsed twice with sterile distilled water. The Congo red test was one of several procedures used to verify the enzyme's activity. For this test, each isolate was separately streaked on CMC agar plates and incubated at 37 °C for 72 h [46]. Filter paper degradation was proposed by the formation of a clear zone of hydrolysis surrounding bacterial colonies. The amount of enzyme needed to release 1 g of glucose per millilitre per minute under normal test conditions is given as one unit of FPCase activity. The acquired data are contrasted with the typical glucose curve [46,47].
2.6. Culture conditions for dye removal through treated wheat straw and potential bacteria
The cultivations were done under semi-solid-state conditions, which are defined as the growth of microorganisms on solid surfaces with very little free water present [48]. The microorganisms receive support as well as carbon, nitrogen, minerals, and other nutrients from the humid insoluble substrates [49]. This study used a synthetic growth medium with a nitrogen restriction, and culture was done in 500 mL flasks with 60 g of powdered wheat straw and 200 mL of dye effluent [50]. In order to provide the perfect environment for the decolorization, the tests were carried out under a variety of initial conditions, including pH and temperature. The pH of the growth media was altered using HCl or NaOH. To test this semi-solid fermentation system's capacity for decolorization under optimum circumstances, five experiment groups were started with MB concentrations ranging from 0.2 to 0.6 g/L. The 5 mL microbial suspension was introduced into each flask at room temperature after they had been stoppered and autoclaved twice for 20 min each at 121 °C in order to assess dynamic changes in enzyme activity. Trials of 35-day fermentation were conducted. During the fermentation process, the humidity was maintained at 75%, which is optimum for the production of lignocellulolytic enzymes and beneficial for the growth of microbial life [51]. After inoculation, fermented straw was gathered in the flask from various sites and well mixed every other day for routine testing. Every experiment was performed three times.
2.7. Characterization of adsorbent
An analysis of morphology Micrographs of untreated and enzyme-treated wheat straw were acquired using a Hitachi S-3400 N Scanning Electron Microscope (SEM) outfitted with a Princeton Gamma Tech (PGT) IMIX digital imaging system and a Prism Intrinsic germanium (IG) detector (SVNIT). To increase conductivity, a gold putter coater was applied to all samples. A resolution of 4 nm was used for all samples. Monitoring was carried out on 0.1–0.005 g of fiber that was fixed on conductive adhesive tape and coated with gold-palladium in at a voltage of 15–20 kV [52].
The Fourier transform infrared spectroscopy (FTIR) method was used to find functional groups and their interactions in both treated and untreated wheat straw. As samples, little fibre sections with moulded biocomposite material were employed (approximate thickness of 0.5 mm). During the research, a SHIMADZU IRAffinity-1S spectrometer was utilized (SVNIT). The range in which the data were collected was 4000–500 cm−1 [53].
2.8. Preparation of dye solution
Merck, a dependable vendor, was the supplier of Methylene Blue. 20 mg of MB were dissolved in 1000 cc of distilled water to create the stock solution. By serially diluting MB solutions from stock solutions, different amounts of dye were added in order to generate a calibration (standard) curve.
2.9. Analysis of MB content
To determine the degree of decolonization, 1 g of fermented straw and 20 mL of ethanol were mixed for 2 h at room temperature. This combination might be used to remove even the pigment that had been absorbed in the fermenting wastes. To quantify the concentration, optical densities (absorbance) of MB solution were measured using a UV/VIS spectrophotometer (Shimadzu 2550) at 660 nm wavelength [54]. The straight-line calibration curve that was first created by graphing absorbance vs concentration of MB in solution was used to evaluate the concentration of methylene [55].
2.10. Plackett-Burman design for the screening of important factors for dye removal
Plackett-Burman design was utilized to screen out medium components and environmental variables impacting isolated bacteria's ability to produce the most dye removal [56]. After the selection of significant process factors, such as medium components and ambient conditions, based on the literature study, submerged fermentation was carried out in line with the planned experiments, and dye removal activity was determined and reported as IU/mL. Important components were identified using a 20-run study design, 14 chosen variables, and 5 dummy variables with two levels for each variable. programThe variables for RSM (CCD) level optimization were further chosen using the Design-Expert programme since they were thought to be crucial components for maximizing dye removal production [57].
Using the central composite design of the response surface approach, some factors are optimized to maximize dye removal (CCD-RSM).
For the maximum dye removal production, shake flask experiments were used to study four significant factors for medium optimization, and RSM's CCD was used for analysis. The following second-order polynomial equation explained the model's behavior:
where Y is the predicted response (dye removal activity), b0 is the model constant, βi, βii, and βij are the linear coefficient, quadratic coefficient, and interaction coefficient, respectively. The independent coded variables of the system are represented by Xi and Xz [58]. The regression analysis was carried out utilising Design-Expert software. The highest accuracy model with a non-significant Lack of Fit test and a p-value (Prob > F) less than 0.05 level of significance was selected. The recommended model terms and statistical significance of the model equation were assessed using fit statistics values such as F value, coefficient of determination (R2), appropriate precision value (signal to noise ratio), and the variability between adjusted and anticipated R2 [59]. To judge the adequacy of the suggested model, several diagnostics and impact plot data for the residual research were also evaluated. The link between the investigative and predicted values was investigated using three-dimensional surface plots and counterplots. The amount of each variable was optimized using Design-Expert software to get the greatest dye removal output. Lastly, validation tests were run to validate the model's optimum outcome [60].
