Abstract
The gut microbiota has a significant role in human health and is affected by many factors. Diet and dietary components have profound impacts on the composition of the gut microbiome and largely contribute to the change in bacterial flora. A high-fiber diet increased dietary fiber (DF) fermentation and the production of short-chain fatty acids (SCFAs), which increased the number of microorganisms. Microbiota-accessible carbohydrates (MACs), a subgroup of fermentable carbohydrates such as DF, are defined as indigestible carbohydrates metabolized by microbes. These carbohydrates are important components to sustain the microbial environment of the complicated digestive tract and avoid intestinal dysbiosis. Each MAC has a unique property and can therefore be used as a sensitive output microbiota modulator to support host homeostasis and modulate health. In addition to the overall health-developing effects, MACs are thought to have a promising effect on the prevention of non-communicable diseases (NCDs), which are major health problems worldwide. The aim of the manuscript was to describe microbiota-accessible carbohydrates and summarize their effects on gut modulation and NCDs.
Keywords: Gut microbiota, Diet, Dietary fiber, Microbiota-accessible carbohydrates, Health, Non-communicable diseases
1. Introduction
The gut microbiota is a dynamic, complex, and spatially heterogeneous ecosystem inhabited by numerous microorganisms, including archaea, bacteria, viruses, and fungi, that interact with each other and the host [1]. 25 different taxa, roughly 2000 genera, and 5000 species have been identified in the gut microbiota [2]. The gut microbiome plays pivotal roles in host immunity, digestion of nutrients, intestinal endocrine function, regulation of neurological signal transmission, elimination of toxins, and the production of numerous compounds that affect the host [3]. While the formation and proliferation of the intestinal microbiome begin at birth, changes in its composition are primarily influenced by genetic, nutritional, and environmental factors [4]. A deeper understanding of the variables influencing the makeup and functionality of this microbial community is necessary given the significance of the gut microbiota for human health [5]. The gut microbiota can be modulated to improve human health and treat or prevent diseases [4].
One of the key points influencing the homeostasis of the gut microbiota is the diet [6]. The microbial ecology in the intestine is significantly shaped by the diet. Due to variations in the availability of macronutrients and micronutrients in the intestine, dietary alterations can have a direct impact on the composition and functionality of the gut microbiota [7]. However, the most determining factor in healthy or unhealthy nutrition is the quality and content of the diet rather than the amount of food consumed [8].
Diet can alter intestinal pH, intestinal permeability, and bacterial metabolites, and therefore inflammation in addition to promoting the growth of specific bacterial groups [8,9]. Food components can modify the immune system in the host by directly influencing the barriers to gut bacteria populations and host metabolites. Each food component directly affects the health of the host by altering the intestinal epithelial barrier, commensal bacteria, and subsequently cell immunophenotypes and their pro- or anti-inflammatory responses [8].
It is well-known that dietary fiber (DF) has a significant effect on the gut microbiota [10]. According to the commonly recognized definition derived by the Codex Alimentarius Commission (CAC) in 2009, DFs are carbohydrate polymers with three or more monomeric units DFs are carbohydrate polymers with three or more monomeric units,which are neither digested nor absorbed in the small intestine [11,12]. A high-DF diet promotes the production of short-chain fatty acids (SCFAs) as a result of DF fermentation, leading to an increase in microbial diversity [7]. Through DFs fermentation, various intestinal bacterial populations can mediate inflammatory functions via SCFAs and endogenous signals to reduce inflammation [13].
A new classification regarding DFs, microbiota-accessible carbohydrates (MACs), are dietary substances that the gut microbiota uses and are crucial in determining the composition of the intestinal microbial ecology [8]. Sonnenburg et al. coined the concept of "microbiota-accessible carbohydrates", which are essentially equivalent to fermentable DFs and are defined as carbohydrates that can be used as growth substrates by intestinal microorganisms that have the necessary enzymatic capacity [14]. This definition excludes insoluble DFs, as they cannot be used by the gut microbiota [15]. MACs are the main source of energy for colonic bacteria [14,16]. MACs can modify the composition and function of the gut microbiota. They can also be used as "gut microbiota modulators" to improve the health of the host [17,18].
Non-communicable diseases (NDCs), commonly known as chronic diseases, are conditions that typically last for a significant amount of time and are brought on by a confluence of genetic, physiological, environmental, and behavioral variables. NDCs, such as obesity, Type 2 diabetes (T2DM), cardiovascular disease, and cancer, cause more than 70.0% of annual global deaths [19]. The 2030 Agenda for Sustainable Development also recognizes NCDs as a major challenge for sustainable development [20].
Nutrition is a key part of keeping people healthy and free of NCDs for longer periods [21]. MACs may also have a promising effect on chronic diseases and immune system [17]. The aim of this review article was to describe MACs and summarize their effects on gut modulation and NCDs.
2. Diet and gut microbiota
The influence of nutrition on gut microbiota modulation is widely accepted. It is well established that nutrition influences microbiota-related disease processes and health-promoting activities [22,23]. There is a mutual relationship between the gut microbiota and diet. Hence, dietary variables rank among the most potent influences on the structure and function of the microbiota [24]. The diet can impact the composition and diversity of the gut microbiota [25]. The effects of dietary changes on microbiota can be both short-term and long-term and vary between individuals [26].
Dietary choices cause differences in the composition of the gut microbiota. While the Western pattern diet reduces the intestinal microbial phyla and genera diversity, the Mediterranean diet positively affects the host's immune functions, as well as microbiota diversity and stability [27]. The composition and functionality of the microbiota can also be altered by nutrients, bioactive substances, and certain dietary patterns, with either good or negative impacts on human health [28]. High consumption of animal proteins, saturated fats, refined carbohydrates, and salt encourage the growth of pathogenic bacteria, which may affect the intestinal barrier and reduction the diversity and function of microorganisms [27]. In contrast, consumption of fruits and vegetables, plant proteins, polyphenols, n-3 fatty acids and DFs provides health benefits through the modulation of the gut microbiota [2].
2.1. What are MACs? What is the difference between DF and MACs?
Nondigestible fermentable carbohydrates (NDFCs), a diverse group of polysaccharides and oligosaccharides that are resistant to digestion and absorption by the host, are metabolically available to the gut microbiota. Prebiotics, fermentable DFs, and MACs, collectively known as nondigestible fermentable carbohydrates (NDFCs), effectively modulate the gastrointestinal microbiome. Although there is no exact distinction between them, these concepts are different from each other [29]. Similar to the varying definitions of DF, prebiotics also have varying definitions. In 2016, the International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus panel recommended "a substrate that is selectively used by host microorganisms while conferring a health benefit"as a definition for prebiotics, and this definition is still in use [30]. However, definitions of prebiotics that are incorrect and based on outdated scientific views also exist [29,[31], [32], [33]].
Most prebiotic carbohydrates are DFs, although not all DFs are considered prebiotics, according to modern criteria [29,34,35]. The classification of DFs as prebiotics is complicated, since a DF that acts as a prebiotic in one host may not act as a prebiotic in another host [30]. Thus, a precise distinction between prebiotic DFs and non-prebiotic DFs is impossible [33]. Many classifications have been created for DF that take into account solubility, viscosity, and fermentability [36]. On the other hand, the fermentability of foods can also change as a result of some food processing techniques [37,38]. This makes it difficult to give a clear definition and to distinguish between terms [33].
Although there is no universally accepted definition of DF, many definitions imply that each form of DF plays a part in controlling the composition of the gut microbiota, which in turn significantly influences host metabolism [39]. Dietary fiber is defined as edible plant parts or analogous carbohydrates that are difficult for humans to digest and absorb in the small intestine, with full or partial fermentation occurring in the large intestine [40]. DFs, such as arabinoxylans, galacto-oligosaccharides (GOS), inulin, and oligofructose, support the growth of various beneficial microorganisms while suppressing potentially damaging ones [41].
The functionality and therapeutic advantages of many forms of DF have been studied. However, the optimal types, amounts, and sources of DFs to be consumed for therapeutic effects are still questionable. Although uncertainties regarding DF intake, a daily intake of 25–35 g of DF for adults is recommended in most countries [42].
Most of the plant polysaccharides are structurally complex [43]. In general, the more complex the polysaccharide, the more enzymes are needed to break it down This requires "carbohydrate active enzymes". However, the human genome encodes 17 glycoside hydrolases (GH) and does not contain polysaccharide lyase (PL). As a result, glycans such as fructo-oligosaccharides (FOS), inulin, lignin, pectin, resistant starch, and inulin reach the large intestine undigested.
The gut microbiome compensates for the lack of GH and PL that are not encoded by the human genome. The gut microbiome can encode 130 GH families and 22 PL families [44]. Glycoconjugates, oligosaccharides, and polysaccharides are broken down into fermentable monosaccharides by these enzymes. A complicated cross-feeding metabolic network is started by fermentation during secondary breakdown, which produces acetate, propionate, formate, butyrate, lactate, and succinate [9]. One of the main jobs of the fecal and colonic microbiota is DF fermentation, which is also a significant source of SCFAs, the products of fermentation. According to current evidence, SCFAs play a key role in how soluble DFs promotes health [36].
