Abstract
The cellular milieu in which malignant growths or cancer stem cells reside is known as the tumour microenvironment (TME). It is the consequence of the interactivity amongst malignant and non-malignant cells and directly affects cancer development and progression. Reactive oxygen species (ROS) are chemically reactive molecules that contain oxygen, they are generated because of numerous endogenous and external factors. Endogenous ROS produced from mitochondria is known to significantly increase intracellular oxidative stress. In addition to playing a key role in several biological processes both in healthy and malignant cells, ROS function as secondary messengers in cell signalling. At low to moderate concentrations, ROS serves as signalling transducers to promote cancer cell motility, invasion, angiogenesis, and treatment resistance. At high concentrations, ROS can induce oxidative stress, leading to DNA damage, lipid peroxidation and protein oxidation. These effects can result in cell death or trigger signalling pathways that lead to apoptosis. The creation of innovative therapies and cancer management techniques has been aided by a thorough understanding of the TME. At present, surgery, chemotherapy, and radiotherapy, occasionally in combination, are the most often used methods for tumour treatment. The current challenge that these therapies face is the lack of spatiotemporal application specifically at the lesion which results in toxic effects on healthy cells associated with off-target drug delivery and undesirably high doses. Nanotechnology can be used to specifically deliver various chemicals via nanocarriers to target tumour cells, thereby increasing the accumulation of ROS-inducing agents at the site of the tumour. Nanoparticles can be engineered to release ROS-inducing agents in a controlled manner to the TME that will in turn react with the ROS to either increase or decrease it, thereby improving antitumour efficiency. Nano-delivery systems such as liposomes, nanocapsules, solid lipid nanoparticles and nanostructured lipid carriers were explored for the up/down-regulation of ROS. This review will discuss the use of nanotechnology in targeting and altering the ROS in the TME.
Keywords: Cancer, Tumour microenvironment, Reactive oxygen species, Nanomedicines, Targeted drug delivery
1. Introduction
Both industrialised and developing nations continue to bear a heavy economic and social burden due to cancer [1]. An estimated 10 million fatalities, or one in every six deaths, were attributable to cancer in 2020, making it a top global cause of death [2]. Cancer is a multifaceted illness, involving intricate genomic alterations that are influenced by interactions between the host and its environment [3]. Cancer is identified by several biological and molecular characteristics, including prolonged proliferative signalling, avoiding growth suppressors, evasion of apoptosis, allowed replicative immorality, effectuated angiogenesis, and triggered invasion and metastasis [4]. The most typical cancers that caused fatalities in 2020 were lung, colorectal, liver, stomach, and breast cancer. These cancers collectively accounted for 5 million deaths [2].
The treatment of cancer typically includes the use of surgery, radiation, and systemic therapy utilising hormone medications, chemotherapy and targeted biological therapies individually or in combination [5].
The cellular environment in which malignancies exist is known as the tumour microenvironment (TME) [5]. Although the TME makeup varies based on the nature of the tumour, commonality exists in that immunological cells, stromal cells, blood arteries, and extracellular matrix are involved [6]. At the beginning of tumour development, components of the TME and cancer cells establish a dynamic and reciprocal connection that promotes cancer survival, local invasion, and metastatic spread [6]. The TME has increasingly been demonstrated to control abnormal tissue function and be essential for the development of cancers [7].
Reactive oxygen species (ROS) are secondary products that are common to a variety of biological functions, including the breakdown of oxygen [8,9]. Hydrogen peroxide (H2O2), superoxide anion (O2−), hypochlorous acid (HOCl), singlet oxygen (1 O2), and hydroxyl radical (•OH) are a few examples of the group of unstable, chemically reactive and partially reduced oxygen derivatives referred to as ROS [10]. These play a role as secondary messengers in cell signalling and are crucial for several biological processes in both healthy and malignant cells [11]. Specifically, ROS plays two roles in cell metabolism viz., between low and moderate concentrations, they serve as signal transmitters that stimulate angiogenesis, invasion, migration and cell proliferation [12]. High concentrations of ROS can activate signalling pathways that promote apoptosis in cancer cells [13]. ROS exert their apoptotic effects by inducing DNA damage through oxidation of DNA bases, DNA strand breaks, and the formation of DNA adducts. The accumulation of DNA damage impairs cancer cells’ ability to replicate and repair their DNA, resulting in cell cycle arrest and ultimately cell death [14]. In addition to their direct effects on cancer cells, ROS also plays a crucial role in modulating the immune response against tumours. ROS stimulate the release of cytokines and chemokines, which attract immune cells to the TME [15]. Furthermore, ROS enhance the antigen presenting capacity of dendritic cells, facilitating the activation of T cells and promoting an overall antitumour immune response [16]. ROS can enhance the efficacy of other cancer treatments, such as radiation therapy and certain chemotherapeutic agents [17]. Radiation for example relies on the production of ROS to induce DNA damage and kill cancer cells. Combining ROS-generating therapies with conventional modalities can lead to synergistic effects, enhancing antitumour efficiency [18,19]. Cancer stem cells are believed to contribute to tumour initiation, progression, and recurrence. These cells often exhibit resistance to conventional therapies, posing a challenge for effective treatment. However, ROS have been found to selectively target cancer stem cells by inducing oxidative stress. By specifically targeting these cells, ROS based therapies hold promise in preventing tumour relapse [[20], [21], [22]].
Due to increased metabolic activity, cellular signalling, peroxisomal activity, mitochondrial dysfunction, oncogene activation, and increased enzymatic activity of oxidases, cyclooxygenases, lipoxygenases, and thymidine phosphorylases, excessive amounts of ROS have been observed in cancer cells as compared to healthy cells [23]. Due to the duplex nature of ROS, methods to upregulate or downregulate ROS in cancer cells seem to hold potential for treatment [9]. Antioxidant and oxidant scavenging systems are typically regarded as advantageous for oxidative stress reduction and cancer prevention and treatment due to their ability to quench ROS levels [24,25]. Through a Fenton reaction, transition metals like iron (Fe) can produce ROS in catalysed reactions [26]. The hydroxyl radical produced by the Fenton reaction, depicted in Equation (1)., which occurs when Fe2+ reacts with hydrogen peroxide can damage DNA and other biomolecules [27,28].
| 1 |
Presently, conventional therapeutic agents lack spatiotemporal application specifically at the lesion, resulting in toxic effects on healthy cells associated with off-target drug delivery and undesirably high doses [29,30]. Advances in both traditional and alternative cancer therapy have been made possible by nanomedicines, which were specifically created to address this problem [30,31].
Nanotechnology has emerged as a promising solution in the realm of antitumour treatments, offering the potential to enhance their effectiveness by directly delivering therapeutic chemicals to tumour cells [32]. This novel approach utilises nanoscale carriers, such as nanoparticles, liposomes, or polymeric micelles, to enable targeted and controlled delivery of therapeutic agents to tumour tissues, while minimising the risk of systemic toxicity [33]. These nanocarriers can specifically target the tumour cells themselves or the TME [34]. To achieve this, various targeting strategies can be employed, such as incorporating ligands or antibodies that recognise specific receptors or antigens that are overexpressed on tumour cells [35,36]. The nanoscale size of these carriers offers distinct advantages over their larger micro or macro counterparts, as they can effectively evade the immune system during circulation. Furthermore, these nanomedicines can penetrate intercellular gaps to escape the compromised tumour blood vessels caused by abnormal angiogenesis and can be readily absorbed by the existing cells within the TME [30].
Herein, the application of medicines with their longest dimension in the nanoscale i.e., <1000 nm applications in cancers are explored. Specifically, nanomedicines targeting the unique ROS associated with the TME are discussed with their unique capabilities of enhancing cancer therapeutic outcomes explored.
2. Reactive oxygen species in the tumour microenvironment
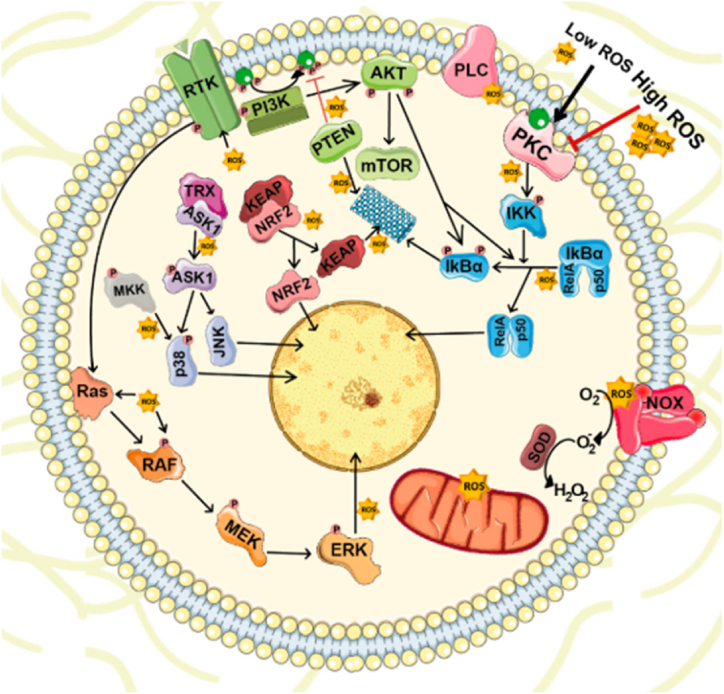
ROS are one of the elements of TME playing a crucial function in tumorigenesis and the progression of a solid tumour to metastatic disease [37]. ROS are crucial secondary messengers in cell signalling and are necessary for numerous biological processes in healthy and malignant cells, including cell proliferation, genomic instability, inflammation, resistance to apoptosis, and metabolic reprogramming as depicted in Fig. 1 [9]. This is achieved by targeting and stimulating several transducer proteins such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), kelch-like ECH-associated protein-nuclear factor erythroid 2-related factor 2 (KEAP1-NRF2), and phosphatidylinositol 3-kinase/AKT (PI3K-AKT) [38]. The principal forms of ROS are superoxide (O2), hydrogen peroxide (H2O2), and hydroxyl radicals, which are all extremely reactive and heterogeneous molecules produced from oxygen. The vast majority of ROS are generated endogenously and contribute to intracellular oxidative stress in significant amounts, as a result of metabolic processes occurring in the mitochondrion or peroxisome [37,39].