3. Results and discussion
3.1. Isolation and screening of potential bacteria
Five possible isolates were found on the nutrient agar plate out of the 15 bacterial isolates, and then the wheat straw enrichment technique was used to confirm the isolates. All of the isolates had cellulase activity on carboxymethylcellulose agar (Fig. 1A), but only two isolates displayed laccase activity (Fig. 1B). PPSUHB 3 had the maximum zone of clearance on all two screening plates as compared to other isolates, demonstrating the greatest lignocellulolytic enzyme activity. On N-agar, isolate PPSUHB3 displayed gram-positive colonies that were medium, round, smooth, convex, opaque, and viscous. It thrived effectively at pH levels ranging from 5 to 8, with pH 7 providing the best growth, Biochemical and it can withstand NaCl concentrations of up to 10% (Table 1). The results were comparable similar to maximum probability to isolate Gram positive bacteria for the laccase activity [61]. Earlier also, CMC agar plate has been reported as the better screening methods to isolate cellase enzyme producing microbes [62]. Furthermore, the dye degradation was considered to be effective techniques to study the laccase activity of bacterial isolated. Although bacteria are less efficient than fungi in degrading lignin, investigating bacteria species for lignin depolymerization potentials is thought to be important for the development of the extracellular enzyme process [63].
Fig. 1.
Screening of lignocellulolytic activity of Bacillus licheniformis PPSUHB3 (A) Cellulase activity, (B) Laccase activity.
Table 1.
Biochemical tests of isolated strains.
| Biochemical tests | Results |
|---|---|
| Gram staining | + Rod |
| Methyl Red test | + |
| Indole production | − |
| Voges-Proskaeur test | + |
| Catalase production | + |
| Citrate production | + |
| Oxidase production | + |
| Urease test | + |
| Nitrate reduction test | + |
3.2. Molecular characterization
The origin of an isolated bacteria was determined using the 16S rRNA sequencing method. Using the 27F and 1492R primers at a 50 °C annealing temperature, a 1.5 kb DNA band size was amplified. Using NCBI-BLAST, the data produced by the sequencing was evaluated. Bacillus licheniformis was discovered to be an isolated lignocellulolytic degrading bacterial species by comparing the sequencing outcomes with the nucleotide sequences of numerous bacterial species using NCBI blast. An 866-bp final sequence from the Bacillus licheniformis isolate PPSUHB3 was produced. The DNA sequence of the Bacillus licheniformis (PPSUHB3) is 99% similar, according to the BLASTX technique [64]. Just a few studies, however, have attempted to produce laccase using Bacillus sp. [65]. Agro-residues were reportedly employed to generate laccase from Bacillus subtilis, according to Ref. [66]. Nevertheless, nothing has been said about the prospective laccase-producing Bacillus sp. that was isolated from dye effluent streams and exploited wheat straw as a potential substrate. The present investigation also shown that Bacillus sp. is more effective at producing cellulase and laccase enzyme by feeding on agricultural waste with degradation of textile dye [61].
3.3. Selection of material
Wheat straw was used in the studies to remove the dye from the aqueous solution of MB because it was close by. Latitude: 21.1702°N and Longitude: 72.8311°E are the coordinates for its location. Each material's solid sample (100 g) was ground into a fine powder. BSI standard sieve sizes 180 and 250 mm were used for the sieving. In order to preserve the manufactured adsorbent materials for future usage, they were oven dried at 60 C for at least 48 h.
3.4. Optimization for production of lignocellulolytic enzyme for wheat straw degradation
3.4.1. pH and temperature optimization
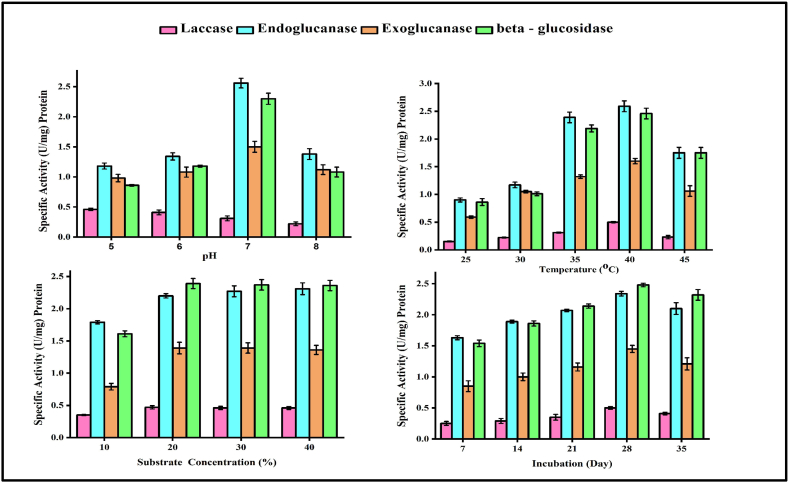
The greatest lignocellulolytic activity generation was found at 35 °C and a constant pH of 7, indicating that a pH of 7 was excellent for enzyme synthesis. Bacillus licheniformis generated the highest levels of 0.31 U/mg laccase, 2.56 U/mg endoglucanase, 1.5 U/mg exoglucanase, and 2.3 U/mg -glucosidase at pH 7. Nevertheless, pH levels above or below 7 significantly reduced the amount of enzyme generated (Fig. 2A and B). That might happen because bacteria's metabolic pathways are overly sensitive to pH, particularly enzymes whose structure and function are substantially affected by shifting ionic pH. Initially, pH 7 was set as the benchmark level for temperature optimization. Bacillus licheniformis produced the most enzymes at 40 °C, including 0.5 U/mg laccase, 2.59 U/mg endoglucanase, 1.6 U/mg exoglucanase, and 2.46 U/mg β-glucosidase. Over 45 °C, however, output drastically reduced. Temperature changes have a substantial impact on enzyme production (Fig. 2A and B). Since atoms move more slowly at lower temperatures, all metabolic activity halts. When the temperature rises, atoms move quicker and enzymes work faster [67]. But, after a certain point, enzymes begin to denature, which can be dangerous. Also, when compared to Ref. [68], who observed that lignocellulolytic enzyme production by Klebsiella sp. was maximum in sorghum at pH 8 and temperature 50 °C [69]. observed that Armillaria tabescens generated the most laccase enzyme at 45 °C.
Fig. 2.
Analytical methods of enzymes (A) pH, (B) Temperature, (C) Substrate concentration, (D) Incubation period.