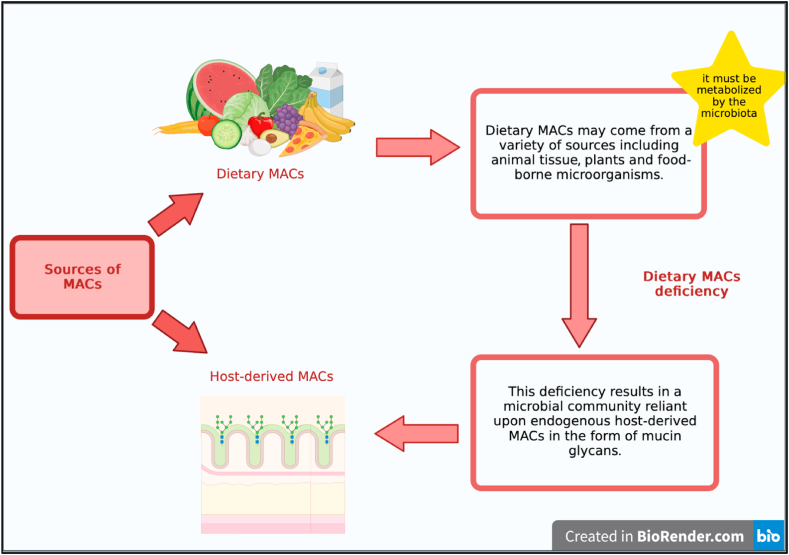
On the other hand, MACs are carbohydrates that can be fermented or "accessed" by an individual's microbiota and used metabolically by gut microbes [14]. MACs can be consumed through a diet or be derived from the host [29]. Dietary MACs can come from a range of sources, including plants, animal tissue,or food-borne microorganisms found in food and must be metabolizable by microbiota [14]. A lack of dietary MACs causes a microbial community to become dependent on endogenous MACs derived from the host, such as mucin glycans [45] (Fig. 1).
Fig. 1.
Main sources of MACs.
Whether or not an NDFC is regarded as a MAC is not just dependent on its physical and chemical properties. This is because the gastrointestinal microbiota of an individual host also should have the enzymatic capacity to metabolize said NDFC [29]. For example, cellulose is not digested by gut microorganisms and is not a MAC [45]. MACs are various oligosaccharide and polysaccharide groups that have significant structural heterogeneity and have various effects on microbial ecology [24].
Because each individual's microbiome determines which carbs are metabolized, the amount of dietary MACs contained in a certain food source varies between individuals [14]. For example, while genes associated with the metabolization of the algal polysaccharide porphyran are expressed in the microbiomes of Japanese individuals, these gene expressions are rarely observed in individuals from North America and Europe. Thus, porphyran would be regarded as a MAC for individuals who host a microbial strain that can metabolize porphyran in their guts, but it would not be regarded as a MAC for individuals who do not have adaptations for metabolizing seaweed [46]. Therefore, the concept of MAC is relevant for efforts aimed at personalizing human nutrition, which can provide individually customized dietary recommendations with the goal of including certain NDFCs known to be accessible by a person's gastrointestinal microbiota [29].
The availability of MACs largely determines the composition and function of the gut microbiota [47]. Each MAC has unique properties and can be used as a sensitive microbiota modulator to support host homeostasis [18]. The intake of different MACs has been associated with the proliferation of different microorganisms. For example, fructans, polydextrose, FOS, and GOS are associated with intestinal Bifidobacteria and Lactobacilli proliferation. On the other hand, resistant starch is associated with Ruminococcus, Eubacterium rectale, and Roseburia proliferation [48]. Because intestinal bacteria vary in their ability to use different MACs as a food source, the effect of MACs on gut microbiota and host physiology varies depending on the type of MAC. However, it remains unclear how each MAC affects the gut microbiota and regulates host physiology [18].
MACs are a resource that modulate the proportion of microbial taxa in the gut. They are also used in the production of metabolic products such as SCFAs [26]. These carbohydrates are functional components that help maintain the microbiome of the complex gastrointestinal tract and prevent intestinal dysbiosis [49].
MACs are the main source of energy for colonic bacteria, and abundance of MACs helps proliferate beneficial bacteria [16]. MACs act as selective agents that can change the microbiota's composition, and they also determine the microbiota's functionality and metabolic output [14]. The main mechanisms of action of MACs are believed to be increasing the production of SCFAs and modulating the gut microbiome [50]. These mechanisms are extremely dependent on the individual host microbiota and persist as long as carbohydrates are consumed [47].
The available data suggest that changes in microbiota composition and diversity caused by dietary MAC are related to host health. Although the mechanisms underlying the connection between microbiota diversity and health remain unknown, it is believed that SCFAs may serve as a possible intermediary of this connection [14]. SCFAs, which are produced by the fermentation of MACs in the colon, serve as intermediaries in various pathways, including local, immune, and endocrine effects, as well as microbiota-gut-brain communication [51].
3. Short-chain fatty acids
The primary metabolite of MAC fermentation in the colon is called SCFAs, which is a fatty acid with a carbon chain length ranging from one to six carbon atoms. In the intestine, 500–600 mmol of SCFAs are produced per day, depending on the amount of fiber in the diet. Acetate (C2), propionate (C3), and butyrate (C4) are the most commonly produced SCFAs in the lumen of the large intestine [52]. Other SCFAs, such as formate, caproate, and valerate, are produced in smaller quantities [53]. Based on the microbiota composition, substrate, and intestinal transit time, each SCFAs's quantity and relative ratio vary [50]. However, in general, the ratio of acetate, propionate, and butyrate in the colon is respectively 60:20:20 [54]. The fermentation of fiber into SCFAs in the colon reduces pH, increases fecal acidification and promotes the growth and taxonomic diversity of the gut microbiome [51]. The SCFAs profile of the human gut is shaped by microbial species. The production of propionate and acetate in the intestine is correlated with the presence of Bacteroides species, whereas butyrate is predominantly produced by Firmicutes [55].
Following their production in the colon, SCFAs that are specifically derived from the intestine are absorbed by the host epithelium. SCFAs are absorbed from the intestinal lumen through two mechanisms: SCFAs can be taken up by epithelial cells by simple diffusion. However, larger amounts of SCFAs can be absorbed into cells through active transport via monocarboxylate transporter 1 (MCT-1) and, to a lesser extent, through sodium-bound monocarboxylate transporter 1 (SMCT-1) [53,56].
SCFAs function as a crucial metabolic fuel because they can be used to create glucose and lipids, which the host uses as energy sources after being absorbed by host cells [53]. SCFAs are used in citric acid cycles and mitochondrial β-oxidation after being absorbed by colonocytes [57]. This provides colonocytes with a crucial source of energy. Butyrate is the primary source of energy for colonocytes. Its anti-inflammatory effects also contribute to maintaining intestinal homeostasis [51]. SCFAs also affect host insulin sensitivity and appetite regulation, and they can help prevent diet-related insulin resistance and obesity. SCFAs can also be used as a substrate for the synthesis of gluconeogenesis and cholesterol [53].
In the cecum and proximal colon, where colonocytes use them as a source of energy, there is a high concentration of SCFAs. However, through the portal vein, SCFAs can enter the peripheral circulation and affect the liver and other tissues [25]. Only a small portion of SCFAs is included in the systemic circulation and reaches other tissues [58]. Although SCFAs are included in the peripheral circulation in small amounts, it is now known that they function as signaling molecules and control various biological processes [25]. SCFAs are associated with various physiological processes, ranging from the regulation of blood pressure and circadian rhythms to natural and adaptive immunity [53,59,60].
SCFAs can have direct or indirect effects on cellular processes such as cell growth, differentiation, and gene expression. SCFAs also act as ligands for G-protein-coupled receptors (GPCRs), including G-protein-coupled receptor 41 (GPR41), G-protein-coupled receptor 43 (GPR43), and G-protein-coupled receptor 109A (GPR109A), and possess anti-inflammatory properties [51]. The activation of these receptors has greatly varying effects depending on the cell in which they are expressed. For example, SCFAs binding to GPCR receptors on enteroendocrine cells induces the secretion of intestinal hormones including glucagon-like peptide 1 (GLP1), aminobutyric acid (GABA), serotonin (5-HT), and peptide YY (PYY), which promotes the indirect signal transmission to the brain via systemic circulation or vagal pathways, whereas the signal transmission in pancreatic cells results in increased insulin secretion [61].
SCFAs also regulate histone deacetylase (HDAC) activity, which affects nuclear factor kappa B (NF-kB) inhibition [62]. SCFAs exert a wide range of effects on T-cell function by directly promoting the differentiation of natural T cells into regulatory T cells (Treg), T helper cell 1 (Th1), and T helper cell 17 (Th17), and indirectly inhibiting the differentiation of natural T cells into T helper cell 2 (Th2) [16]. Through GPR43, SCFAs affect the differentiation of Treg cells and the production of interleukin-10 (IL-10). They also regulate the function of dendritic cells (DC) [62]. By regulating the levels of metabolites and components associated with microbiota, including SCFAs and endotoxins, butyrate inhibits the expression levels of interleukin-1 (IL-1), interleukin-6 (IL-6), and Monocyte chemoattractant protein-1/Chemokine (CC-motif) ligand 2 (MCP1/CCL2) in the liver, as well as Toll Like Receptor 4 (TLR4) in adipose tissue [63]. Butyrate also affects the anti-inflammatory cytokine production, plasma B -cell proliferation, and antibody production [64]. It also supports the immune system's natural defense against pathogenic bacteria by activating antimicrobial molecules [65]. These functions of SCFAs affect their immunomodulatory potential and maintain a balance between pro- and anti-inflammatory processes [58].
SCFAs, whose circulating concentrations can be efficiently manipulated by diet, have both direct and indirect effects on the brain [53]. SCFAs can modulate the enteric nervous system, which affects intestinal motility and the gut-brain axis [65]. SCFAs can influence blood-brain barrier (BBB) integrity by upregulating the expression of tight junction proteins after passing through endothelial cells' MCTs [61].