Fig. 1.
ROS-mediated cellular signalling: Intracellular ROS at low concentrations serve as crucial secondary messengers that target and stimulate transducer proteins such as NF-κB, MAPKs, KEAP1-NRF2, and PI3K-AKT, which are essential for cell survival, proliferation, and differentiation under normal or healthy cell conditions. Obtained and reproduced from Ref. [38] and MDPI (Basel: Switzerland) in accordance with Creative Commons Attribution 4.0 International (CC BY 4.0).
The mitochondria are the powerhouse of eukaryotic cells and the primary source of adenosine triphosphate (ATP), an energy-dense substance that powers essential cellular processes like force production, protein biosynthesis, folding, and breakdown, as well as the development and sustentation of membrane potentials [40]. During oxidative phosphorylation, ROS are primarily generated in the electron transport chain (ETC) on the inner mitochondrial membrane. ROS are first produced as O2, which can then be converted into H2O2 [9,37]. The three most significant sites for the production of ROS within the mitochondria are complexes I, II, and III. O2 is produced by complexes I and II in the mitochondrial matrix, while complex III also creates O2 in the intermembrane space (IMS). Superoxide dismutase protein 2 (MnSOD; SOD2) converts O2 produced in the mitochondrial matrix to H2O2. O2 produced by complex III can cross the outer mitochondrial membrane affecting cellular signalling, which results in ROS generated by complex III accessing and entering the cytoplasmic matrix, it is changed into H2O2 by the superoxide dismutase protein 1 (CnZnSOD; SOD1) [9,39]. A summary of the ROS production pathway in the mitochondria is shown in Fig. 2.
Fig. 2.
A depiction of the ROS production pathway in the mitochondrion. Obtained and reproduced from Ref. [41] SPIE Digital Library in accordance with Creative Commons Attribution 3.0 Unported License (CC BY-NC 3.0).
The imbalance between production and accumulation of ROS can lead to and affect tumour formation. This occurs through a variety of cellular and molecular processes, including altering the TME such as causing intracellular oxidative stress, which affects DNA impairment and genomic instability, and changing cell signalling, which converts healthy cells into cancerous and neoplastic cells. Numerous cancer cells have been found to produce more ROS in response to hypoxia [42].
The concentration of ROS equates to different impacts on biological function. ROS function as intracellular secondary messengers at low concentrations. Due to their ability to boost metabolism, signalling and suppress antioxidants, which aid in oncogenesis, controlled levels of ROS are advantageous to malignant cells. Conversely, excessive ROS levels can cause the induction of apoptosis and DNA damage that results in cell death. Cancer cells safeguard themselves against immoderate intracellular ROS by activating the transcription of antioxidant enzymes in reaction to oxidative stress [9,37]. The general effects of ROS as a function of concentration are summarised in Fig. 3.
Fig. 3.
Generation of ROS and their effects. Obtained and reproduced with changes from Ref. [9] and John Wiley & Sons Australia (Ltd) in accordance with Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0).
For a long time, ROS have been identified as a therapeutic target with some success because of the well documented function they play in the initiation and progression of malignancies [43]. ROS may be involved in therapeutic failure for a variety of reasons, hence a deeper awareness of the makeup of ROS in tumours and their surrounding environments is required. The different components that make up ROS have unique properties, that have various impacts at various stages of cancer as their origins and concentrations can vary at various stages of cancer progression. The variation in threshold levels of ROS in various sub-populations of the tumour microenvironment adds to this complexity and heterogeneity [37]. ROS could serve as a bridge connecting the tumour and immunological microenvironment. To control the cross-talk between the tumour and the microenvironment and enhance the prognosis for cancer making ROS an attractive therapeutic target [39].
Antioxidant-based therapy, which lowers the amount of ROS to evade oncogene activation, or ROS therapy, which raises the amount of ROS above the vulnerable amount to specifically target and kill cancer cells, are two ROS-modulating methods for cancer treatment. Of the two, pro-oxidative therapy is the most favoured and frequently used in clinical settings. Numerous pro-oxidative substances can raise the concentration of ROS above the threshold, either directly by producing ROS or obliquely by preventing the natural antioxidant defence system of cells from functioning correctly. Although, the cytotoxicity of the pro-oxidative agents has an impact on non-cancerous tissues and their limited solubility prevents them from being effective in clinical settings [44].
3. Current nanotechnology techniques used to target ROS
The application of nanoparticles for diagnosis, monitoring, control, prevention, and therapy is known as nanomedicine, a medical application of nanotechnology.
Thioketal (TK), thioether, aryl-boronic ester, vinyl-di-thioether, peroxalate ester, and phenylboronic acid/esters are chemical moieties that react with ROS causing ROS-induced structural cleavage [45]. These moieties are combined inside the main polymeric chain or are incorporated in the side chain for amphiphilic polymers that are linear or branched. These amphiphilic polymers are utilised in the formulation or design of different ROS-responsive drug delivery systems such as polymeric nanoparticles, polymersomes, core-shell micelles, polymeric micelles, lipid-polymer hybrid nanoparticles, core-cross-linked micelles, hydrogels, nanogels, mesoporous silica nanoparticles, silver nanoparticles, and prodrugs [[45], [46], [47]].
ROS can be generated through a variety of agents and technologies. These include natural enzymes, chemotherapeutics, metal peroxide nanoparticles, photosensitisation, and ionising radiation [48]. Numerous enzymes are involved in producing ROS, with NADPH oxidase (NOX) being particularly significant for its role in the respiratory burst process. The NOX family compromises seven members: NOX 1–5 and dual oxidases 1 and 2 (DUOX 1 and 2). When activated, these enzymes use NADPH or NADH as an electron donor to convert dioxygen into superoxide anion [49]. The generation of ROS within cancer cells is a common effect of many chemotherapeutic agents. This process is believed to play a crucial role in inducing cell death, especially in cancer cells that have already experienced an elevated ROS level. An example of such chemotherapeutics are anthracyclines, including doxorubicin, daunorubicin, and epirubicin [50]. Photodynamic Therapy (PDT) necessitates the introduction of a photosensitizer into the cellular tissue. These PS molecules have the capability to transfer energy from their excited state, resulting from light absorption, to molecular oxygen, thereby generating ROS [51,52]. Ionising radiation induces oxidative stress primarily through the generation of ROS via radiolysis of water molecules [53].
A summary of the current nanomaterials that can be exploited in targeting ROS is provided in Fig. 4.
Fig. 4.
Nanotechnology materials used to target ROS.
3.1. Polymeric nanomaterials
Polymeric nanomaterials have been utilised in various ailments including choroidal neovascularisation, age-related macular degeneration, breast and lung cancer, tuberculosis, and human immunodeficiency virus [[54], [55], [56]]. They have found use due to their versatility in manufacturing techniques as well as their ability for on-target drug delivery [57]. Furthermore, polymeric nano drug delivery has the versatility of delivering API of both hydrophobic and hydrophilic drugs [58].
3.1.1. Polymeric micelles
Nanoscale core-shell structures known as polymeric micelles are created by amphiphilic block copolymers [59]. They have a hydrophobic core which serves as a small-scale repository to enclose lipophilic medicines, proteins, or DNA, while the hydrophobic frame borders the biological matter in these typically spherical structures [60]. They hold the potential to gain desired biopharmaceutical and pharmacokinetic features of medications and boost their bioavailability because of their nanoscopic size, capacity to solubilise hydrophobic pharmaceuticals in high quantities, and ability to accomplish site-specific delivery [61]. Smaller size allows for passive targeting of solid tumours (even those with limited permeability), more effective cellular internalisation, and strong solubilisation capabilities [62].
Instead of improving the sensitivity of stimuli-receptive materials, it was envisaged that enhancing tumour stimulation by modulating the disparities between the TME and normal physiology may drive the usefulness of the developed tumour-receptive materials. H2O2-responsive chemotherapeutic drug (CPT) delivery nanocarriers augmented with palmitoyl ascorbate (PA) via the generation of polymer prodrug-PA hybrid micelles (HPMs) were designed. Tactical integration of PA specifically increased tumour H2O2 levels via H2O2 production. Excess H2O2 and produced CPT have been shown to efficiently enter cells and exhibit synergistic in vitro cytotoxicity against tumours. Hence, by systemically administering the self-sufficient H2O2-responsive nanocarriers, effective in vivo synergistic oxidation-chemotherapy may be accomplished [63].