3.4.2. Substrate concentration & incubation period optimization
According to enzyme kinetics, increasing the concentration of substrate reduces enzyme production to some extent, however this diminishes at high concentrations [70]. At a substrate concentration of 20%, Bacillus licheniformis as a maximum output of 0.47 U/mg laccase, 2.2 U/mg endoglucanase, 1.39 U/mg exoglucanase, and 2.37 U/mg β-glucosidase. Cellulomonas and Bacillus bacteria strains generated small levels of endoglucanase enzyme at 0.5% of the substrates, progressively rising to 2% of the substrates [71,72].
The whole lignocellulolytic production began 7 days after inoculation and increased to 35 days. Bacillus licheniformis isolate generated the most 0.5 U/mg laccase, 2.34 U/mg endoglucanase, 1.45 U/mg exoglucanase, and 2.48 U/mg β-glucosidase at 28 days (Fig. 2C and D). Bacterial growth will be halted during the final phase of the growth curve due to limited nutritional availability, which hampers the metabolic enzymes and normal functioning of bacterial physiology [73]. It was discovered that cellulase enzyme production was highest during the incubation period of 28 days, after which B. licheniformis ceased production. The peak laccase activity in Ganoderma sp. was seen on the tenth day of incubation. After 12 days in a stationary environment, Pleurotus florida was said to have generated a significant amount of laccase (4.60 IU/ml) in malt extract broth [74]. The optimization of lignocellulolytic enzyme production may lead to efficient degradation of wheat straw. This may lead to generate a more porous WS material. This porous WS material is act as best absorbent material for dye removal from textile effluent which was supported by previous studies.
3.5. Characterization of adsorbent
3.5.1. Scanning electron microscope (SEM)
SEM was used to examine the morphological traits and surface traits of wheat straw that had been treated with Bacillus licheniformis, 5 N NaOH, and 5 N HCL, as well as straw that had not been treated (Control) (Fig. 3). The potential variations between the morphological features before and after acid/alkali and microbial treatment were examined using these Fig. 3A, its show the untreated (Control) wheat straw's asymmetrical, flexible, and smooth surface. The material appears to be less porous because there aren't many pores and porosities despite the presence of both.
Fig. 3.
(A) Control (B) 5 N NaOH (C) 5 N HCL and (D) Bacillus licheniformis PPSUHB3.
As shown in Fig. 3B and C, wheat straw treated with 5 N NaOH and 5 N HCL had enhanced physical features. The surface of the bacterial treated wheat straw is rougher than other chemically treated wheat straw (Fig. 3D). It is easy to observe the pores and cavities, and the depth and more open structure of the holes hints at a more porous structure in the treated wheat straw. Such characteristics are essential for the adsorption mechanism because they facilitate better dye molecule dispersion and improve access to active areas [75]. Alkali/acid and microbe treatment led to pore cracking and increased surface roughness. Additionally, it results in open compressed structures that demonstrate the removal of some of the outer non-cellulose layer of the adsorbent, which is made up of hemicellulose, pectin, lignin, wax, and other residues. These well-known materials that act as a barrier on the surface of natural fibres include wax and pectin [76].
3.5.2. Fourier transform infrared spectroscopy (FTIR)
The analysis of FTIR spectra was primarily utilized to emphasize the existence of functional groups that might bind dye (carbonyl, hydroxyl, and carboxyl). Fig. 4 compares the FTIR spectra of wheat straw that had been untreated, treated, and mostly linked to microbial treatment. It is clear from Fig. 4 that some of the peaks have shifted as a result of the wheat straw's 5 N NaOH, 5 N HCL and Microbial treated modification. Before treatment, the peak at 1061 cm−1, 1172 cm−1, 1648 cm−1, 1663 cm−1, 1718 cm−1, 2254 cm−1, 2899 cm−1 and 3436 cm−1 indicated the existence of (pH bend) Phosphines, (C–N) Amine, (C=C) Alkane, (C–H) Alkene, (C=O) Carboxylic acid, (C=C) Alkyne, (C–H) Alkane and (O–H) Alcohol functional group. In addition, Table 2 showed that, some new bands emerged, some remained, and others were displaced. The possibility that (N=O) Nitro, (CN) Nitrile, and (N–H) Amine group may have contributed to the adsorption of MB onto wheat straw is justified by this [54].
Fig. 4.
Fourier transform infrared spectroscopy (FTIR).
Table 2.
FTIR characteristics of a wheat straw.
| IR Peak | Before adsorption (Control) | Treatment with HCL | Treatment with NaOH | Treatment with Bacteria (PPSUHB3) | Assignment |
|---|---|---|---|---|---|
| 1 | 1061 | 1061 | 1061 | – | (Ph bend) Phosphines |
| 2 | – | – | – | 1172 | (C–N) Amine |
| 3 | – | – | 1590 | – | (N=O) Nitro |
| 4 | 1648 | 1655 | – | – | (C=C) Alkane |
| 5 | – | – | – | 1654 | (C=O) Carboxylic acid |
| 6 | – | – | – | – | (C–H) Alkene |
| 7 | – | – | – | 1727 | (C=O) Carboxylic acid |
| 8 | – | – | – | 2127 | (C=C) Alkyne |
| 9 | – | – | – | 2252 | (C=C) Alkyne |
| 10 | – | – | 2360 | – | (C≡N) Nitrile |
| 11 | – | – | – | 2554 | (O–H) Alcohol |
| 12 | 2899 | 2899 | 2917 | 2899 | (C–H) Alkane |
| 13 | – | 3334 | – | – | (N–H) Amine |
| 14 | 3436 | – | 3454 | (O–H) Alcohol |
Increased inner surface area, disruption of the lignin structure, increased inner surface area, a decrease in polymerization, and crystallinity can all result from the change of 5 N NaOH, 5 N HCL, and Microbial treatment. The MB dye molecules may have had ion-exchange processes that increased their capacity for adsorption following exposure to acidic or alkaline or bacterial solutions. The analysis of the FTIR spectra showed that the surface of the wheat straw contains a significant amount of hydroxyl and carboxyl groups. This showed how these functional groups could potentially operate as active sites for interacting with dye molecules [26].