Mucin-2 glycoprotein (MUC2) secretions constitute the main component of the colonic mucus layer, which is a dynamic and chemically complicated structure [66]. The mucus layer of the colon is an alternate source of host-derived glycans and contributes to the colonization of bacteria in the human intestine [67]. This layer functions as a physical barrier to protect the host from the intestine's high bacterial load [68]. Mucus and mucin glycosylation play a key role in shaping the microbiota and mediating host health by allowing the selection of the most suitable microbial species [69]. A compromised intestinal barrier may allow microbial metabolites to pass from the intestine into the systemic circulation, which can lead to metabolic diseases [68].
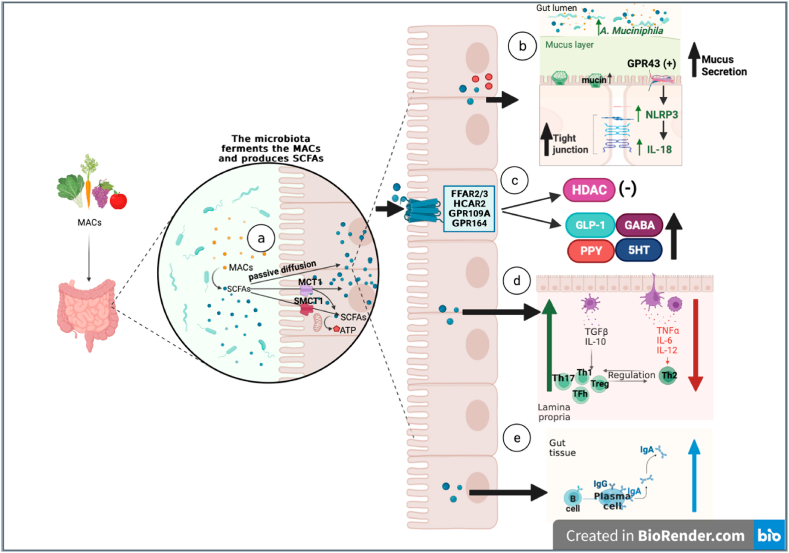
SCFAs have significant effects on the mucosal homeostasis of the intestine. By inducing intracellular or extracellular processes, SCFAs have positive effects on intestinal epithelial cells (IECs) and immune cells [51]. They fortify intestinal barrier integrity by supporting epithelial cell function [65]. SCFAs affect the inflammatory activation of absent in melanoma 2 (AIM2) and Nucleotide-binding oligomerization domain-like receptor 3 (NLRP3), which impacts the generation of interleukin-18 (IL-18) and improves epithelial barrier function. The secretion of IL-18 and the inflammatory activation of Nucleotide-binding oligomerization domain-like receptor 6 (NLRP6) regulate the synthesis of intestinal antimicrobial peptides (AMPs) [62]. SCFAs improve intestinal health through various local effects, including preserving intestinal barrier integrity, mucus production, and protection against inflammation, as well as reducing the risk of colorectal cancer [64]. The mechanisms of action of SCFAs are summarized in Fig. 2.
-
a.
Following intestinal bacterial hydrolysis or fermentation of MACs, SCFAs are absorbed by colonocytes either by passive diffusion or by active transport via MCTs (MCT1 or SCMT1). SCFAs are the main energy source for colonists and contribute to ATP production.
-
b.
The increase of SCFAs in the lumen increases the abundance of Akkermansia muciniphila. An increase in mucin and mucus synthesis has protective effects on tight junction integrity. The production of SCFAs increases the activation of NLRP3, resulting in the activation of GPR43 on epithelial cells, which increases the production of the epithelial healing cytokine IL-18. Additionally, ATP contributes to tight junction integrity.
-
c.
Through the stimulation of receptors on the membrane, SCFAs promote secretion of gut hormones such as GLP1, PYY, GABA and 5-HT. On the other hand, SCFAs inhibit HDAC.
-
d.
SCFAs stimulate the release of IL-10and inhibit the release of TNF-α, IL-6, and IL-12. However, it increases the growth of Treg, Th1, Th17, and Tfh and decreases Th2 development.
-
e.
SCFAs contribute to the direct activation of B cells and the production of IgA and IgG. Additionally, it leads to the plasma cell-mediated production of IgA.
Fig. 2.
Metabolic functions of short-chain fatty acids (SCFAs).
(MACs: Microbiota-accessible carbohydrates, SCFAs: Short chain fatty acids, MCT: H + -linked monocarboxylate transporters, MCT1: Monocarboxylate transporter 1,SCMT1: Sodium dependent monocarboxylate transporter 1, NLRP3: Nucleotide-binding oligomerization domain 3, GPR43: G-protein-coupled receptor 43, IL-18: Interleukin 18, GLP1: Glucagon-like peptide 1, PYY: Peptide YY, GABA: γ-aminobutyric acid, 5-HT: Serotonin, HDAC: Histone deacetylases, IL-10: Interleukin 10, TNF-α: Tumor necrosis factor alfa, IL-6: Interleukin 6,IL-12: Interleukin 12, Treg: Regulatory T cell,Th1: T helper 1 cell, Th17: T helper 17 cell, Tfh: T follicular helper cell,Th2: T helper 2,IgA: Immunoglobulin A,IgG: Immunoglobulin G).
4. Amounts of MACs in diets
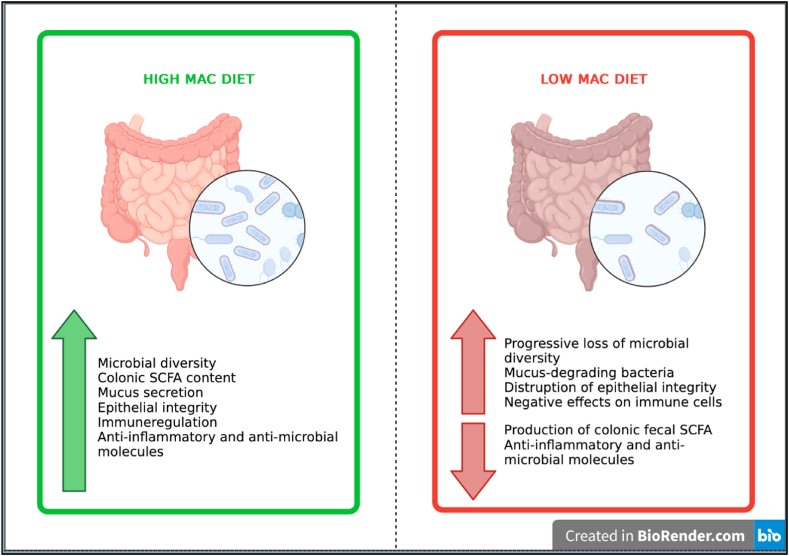
The diet composition, particularly the availability of MACs, has a significant effect on the gut microbiota [16]. It is thought that dietary MAC-induced changes in microbiota composition and diversity are linked to host health [50]. Fig. 3 summarizes the metabolic changes caused by low MAC intake and high MAC intake via diet.
Fig. 3.
Certain Changes in Gut Microbiota by the high MAC diet and the low MAC diet.
4.1. Low MAC diets
The gut microbiome is resistant to short-term degradation. It seems likely that microbiome adaptation can be largely reversed on short-term scales. On the other hand, a consequence of long-term and intergenerational low uptake of MAC also leads to a decrease in bacterial diversity and an irreversible loss of bacterial strains [14]. In a prior study, it was observed that changes in mice's microbiota, which were hosting strains found in human microbiota and fed with a diet low in MACs, were largely reversible within a single generation. However, a low-MAC diet over several generations causes a progressive loss of diversity that cannot be recovered by reintroducing dietary MACs [70]. Another study similarly showed that a diet with a low MAC content resulted in a reduction in a large number of specific bacterial taxa and reduced diversity, this effect persisting even after reintroduction of MACs [71]. Although comparable research on people has not been undertaken, it is known that, similar to mice, consuming a diet low in MACs lowers the diversity of bacteria in the human microbiome [16].
The changes caused by the modern lifestyle affect in the function and composition of microbiota [72]. In addition to the loss of microbial species across generations in industrialized countries, the lack of MACs required to feed this community causes severe functional changes, such as a decline in SCFAs production [14]. Low MAC consumption has harmful effects not only on the gut microbiota but also on the entire host. Low MAC consumption facilitates the development of diseases and increases mortality [70].
The gut microbiomes of cities and industrialized societies are different from those of traditional societies, according to data collected over time. It is observed that traditional societies have higher intestinal microbial diversity than urban-industrialized societies and have a lower rate of NCDs [70,73,74]. For example, the Yanomami subjects of South America are a group of people who live in a relatively isolated and remote area. Their microbiome has the most bacterial diversity and genetic functions ever found in a human group. Dietary practices in industrialized societies may eliminate potentially useful microorganisms and their encoded functions [75]. It has been shown that the Tanzanian Hadza, a hunter-gatherer community, has a higher microbial richness and diversity than the Italian urban groups [76]. In a study, the fecal microbiota of children from Europe and children from a remote African village in Burkina Faso, where the diet is high in DF and similar to the diets of early human settlements, have been compared. It has been revealed that the gut microbiota of the two groups differ significantly [73]. In another study, fecal samples were acquired from traditional agrarian and hunter-gatherer communities in Peru and from an urban-industrialized population in the United States of America. It was shown that the rural groups had higher levels of microbial richness [77]. In one study, the fecal microbiota of adults in two non-industrialized regions of Papua New Guinea was compared with that of a community in the United States. Researchers found that the Papua New Guineans hosted microbial communities with higher bacterial diversity, very different species abundance profiles and bacterial lineages, and lower interpersonal variation [74].