In a study, polypropylene sulphide (PPS)-PNIPAm block copolymers were created by conjoining an atom transfer radical polymerization (ATRP) for PNIPAm and a live anionic ring-opening polymerization for PPS. Systematically investigated were the creation of polymeric micelles, medicine release in reaction to external stimuli, as well as the absorption and effectiveness of drug-loaded micelles in human breast cancer cell lines (MCF-7). Precise observation was paid regarding how ROS affected the absorption of anticancer drugs into cells, their release into cells, and their cytotoxicity in PPS-PNIPAm micelles. The study as a whole demonstrated that the novel dual responsive polymeric drug delivery system has the potential for treating cancer connected to temperature and ROS overproduction [64].
Heme oxygenase-1 (HO-1) is an antioxidant defence mechanism which plays a crucial function in tumour formation by providing the antioxidant bilirubin to safeguard cancer cells undergoing stressful circumstances. Therefore, it stands to reason that the interaction of ROS production with HO-1 suppression would increase oxidative stress and have synergistic anticancer effects, opening new possibilities for targeted anticancer therapy. Noh et al. created the molecularly engineered polymer known as cinnamaldehyde (CA) and zinc protoporphyrin (ZnPP) (CZP), which contains the HO-1 inhibitor ZnPP and ROS-generating CA in its backbone to institute directed anticancer treatment grounded on increased oxidative stress. CZP could also construct sturdy micelles in aqueous solutions. Additionally, CZP micelles were able to drastically reduce tumour growth unaccompanied by body weight loss, tumour recurrence, or obvious organ damage. The study's findings demonstrated synergistic effects of ROS production and HO-1 inhibition can increase oxidative stress to a point at which cancer cells could be destroyed. CZP micelles that increase oxidative stress may be a viable anticancer therapy [65].
An investigation that investigated the possibility of employing a poly (ε-caprolactone) (PCL) scaffold with oligoproline crosslinks in an in vivo model for perennial tissue production uses was conducted. PCL was recognized for its gradual in vivo long-term degradation by hydrolysing ester linkages, providing a structure that made the scaffold more prone to ROS degradation quickly. According to the study's hypothesis, the initial inflammatory host reaction would damage the embedded scaffold by producing too much ROS and would drive cell infiltration into the scaffold, improving neovascularisation and engraftment at the site of implantation [66].
A possible approach to improve cancer treatment is in situ mitochondrial ROS amplification. It was reported that cancer cell and mitochondria binary targeting poly-prodrug nanoreactors (DT-PNs) were covalently bound with a significant amount of recurring camptothecin (CPT) units, which released initial free CPT in the presence of intracellular mitochondrial ROS (mtROS) [67]. When the mitochondria were actively targeted, intracellular upregulation of mtROS in cancer cells induced initial free CPT release in mitochondria. The release of CPT additionally triggered the circulating rise of mtROS, leading to amplification of high-dosage CPT release and an ultimate mtROS burst, which encouraged for protracted high oxidative stress to proficiently cause cancer cell apoptosis [67].
Pan et al. demonstrated that ROS-responsive prodrug nanoparticles were an efficient approach to formulate nanomedicine for cancer chemotherapy by conjugation of polyethylene glycol (mPEG) 2000 to doxorubicin (DOX) through a ROS cleavable moiety TK. The highly potent ROS in tumour had the ability to activate the dissolution of TK moieties and the doxorubicin secretion for the suppression of cancer cell growth. In human hepatocarcinoma (HepG2) tumour-bearing nude mice, the ROS-responsive PEG-doxorubicin (PEG-DOX) prodrug showed remarkable anticancer activity, substantially increased tumour cell death, and decreased the systemic toxicity of DOX. The research demonstrated that the ROS-responsive prodrug nanoparticles are a useful method for creating nanomedicine for chemotherapy [68].
Shim and co-workers created a new ROS-responsive, cationic, water-soluble polymer made of degradable TK links that are easily separated in ROS-rich settings. The effectiveness and safety of gene transfer in cancer cells were demonstrated by utilising the intracellular ROS in cancer cells as a distinctive cancer-linked signal to facilitate intracellular gene conveyance. The ROS-severable TK polymeric porter was shown to improve the efficacy of gene transportation in cancer cells by enabling the liberation of the nucleic acids that were encapsulated in reaction to increased concentrations of intracellular ROS. To transport genes specifically to cancer, they additionally functionalised this polymer using a cancer-targeting peptide. By preventing generic accretion in healthy cells, the modification could improve the effectiveness of gene delivery and hence lessen the possibility of systemic toxicity [69].
One of the leading causes of cancer-related mortality across the globe is colorectal cancer. In a study that aimed to develop a carriage system for targeted therapy of colorectal cancer, A pH and ROS cascade-reactive drug carrier to circumvent multidrug resistance (MDR) in colorectal cancer was designed and formulated. The pH/ROS cascade-receptive and self-advancing drug-producing nanoparticle system (PLP-NPs) consisted of a ROS-sensitive polymeric paclitaxel (PTX) prodrug, a pH-sensitive poly(l-histidine) (Phis), and beta-lapachone (Lapa), a ROS-developing agent. PLP-NPs were taken up by lysosomes after entering cancer cells through the endocytic pathway. Under lysosomal acidic conditions, Phis was protonated and released PLP-NP. At the same time, Lapa was released and selectively increased intracellular ROS levels in cancer cells via the NQO1-mediated redox cycle. This increased therapeutic effectiveness against MDR colon cancer by encouraging the production of PTX and blocking ATP-dependent drug effluence [70].
3.1.2. Nanospheres
Nanospheres are spherical particles that range in size from 10 to 200 nm in diameter. Compared to bigger spheres made of the same material, they show certain new, improved size-dependent features. The drug is essentially dispersed, trapped, encased, or attached to the polymer matrix [71].
Due to their advantageous features, nano biopolymers including chitosan, poly(lactic-co-glycolic acid), and bovine serum albumin (BSA) are now often employed in nanobiotechnology. In a study by Hemlata et al., BSA-nanosphere-encapsulated C. prophetarum fruit extract was prepared at various pH levels of 5, 7, and 9, and the nanospheres' in vitro anticancer efficacy was assessed on various cancer cell lines. The Cp-BSA nanospheres were discovered to induce death of mitochondria-mediated by ROS in various human cancer cell lines as compared to non-cancerous cell lines, indicating that they may be employed as anticancer [72].
3.1.3. Polymersomes
Polymersomes are a type of synthetic self-fabricated nanovesicle formed from amphiphilic block copolymers [73,74]. A typical polymersome is a hollow sphere with an aqueous solution at its centre and a bilayer membrane surrounding it [[74], [75], [76]]. Numerous stimuli-receptive polymersomes have been established to attain controlled release, and these include pH-responsive polymersomes, temperature responsive polymersomes, enzyme responsive polymersomes, redox responsive polymersomes, photo, voltage, magnetic or electrical field polymersomes [74,77].
Different anticancer drugs depending on their solubility, including those for brain, breast, lung, pancreatic, prostate, colorectal, or ovarian cancer, can either be added directly to the reservoir or integrated into the membrane for administration to any malignant site. Docetaxel and paclitaxel, two taxane derivatives, have also been studied after being loaded into polymersomes. Breast, liver, lung, neck, prostate, and brain carcinoma are only a few of the cancer types that have shown to have stronger anticancer activity [74]. However, it is important to take into account the biosafety of polymersomes by determining their toxicity, specifically, on vital organs after systemic circulation [74,77].
3.1.4. Nanogels
Due to their hydrogel-like characteristics and minuscule particle size, which provide them with an edge over macro-scale gels in terms of quick reaction to environmental changes, nanogels have been extensively used as drug carriers [78]. High drug loading content, good biocompatibility, prolonged circulation period, particular ligands recognized by targeted cells, and stimulus-sensitive breakdown properties are design features of nanogels used as drug carriers [79].
Premised on the brand-new monomer 4-selenoctane1,8-diyl bis(propylphosphatelane), a ROS-responsive PEGylated polyphosphoester nanogel was created by Zhang and his team. The nanogels had exceptional stability due to the hydrophilicity of mPEG and polyphosphoester as well as the crosslinking structure. The nanogels also showed a good ability to capture water-soluble doxorubicin hydrochloride, and the selenide groups in the nanogels gave the nanogels their ROS receptiveness, which led to an effective drug release into the cells [80].
3.1.5. Miscellaneous
In a study by Wang et al. a triple-negative breast cancer (TNBC) mouse imitation was used to test the effects of arsenic sulphide (e-As4S4) on a type of metastatic solid tumour and to elucidate its core mechanisms. The novel e-As4S4 was manufactured by using co-rotating twin-screw extrusion with the excipient Soluplus®. The technology was applied to the lab-developed TNBC mouse model and demonstrated that taking e-As4S4 orally ensued in the accrual of arsenic in the target tissue. Consequently, angiogenesis was decreased and inflammasome in the TME by reduction of the level of ROS. As a result, the lifespan of breast cancer mice was also lengthened, and tumour spread to the liver and lung was significantly lowered [81].
3.2. Inorganic nanomaterials
An inorganic nanomaterial can consist of a metal or non-metal element or exist as an oxide, hydroxide, chalcogenide, or phosphate compound [82]. In contrast to organic compounds, inorganic nanoparticles are non-toxic, lipophobic, biocompatible, and relatively sturdy [83]. Inorganic nanoparticles have garnered a lot of interest throughout the preclinical research phase as prospective diagnostic and therapeutic systems in oncology for a range of uses, including tumour imaging, tumour drug delivery, or radiation augmentation [84].