3.6. Screening of significant variables for dye removal using Plackett-Burman design
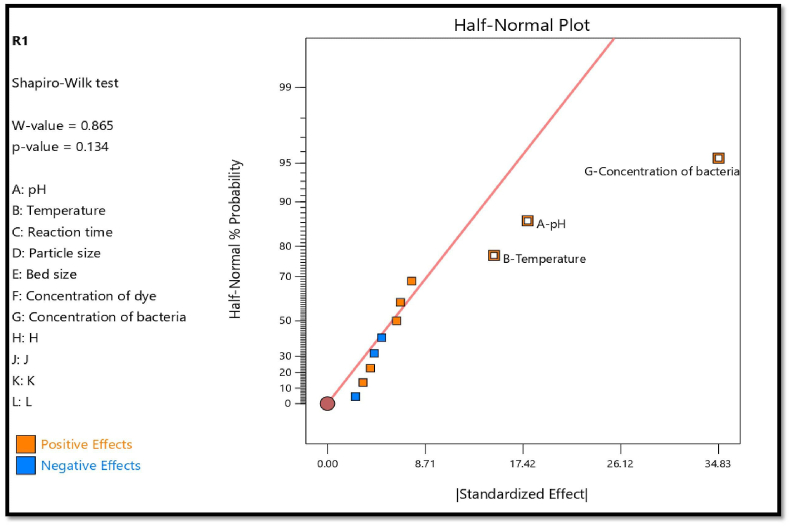
The 20 experimental runs with 5 dummy variables were used to investigate 14 process factors using the Plackett-Burman design in order to choose the significant variables for the dye removal [77]. Experimental design with the recorded response (dye removal activity) is shown in (Table 3). The process variables that have the greatest beneficial impacts include pH, temperature, reaction time, portion size, bed size, dye concentration, and bacteria concentration, according to the statistical analysis of the dye removal data (Table 4). The Pareto chart (Fig. 5) depicts the order of relevance of these variables, with factors coloured orange (dark colour bar) showing a positive effect and factors coloured blue (light colour bar) showing a negative effect on the dye removal. The half-normal plot of effects shows that among various bacteria concentrations, pH and temperature have a much greater impact on dye removal than other parameters (Fig. 6). Nevertheless, all the other parameters have normal distributions along the straight line and are outliers that lie below or to the right of the straight line, therefore they have less of an effect on dye removal production. The Model F-value of 22.43 in the ANOVA table demonstrates the model's significance. Just 0.03% of the time may noise be the cause of an F-value this high. When the P-value is less 0.0500, model terms are deemed significant. In this case, key model terms include A, B, and G. If the value is more than 0.1000, the model terms are not significant (Table 5) [78].
Table 3.
Factorial data along with significant variables for the dye removal by Plackett Burman Design.
| Factor | Name | Units | Type | Subtype | Minimum | Maximum | Coded Low | Coded High | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|---|---|
| A | pH | Numeric | Continuous | 5.00 | 8.00 | −1 ↔ 5.00 | +1 ↔ 8.00 | 6.50 | 1.57 | |
| B | Temperature | °C | Numeric | Continuous | 25.00 | 55.00 | −1 ↔ 25.00 | +1 ↔ 55.00 | 40.00 | 15.67 |
| C | Reaction time | Min | Numeric | Continuous | 5.00 | 60.00 | −1 ↔ 5.00 | +1 ↔ 60.00 | 32.50 | 28.72 |
| D | Particle size | Micron | Numeric | Continuous | 150.00 | 250.00 | −1 ↔ 150.00 | +1 ↔ 250.00 | 200.00 | 52.22 |
| E | Bed size | gm | Numeric | Continuous | 1.0000 | 5.00 | −1 ↔ 1.00 | +1 ↔ 5.00 | 3.00 | 2.09 |
| F | Concentration of dye | % | Numeric | Continuous | 1.0000 | 10.00 | −1 ↔ 1.00 | +1 ↔ 10.00 | 5.50 | 4.70 |
| G | Concentration of bacteria | % | Numeric | Continuous | 1.0000 | 5.00 | −1 ↔ 1.00 | +1 ↔ 5.00 | 3.00 | 2.09 |
| H | H | Numeric | Continuous | −1.0000 | 1.0000 | −1 ↔ −1.00 | +1 ↔ 1.00 | 0.0000 | 1.04 | |
| J | J | Numeric | Continuous | −1.0000 | 1.0000 | −1 ↔ −1.00 | +1 ↔ 1.00 | 0.0000 | 1.04 | |
| K | K | Numeric | Continuous | −1.0000 | 1.0000 | −1 ↔ −1.00 | +1 ↔ 1.00 | 0.0000 | 1.04 | |
| L | L | Numeric | Continuous | −1.0000 | 1.0000 | −1 ↔ −1.00 | +1 ↔ 1.00 | 0.0000 | 1.04 |
Table 4.
Response surface methodology–box–Behnken design.
| Factor | Name | Units | Type | Subtype | Minimum | Maximum | Coded Low | Coded High | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|---|---|
| A | pH | Numeric | Continuous | 5.00 | 8.00 | −1 ↔ 5.61 | +1 ↔ 7.39 | 6.50 | 0.7562 | |
| B | Temp | C | Numeric | Continuous | 25.00 | 55.00 | −1 ↔ 31.08 | +1 ↔ 48.92 | 40.00 | 7.56 |
| G | Conc of Bact | % | Numeric | Continuous | 1.0000 | 5.00 | −1 ↔ 1.81 | +1 ↔ 4.19 | 3.00 | 1.01 |
Fig. 5.
Pareto chart for variable parameter having greatest effect on experiment.
Fig. 6.
Half-normal plot for variable parameter having greatest effect on experiment.
Table 5.