Changes in the microbiota induced by MACs can directly affect epithelial integrity. In low MAC environments, certain bacteria use the mucus layer as an energy source and disrupt the epithelial barrier by thinning the mucus layer. The overgrowth of bacteria that metabolize mucus and the decrease in bacteria that metabolize MAC decrease intestinal bacterial diversity [16]. Therefore, diets deficient in MACs cause the inner mucus layer to get thinner due to reduced MUC2 production, and bacteria can pierce deeper into the epithelium. This could lead to the mucosal barrier disruption, which in turn increases inflammation and susceptibility to pathogens [78]. This MAC-induced condition has been associated with increased sensitivity to Citrobacter rodentium, a pathogen that targets the epithelium, which indicates the importance of dietary MACs in fighting against gastrointestinal infections [66].
To maintain intestinal homeostasis, IECs create a physical and metabolic barrier between the host tissue and commensal microorganisms. Secretory IECs support this function through secretion of mucins and antimicrobial peptides [79]. Epithelial integrity has paramount importance for both intestinal and host health. SCFAs production modulates epithelial cells and the immune system [80]. Low MAC consumption causes impaired epithelial integrity and is associated with epithelial cytokine expression changes [16,81].
MACs can affect both bacterial-epithelial and epithelial-immune interactions [82]. An important consequence of low MAC consumption is a decrease in SCFAs production [29]. Low SCFAs production leads to decreases in the release of anti-inflammatory cytokines, whereas the release of pro-inflammatory cytokines increases [83]. Epithelial damage is caused by the production of pro-inflammatory cytokines (such as IL-1, interleukin-17 (IL-17), tumor necrosis factor alpha (TNF-α), or interferon gamma (INF-γ)) by natural and adaptive inflammatory cells that infiltrate the lamina propria (LP) [51]. Low SCFAs production also disrupts the activation of GPR43 on epithelial cells, which in turn increases the production of the pro-Th2 cytokine thymic stromal lymphopoietin (TSLP) while decreasing activation of NLRP3 and, ultimately, decreases the production of the epithelium-healing cytokine IL-18 [16]. In one study, mice fed a diet low in MACs had increased epithelial TSLP expression, which triggered an immune response and allergies. In the aforementioned study, this effect was reversed with the administration of a diet high in MACs [84]. On the other hand, mice fed with a MAC-free diet produced significantly less IL-18 [85,86].
Low IL-18 production caused by diets low in MACs can also affect immune cells. SCFAs deficiency disrupts Treg, Th1 cells, and Th17 cells while increasing Th2 cells. SCFAs deficiency also interferes with the production of IgA and immunoglobulin G (IgG) by reducing the direct activation of B cells and the indirect stimulation of T follicular helper cells (Tfh) [16].
In a mice model, it has been observed that diets low in MACs exacerbate gout and that acetate promotes recovery from neutrophilic inflammation via GPR43. This condition has been linked to enhanced neutrophil apoptosis mediated by caspase in mice [87,88]. Mice that produce low amounts of SCFAs due to low MAC consumption or microbial insufficiencies have poorer homeostatic and pathogen-specific antibody responses and are more sensitive to pathogens [89]. The amount of MAC in the diet changes the composition of intestinal and lung microbiota, particularly by changing the ratio of Firmicutes to Bacteroidetes. A diet containing low DFs/MACs reduces SCFAs levels and exacerbates allergic airway disease [90]. A higher consumption of SCFAs or MACs relieves this immune deficiency and lowers the severity of inflammation [89,90].
In comparison to a high-MAC diet, the most consistent effects of a low-MAC diet include a rise in Proteobacteria and a decrease in Bacteroidetes, as well as a reduction in bacterial diversity [84,89].
4.2. High -MAC diets
Increased dietary MAC consumption has been associated with the composition and diversity of microorganisms [14]. Increased dietary MAC consumption, sourced from a healthy diet and processed foods, supports the increase in beneficial microorganisms [91]. An umbrella review examined the associations between 14 dietary interventions and cardiovascular risk factors. It has been cited as the most important dietary intervention of the MAC recommendation [17].
Diets high in MACs can change the composition of the microbiota in humans within days or weeks [55]. Previous studies also support the idea that MACs have a role in modulating the gut microbiota [92,93]. It has been shown that feeding mice white button mushrooms high in DF for six weeks increased the number of Ruminococcaceae and Lachnospiraceae in the mouse fecal microbiome [94]. A high consumption of MAC has been linked to an increase in the fecal abundance of Bacteroidetes, particularly Prevotella, and the eradication of Firmicutes in children who are otherwise healthy. Children who have high MAC consumption also have greater amounts of SCFAs, which is connected with a lower prevalence of fecal infections, including Escherichia and Shigella, in their guts [95]. Clostridioides difficile can be suppressed by adding a complex mixture of MACs to the diet or by adding inulin alone as a source of MAC for a simplified diet [96].
SCFAs affect the T lymphocytes of the developing fetal immune system, resulting in a tolerogenic condition and a decreased risk of asthma in the baby [92]. The activation of T cells that produce Th1, Th17, and IL-10 by acetate and butyrate may play a crucial role in the protective effects of high MAC consumption against Citrobacter rodentium infections [97]. In one study, it was concluded that the methanolic extract of Hemidesmus indicus protected against oxidative stress, hyperlipidemia, and liver damage [98]. In another study, IgA production was shown to be significantly increased in mice fed with a diet containing a high amount of MACs compared to mice fed with a MAC-free diet. The production of TFh, IgA, and B cells in the intestine all increased in response to high MAC consumption [84].
In mice, high DF/MAC consumption improves oral tolerance and protects against food allergies. High DF/MAC consumption changes intestinal microbial ecology and increases the production of SCFAs, especially butyrate and acetate [84]. High-DF or acetate content suppresses the expression of genes in the embryonic lungs of mice that are associated with both human asthma and mouse allergic reactivity [99].
In a previous study, a diet rich in MACs was found to strengthen the endurance exercise capacity in mice. In contrast, antibiotic administration and a low-MAC diet modulated muscle fuel availability and impaired exercise capacity [93]. In another study, the effect of high MAC consumption on the microbiota-gut-brain axis as well and the cognitive performance of mice with obesity caused by a high-fat diet was evaluated. High MAC intake by the aforementioned mice has prevented gut microbiota dysbiosis, disruption of the colonic mucus barrier, endotoxemia, and cognitive impairment. It has also decreased systemic and colonic inflammation and an increase in serum SCFAs levels [100].
In addition to studies on high MAC intake through diet, other studies that increase the amount of MACs in foods have also been conducted. For example, whole grains have low MAC content. However, food processing affects the availability of carbohydrates to the microbiome, and processing can increase the MAC content in whole grains [101,102]. Baking bread is the most popular method of cereal preparation [103]. In a previous study, sourdough bread and other breads were found to enhance the amount of beneficial microorganisms belonging to the families Ruminococcaceae and Lachnospirae that ferment complex carbohydrates into SCFAs. Increased resistant starch content combined with small changes in the cell wall matrix caused by sourdough bread baking increased MAC quality, both in terms of complexity and variety [100].
5. MACs and certain non-communicable diseases (NCDs)
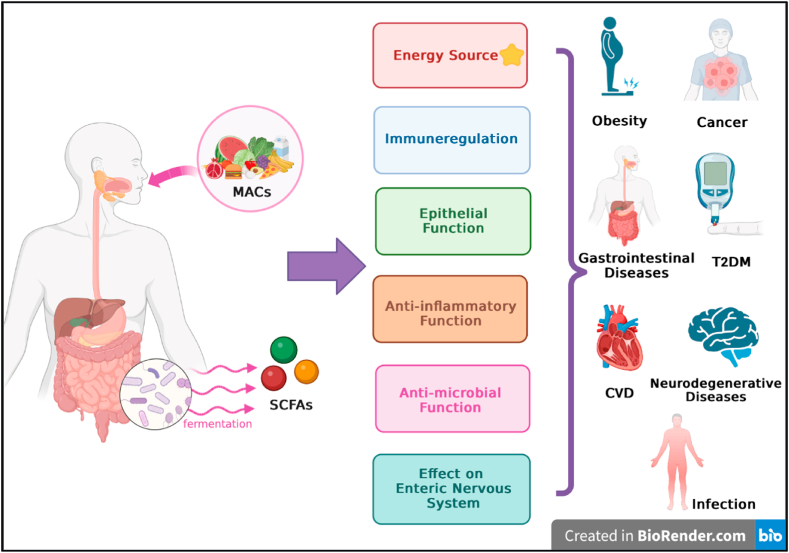
The majority of global annual deaths are caused by NCDs, and the incidence of these diseases is increasing. Healthy nutrition and modulation of dietary content can have a positive effect on combating the global burden of NCDs [104]. Defining a framework for nutrition research is the initial step in efforts to lower the risk of diet-related chronic diseases. Dietary patterns are a valuable nutritional assessment method for examining the association between food and the risk of chronic disease at the population level [105]. The content and variety of MACs in the diet has gradually decreased, particularly because of Westernization and the transition to foods rich in refined simple sugars [106,107]. Many factors associated with dysbiosis, such as the modern lifestyle and increased intestinal permeability, facilitate bacterial translocation in the host [6,16]. It is thought that the dysbiotic microbiota that is proliferating due to lower MAC levels may exacerbate the development of NCDs [16]. Dysbiotic microbiota, which is associated with NCDs, also leads to a substantial decrease in intestinal microbial biodiversity [6]. Because of this mutual interaction, low microbial diversity also contributes to obesity and diabetes [108]. It is thought that by regulating the microbiota in the gut, one might gradually improve the host's metabolic and immunological functioning, hence assisting in the prevention of chronic diseases or in the improvement of their symptoms [109]. Higher abundances of MAC-digesting bacteria and the resulting increase in SCFAs have positive effects [108]. MACs also have preventive effects in chronic diseases, based on their synergism with other nutritional components in regulating intestinal function [109]. Additionally, MACs have effects that improve the main metabolic risk factors associated with NCDs, which are increased blood pressure, overweight or obesity, hyperglycemia, and hyperlipidemia (Fig. 4) [110,111].