A novel bioactive copper-olsalazine (Cu-Olsa) nanoMOF as a nanodrug for the treatment of colorectal cancer was introduced by Li and colleagues. The unique characteristic of this nanoMOF was its inherent enzyme-like catalytic activity, which enabled the generation of cancericidal species such as ·OH and 1O2 by utilising the abundant H2O2 present within tumours. Upon entering cancer cells, the Cu-Olsa nanoMOF dissociated into small molecular copper-organic complexes and olsalazine. This dissociation led to the inhibition of COX-2, an enzyme associated with inflammation and cancer progression, and facilitated epigenetic modulation within the cells. These combined effects resulted in the selective inhibition of colorectal cancer growth and metastasis [85].
Zhang et al. developed a nanoparticle called PCFD, which incorporated the anticancer drug DOX and an iron coordination polymer. This nanoparticle was designed for efficient chemo-dynamic cancer therapy, utilising a cinnamaldehyde-based organic ligand that replenishes ROS. The functional ligand had the ability to release cinnamaldehyde in response to ROS levels, supplementing intracellular H2O2 and depleting GSH and depleting glutathione (GSH) through a thiol-Michael addition reaction. Together with the ROS upregulation triggered by DOX and the GSH depletion enabled by Fe3+, this mechanism facilitated efficient release of DOX and enhanced the Fenton reaction. Consequently, this approach induced redox dyshomeostasis and lead to cancer cell death through concurrent apoptosis-ferroptosis pathways. Both in vitro and in vivo studies demonstrated that the PCFD nanoparticles, exhibited significantly better anticancer effects compared to nanoparticles that consume ROS. This study offers a straightforward and effective strategy for designing nano-platforms that amplify ROS levels for improved cancer treatment [86].
A strategy for photo-enhanced nano-catalytic tumour therapy using a nanocomposite called Fe3O4@MIL-100 (IFM) was presented by Cun et al. The IFM nanocomposite was decorated with IR-780 and designed to deplete GSH while generating highly cytotoxic ·OH radicals from tumoural H2O2. The therapy involved the selective upregulation of tumoural H2O2 with β-lapachone and localised hyperthermia through near-infrared light irradiation. In 4T1 cancer cells, the IFM nanocomposite exhibited potent anti-proliferative effects by inducing redox dyshomeostasis, concurrent apoptosis, and ferroptosis. In vivo studies demonstrated successful combinational therapy guided by photoacoustic and fluorescence imaging, resulting in a high tumour inhibition rate of 96.4%. This approach provides a promising strategy for targeted and efficient tumour treatment through H2O2 amplification and hyperthermia [87].
Mesoporous silica nanoparticles (MSN) have shown promising applications in nanomedicine. Specifically, the ROS-responsive MSN show accentuated therapeutic effectiveness and fewer adverse effects compared to other conventional methods and they have been used in a number of applications [88].
For example, a ROS reactive free blockage regulated system was formulated by Cheng et al. by controlling the wetting behaviour of nanopores on MSNs. Upon stimulation by ROS, the hydrophobic phenyl sulphide group in the internal phase of the nanopores that was preserved from being wetted by water initially, was oxidized and the nanopores were gradually wetted causing the release of doxorubicin from the nanopores. They showed that the wettability-established free-blockage regulated release system was easy and efficient, and it can be activated by intracellular biological signals, generating novel avenues for cancer treatment and drug delivery [89].
Two explored considerations for tumour therapy are the conveyance of therapeutic proteins and the obliteration of adverse effects linked to standard chemotherapeutic drugs. In a study, Pei et al. looked at the design of a yolk-shell nanoplatforms activated by reactive oxygen species (ROS) for the tumour-specific co-delivery of cytochrome c (Cyt c) prodrug and DOX, where the intracellular ROS-trigger could easily reimpose the bioactivity of Cyt c and institute the sequential discharge of doxorubicin. The experimental findings repeatedly showed that Cyt c and DOX could be administered with heightened selectivity and efficacy to the tumour site, and that the combination of the two agents produced a more effective curative behaviour than either agent alone [90].
To deliver DOX and -tocopheryl succinate (-TOS) to specific locations, thioketal-bonded hollow MSNs that are ROS-cleavable were coated with carboxymethyl chitin via electrostatic interaction, and the exterior was further imbedded with glucose-regulated protein 78 binding peptide. The nano-system may have the ability to directly attack murine mammary cancer (4T1) cells, resulting in cell apoptosis in vitro and inhibiting tumour development in vivo in BALB/c animals harbouring 4T1 as well as diminished adverse effects. This proved that the nano-system had a potent antitumour effect and that the released DOX was pharmacologically active to cause tumour cells to die [91].
Liao et al. created a dual ROS-responsive nanocarrier system that could self-regulate the level of ROS and respond progressively to endogenous ROS, occasioning efficient and targeted medicine release in cancer cells. Doxorubicin and MSNs were joined by TK bonds (M-TDOX). Β-lapachone (Lap) was put into M-TDOX to stimulate the generation of ROS. Triphenylphosphonium-reconstructed chitosan was then overlayed to create nanocarriers that could only be delivered to the mitochondria. Lap was first introduced into cancer cells where it elevated ROS levels surrounding mitochondria in response to endogenous ROS, producing tumour-specific release of DOX and enhanced oxidative stress that led to both in vivo and in vitro apoptosis of cancer cells [92].
3.3. Lipid drug delivery systems
Enhancing the bioavailability of medications is the main goal of lipid-based formulations. This technology also aims to solve problems associated with the solubility of poorly water-soluble medicines. In addition, evidence suggests that lipid-based formulations can alter the biodistribution of a drug's toxicity by shifting it away from sensitive organs [93].
Self-emulsifying drug delivery, micellar systems, and lipid solutions are a few of the physically diverse systems that make up lipid based drug delivery systems [94].
3.3.1. Ufasomes
Unsaturated fatty acid vesicles (ufasomes) are pH-constrained mixtures of sealed lipid bilayers made up of fatty acids and their ionized species (soap) [95]. In vitro and in vivo, n-3 polyunsaturated fatty acids (n-3 PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to have anti-cancer properties. The primary anti-cancer effects of n-3 PUFA EPA were investigated in a study. Initially, the ideal dose and duration needed for effects in PC3 cells were characterised. Next, western blotting and antibody arrays were used to examine protein expression. The ROS inhibitor N-acetyl cysteine (NAC) was utilised to elucidate the consequences of ROS generation brought on by EPA [96].
3.3.2. Liposomes
ROS has been used in photodynamic therapy (PDT) to destroy cancer cells during therapy. For PDT-activated chemotherapy, a new liposome (LCT) was created, that included photosensitizer (PS) and bio reductive prodrugs. The lipid bilayer was loaded with Cyl, an iodinated cyanine dye that may produce increased ROS and heat, and tirapazamine (TPZ), a hypoxia-activated prodrug, which was enclosed in the lipophobic nucleus. Cyl could generate ROS and heat at the same time for PDT and photothermal treatment upon the application of the proper near-infrared (NIR) irradiation (PTT). Synergistic PDT/PTT/chemo/immunotherapy was used to eradicate cancer cells. Findings from both in vitro and in vivo studies showed that LCT had improved anticancer effectiveness than conventional PDT or chemotherapy [97].
Gold nanoparticles (AuNPs) and emissive graphene quantum dots (GQDs) were deposited into a multifunctional liposome-based nano theranostic by Prasad et al. To display photo-triggered chemotherapy, doxorubicin hydrochloride was also enveloped in NFGL and conjugated with folic acid-targeting ligands. Attributable to the heat of the heat and reactive oxygen species produced, NFGL nanohybrids showed that tumour elimination by near-infrared light (NIR, 750 nm) is possible (ROS). Additionally, as contrasted to GQDs loaded liposomes, NFGL nanohybrids showed outstanding ROS scavenging capabilities, which was supported by an anticancer investigation [98].
Diethyldithiocarbamate-copper (Cu(DDC)2) containing liposomes enclosed with hyaluronic acid (HA), being able to specifically target pancreatic cancer stem cells (CSC) marker CD44 receptor was produced by the ion gradient method. To comprehend how Cu(DDC)2 liposomes work, the ROS level neutralisation assay was conducted in the presence of N-acetyl-l-cysteine [99]. Increased ROS-mediated anticancer activity of HA-coated liposomes was shown in in vitro tests on pancreatic CSCs sourced from pancreatic ductal adenocarcinoma (PDAC) cell lines or patients. Metals including iron, copper, zinc, gold, and disulfiram (DSF) can form stable complexes with DSF and dithiocarbamates [99]. The resultant complexes inhibit proteasome, and metalloproteinase, and have ROS generation induction abilities [100]. According to research, Cu(DDC)2 is an ionophore complex that can cause an increase in ROS caused by copper, which can encourage a mitochondrial-mediated cell death program [101].
A combination therapy nano-system was created by co-encapsulating pB-DOX, a ROS-responsive drug, Indocyanine Green (ICG), a ROS trigger and photosensitizer, in polyethylene glycol adapted liposomes (Lipo/pB-DOX/ICG). Normal HEK-293 cells were utilised to assess the nano-system's safeness, while human breast cancer cells from the MCA-MB-231 subcutaneous tumour model were used to quantify cellular absorption, intracellular ROS generation capability, target cell toxicity, and combined therapy impact. Lipo/pB-DOX/ICG showed greater safety on normal cells as compared to DOX-HCI. Lipo/pB-DOX/ICG was substantially more harmful to target cells than DOX-HCI, Lipo/pB-DOX, and Lipo/ICG. For PDT by laser irradiation, Lipo/pB-DOX/ICG created a significant quantity of ROS after being endocytosed by MBA-MB-231 cells, and pB-DOX was transformed to DOX by ROS for chemotherapy [102].