ANOVA for selected factorial model.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 5254.25 | 3 | 1751.42 | 22.43 | 0.0003 | significant |
| A-pH | 954.08 | 1 | 954.08 | 12.22 | 0.0081 | |
| B-Temperature | 660.08 | 1 | 660.08 | 8.45 | 0.0197 | |
| G-Concentration of bacteria | 3640.08 | 1 | 3640.08 | 46.62 | 0.0001 | |
| Residual | 624.67 | 8 | 78.08 | |||
| Cor Total | 5878.92 | 11 |
3.7. Using the central composite design of the response surface approach, some factors are optimized to maximize dye removal (CCD-RSM)
Design-Expert was used to analyze the combined influence of the process factors and find the best concentration of each variable using a central composite design of response surface approach. From the Plackett-Burman design 13.0.1.0, process variables that had a substantial influence on dye removal were selected [79]. Each numeric factor in this CCD experiment was adjusted to 5 levels (rotatable with 6 repetitions at the center point), and 30 iterations of the experimental design were done with both experimental and projected responses. The Design-Expert programme has recommended a quadratic model based on the model statistics' F-value (57.83). where the likelihood of a greater F-value owing to noise is 0.01%. The determination coefficient, which measures how well the model fits the data (R2 = 0.9812), indicates a stronger connection between the observed and expected responses. Similar to this, as the difference between the adjusted R2 of 0.9642 and the projected R2 of 0.9136 is less than 0.2, they are reasonably in agreement. Second, a model that has been suggested with a signal to noise ratio of 27.252 is deemed to be sufficiently accurate for the investigation. The F-value for the lack of fit of 0.9895 indicates that the lack of fit is not significant in comparison to the pure error (non-significant Lack of Fit is good for the model to fit). A significant Lack of Fit F-value has a 50.45% possibility of being caused by noise (Table 6) [80]. All of these fit statistical data attest to the quadratic model's suitability for the increased dye removal. Also, several Design-Expert software diagnostic and impact tools were used to test the suitability of the proposed model [81]. The normal probability plot displayed a straight line, indicating that the residuals were distributed normally. For the purpose of determining if variance remained constant, the residuals vs expected response values plot was examined. This plot displayed a random scatter, therefore expanding variance from lower to higher projected scores was not seen. The residual versus run plot did not reveal any trends. A box-cox plot was investigated in order to apply the proper power transformation. The graphic displays a 95% confidence interval lambda value range of 0.1800–1.74, with a best lambda value of 0.7600. No power transformation was proposed since the suggested model contains one lambda value that is both close to the optimal lambda value and falls within the provided range. All these diagnostics data support the adequacy of the suggested model [80]. This model was used to produce predictions about the response for certain values of each element using the second-order polynomial equation in terms of coded factors. Comparing the factor coefficients is helpful for determining the factors' relative influence:
Table 6.
ANOVA quadratic model.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 1950.28 | 9 | 216.70 | 57.83 | <0.0001 | significant |
| A-pH | 33.70 | 1 | 33.70 | 9.00 | 0.0134 | |
| B-Temp | 776.82 | 1 | 776.82 | 207.32 | <0.0001 | |
| C-Conc of Bact | 11.86 | 1 | 11.86 | 3.17 | 0.1056 | |
| AB | 112.50 | 1 | 112.50 | 30.02 | 0.0003 | |
| AC | 40.50 | 1 | 40.50 | 10.81 | 0.0082 | |
| BC | 8.00 | 1 | 8.00 | 2.14 | 0.1747 | |
| A2 | 42.87 | 1 | 42.87 | 11.44 | 0.0070 | |
| B2 | 785.23 | 1 | 785.23 | 209.57 | <0.0001 | |
| C2 | 254.16 | 1 | 254.16 | 67.83 | <0.0001 | |
| Residual | 37.47 | 10 | 3.75 | |||
| Lack of Fit | 18.64 | 5 | 3.73 | 0.9895 | 0.5045 | not significant |
| Pure Error | 18.83 | 5 | 3.77 | |||
| Cor Total | 1987.75 | 19 |
Dye removal activity = +1950.28 + 33.70 A − 776.82 B + 11.86 C − 112.50 AB − 40.50 AC + 8.00 BC − 42.87 A2 − 785.23 B2 − 254.16 C2
A two-dimensional contour plot and three-dimensional surface plot were created by plotting the representation of response against the combination of any two numeric factors while keeping other parameters constant at their mid-value in order to study the interaction effect of the chosen process variables (Fig. 7). Fig. 7A demonstrates that a dye removal was maximum at pH 6 & 40 °C temperature considering two factor only. Similar to this, Fig. 7B shows that the highest dye removal occurs at a pH midpoint and a 3% of bacterial concentration. It is evident that when the bacterial concentration are increased, the dye clearance rate increases. Fig. 7C indicated that temperature was very crucial factor when it is related with concentration of bacteria for dye removal. Furthermore, the Design-Expert software's optimum solution is displayed in the desirability plot (Fig. 8). The medium must be modified by adding pH 6.36823, temperature 44.6645, bacteria concentration 3.04204, and dye removal 55.89 for maximal dye removal production. Using Bacillus licheniformis and the modified selective medium with the recommended solution, laboratory flask tests were conducted in triplicates to validate the suggested optimized solution [80]. Data from the anticipated response trials were compared with the findings from the validation studies. The experimental response data mean (307 IU/mL) fell within the dye removal's 95% prediction interval (289–335 IU/mL). The fundamental composite design tool of response surface approach was used to obtain a more than twice (2.22) increase in the dye removal production when compared to the baseline medium [82].
Fig. 7.
(A): 3D and 2D interaction plot of temperature & pH for laccase activity, (B): 3D and 2D interaction plot of pH & concentration of bacteria for laccase activity, (C): 3D and 2D interaction plot of temperature & concentration of bacteria for laccase activity.
Fig. 8.
Desirability plot shows the optimum solution suggested by the software.