Fig. 4.
MACs and their promising effects on non-communicable diseases (NCDs).
5.1. Obesity
The gut microbiota of obese and lean people are different in composition, suggesting that microbiome dysbiosis play a role in changes in body mass index [112]. Obese and lean human subjects have different microbiota compositions. Obese subjects had a higher Firmicutes/Bacteroidetes ratio and Actinobacteria ratio, less diversity, and different representations of bacterial genes and metabolic pathways [113].
Compared to lean mice, the intestinal microbiome of obese mice has a larger number of enzymes that degrade carbohydrates that cannot be digested by the host. This enzyme richness increases SCFAs production [110,114]. Microbial products such as SCFAs, can regulate appetite, lipogenesis, gluconeogenesis, inflammation, and other metabolic activities [115]. SCFAs have a significant impact on the inflammation caused by obesity. SCFAs affect appetite through substrates for lipid storage and G-protein-coupled receptor signaling, in addition to serving as the main source of energy for colonocytes [110]. A systematic review of seven human clinical trials with a total of 246 obese cases and 198 healthy controls demonstrated that obese subjects had higher amounts of fecal acetate, propionate, butyrate, and other SCFAs than lean subjects [116]. There are contradictory findings concerning the association between SCFAs and obesity in the literature [117]. For example, in a number of interventional studies, it has been observed that high SCFAs levels was positively correlated with weight loss and insulin sensitivity, modulated through the activation of PYY and GLP-1 by SCFAs [118]. PYY modulates appetite and satiety by either delaying gastric emptying or inhibiting neuropeptide Y (NPY) and stimulating proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (ARC) [119]. On the other hand, it has been suggested that the regulating effects of SCFAs on appetite and energy expenditure become ineffective in the context of obesity, so that obese people do not benefit from high levels of SCFAs [117].
According to a study, l-arabinose and sucrose, two MACs that affect diet-related obesity and gut microbiota metabolism, work together on certain bacteria, such as Bacteroides, to synergistically increase acetate and propionate concentrations in the intestine, thereby preventing diet-related obesity [18]. Acetate can directly modulate satiety through a primary mechanism in the central nervous system, whereas propionate can influence body weight regulation through sympathetic nervous system activity and activation of intestinal gluconeogenesis [119]. It has been shown in inulin intervention studies in obese children that inulin reduced overall fat masses and body weight gain [120].
MACs that complement the fight against malfunction offer a promising approach to improving Westernized dietary patterns and preventing obesity-related neurodegenerative diseases [110]. It has been suggested that adding DF to existing food products is an effective way to boost MAC content, microbiome diversity, and fermentation end product output [121].
5.2. Type 2 diabetes (T2DM)
Substantial evidence suggests that the gut microbiota play a role in T2DM [17]. The microbiota composition of T2DM patients and healthy controls differs significantly [122]. T2DM patients' gut microbiota and fecal metabolites are characterized by an abundance of Proteobacteria, a much higher ratio of Firmicutes/Bacteroidetes than in healthy individuals, and significantly irregular SCFAs concentrations [123]. In one of the first large-scale microbiota investigations conducted on persons with T2DM, it was found that T2DM patients had significantly decreased abundance of butyrate-producing bacteria, such as Roseburia intestinalis and Faecalibacterium prausnitzii, compared with healthy controls. Additionally, it was found that the microbiota of T2DM patients included a much larger number of harmful bacteria, such as Desulfovibrio, Clostridiales, and Bacteroides caccae [15,124]. Another study found that people with diabetes have lower levels of butyrate-producing bacteria and a higher frequency of opportunistic infections [124]. The abundance of Bifidobacteriaceae and the diversity of gut microbiota have been found to be significantly lower in T2DM patients than in healthy subjects, and although GOS consumption has not improved the glucose tolerance of these patients, it has significantly improved their Bifidobacteriaceae infections [125].
Dietary intervention in patients with T2DM patients modulates the gut microbiota and improves glucose control [126]. Generally, the role of DF in physically encasing/retaining nutrients is linked to the main mechanism of action of MACs in the regulation of blood glucose in patients with T2DM. This slows the rate of digestion of plant tissues and reduces the increase in blood glucose [127,128]. According to the available studies, MAC consumption improves glycemic control, blood pressure, blood lipid concentrations, obesity, and inflammation in patients with T2DM patients. These effects may be attributable to SCFAs, the primary bacterial byproducts of MAC fermentation. SCFAs can decrease metabolic inflammation, improve glycemic control and modulate satiety, preventing the progression of T2DM [17]. One study has shown that increasing the amount of indigestible carbohydrates reaching the intestines may be sufficient to improve T2DM patients' metabolic parameters [129]. The consumption of a diet with high DF/MAC content can decrease body weight and improve hemoglobin A1c (HbA1c) levels [130]. SCFAs can activate the secretion of both GLP-1 and PYY by enteroendocrine L-cells [131]. Through increased GLP-1 production, this increases glucose usage and insulin sensitivity, reducing HbA1c. SCFAs also reduce microbes that produce metabolically harmful compounds such as indole and hydrogen sulfide [129,131]. Another study found that a milk powder supplement fortified with inulin and resistant dextrin improved islet B cell function, decreased blood glucose, and reduced blood pressure in elderly T2DM patients compared with the placebo group [132]. The results of the aforementioned studies provide supporting evidence that consuming a diet rich in DFs/MACs may have positive effects on T2DM diagnoses and the course of the disease [17].
5.3. Cardiovascular diseases
It has been reported that patients with T2DM patients have a greater incidence of hypertension. Hypertension is a serious risk factor for cardiovascular disease, which is linked to mortality and morbidity in patients with DM patients [133]. Prior research has shown that a 10 mmHg reduction in systolic blood pressure reduces the risk of cardiovascular disease by 20%, heart failure by 28%, stroke by 27%, and the chance of mortality from any cause [134]. According to a meta-analysis conducted in 2015, people who ate a high-DF diet had reductions in both their systolic and diastolic blood pressure, with the latter effect being more pronounced in people who consumed more beta-glucan [135]. A similar study compared the serum cholesterol levels and blood pressure of participants who consumed high amounts of soluble viscous DF with those receiving a placebo. As a result of the study, decreases in blood pressure and cholesterol levels were observed in the group with high soluble viscous DF consumption [130].
In other meta-analyses and an umbrella review, it has been shown that high DF consumption improves cardiovascular risk factors. The correlation between MACs and cardiovascular risk factors was supported by evidence ranging from moderate to high quality. Increased consumption of MAC reduces LDL and TG levels while increasing HDL levels [17]. In a study conducted on the psyllium- and placebo-administered groups of patients, a decrease in total cholesterol, TG, and LDL levels, and an increase in HDL levels were observed in the psyllium group, whereas no significant changes were observed in the placebo group [136]. Consumption of psyllium effects intravascular processing of lipoproteins by decreasing the cholesteryl ester transfer protein activity through changes in VLDL composition [136,137]. It is well known that oats can prevent induced hyperlipidemia in rats and humans. In a study, oat flakes were found to positively affect the lipid metabolism of rats fed a high-fat diet. In the aforementioned study, four different types of oats exhibited distinct nutritional qualities and hypolipidemic effects. β-Glucan extracted from the Bayou-1 variety of said oats were the most effective in preventing serum lipid levels from increasing [138].
There is growing evidence that SCFAs may play a role as a metabolic tool in the prevention and treatment of obesity and obesity associated cardiometabolic risk factors, such as insulin resistance [119]. SCFAs have the ability to improve adipose tissue's lipid storage capacity, which in turn lowers the amount of circulating TG and prevents ectopic fat storage. SCFAs may also reduce inflammation in adipose tissue, possibly via lowering levels of CRP and TNF-a as well as downregulating ectopic fat deposits in non-adipose tissues, all of which are related to insulin resistance remission [17].
Glutathione (GSH), a marker of oxidative stress, plays a crucial role in detoxifying oxygen radicals and can prevent cellular damage resulting from oxidative stress. Reduced GSH levels have been linked to cardiovascular disease [139]. A study has shown that mice fed with resistant starch have higher circulating GSH levels, suggesting that they may be exposed to less oxidative stress [140].
5.4. Gastrointestinal diseases
Diseases related to intestinal metabolism have become an important public health problem due to their widespread prevalence and harmful effects. Functional oligosaccharides promote the proliferation of probiotics, regulate immunological responses, and increase SCFAs production by enabling the metabolism of intestinal bacteria [141]. High psyllium intake can alleviate the symptoms of chronic constipation and reduce body weight, glycemic, and cholesterol levels in patients with T2DM and chronic constipation [136]. In one study, the symptoms, glycemia lipids, and weight of chronic constipation patients with T2DM who consumed high amounts of flaxseed or psyllium were compared with those of patients who took a placebo. Because of the aforementioned study, both nutritional substances provide positive effects to constipated patients with T2DM, but flaxseed is superior to psyllium when it comes to relieving constipation and managing weight, glycemic, and lipid levels. However, these benefits of flaxseed and psyllium were not sustained over the long term [142].