3.4. Amino acids
Recent developments in genetic and chemical engineering have made it possible to modify protein activity within cells without harming them. For the therapy of disease, the creation of novel strategies to alter protein activities without the assistance of an external stimulus is required, particularly modifications that are responsive to innate diseased microenvironments. Wang et al. presented a practical chemical method to produce a protein (RNase A) that is reactive to ROS and can be utilised for targeted cancer treatment. In the research, protein lysine was blocked and momentarily rendered inactive by the coupling of RNase A with 4-nitrophenyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzyl carbonate (NBC). However, when hydrogen peroxide, a significant intracellular ROS, was used to treat RNase A-NBC, the NBC conjugation was effectively broken down and the RNase A activity was recovered. High quantities of intracellular ROS could thereby reactivate RNase A-NBC inside tumour cells, restoring RNase A's cytotoxicity for cancer therapy [103].
Another study developed a (ROS)-activated smart theranostic prodrug system for treating metastatic cancer on a ROS-active site connected with a targeting group and an anticancer drug. This system was based on the combination of leucomethylene (LMB) and the (2-amino-1,3-phenylene) dimethanol group, which was linked to both the targeting group and the anticancer drug. Using biotin as the target, the enhanced prodrug (Bio-(8)-MB-CPT) demonstrated significant ROS sensitivity, selective targeting capacity towards cervical cancer cells, and extremely effective drug release (up to 92%) in vitro. The prodrug also pointedly increased anticancer activity in vivo, totally eradicated the tumour, and did so with no evident adverse effects (tumour inhibition reached up to 99.9%) [104].
3.5. Nanosponges
The nanosponges’ drug transport system is an exceptionally tiny network with nano-formulation that enables a broad array of drugs to be entrapped, suspended, or encapsulated before being integrated as a new dosage form. This is due to the globular colloid structure, inclusion and non-inclusion behaviour they display. Nanosponges have been shown to have the greatest ability to saturate drugs with little water solubility. Nanosponges can enhance therapeutic properties like bioavailability, solubility, and permeability while encasing hydrophilic and hydrophobic medicinal components in their porous structure to provide sustained release. They exhibit a variety of sizes (1 m or less than 1), three-dimensional structures, and good concavity polarity [105].
An investigation with a straightforward technique to make fluorescent traceable prodrug nanosponges for the tumour-specific pH/hypoxia dual-triggered drug administration used a high-performance synthesis of isocyanate groups. The proposed prodrug nanosponges were projected to be identified in the human body due to their peculiar shape and to disintegrate only when applied selectively for theranostic purposes in an acidic and hypoxic tumour intracellular milieu. The suggested doxorubicin conjugated carbon quantum dots-based nanosponges displayed good acid/glutathione binary-triggered medicine discharge because of the binary triggered breakdown within the tumour. The combined medication was consequently liberated with an improved selective inhibition of tumour cell proliferation when compared to free doxorubicin [106].
3.6. Miscellaneous
Wang et al. developed a poly-prodrug for chemo/chemo dynamic treatment that consists of pH-responsive (PEG)-block-poly diisopropylaminoethyl methacrylate block-poly dopamine (PEG-PDPA-PDA) and ROS-responsive PEG-block-poly TK doxorubicin (DOX) prodrug (PEG-PtkDOX). This nanomedicine demonstrated promise in its ability to boost anticancer activity via a cascade of ROS production and drug release [107]. The increased permeability and retention (EPR) impact of the pH/ROS dual-responsive nanomedicine allowed for efficient tumour accumulation. A pH-induced breakdown of β-lapachone could occur in the acidic intracellular environment. The medication was effectively able to produce H2O2, which was then transformed by the Fenton reaction into extremely dangerous hydroxyl radicals. The TK linker was subsequently cleaved by ROS, releasing doxorubicin from the poly-prodrug [107].
Uthaman et al. created an innovative TME-reactive photodynamic treatment system using self-quenching polysaccharide NPs and a ROS-sensitive cascade. To create a self-quenchable GC-TK-PhA with both TME ROS-reactive photo-activity and cascade production of the PSs for improved photodynamic therapy, pheophorbide A (PhA) was coalesced to a water-soluble glycol chitosan (GC) through a ROS-sensitive TK linker [108].
In a different study, amphiphilic PEG-TK-DOX conjugates that function as prodrug-type nanocarriers to increase DOX payload were created by combining the anticancer drug DOX with PEG and a ROS-receptive decomposable TK linker. When PEG-TK-DOX self-assembled, PhA, a PS, was efficiently loaded due to the interaction between PhA and DOX. The NP system was anticipated to demonstrate spatiotemporally regulated site precise release of DOX and PhA following improved passive accumulation in tumours via EPR. During the following stage of photodynamic therapy, endogenous early-stage ROS and exogenous ROS were produced in a cascade-like fashion, which resulted in another ROS cascade [109]. A summary of current nanotechnology approaches to target the ROS are provided in Table 1.
Table 1.
Summary of current nanotechnology approaches used to target ROS.
| Nanomaterial | Active Pharmaceutical Ingredient | Study model | Study outcome | Type of cancer | Mechanism | Ref |
|---|---|---|---|---|---|---|
| Polymeric nanoparticles | Arsenic sulphide | Female BALB/c mice | Decreased angiogenesis and inflammasomes | Breast cancer | Reduction of the level of ROS by manufacturing novel e-As4S4 by using co-rotating twin-screw extrusion with the excipient Soluplus® | [81] |
| Conatumumab and irinotecan | HT-29 cells, NCM460 cells, BALB/c nude mice | Targeted cancer treatment | Colorectal cancer | ROS sensitive linker, TK, is directly conjugated to the phenolic hydroxyl group of SN-38 and the carboxyl group of stearic acid | [110] | |
| Polymeric micelles | Camptothecin | MCF-7 human breast cancer cell line | Synergistic oxidation chemotherapy | Breast cancer | Integration of PA specifically increased tumour H2O2 levels via H2O2 production | [63] |
| Polyproylene sulphide- PNIPAm | MCF-7 human breast cancer cell line | Treating cancer connected to temperature and ROS overproduction | Breast cancer | An atom transfer radical polymerization (ATRP) for PNIPAm and a live anionic ring-opening polymerization for PPS were conjoined | [64] | |
| Cinnamaldehyde and Zinc Protoporphyrin | A549 cells | Synergistic anticancer effects | Lung cancer | CA and (ZnPP) (CZP), which contains the HO-1 inhibitor ZnPP and ROS-generating CA in its backbone to institute anticancer treatment | [65] | |
| Poly (ε-caprolactone) (PCL) with oligoproline |
Mouse bone marrow-derived macrophages | Improved neovascularisation and engraftment | Lung cancer, breast cancer | Crosslinks damage the embedded scaffold by generating ROS and would drive cell infiltration into the scaffold | [66] | |
| Camptothecin polydrug | 4T1 rat breast cancer cells, MCF-7 human breast cancer cells, U87 human glioblastoma cells and female BALB/c mice | Cancer cell apoptosis | Breast cancer | Intracellular upregulation of mtROS in cancer cells | [67] | |
| Doxorubicin | HepG2 cells, HepG2 tumour-bearing mice | Increased tumour cell death | Liver cancer | By conjugation of (mPEG) 2000 to DOX through a ROS cleavable moiety TK | [68] | |
| Nucleic acids | PC3 cells | Improved gene delivery | Prostate cancer | Through enabling the liberation of the nucleic acids that were encapsulated in reaction to increased concentrations of intracellular ROS | [69] | |
| Paclitaxel | HCT-8/PTX tumour bearing mice | Increased therapeutic efficacy | Colon cancer | Lapa was released and selectively increased intracellular ROS levels in cancer cells via the NQO1-mediated redox cycle | [70] | |
| Mesoporous silica nanoparticles | Doxorubicin | MCF-7 cells, HUVEC cells | Increased drug delivery | Breast cancer | Upon stimulation by ROS, nanopores were gradually wetted causing the release of doxorubicin | [89] |
| Doxorubicin and cytochrome c | HepG2 cells, human endothelial cells, LO2 cells | Sequential release of doxorubicin and cytochrome c | Liver cancer | Tumour-specific co-delivery of Cyt c prodrug and DOX | [90] | |
| Doxorubicin and tocopheryl succinate | 4T1 cell, 3T3 cells, 4T1-bearing BALB/c mice | Antitumour efficacy | Breast cancer | TK-bonded hollow MSNs that are ROS-cleavable were coated with carboxymethyl chitin via electrostatic interaction | [91] | |
| Amino acids | RNase A | HeLA cervical cancer cells, B16F10 melanoma cells, PC-3 prostate cancer cells, MDA-MB-231 breast cancer cells | Restoring RNase cytotoxicity | Prostate cancer, cervical cancer, lung cancer | NBC conjugation was effectively broken down and the Rnase A activity was recovered | [103] |
| Bio-8-MB-CPT | HeLA, NIH3T3 cells, Hela-tumour bearing mice | 99.9% tumour inhibition | Metastatic cancer, cervical cancer | High quantities of intracellular ROS reactivated RNase A-NBC inside tumour cells, through the coupling of RNase A with 4-NBC | [104] | |