4. Conclusion
The outcome of bacteria decolorizing hues using wheat straw is the most rapid rate of disintegration of methylene blue (MB). These findings demonstrated that bacterial treated wheat straw had higher concentrations of dye removal capacity than bacteria operating alone. When compared to other isolates, PPSUHB3 showed the most noticeable decline. On the basis of 16s rDNA sequencing, it was projected that the isolate PPSUHB3 belonged to the genus Bacillus licheniformis, which has a strong ability to break down wheat straw and colour by creating effective enzyme. After optimization using WS absorbent, the isolate had the maximum decolorization percentage of MB dye, which was confirmed by FTIR and SEM. The porosity structure of the treated wheat straw was identified using SEM analysis. The FTIR spectra, however, demonstrated that the activation procedure was successful and that the wheat straw had transitioned into an amorphous state. Plackett-Burman design revealed that pH, temperature and the concentration of bacteria all significantly affect dye removal. The central composite design (CCD) of response surface technique was used to find the appropriate concentrations of these selected bioprocess nutritional components (RSM). According to the ANOVA results, the optimal conditions led to a dye removal of up to 55.89% at pH 6.36, Temperature 44.6 °C, and Bacteria Concentration 3.04%. The adsorbent dosage had the most influence out of all the independent factors examined. The verification experiment performed under ideal conditions showed that the experimental and projected MB removal roughly matched. So, as an inexpensive adsorbent for the removal of contaminants from an aqueous solution, wheat straw generated from agricultural waste is an excellent option.
Author contribution statement
Binal Y. Patel; Hiren K Patel: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.M-Ridha M.J., et al. Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alex. Eng. J. 2020;59(5):3551–3563. [Google Scholar]
- 2.El-Mekkawi S.A., et al. Application of response surface methodology for color removing from dyeing effluent using de-oiled activated algal biomass. Bull. Natl. Res. Cent. 2021;45(1):1–10. [Google Scholar]
- 3.Gokulan R., Balaji S., A i P.J., Sivaprakasam M.S. Optimization of remazol black B removal using biochar produced from Caulerpa scalpelliformis using response surface methodology. Adv. Mater. Sci. Eng. 2021:1–8. [Google Scholar]
- 4.Darwesh O., et al. Bioremediation of textile reactive blue (RB) azo dye residues in wastewater using experimental prototype bioreactor. Res. J. Pharmaceut. Biol. Chem. Sci. 2014;5(4):1203–1219. [Google Scholar]
- 5.Ikram M., Zahoor M., Batiha G.E.-S. Biodegradation and decolorization of textile dyes by bacterial strains: a biological approach for wastewater treatment. Z. Phys. Chem. 2021;235(10):1381–1393. [Google Scholar]
- 6.Elgarahy A., et al. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Cleaner Eng. Technol. 2021;4 [Google Scholar]
- 7.Januário E.F.D., et al. Performance of a hybrid coagulation/flocculation process followed by modified microfiltration membranes for the removal of solophenyl blue dye. Chem. Eng. Process.-Process Intensific. 2021;168 [Google Scholar]
- 8.Jawad S.F., et al. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2021. Dye removal from textile wastewater using solar-powered electrocoagulation reactor. [Google Scholar]
- 9.Xiao W., et al. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021;284 [Google Scholar]
- 10.Augustowski D., et al. Efficiency boost in dye-sensitized solar cells by post-annealing uv-ozone treatment of tio2 mesoporous layer. Materials. 2021;14(16):4698. doi: 10.3390/ma14164698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradihamedani Recent advances in dye removal from wastewater by membrane technology: a review. Polym. Bull. 2022;79(4):2603–2631. [Google Scholar]
- 12.Ledakowicz S., Paździor K. Recent achievements in dyes removal focused on advanced oxidation processes integrated with biological methods. Molecules. 2021;26(4):870. doi: 10.3390/molecules26040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magdalane C.M., et al. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: investigation of photocatalytic performance of Congo red degradation dye. Surface. Interfac. 2021;25 [Google Scholar]
- 14.Dheyab M.A., et al. Sonochemical-assisted synthesis of highly stable gold nanoparticles catalyst for decoloration of methylene blue dye. Inorg. Chem. Commun. 2021;127 [Google Scholar]
- 15.Darwesh O.M., et al. Bioremediation of textile reactive blue azo dye residues using nanobiotechnology approaches. Res. J. Pharmaceut. Biol. Chem. Sci. 2015;6(1):1202–1211. [Google Scholar]
- 16.Domenzain-Gonzalez J., et al. Photocatalytic membrane reactor based on Mexican natural zeolite: RB5 dye removal by photo-Fenton process. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 17.Zhang Z.-Q. Magnolia Press; 2011. Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness. [DOI] [PubMed] [Google Scholar]
- 18.Tan I., Ahmad A.L., Hameed B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: batch and fixed bed studies. Desalination. 2008;225(1–3):13–28. [Google Scholar]
- 19.Vadivelan V., Kumar K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005;286(1):90–100. doi: 10.1016/j.jcis.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Singh S., Pakshirajan K. Enzyme activities and decolourization of single and mixed azo dyes by the white-rot fungus Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2010;64(2):146–150. [Google Scholar]
- 21.Syed M., et al. A simple method to screen for azo-dye-degrading bacteria. Environ. Biol. 2009;30(1):89–92. [PubMed] [Google Scholar]
- 22.Han Q., et al. Biochar derived from agricultural wastes as a means of facilitating the degradation of azo dyes by sulfides. Catalysts. 2021;11(4):434. [Google Scholar]
- 23.Selvaraj V., et al. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021;1224 [Google Scholar]