The decreased diversity of the gut microbiota in patients with inflammatory bowel diseases (IBD) patients is a significant feature [143]. The modern Western diet is also considered an environmental factor in the cause of IBD [144]. Functional oligosaccharides may regulate inflammatory cytokines to alleviate intestinal diseases [141]. Evidence suggests that inulin's ability to modify intestinal barrier function and deliver anti-inflammatory benefits through modulating dysbiosis of the gut microbiota and metabolic butyrate help alleviate colon inflammation [143].
The mucus layer in the colon is an early barrier that bacteria must infiltrate. A microbiota that is not adequately supplied with DF results in the thinning of this barrier, indicating that DF consumption has significant implications on susceptibility to gastrointestinal pathogens. In this context, patients with IBD patients have higher levels of mucolytic bacteria [66]. Bifidobacterium and other symbiotic bacteria ferment indigestible oligosaccharides, which result in the production of SCFAs, vitamins, and various other small molecules beneficial to the structural integrity of the intestinal epithelial barrier [141]. Prebiotics increase the secretion of GLP-2 by increasing L-cell activity and Akkermansia muciniphila count, which can strengthen the intestinal barrier and improve intestinal tight junctions. Prebiotics can also increase endogenous 2-arachidonoylglycerol (2-AG) levels and reduce systemic inflammation and metabolic endotoxemia by increasing Akkermansia muciniphila counts [145]. Peptic polysaccharides (PPSs) can efficiently manage the dysbiosis of the gut microbiota, which is one of the anti-IBD modes of action. Additionally, PPSs increase the diversity of gut microbiota while decreasing the intestinal bacterial load [143].
Ulcerative colitis, a type of IBD, is characterized by the infiltration of inflammatory cells into the colon mucosa. According to the available evidence, GOS may can reduce the harm caused by colitis [146]. Patients with ulcerative colitis who received 2.8-g GOS per day for six weeks showed an improvement in stool consistency and a reduction in the incidence and severity of loose stools [147]. GOS treatment inhibits the activation of the NF-kB signaling pathway, significantly decreasing the secretion of IL-6, IL-13, IL-18, and IL-33, as well as the expression of mRNA in the colon, and induces a Th17/Treg imbalance by regulating the intestinal flora. All of these findings suggest that GOS protect mice from dextran sulfate sodium-induced colitis [146]. In clinical trials, FOS and inulin have improved the course of IBD, possibly by repairing intestinal penetrations or through direct interactions with gut microbes or immune cells [148]. Efforts to identify optimal combinations of DF to be consumed and the minimum intake required for restoring the integrity and elasticity of the colonic mucus layer are of critical importance [66].
5.5. Neurodegenerative diseases
The human gut microbiome can have a big effect on the activity of the central nervous system in a number of ways. These include the physiological effects of microbiota, changes in the way the intestinal barrier works, and changes in the activity of neurons in the periphery. The gut-brain axis controls immunological activity, which is considered a crucial component of neurodegenerative diseases [149]. Research on the gut-brain axis suggests that the gut microbiota plays a significant role in the coordination of brain development and behavior, and that metabolites that are mediated by the gut microbiota are a primary regulator of these interactions [150].
Lipopolysaccharide (LPS) is the primary component of gram-negative bacteria's outer cell wall and is modulated by stress via changes in the gut microbiota [151]. LPS has triggered BBB degradation, which has been linked to a number of neurodegenerative diseases [152]. Toll-like receptors (TLRs) identify LPS and signal for the production of inflammatory cytokines such TNFα, IFNγ, and IL-6, which impact the hypothalamic-pituitary-adrenal (HPA) axis and its metabolites [153]. By crossing the BBB and altering neurotransmitter metabolism, LPS affects the activation of the HPA axis, the disruption of synaptic plasticity, activation of afferent nerve DFs, and the development of depression [154]. Decreases in tight junction proteins increase BBB permeability, but high MAC intake can correct this deficit [100].
Eucommia cortex polysaccharide (EP) intake effectively inhibits intestinal dysbiosis, thereby reducing neuroinflammation and serum endotoxin. Additionally, high EP consumption lowers the levels of glutamic acid and quinolinic acid in the hippocampus and aids in treating metabolic syndrome, which is a result of an obesogenic diet. The recovery of adult behavioral impairments and neurogenesis deficits in mice on an obesogenic diet has been facilitated by these effects [150].
The high consumption of MAC significantly increases the abundance of the Bacteroidetes phylum and decreases the abundance of the Proteobacteria phylum. Studies indicate that High MAC intakes improve the diversity and composition of the gut microbiota, which may contribute to the prevention of cognitive decline in mice fed a high-fat, low-DF diet [100]. Resistant dextrin increased the abundance of Bifidobacterium. Bifidobacterium and Lactobacillus metabolize glutamate to generate GABA, which can alter the expression of GABA receptors in key brain regions associated with central nervous system stress. Additionally, Bifidobacterium can improve mental health by increasing tryptophan levels, a precursor of serotonin, which increases the availability of serotonin [154]. PYY is associated with anxiety, and it can increase resistant dextrin GLP2 and PYY neuropeptide levels, which can improve anxiety [154,155]. It has also been reported that some types of inulin are antidepressant-like in behavioral patterns of depression [156].
5.6. Cancer
Even though cancer is caused by plenty of factors, diet is an important modifiable risk factor. DFs have been known for a long time as an essential dietary component that can influence cancer risk. DFs and colorectal cancer (CRC) have been the subject of much research [157]. There is conflicting information about the link between DF intake and the risk of colorectal adenoma (CRA), which is a precursor to colorectal cancer. A meta-analysis of case-control and cohort studies has been used to look into this link. This meta-analysis backs up the idea that a high intake of DF, especially DF from fruits and grains, is linked to a lower risk of CRA [158].
It has been indicated that fecal SCFAs concentrations are not correlated with the presence of adenomas or carcinomas, have poor predictive ability, and do not aid in the diagnosis of a person with one of these lesions [159]. SCFAs induce apoptosis in cancerous cells, regulate tumor suppressor gene expression via histone deacetylase inhibition and regulate cellular glucose metabolism [160]. On the other hand, comparing the fecal SCFAs concentrations of healthy people with those of people with carcinoma or adenoma showed that there is no link between fecal SCFAs concentrations and tumor load or the bacteria in the feces [159].
MAC-microbiota interactions can affect the colonic environment and prevent and control cancer independently from the potential effects of SCFAs [157]. Low MAC intake may result in intestinal mucosa glycans as a food source by microorganisms [161]. This weakens the epithelial mucosa, increases the microbial invasion of commensal bacteria, and triggers inflammation in gnotobiotic mice's epithelial cells [66]. Similarly, mice fed a Western diet low in DF showed increased microbial infiltration and changed MUC2 protein levels in the mucus layer [162]. Although it has not been proven that these changes are directly related to cancer, individuals with ulcerative colitis have an increased risk of developing CRC ranging from 2% to 18%, depending on the duration of the disease [163]. The literature indicate that MAC-mediated microbiota interactions may alter mucus production and mucosal immune responses to anti-inflammatory and, subsequently, tumor suppressor profiles. However, the significance of these mechanisms in human populations has not yet been determined [157].
The results of studies examining the association between cereal DF consumption and the risk of various types of cancer have been inconsistent. Although cereal DF consumption significantly reduces the risk of gastrointestinal and head and neck cancer, it did not significantly reduce the risk of breast and colorectal cancer. On the other hand, no relationship has been found between female and genitourinary cancers and cereal DF consumption [164].
Inflammation, insulin resistance, hyperinsulinemia, and increased concentrations of circulating adrenergic or estrogenic hormones can stimulate prostate carcinogenesis. It has been shown that MAC intake plays a potentially protected role against prostate carcinogenesis through direct or indirect modulation of these mechanisms [165].
The biological mechanisms by which MAC exerts its anticarcinogenic effects are not yet fully understood, despite important developments regarding the role of microbiomes [166].
6. MACs and Clostridioides difficile infection
Clostridioides difficile (CD) is an opportunistic diarrheal pathogen. The most important risk factor for Clostridioides difficile infection (CDI) is the dysbiosis of the gut microbiota, which is typically brought on by antibiotic use. Hence, methods targeted at understanding and reducing CD pathogenesis may include treatments aimed at positively altering the composition and function of the gut microbiome [167].
Dietary fat increases the mortality rate related to CD [96]. In one study, the introduction of exogenous pig mucin glycans decreases host mucus consumption in the lack of dietary MACs and suppresses CD abundance [168]. Diets containing MAC mixtures such as inulin, xanthan gum, FOS have also been used to reduce CD burden below detection levels. This effect can also be observed by adding a complex mixture of MACs to the diet or adding inulin alone as a source of MAC for a simplified diet [96]. MACs are a particularly productive avenue for diet-oriented studies on CD. Inulin and FOS have different effects on CD loads in mice. There is a possibility that sources of MAC that eliminate CDI may not do so in a different microbiome and host [167]. The health effects of MACs are summarized in Table 1.
Table 1.