| Nanogels | Doxorubicin hydrochloride | A549, HEK293 cell lines | Effective drug release into the cells | Lung cancer | ROS-responsive PEGylated polyphosphoester nanogel was created which was ROS receptive | [80] |
| Nanospheres | C.prophetarum fruit | A549, HepG2, HEK293, MCF-7 cell lines | Anticancer efficacy | Lung cancer, liver cancer, breast cancer | Cp-BSA nanospheres were prepared using a desolvation method | [72] |
| Ufasomes | Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) | PC3 cellsl | Anticancer effects | Prostate cancer | ROS generation brought on by EPA | [96] |
| Liposomes | Tirapazamine, cyanine dye | 4T1 breast cancer cells, BALB/c mice | Eradication of cancer cells | Breast cancer | The lipid bilayer was loaded with Cyl, an iodinated cyanine dye that may produce increased ROS and heat | [97] |
| Doxorubicin hydrochloride | 4T1 breast cancer, MCF-7 cell lines, tumour bearing mice | Tumour elimination by near-infrared light | Breast cancer | NFGL nanohybrids showed tumour elimination by near infrared light | [98] | |
| Diethyldithiocarbamate-copper | Pancreatic cancer cell lines, SW1990, PANC-1, BXPC-3 | Target pancreatic cancer stem cells | Pancreatic cancer | Generation of metals to form stable complex DSF and dithiocarbamates. Complexes have ROS generation induction abilities | [100] | |
| Indocyanine Green, Doxorubicin | MDA-MB-231, HEK293 cell lines | Quantifying cellular absorption, intracellular ROS generation capability, target cell toxicity, and combined therapy impact | Breast cancer | ROS generation by combination therapy and by co-encapsulating pB-DOX | [102] | |
| Nanosponges | Doxorubicin | HepG2 cell line | Enhanced selective suppression of tumour cell proliferation | Liver cancer | Fluorescent traceable prodrug nanosponges were made for the tumour-specific pH/hypoxia dual-triggered drug administration and used a high-performance synthesis of isocyanate groups | [106] |
| Miscellaneous | Doxorubicin | A549 cell line, A549 tumour bearing mice | Increased antitumour efficacy. | Lung cancer | Anticancer activity was boosted via a cascade of ROS production and drug release | [107] |
| Pheophorbide | Mouse colon cancer CT-26 cell line | Photodynamic therapy | Colon cancer | TME-reactive photodynamic treatment system using self-quenching polysaccharide NPs and a ROS-sensitive cascade | [108] | |
| Doxorubicin and Pheophorbide | Male inbred BALB/c nude mice | Target specific release | Colon cancer | Endogenous early-stage ROS and exogenous ROS were generated in a cascade-like fashion, which resulted in another ROS cascade | [109] | |
| Doxorubicin | 4T1 female BALB/c nude mice | Apoptosis of cancer cells | Breast cancer | Excess H2O2 and produced CPT efficiently entered tumour cells | [63] |
4. Future perspectives and conclusion
Currently cancer treatment faces numerous challenges, ranging from drug resistance to systemic toxicity.
Nano-systems, a cutting-edge application of nanotechnology, have revolutionised cancer therapy by improving the targeting of ROS in the TME. These advanced systems enable enhanced precision in the therapeutic delivery, minimising the toxicity on healthy cells while increasing the specificity of treatment. By directly manipulating ROS levels, whether by increasing or reducing them, nano-systems have emerged as a novel approach to induce apoptosis in cancer cells.
Looking to the future, the field of cancer therapy holds immense potential for further advancements in nanotechnology. Continued research and development efforts will likely lead to the integration of smart nano-systems, enabling real-time monitoring and adaptive treatment strategies. Personalised medicine approaches will play a pivotal role, tailoring therapies to individual patients based on their unique TME characteristics.
In addition, combining nano-systems with other treatment modalities, such as immunotherapy or gene therapy, holds promise for synergistic effects and improved outcomes. The tantalising potential of combining the immunotherapy to improve outcomes while utilising the advantages of nano-systems could improve the outcomes of the treatment modalities.
An additional potentially tantalising aspect is to utilise biomimetic approaches to improve the geospatial treatment aspects. There is a great opportunity to explore the use of body cell as trojan horses to target TME where the nano-systems could subsequently elicit their improved effects [111,112].
The field of cancer treatment has undergone remarkable transformations in recent years, shifting from a tumour-centric approach to a comprehensive understanding of the TME. The TME, consisting of a complex network of cells that interact with cancer cells, plays a crucial role in malignant progression. Within this intricate system, the regulation, of ROS emerges as a promising avenue for therapeutic intervention. Both normal and cancer cells rely on controlled ROS levels for their cellular processes, making ROS modulation an attractive target for cancer treatment.
As researchers continue to unravel the intricate interactions between nano-systems and cancer cells, we can anticipate the emergence of novel treatment strategies that revolutionise the way cancer is combated. By harnessing the power of nanotechnology, we may be one step closer to achieving more effective and personalised cancer therapies, thereby improving patient outcomes, and ultimately paving the way towards a future where cancer becomes a manageable condition.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Arneth B. Tumor microenvironment,” Med. 2020;56(1) doi: 10.3390/medicina56010015. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Cancer,” Cancer Fact Sheet. 2022 https://www.who.int/news-room/fact-sheets/detail/cancer [Google Scholar]
- 3.Abbas Z., Rehman S. An overview of cancer treatment modalities. Neoplasm. 2018 doi: 10.5772/intechopen.76558. [DOI] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Wang J.J., Lei K.F., Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018;22(12):3855–3864. doi: 10.26355/eurrev-201806-15270. [DOI] [PubMed] [Google Scholar]
- 6.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. 2020;30(16):R921. doi: 10.1016/j.cub.2020.06.081. –R925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roma-Rodrigues C., Mendes R., Baptista P.V., Fernandes A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20(4) doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. Jan. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H., Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112(10):3945–3952. doi: 10.1111/cas.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Z., Chang H., Li H., Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22(11):1321–1335. doi: 10.1007/s10495-017-1424-9. [DOI] [PubMed] [Google Scholar]
- 11.Chio I.I.C., Tuveson D.A. ROS in cancer: the burning question. Trends Mol. Med. May 2017;23(5):411–429. doi: 10.1016/j.molmed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. Dec. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivas U.S., Tan B.W.Q., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris G., Gevezova M., Sarafian V., Maes M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022;19(10):1079–1101. doi: 10.1038/s41423-022-00902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W., Shen P., Song Y., Huang Y., Tu S. Reactive oxygen species in autoimmune cells: function, differentiation, and metabolism. Front. Immunol. 2021;12(February):1–16. doi: 10.3389/fimmu.2021.635021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perillo B., et al. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020;52(2):192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Jiang H., Van De Gucht M., De Ridder M. Hypoxic radioresistance: can ROS be the key to overcome it? Cancers. 2019;11(no. 1) doi: 10.3390/cancers11010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard D., Sebastian S., Le Q.V.C., Thierry B., Kempson I. Chemical mechanisms of nanoparticle radiosensitization and radioprotection: a review of structure-function relationships influencing reactive oxygen species. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi X., Zhang Y., Zheng J., Pan J. Reactive oxygen species in cancer stem cells. Antioxidants Redox Signal. 2012;16(11):1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cieślar-Pobuda A., Yue J., Lee H.C., Skonieczna M., Wei Y.H. ROS and oxidative stress in stem cells. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/5047168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saya H. Redox regulation in cancer stem cells. Nihon Rinsho. 2015;73(5):790–794. [PubMed] [Google Scholar]
- 23.Kumari S., Badana A.K., Murali Mohan G., Shailender G., Malla R.R. Reactive oxygen species: a key constituent in cancer survival. Biomark. Insights. 2018;13 doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamihara Y., et al. The iron chelator deferasirox induces apoptosis by targeting oncogenic Pyk2/β-catenin signaling in human multiple myeloma. Oncotarget. Sep. 2016;7(39):64330–64341. doi: 10.18632/oncotarget.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matés J.M., Sánchez-Jiménez F.M. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000;32(2):157–170. doi: 10.1016/S1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 26.Kutoka P.T., Seidu T.A., Baye V., Khamis A.M., qizi Omonova C.T., Wang B. Current nano-strategies to target tumor microenvironment (TME) to improve anti-tumor efficiency. OpenNano. 2022;7 doi: 10.1016/j.onano.2022.100041. [DOI] [Google Scholar]
- 27.Beyersmann D., Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008;82(8):493–512. doi: 10.1007/s00204-008-0313-y. Aug. [DOI] [PubMed] [Google Scholar]