- 24.Kerkez D.V., et al. Three different clay-supported nanoscale zero-valent iron materials for industrial azo dye degradation: a comparative study. J. Taiwan Inst. Chem. Eng. 2014;45(5):2451–2461. [Google Scholar]
- 25.Wu J., et al. Brewer's grains with different pretreatments used as bio-adsorbents for the removal of Congo red dye from aqueous solution. Bioresources. 2020;15(3):6928–6940. [Google Scholar]
- 26.Ali A., et al. Preparation of high-performance adsorbent from low-cost agricultural waste (Peanut husk) using full factorial design: application to dye removal. Biointerface Res. Appl. Chem. 2020;10:6619–6628. [Google Scholar]
- 27.Xiao X., et al. Biodecolorization of Naphthol Green B dye by Shewanella oneidensis MR-1 under anaerobic conditions. Bioresour. Technol. 2012;110:86–90. doi: 10.1016/j.biortech.2012.01.099. [DOI] [PubMed] [Google Scholar]
- 28.Ashori A., Nourbakhsh A. Bio-based composites from waste agricultural residues. Waste Manag. 2010;30(4):680–684. doi: 10.1016/j.wasman.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Bansal N., et al. Production of cellulases from Aspergillus Niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manag. 2012;32(7):1341–1346. doi: 10.1016/j.wasman.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Biswas A., et al. Conversion of agricultural residues to carboxymethylcellulose and carboxymethylcellulose acetate. Industrial Crops. 2014;60:259–265. [Google Scholar]
- 31.Chen Y., et al. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour. Technol. 2017;243:347–355. doi: 10.1016/j.biortech.2017.06.100. [DOI] [PubMed] [Google Scholar]
- 32.Taccari M., et al. Effect of Phanerochaete chrysosporium inoculation during maturation of co-composted agricultural wastes mixed with olive mill wastewater. Waste Manag. 2009;29(5):1615–1621. doi: 10.1016/j.wasman.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Zeng G., et al. Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour. Technol. 2010;101(1):222–227. doi: 10.1016/j.biortech.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Myers R.H., et al. Response surface methodology: a retrospective and literature survey. J. Qual. Technol. 2004;36(1):53–77. [Google Scholar]
- 35.Bari M.N., et al. Improvement of production of citric acid from oil palm empty fruit bunches: optimization of media by statistical experimental designs. Bioresour. Technol. 2009;100(12):3113–3120. doi: 10.1016/j.biortech.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Mohan S., et al. Synthesis of CuO nanoparticles through green route using Citrus limon juice and its application as nanosorbent for Cr (VI) remediation: process optimization with RSM and ANN-GA based model. Process Saf. Environ. Protect. 2015;96:156–166. [Google Scholar]
- 37.Ahmad A., et al. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015;5(39):30801–30818. [Google Scholar]
- 38.Cuprys A., et al. Potential of agro-industrial produced laccase to remove ciprofloxacin. Environ. Sci. Pollut. Res. 2021:1–10. doi: 10.1007/s11356-021-13578-2. [DOI] [PubMed] [Google Scholar]
- 39.Bandounas L., et al. Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 2011;11(1):94. doi: 10.1186/1472-6750-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrow G.J. 1993. Cowan and Steel's Manual for the Identification of Medical Bacteria. Cowan and Steel's Manual for the Identification of Medical Bacteria. [Google Scholar]
- 41.Kurniawan S.B., et al. Isolation and characterisation of bioflocculant-producing bacteria from aquaculture effluent and its performance in treating high turbid water. J. Water Process Eng. 2021;42 [Google Scholar]
- 42.Nadia A., et al. IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2020. The effectiveness of activated carbon as adsorbent in the oil purification process fish by-product of the fish canning industry. [Google Scholar]
- 43.Wayland H.A., et al. Advanced cellulosic materials for treatment and detection of industrial contaminants in wastewater. Chem. Select. 2016;1(15):4472–4488. [Google Scholar]
- 44.Mohammad Y., et al. Adsorption of phenol from refinery wastewater using rice husk activated carbon. Iranian J. Energy Environ. 2014;5(4) [Google Scholar]
- 45.Sharma S., Saxena R., Gaur G. Study of removal techniques for azo dyes by biosorption: a review. IOSR J. Appl. Chem. 2014;7(6) [Google Scholar]
- 46.Rawway M., Ali S.G., Badawy A.S. Isolation and identification of cellulose degrading bacteria from different sources at Assiut Governorate (Upper Egypt) Ecol. Health Environ. 2018;6:15–24. [Google Scholar]
- 47.Dobrzyński J., Wróbel B., Górska E.B. Cellulolytic properties of a potentially lignocellulose-degrading Bacillus sp. 8E1A strain isolated from bulk soil. Agronomy. 2022;12(3):665. [Google Scholar]
- 48.Rivela I., Rodríguez Couto S., Sanromán A. Extracellular ligninolytic enzyme production by Phanerochaete chrysosporium in a new solid-state bioreactor. Biotechnol. Lett. 2000;22:1443–1447. [Google Scholar]
- 49.Nigam P., et al. Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour. Technol. 2000;72(3):219–226. [Google Scholar]
- 50.Eslami H., et al. Biodegradation of methylene blue from aqueous solution by bacteria isolated from contaminated soil. J. Adv. Environ. Health Res. 2017;5(1):10–15. [Google Scholar]
- 51.Mathews S.L., Pawlak J., Grunden A.M. Bacterial biodegradation and bioconversion of industrial lignocellulosic streams. Appl. Microbiol. Biotechnol. 2015;99:2939–2954. doi: 10.1007/s00253-015-6471-y. [DOI] [PubMed] [Google Scholar]
- 52.George R., et al. In vivo analysis of dermal and systemic absorption of silver nanoparticles through healthy human skin. Australas. J. Dermatol. 2014;55(3):185–190. doi: 10.1111/ajd.12101. [DOI] [PubMed] [Google Scholar]
- 53.Ternero-Hidalgo J., et al. A simultaneous operando FTIR & Raman study of propane ODH mechanism over V-Zr-O catalysts. Catal. Today. 2022;387:197–206. [Google Scholar]
- 54.Alam M.Z., Bari M.N., Kawsari S. Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environ. Sustain. Indicators. 2022;14 [Google Scholar]
- 55.Ibupoto A.S., et al. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018;136:744–752. [Google Scholar]