Summary of the health effects of MACs.
| MACs | Health Effects | References |
|---|---|---|
| l-arabinose and Sucrose |
|
[119] |
| Inulin and Resistant dextrin |
|
[132] |
| Psyllium |
|
[135] |
| Β-Glucan |
|
[138] |
| Resistant starch |
|
[140] |
| Inulin |
|
[143] |
| Pectic polysaccharides |
|
[143] |
| Galacto-oligosaccharides (GOS) |
|
[146,147] |
| Eucommia cortex polysaccharide (EP) |
|
[150] |
| Resistant dextrin |
|
[154] |
| Inulin, Xanthan gum and Fructo-oligosaccharides (FOS) |
|
[167] |
7. Conclusions
The gut microbiota is important due to its regulatory roles in health and disease. Dietary variables are among the most effective modulators of microbiota composition and function due to the reciprocal interaction between nutrition and gut microbiota. MACs are defined as carbohydrates that can be digested by bacteria. MACs are the primary energy source for colonic bacteria, and abundance of MACs promotes the proliferation of beneficial bacteria. Each MAC has unique properties and can be used as a sensitive microbiota modulator to support host homeostasis. Most of the effects of MACs on metabolism occur through SCFAs mechanisms. SCFAs serve as mediators in multiple pathways, such as intestinal homeostasis, endocrine effects, immunoregulation, and microbiota-gut-brain communication. MACs show health-enhancing effects with these metabolic functions. MACs are also effective on metabolic risk factors such as increased blood pressure, obesity, hyperglycemia, and hyperlipidemia. MACs are also thought to have positive effects on preventing NCDs and slowing down the course of disease. Based on the current data, it is thought that high MAC-containing diet consumption and personalized nutrition will have an impact on microbiota modulation and health.
Funding
Not applicable.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgement
Figures were created with BioRender.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19888.
Contributor Information
Gamze Ayakdaş, Email: gamze.ayakdas@acibadem.edu.tr.
Duygu Ağagündüz, Email: duyguturkozu@gazi.edu.tr.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 5-HT
Serotonin
- AIM2
Absent in melanoma 2
- AMPs
Antimicrobial peptides
- ARC
Arcuate nucleus
- BBB
Blood-brain barrier
- CCL2
Chemokine (CC-motif) ligand 2
- CD
Clostridioides difficile
- CDI
Clostridioides difficile infection
- CRA
Colorectal adenoma
- CRC
Colorectal cancer
- CRP
C-reactive protein
- DC
Dentritic cells
- DF
Dietary fiber
- EP
Eucommia cortex polysaccharide
- FOS
Fructo-oligosaccharides
- GABA
Aminobutyric acid
- GLP-1
Glucagon-like peptide-1
- GLP-2
Glucagon-like peptide-2
- GOS
Galacto-oligosaccharides
- GPCRs
G-protein-coupled receptors
- GPR109A
G-protein-coupled receptor 109A
- GPR41
G-protein-coupled receptor 41
- GPR43
G-protein-coupled receptor 43
- GSH
Glutathione
- HbA1c
Hemoglobin A1c
- HDAC
Histone deacetylase
- HDL
High-density lipoprotein
- HPA
Hypothalamic-pituitary-adrenal
- IBD
Inflammatory bowel diseases
- IECs
Intestinal epithelial cells
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL-13
interleukin 13
- IL-17
interleukin-17
- IL-18
Interleukin-18
- IL-33
interleukin 33
- INF-γ
interferon gamma
- ISAPP
International Scientific Association of Probiotics and Prebiotics
- LDL
Low-density lipoprotein
- LP
lamina propria
- LPS
Lipopolysaccharide
- MACs
Microbiota-accessible carbohydrates
- MCP1
Monocyte chemoattractant protein-1
- MCT-1
Monocarboxylate transporter 1
- MUC2
Mucin-2 glycoprotein
- NCDs
Non-communicable diseases
- NDFCs
Nondigestible fermentable carbohydrates
- NF-kB
nuclear factor kappa B
- NLRP3
Nucleotide-binding oligomerization domain-like receptor 3
- NLRP6
Nucleotide-binding oligomerization domain-like receptor 6
- NPY
Neuropeptide Y
- POMC:
Proopiomelanocortin
- PPSs
Peptic polysaccharides
- PYY
Peptide YY
- SCFAs
Short-chain fatty acids
- SCMT-1
Sodium-bound monocarboxylate transporter 1
- T2DM
Type 2 diabetes
- Tfh
T follicular helper cells
- TG
Triacylgylcerol
- Th1
T helper cell 1
- Th17
T helper cell 17
- Th2
T helper cell 2
- TLR4
Toll Like Receptor 4
- TLRs
Toll-like receptors
- TNF-α
Tumor necrosis factor alpha
- Treg
regulatory T cells
- TSLP
Thymic stromal lymphopoietin
- VLDL
Very low-density lipoprotein
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Chen Y., Zhou J., Wang L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berding K., et al. Diet and the microbiota–gut–brain Axis: sowing the seeds of good mental health. Adv. Nutr. 2021;12(4):1239–1285. doi: 10.1093/advances/nmaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 4.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie Leeuwenhoek. 2020;113(12):2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 5.Wastyk H.C., et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–4153 e14. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klement R.J., Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina. 2019;55(4):84. doi: 10.3390/medicina55040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubert C., et al. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol. Dis. 2020;134 doi: 10.1016/j.nbd.2019.104621. [DOI] [PubMed] [Google Scholar]
- 8.García-Montero C., et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients. 2021;13(2) doi: 10.3390/nu13020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A.S., et al. Diet and the human gut microbiome: an international review. Dig. Dis. Sci. 2020;65(3):723–740. doi: 10.1007/s10620-020-06112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Menezes E.W., et al. Codex dietary fibre definition – justification for inclusion of carbohydrates from 3 to 9 degrees of polymerisation. Food Chem. 2013;140(3):581–585. doi: 10.1016/j.foodchem.2013.02.075. [DOI] [PubMed] [Google Scholar]
- 12.Codex Alimentarius Commission (CAC) 2009. Report of the 31st session of the codex committee on nutrition and foods for special dietary uses, Düsseldorf, Germany (2009) 2–6 November; pp. 1–3. in ALINORM 10/33/26. [Google Scholar]
- 13.Hills R.D., Jr., et al. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11(7) doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnenburg E.D., Sonnenburg J.L. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metabol. 2014;20(5):779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin P., et al. Dietary fibre modulates the gut microbiota. Nutrients. 2021;13(5):1655. doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daien C.I., et al. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: an overview. Front. Immunol. 2017;8:548. doi: 10.3389/fimmu.2017.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu B., et al. Higher intake of microbiota-accessible carbohydrates and improved cardiometabolic risk factors: a meta-analysis and umbrella review of dietary management in patients with type 2 diabetes. Am. J. Clin. Nutr. 2021;113(6):1515–1530. doi: 10.1093/ajcn/nqaa435. [DOI] [PubMed] [Google Scholar]
- 18.Tomioka S., et al. Cooperative action of gut-microbiota-accessible carbohydrates improves host metabolic function. Cell Rep. 2022;40(3) doi: 10.1016/j.celrep.2022.111087. [DOI] [PubMed] [Google Scholar]
- 19.Organization W.H. 2022. Noncommunicable Diseases Progress Monitor; p. 2022. [Google Scholar]
- 20.Cf O. United Nations; New York, NY, USA: 2015. Transforming Our World: the 2030 Agenda for Sustainable Development. [Google Scholar]
- 21.Ruthsatz M., Candeias V. Non-communicable disease prevention, nutrition and aging. Acta Biomed. 2020;91(2):379–388. doi: 10.23750/abm.v91i2.9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merra G., et al. Influence of mediterranean diet on human gut microbiota. Nutrients. 2020;13(1) doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David L.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentile C.L., Weir T.L. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 25.Makki K., et al. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenburg J.L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinninella E., et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos S., Martín M.Á. Impact of diet on gut microbiota. Curr. Opin. Food Sci. 2021;37:83–90. [Google Scholar]
- 29.Deehan E.C., et al. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol. Spectr. 2017;5(5) doi: 10.1128/microbiolspec.bad-0019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson G.R., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 31.Katsnelson A. Core Concept: prebiotics gain prominence but remain poorly defined. Proc. Natl. Acad. Sci. U. S. A. 2016;113(50):14168–14169. doi: 10.1073/pnas.1618366113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bindels L.B., et al. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015;12(5):303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 33.Verspreet J., et al. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 2016;7:167–190. doi: 10.1146/annurev-food-081315-032749. [DOI] [PubMed] [Google Scholar]
- 34.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan Z.-W., Yu E.-Z., Feng Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules. 2021;26(22):6802. doi: 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu J., et al. In vitro digestion and colonic fermentation of UHT treated faba protein emulsions: effects of enzymatic hydrolysis and thermal processing on proteins and phenolics. Nutrients. 2023;15(1):89. doi: 10.3390/nu15010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Mantrana I., et al. Expression of bifidobacterial phytases in Lactobacillus casei and their application in a food model of whole-grain sourdough bread. Int. J. Food Microbiol. 2016;216:18–24. doi: 10.1016/j.ijfoodmicro.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Stephen A.M., et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30(2):149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 40.Park Y., et al. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011;171(12):1061–1068. doi: 10.1001/archinternmed.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Q., et al. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12(2) doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gill S.K., et al. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18(2):101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 43.Sonnenburg J.L., Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaoutari A.E., et al. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11(7):497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 45.Paone P., Cani P.D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hehemann J.H., et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464(7290):908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 47.Valdes A.M., et al. Role of the gut microbiota in nutrition and health. Bmj. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponzo V., et al. Diet-gut microbiota interactions and gestational diabetes mellitus (GDM) Nutrients. 2019;11(2) doi: 10.3390/nu11020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tingirikari J.M.R. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018;9(10):5059–5073. doi: 10.1039/c8fo01296b. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N., et al. Functional oligosaccharide fermentation in the gut: improving intestinal health and its determinant factors-A review. Carbohydr. Polym. 2022;284 doi: 10.1016/j.carbpol.2021.119043. [DOI] [PubMed] [Google Scholar]
- 51.Parada Venegas D., et al. Short chain fatty acids (SCFAs)-Mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalile B., et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 53.O'Riordan K.J., et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022;546 doi: 10.1016/j.mce.2022.111572. [DOI] [PubMed] [Google Scholar]