- 28.Bystrom L.M., Guzman M.L., Rivella S. Iron and reactive oxygen species: friends or foes of cancer cells? Antioxidants Redox Signal. Apr. 2014;20(12):1917–1924. doi: 10.1089/ars.2012.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp J.A., Shim M.S., Heo C.Y., Kwon Y.J. ‘Combo’ nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. Mar. 2016;98:3–18. doi: 10.1016/j.addr.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Kwon S., Ko H., You D.G., Kataoka K., Park J.H. Nanomedicines for reactive oxygen species mediated approach: an emerging paradigm for cancer treatment. Acc. Chem. Res. 2019;52(7):1771–1782. doi: 10.1021/acs.accounts.9b00136. [DOI] [PubMed] [Google Scholar]
- 31.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessale M., Mengistu G., Mengist H.M. Nanotechnology: a promising approach for cancer diagnosis, therapeutics and theragnosis. Int. J. Nanomedicine. 2022;17(August):3735–3749. doi: 10.2147/IJN.S378074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavas S., Quazi S., Karpiński T.M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 2021;16(1) doi: 10.1186/s11671-021-03628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Yang J., Sun X. Reactive oxygen species-based nanomaterials for cancer therapy. Front. Chem. 2021;9(April):1–12. doi: 10.3389/fchem.2021.650587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das M., Mohanty C., Sahoo S.K. Ligand-based targeted therapy for cancer tissue. Expet Opin. Drug Deliv. 2009;6(3):285–304. doi: 10.1517/17425240902780166. [DOI] [PubMed] [Google Scholar]
- 36.Marques A.C., Costa P.J., Velho S., Amaral M.H. Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J. Contr. Release. 2019;320(December):180–200. doi: 10.1016/j.jconrel.2020.01.035. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Saikolappan S., Kumar B., Shishodia G., Koul S., Koul H.K. Reactive oxygen species and cancer: a complex interaction. Cancer Lett. 2019;452(February):132–143. doi: 10.1016/j.canlet.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-De-Diego C., Antonio Valer J., Pimenta-Lopes C., Luis Rosa J., Ventura F. Interplay between BMPs and reactive oxygen species in cell signaling and pathology. Biomolecules. 2019;9:10. doi: 10.3390/biom9100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J., Matosevic S. Functional and metabolic targeting of natural killer cells to solid tumors. Cell. Oncol. 2020;43(4):577–600. doi: 10.1007/s13402-020-00523-7. [DOI] [PubMed] [Google Scholar]
- 40.Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13(1):1–11. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desa D.E., Nichols M.G., Smith H.J. Aminoglycosides rapidly inhibit NAD(P)H metabolism increasing reactive oxygen species and cochlear cell demise. J. Biomed. Opt. 2018;24(5):1. doi: 10.1117/1.jbo.24.5.051403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moldogazieva N.T., Lutsenko S.V., Terentiev A.A. Reactive oxygen and nitrogen species–induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78(21):6040–6047. doi: 10.1158/0008-5472.CAN-18-0980. [DOI] [PubMed] [Google Scholar]
- 43.Lu T., Gabrilovich D.I. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin. Cancer Res. 2012;18(18):4877–4882. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somu P., Mohanty S., Paul S. A detailed overview of ROS-modulating approaches in cancer treatment. Handb. Oxidative Stress Cancer Ther. Asp. 2022;(–22):1. doi: 10.1007/978-981-16-1247-3_213-1. [DOI] [Google Scholar]
- 45.Mirhadi E., Majeed M., Kesharwani P., Sahebkar A. Reactive oxygen species-responsive drug delivery systems: a new approach in nanomedicine. Curr. Med. Chem. Jan. 2022;29(25):4320–4323. doi: 10.2174/0929867329666220127110654. [DOI] [PubMed] [Google Scholar]
- 46.Zhou M., Wen L., Wang C., Lei Q., Li Y., Yi X. Recent advances in stimuli-sensitive amphiphilic polymer-paclitaxel prodrugs. Front. Bioeng. Biotechnol. 2022;10(April):1–9. doi: 10.3389/fbioe.2022.875034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinaldi A., et al. Applications of the ROS-responsive thioketal linker for the production of smart nanomedicines. Polymers. 2022;14:4. doi: 10.3390/polym14040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y., Pan Q., Gao W., Pu Y., Luo K., He B. Reactive oxygen species-upregulating nanomedicines towards enhanced cancer therapy. Biomater. Sci. 2022;11(4):1182–1214. doi: 10.1039/d2bm01833k. [DOI] [PubMed] [Google Scholar]
- 49.Villalpando-Rodriguez G.E., Gibson S.B. vol. 2021. Hindawi Limited; 2021. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. (Oxidative Medicine and Cellular Longevity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H., et al. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018;37(1) doi: 10.1186/s13046-018-0909-x. BioMed Central Ltd., Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tada D.B., Baptista M.S. vol. 3. MAY. Frontiers Media S. A; 2015. Photosensitizing nanoparticles and the modulation of ROS generation. (Frontiers in Chemistry). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B., Lin L., Lin H., Wilson B.C. Photosensitized singlet oxygen generation and detection: recent advances and future perspectives in cancer photodynamic therapy. J. Biophot. Dec. 2016;9(11–12):1314–1325. doi: 10.1002/jbio.201600055. [DOI] [PubMed] [Google Scholar]
- 53.Datta K., Suman S., Kallakury B.V.S., Fornace A.J. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042224. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begines B., et al. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials. 2020;10(7):1–41. doi: 10.3390/nano10071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banyal S., Malik P., Tuli H.S., Mukherjee T.K. Advances in nanotechnology for diagnosis and treatment of tuberculosis. Curr. Opin. Pulm. Med. 2013;19(3):289–297. doi: 10.1097/MCP.0b013e32835eff08. [DOI] [PubMed] [Google Scholar]
- 56.Aguilera-Correa J.J., Esteban J., Vallet-Regí M. Inorganic and polymeric nanoparticles for human viral and bacterial infections prevention and treatment. Nanomaterials. 2021;11(1):1–26. doi: 10.3390/nano11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Contr. Release. Jan. 2001;70(1–2):1–20. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 58.Zielinska A., et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:16. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paprikar A., Soni A., Kaushal N., Lin S. Polymeric micelles for drug delivery. Nanotechnol. Life Sci. 2021:345. doi: 10.1007/978-3-030-84262-8_12. –372. [DOI] [Google Scholar]
- 60.Aliabadi H.M., Lavasanifar A. Polymeric micelles for drug delivery. Expet Opin. Drug Deliv. 2006;3(1):139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 61.Xu W., Ling P., Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013;2013(1):1–15. doi: 10.1155/2013/340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghezzi M., et al. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J. Contr. Release. 2021;332(February):312–336. doi: 10.1016/j.jconrel.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 63.Li J., et al. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J. Contr. Release. 2016;225:64–74. doi: 10.1016/j.jconrel.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 64.Tang M., et al. Polymeric micelles with dual thermal and reactive oxygen species (ROS)-responsiveness for inflammatory cancer cell delivery. J. Nanobiotechnol. 2017;15(1):1–11. doi: 10.1186/s12951-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noh J., Jung E., Lee J., Hyun H., Hong S., Lee D. Engineered polymeric micelles for combinational oxidation anticancer therapy through concurrent HO-1 inhibition and ROS generation. Biomacromolecules. 2019;20(2):1109–1117. doi: 10.1021/acs.biomac.8b01802. [DOI] [PubMed] [Google Scholar]
- 66.Lee S.H., et al. ROS-cleavable proline oligomer crosslinking of polycaprolactone for pro-angiogenic host response. J. Mater. Chem. B. 2014;2(41):7109–7113. doi: 10.1039/c4tb01094a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W., Hu X., Shen Q., Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019;10(1):1–14. doi: 10.1038/s41467-019-09566-3. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan Q., Deng X., Gao W., Chang J., Pu Y., He B. ROS triggered cleavage of thioketal moiety to dissociate prodrug nanoparticles for chemotherapy. Colloids Surf. B Biointerfaces. 2020;194 doi: 10.1016/j.colsurfb.2020.111223. Oct. [DOI] [PubMed] [Google Scholar]
- 69.Shim M.S., Xia Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew. Chem. Int. Ed. 2013;52(27):6926–6929. doi: 10.1002/anie.201209633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang N., Zhao Y., Ge N., Qian L. A pH/ROS cascade-responsive and self-accelerating drug release nanosystem for the targeted treatment of multi-drug-resistant colon cancer. Drug Deliv. Jan. 2020;27(1):1073–1086. doi: 10.1080/10717544.2020.1797238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh A., Garg G., Sharma P.K. Nanospheres: a novel approach for targeted drug delivery system. Int. J. Pharmaceut. Sci. Rev. Res. 2010;5(3):84–88. [Google Scholar]
- 72.Hemlata S. Gupta, Tejavath K.K. ROS-mediated apoptosis induced by BSA nanospheres encapsulated with fruit extract of cucumis prophetarum in various human cancer cell lines. ACS Omega. Apr. 2021;6(15):10383–10395. doi: 10.1021/acsomega.1c00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crayton S.H., et al. 3.20 Molecular imaging. 2017;3(October 2016) doi: 10.1016/B978-0-12-803581-8.10222-X. [DOI] [Google Scholar]