- 56.Jampala P., et al. Investigation on the effect of carbon and nitrogen sources for the production of cellulosome by Trichoderma reesei NCIM 1186 using saturated placket Burman design. Biosci. Biotechnol. Res. Asia. 2015;12(2):1577–1586. [Google Scholar]
- 57.Venkataraghavan R., Thiruchelvi R., Sharmila D. Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using Plackett-Burman design and response surface methodology. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baskar G., Renganathan S. Optimization of L‐asparaginase production by Aspergillus terreus MTCC 1782 using response surface methodology and artificial neural network‐linked genetic algorithm. Asia Pac. J. Chem. Eng. 2012;7(2):212–220. [Google Scholar]
- 59.Montgomery D.C. John Wiley & Sons; 2017. Design and Analysis of Experiments by John Wiley & Sons. [Google Scholar]
- 60.Daâssi D., et al. Sawdust waste as a low-cost support-substrate for laccases production and adsorbent for azo dyes decolorization. J. Environ. Health Sci. Eng. 2016;14:1–12. doi: 10.1186/s40201-016-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chukwuma O.B., et al. Isolation and characterization of lignocellulolytic bacteria from municipal solid waste landfill for identification of potential hydrolytic enzyme. Fermentation. 2023;9(3):298. [Google Scholar]
- 62.da Silva R.N., de Andrade Melo L.F., Finkler C.L.L. Optimization of the cultivation conditions of Bacillus licheniformis BCLLNF-01 for cellulase production. Biotechnol. Rep. 2021;29 doi: 10.1016/j.btre.2021.e00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., et al. Isolation and screening of microorganisms for the effective pretreatment of lignocellulosic agricultural wastes. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/5514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saravanan A., et al. Identification and sequencing of bacteria from crop field: application of bacteria—agro-waste biosorbent for rapid pesticide removal. Environ. Technol. Innov. 2022;25 [Google Scholar]
- 65.Lu L., et al. Characterization and dye decolorization ability of an alkaline resistant and organic solvents tolerant laccase from Bacillus licheniformis LS04. Bioresour. Technol. 2012;115:35–40. doi: 10.1016/j.biortech.2011.07.111. [DOI] [PubMed] [Google Scholar]
- 66.Muthukumarasamy N.P., et al. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem. Res. Internat. 2015;2015 doi: 10.1155/2015/765190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao B., et al. Lignocellulolytic enzyme cocktail produced by plant endophytic Chaetomium globosum exhibits a capacity for high-efficient saccharification of raw rice straw. Ind. Crops Prod. 2023;196 [Google Scholar]
- 68.Waghmare P., et al. Production and characterization of cellulolytic enzymes by isolated Klebsiella sp. PRW-1 using agricultural waste biomass. Emir. J. Food Agric. 2014:44–59. [Google Scholar]
- 69.He J.Y., et al. Advanced Materials Research. Trans Tech Publ; 2014. Efficient production of laccase from armillariella tabescens by solid state fermentation. [Google Scholar]
- 70.Benatti A.L.T., Polizeli M.d.L.T.M. Lignocellulolytic biocatalysts: the main players involved in multiple biotechnological processes for biomass valorization. Microorganisms. 2023;11(1):162. doi: 10.3390/microorganisms11010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Immanuel G., et al. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Technol. 2006;3:25–34. [Google Scholar]
- 72.da Rosa-Garzon N.G., et al. Valorization of agricultural residues using Myceliophthora thermophila as a platform for production of lignocellulolytic enzymes for cellulose saccharification. Biomass Bioenergy. 2022;161 [Google Scholar]
- 73.Acharya S., Chaudhary A. Optimization of fermentation conditions for cellulases production by Bacillus licheniformis MVS1 and Bacillus sp. MVS3 isolated from Indian hot spring. Braz. Arch. Biol. Technol. 2012;55:497–503. [Google Scholar]
- 74.Sivakumar R., et al. Isolation, screening and optimization of production medium for thermostable laccase production from Ganoderma sp. Int. J. Eng. Sci. Technol. 2010;2(12):7133–7141. [Google Scholar]
- 75.Boadu K., et al. Comparative studies of the physicochemical properties and heavy metals adsorption capacity of chemical activated carbon from palm kernel, coconut and groundnut shells. J. Appl. Sci. Environ. Manag. 2018;22(11):1833–1839. [Google Scholar]
- 76.Vitas S., et al. Porosity and pore size distribution of native and delignified beech wood determined by mercury intrusion porosimetry. Materials (Basel) 2019;12(3):416. doi: 10.3390/ma12030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boateng I.D., Yang X.-M. Process optimization of intermediate-wave infrared drying: screening by Plackett–Burman; comparison of Box-Behnken and central composite design and evaluation: a case study. Ind. Crops Prod. 2021;162 [Google Scholar]
- 78.Rosly N.Z., et al. Adsorption of methylene blue dye by calix [6] arene-modified lead sulphide (Pbs): optimisation using response surface methodology. Int. J. Environ. Res. Publ. Health. 2021;18(2):397. doi: 10.3390/ijerph18020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mustapha M.U., Halimoon N., Johari W.L.W. Optimization of carbofuran insecticide degradation by Enterobacter sp. using response surface methodology (RSM) J. King Saud Univ. Sci. 2020;32(3):2254–2262. [Google Scholar]
- 80.Dhingani R., Shah G., Joshi B. Sequential optimization of bioprocess nutritional parameters for maximum l-asparaginase production from Pseudomonas aeruginosa BGR1I1. Biocatal. Biotransform. 2021:1–10. [Google Scholar]
- 81.Rosly N.Z., et al. Adsorption of methylene blue dye by calix [6] arene-modified lead sulphide (Pbs): optimisation using response surface methodology. Int. J. Environ. Res. Publ. Health. 2021;18(2):397. doi: 10.3390/ijerph18020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venil C.K., et al. 2009. Production of L-Asparaginase by Serratia marcescens SB08: Optimization by Response Surface Methodology. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.