- 74.Singh V., Md S., Alhakamy N.A., Kesharwani P. Taxanes loaded polymersomes as an emerging polymeric nanocarrier for cancer therapy. Eur. Polym. J. 2022;162(September 2021) doi: 10.1016/j.eurpolymj.2021.110883. [DOI] [Google Scholar]
- 75.Lee J.S., Feijen J. Polymersomes for drug delivery: design, formation and characterization. J. Contr. Release. 2012;161(2):473–483. doi: 10.1016/j.jconrel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Madkour L.H. Advanced drug delivery systems: new nanomedication technologies. Nucleic Acids as Gene Anticancer Drug Deliv. Ther. 2019:1. doi: 10.1016/b978-0-12-819777-6.00001-9. –29. [DOI] [Google Scholar]
- 77.Sharma A.K., et al. Emerging era of ‘somes’: polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020;10(5):1171–1190. doi: 10.1007/s13346-020-00789-2. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H., Zhai Y., Wang J., Zhai G. New progress and prospects: the application of nanogel in drug delivery. Mater. Sci. Eng. C. 2016;60:560–568. doi: 10.1016/j.msec.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 79.Du X., Gao Y., Kang Q., Xing J. Design and applications of tumor microenvironment-responsive nanogels as drug carriers. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.771851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Ma C., Zhang S., Wei C., Xu Y., Lu W. ROS-responsive selenium-containing polyphosphoester nanogels for activated anticancer drug release. Mater. Today Chem. Sep. 2018;9:34–42. doi: 10.1016/j.mtchem.2018.04.002. [DOI] [Google Scholar]
- 81.Wang T., et al. Inhibition of murine breast cancer metastases by hydrophilic As4S4 nanoparticles is associated with decreased ROS and HIF-1α downregulation. Front. Oncol. 2019;9(APR):333. doi: 10.3389/fonc.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi Y., Lee S.Y. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nat. Rev. Chem. 2020;4(12):638–656. doi: 10.1038/s41570-020-00221-w. [DOI] [PubMed] [Google Scholar]
- 83.Paul W., Sharma C., Chitra S. Biointegration of Medical Implant Materials: Science and Design. Woodhead Publishing; 2010. Inorganic nanoparticles for targeted drug delivery; pp. 204–235. [DOI] [Google Scholar]
- 84.Egusquiaguirre S.P., Pedraz J.L., Hernández R.M., Igartua M. Nanotherapeutic platforms for cancer treatment: from preclinical development to clinical application. Nanoarchitectonics Smart Deliv. Drug Target. 2016:813–869. doi: 10.1016/B978-0-323-47347-7.00029-X. Jan. [DOI] [Google Scholar]
- 85.Li J., et al. Copper-olsalazine metal-organic frameworks as a nanocatalyst and epigenetic modulator for efficient inhibition of colorectal cancer growth and metastasis. Acta Biomater. 2022;152:495–506. doi: 10.1016/j.actbio.2022.08.076. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z., et al. A reactive oxygen species-replenishing coordination polymer nanomedicine disrupts redox homeostasis and induces concurrent apoptosis-ferroptosis for combinational cancer therapy. Acta Biomater. 2022;151:480–490. doi: 10.1016/j.actbio.2022.07.055. [DOI] [PubMed] [Google Scholar]
- 87.Cun J.E., et al. Photo-enhanced upcycling H2O2 into hydroxyl radicals by IR780-embedded Fe3O4@MIL-100 for intense nanocatalytic tumor therapy. Biomaterials. 2022;287(July) doi: 10.1016/j.biomaterials.2022.121687. [DOI] [PubMed] [Google Scholar]
- 88.Daund V., Chalke S., Sherje A.P., Kale P.P. ROS responsive mesoporous silica nanoparticles for smart drug delivery: a review. J. Drug Deliv. Sci. Technol. 2021;64 doi: 10.1016/j.jddst.2021.102599. [DOI] [Google Scholar]
- 89.Cheng Y., et al. Free-blockage mesoporous anticancer nanoparticles based on ROS-responsive wetting behavior of nanopores. Small. 2017;13(40):1–9. doi: 10.1002/smll.201701942. [DOI] [PubMed] [Google Scholar]
- 90.Pei Y., et al. An autonomous tumor-targeted nanoprodrug for reactive oxygen species-activatable dual-cytochrome c/doxorubicin antitumor therapy. Nanoscale. 2018;10(24):11418–11429. doi: 10.1039/c8nr02358a. [DOI] [PubMed] [Google Scholar]
- 91.Ding X., et al. A pH/ROS-responsive, tumor-targeted drug delivery system based on carboxymethyl chitin gated hollow mesoporous silica nanoparticles for anti-tumor chemotherapy. Carbohydr. Polym. 2020;245 doi: 10.1016/j.carbpol.2020.116493. Oct. [DOI] [PubMed] [Google Scholar]
- 92.Liao J.X., Huang Q.F., Li Y.H., Zhang D.W., Wang G.H. Chitosan derivatives functionalized dual ROS-responsive nanocarriers to enhance synergistic oxidation-chemotherapy. Carbohydr. Polym. 2022;282(January) doi: 10.1016/j.carbpol.2021.119087. [DOI] [PubMed] [Google Scholar]
- 93.Zubair A.-H.A., Sheshe S.M., Bashir M.R., Sade S.M. Lipid based drug delivery system: a review. J. Appl. Life Sci. Int. 2021;(August):33–46. doi: 10.9734/jalsi/2021/v24i330228. [DOI] [Google Scholar]
- 94.Shrestha H., Bala R., Arora S. Lipid-based drug delivery systems. J. Pharm. (Lahore) 2014:1–10. doi: 10.1155/2014/801820. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel D.M., Jani R.H., Patel C.N. Ufasomes: a vesicular drug delivery. Sys. Rev. Pharm. 2011;2(2):72–78. doi: 10.4103/0975-8453.86290. [DOI] [Google Scholar]
- 96.Oono K., Ohtake K., Watanabe C., Shiba S., Sekiya T., Kasono K. Contribution of Pyk2 pathway and reactive oxygen species (ROS) to the anti-cancer effects of eicosapentaenoic acid (EPA) in PC3 prostate cancer cells. Lipids Health Dis. 2020;19(1):1–12. doi: 10.1186/s12944-019-1122-4. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong Y., et al. Iodinated cyanine dye-based nanosystem for synergistic phototherapy and hypoxia-activated bioreductive therapy. Drug Deliv. 2022;29(1):238–253. doi: 10.1080/10717544.2021.2023701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prasad R., et al. Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near‐infrared light mediated cancer therapy. Commun. Biol. 2020;3(1) doi: 10.1038/s42003-020-1016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marengo A., et al. Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochim. Biophys. Acta Gen. Subj. 2019;1863(1):61–72. doi: 10.1016/j.bbagen.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 100.Han J., Liu L., Yue X., Chang J., Shi W., Hua Y. A binuclear complex constituted by diethyldithiocarbamate and copper(I) functions as a proteasome activity inhibitor in pancreatic cancer cultures and xenografts. Toxicol. Appl. Pharmacol. Dec. 2013;273(3):477–483. doi: 10.1016/j.taap.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 101.Dalla Pozza E., et al. Gemcitabine response in pancreatic adenocarcinoma cells is synergistically enhanced by dithiocarbamate derivatives. Free Radic. Biol. Med. Apr. 2011;50(8):926–933. doi: 10.1016/j.freeradbiomed.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Yi H., et al. ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J. Nanobiotechnol. 2021;19(1) doi: 10.1186/s12951-021-00877-6. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang M., Sun S., Neufeld C.I., Perez-Ramirez B., Xu Q. Reactive oxygen species-responsive protein modification and its intracellular delivery for targeted cancer therapy. Angew. Chem. Dec. 2014;126(49):13662–13666. doi: 10.1002/ange.201407234. [DOI] [PubMed] [Google Scholar]
- 104.Liu L., et al. A smart theranostic prodrug system activated by reactive oxygen species for regional chemotherapy of metastatic cancer. Angew. Chem. Mar. 2022;134(12) doi: 10.1002/ange.202116807. [DOI] [PubMed] [Google Scholar]
- 105.Jani R.K., Patel N., Patel Z., Chakraborthy G.S., Upadhyay V. Nanosponges as a biocatalyst carrier — an innovative drug delivery technology for enzymes, proteins, vaccines, and antibodies. Biocatal. Agric. Biotechnol. 2022;42(February) doi: 10.1016/j.bcab.2022.102329. [DOI] [Google Scholar]
- 106.Chen W., Xie P., Pei M., Li G., Wang Z., Liu P. Facile construction of fluorescent traceable prodrug nanosponges for tumor intracellular pH/hypoxia dual-triggered drug delivery. Colloids Interface Sci. Commun. 2022;46 doi: 10.1016/j.colcom.2021.100576. [DOI] [Google Scholar]
- 107.Wang S., et al. Enhanced antitumor efficacy by a cascade of reactive oxygen species generation and drug release. Angew. Chem. Int. Ed. 2019;58(41):14758–14763. doi: 10.1002/anie.201908997. [DOI] [PubMed] [Google Scholar]
- 108.Uthaman S., Kim Y., Lee J.Y., Pillarisetti S., Huh K.M., Park I.K. Self-quenched polysaccharide nanoparticles with a reactive oxygen species-sensitive cascade for enhanced photodynamic therapy. ACS Appl. Mater. Interfaces. 2020;12(25):28004–28013. doi: 10.1021/acsami.0c06311. [DOI] [PubMed] [Google Scholar]
- 109.Kim Y., Uthaman S., Pillarisetti S., Noh K., Huh K.M., Park I.K. Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy. Acta Biomater. 2020;108:273–284. doi: 10.1016/j.actbio.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., et al. Combined and targeted drugs delivery system for colorectal cancer treatment: conatumumab decorated, reactive oxygen species sensitive irinotecan prodrug and quercetin co-loaded nanostructured lipid carriers. Drug Deliv. 2022;29(1):342–350. doi: 10.1080/10717544.2022.2027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Witika B.A., Matafwali S.K., Makoni P.A. Nanotechnology Principles in Drug Targeting and Diagnosis. Elsevier; 2023. Macrophage-mimetic nanomedicines for the treatment of diseases; pp. 63–89. [DOI] [Google Scholar]
- 112.Hou S., et al. Tumor microenvironment responsive biomimetic copper peroxide nanoreactors for drug delivery and enhanced chemodynamic therapy. Chem. Eng. J. Jul. 2021;416 doi: 10.1016/j.cej.2021.129037. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.