- 54.Martin-Gallausiaux C., et al. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021;80(1):37–49. doi: 10.1017/S0029665120006916. [DOI] [PubMed] [Google Scholar]
- 55.Portincasa P., et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int. J. Mol. Sci. 2022;23(3):1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Hee B., Wells J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J. Lipid Res. 2016;57(6):943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratajczak W., et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs) Acta Biochim. Pol. 2019;66(1):1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 59.Tahara Y., et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep. 2018;8(1):1395. doi: 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pluznick J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017;19(4):25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markowiak-Kopeć P., Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu T., et al. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr. Opin. Pharmacol. 2022;62:36–42. doi: 10.1016/j.coph.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Mirzaei R., et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 65.Blaak E.E., et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes. 2020;11(5):411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 66.Desai M.S., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schroeder B.O. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterology Report. 2019;7(1):3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reimer R.A. Establishing the role of diet in the microbiota–disease axis. Nat. Rev. Gastroenterol. Hepatol. 2019;16(2):86–87. doi: 10.1038/s41575-018-0093-7. [DOI] [PubMed] [Google Scholar]
- 69.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonnenburg E.D., et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehlbaum S., et al. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int. J. Mol. Sci. 2018;19(10):3097. doi: 10.3390/ijms19103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song M., Chan A.T. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin. Gastroenterol. Hepatol. 2019;17(2):275–289. doi: 10.1016/j.cgh.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Filippo C., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107(33) doi: 10.1073/pnas.1005963107. 14691-14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martínez I., et al. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11(4):527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 75.Clemente J.C., et al. The microbiome of uncontacted Amerindians. Sci. Adv. 2015;1(3) doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schnorr S.L., et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014;5(1):3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obregon-Tito A.J., et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015;6(1):6505. doi: 10.1038/ncomms7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Earle K.A., et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe. 2015;18(4):478–488. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 80.Barbara G., et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14(1):9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goverse G., et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J. Immunol. 2017;198(5):2172–2181. doi: 10.4049/jimmunol.1600165. [DOI] [PubMed] [Google Scholar]
- 83.Li M., et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Tan J., et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016;15(12):2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 85.Macia L., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6(1):6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 86.Lamkanfi M., Dixit Vishva M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Vieira A.T., et al. Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J. Leukoc. Biol. 2017;101(1):275–284. doi: 10.1189/jlb.3A1015-453RRR. [DOI] [PubMed] [Google Scholar]
- 88.Gupta G., et al. Current update on the protective effect of naringin in inflammatory lung diseases. EXCLI journal. 2022;21:573–579. doi: 10.17179/excli2022-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim M., et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20(2):202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trompette A., et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 91.Sonnenburg E.D., Sonnenburg J.L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 2019;17(6):383–390. doi: 10.1038/s41579-019-0191-8. [DOI] [PubMed] [Google Scholar]
- 92.Gray L.E., et al. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front. Immunol. 2017;8:365. doi: 10.3389/fimmu.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hawley J.A. Microbiota and muscle highway - two way traffic. Nat. Rev. Endocrinol. 2020;16(2):71–72. doi: 10.1038/s41574-019-0291-6. [DOI] [PubMed] [Google Scholar]
- 94.Alan P.-A., et al. Cereal bran and wholegrain as a source of dietary fibre: technological and health aspects. Int. J. Food Sci. Nutr. 2012;63(7):882–892. doi: 10.3109/09637486.2012.676030. [DOI] [PubMed] [Google Scholar]
- 95.Han Y., Xiao H. Whole food-based approaches to modulating gut microbiota and associated diseases. Annu. Rev. Food Sci. Technol. 2020;11:119–143. doi: 10.1146/annurev-food-111519-014337. [DOI] [PubMed] [Google Scholar]
- 96.Hryckowian A.J., et al. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat Microbiol. 2018;3(6):662–669. doi: 10.1038/s41564-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiminez J.A., et al. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016;8(1):67. doi: 10.1186/s13099-016-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkateshan S., et al. Anti-oxidant and anti-hyperlipidemic activity of Hemidesmus indicus in rats fed with high-fat diet. Avicenna J Phytomed. 2016;6(5):516–525. [PMC free article] [PubMed] [Google Scholar]
- 99.Thorburn A.N., et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015;6(1):7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 100.Shi H., et al. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J. Neuroinflammation. 2020;17(1):77. doi: 10.1186/s12974-020-01760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith C., Van Haute M.J., Rose D.J. Processing has differential effects on microbiota-accessible carbohydrates in whole grains during in vitro fermentation. Appl. Environ. Microbiol. 2020;86(21) doi: 10.1128/AEM.01705-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Awika J.M., Rose D.J., Simsek S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018;9(3):1389–1409. doi: 10.1039/c7fo02011b. [DOI] [PubMed] [Google Scholar]
- 103.Reicks M., et al. Total dietary fiber intakes in the US population are related to whole grain consumption: results from the National Health and Nutrition Examination Survey 2009 to 2010. Nutr. Res. 2014;34(3):226–234. doi: 10.1016/j.nutres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Noce A., Romani A., Bernini R. Dietary intake and chronic disease prevention. Nutrients. 2021;13(4):1358. doi: 10.3390/nu13041358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neuhouser M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019;70:3–6. doi: 10.1016/j.nutres.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soverini M., et al. Variation of carbohydrate-active enzyme patterns in the gut microbiota of Italian healthy subjects and type 2 diabetes patients. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lutsiv T., et al. Relandscaping the gut microbiota with a whole food: dose–response effects to common bean. Foods. 2022;11(8):1153. doi: 10.3390/foods11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swain Ewald H.A., Ewald P.W. Natural selection, the microbiome, and public health. Yale J. Biol. Med. 2018;91(4):445–455. [PMC free article] [PubMed] [Google Scholar]
- 109.Song X., et al. Rediscovering the nutrition of whole foods: the emerging role of gut microbiota. Curr. Opin. Food Sci. 2022;48 [Google Scholar]
- 110.Mayengbam S., et al. Impact of dietary fiber supplementation on modulating microbiota-host-metabolic axes in obesity. J. Nutr. Biochem. 2019;64:228–236. doi: 10.1016/j.jnutbio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Peng X., et al. Lactobacillus plantarum NDC 75017 alleviates the learning and memory ability in aging rats by reducing mitochondrial dysfunction. Exp. Ther. Med. 2014;8(6):1841–1846. doi: 10.3892/etm.2014.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riva A., et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017;19(1):95–105. doi: 10.1111/1462-2920.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tseng C.-H., Wu C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019;118:S3–S9. doi: 10.1016/j.jfma.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 114.Turnbaugh P.J., et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 115.Cuevas-Sierra A., et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv. Nutr. 2019;10(suppl_1):S17–S30. doi: 10.1093/advances/nmy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim K.N., Yao Y., Ju S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11(10):2512. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moszak M., Szulińska M., Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—a review. Nutrients. 2020;12(4):1096. doi: 10.3390/nu12041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chambers E.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 120.Nicolucci A.C., et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153(3):711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 121.Santos-Paulo S., et al. The gut microbiota as a therapeutic target for obesity: a scoping review. Nutr. Res. Rev. 2022;35(2):207–220. doi: 10.1017/S0954422421000160. [DOI] [PubMed] [Google Scholar]
- 122.Larsen N., et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao L., et al. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine. 2019;66(3):526–537. doi: 10.1007/s12020-019-02103-8. [DOI] [PubMed] [Google Scholar]
- 124.Qin J., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 125.Gonai M., et al. Galacto-oligosaccharides ameliorate dysbiotic Bifidobacteriaceae decline in Japanese patients with type 2 diabetes. Benef. Microbes. 2017;8(5):705–716. doi: 10.3920/BM2016.0230. [DOI] [PubMed] [Google Scholar]
- 126.Houghton D., et al. Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with type 2 diabetes. Diabetologia. 2018;61(8):1700–1711. doi: 10.1007/s00125-018-4632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ojo O., et al. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12(11):3239. doi: 10.3390/nu12113239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McRorie J.W., McKeown N.M. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017;. 117(2):251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 129.Zhao L., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 130.Reimer R.A., et al. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2021;60(3):1237–1251. doi: 10.1007/s00394-020-02328-8. [DOI] [PubMed] [Google Scholar]
- 131.Cani P.D. Targeting gut microbiota with a complex mix of dietary fibers improves metabolic diseases. Kidney Int. 2019;95(1):14–16. doi: 10.1016/j.kint.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 132.Cai X., et al. Milk powder Co‐supplemented with inulin and resistant dextrin improves glycemic control and insulin resistance in elderly type 2 diabetes mellitus: a 12‐week randomized, double‐blind, placebo‐controlled trial. Mol. Nutr. Food Res. 2018;62(24) doi: 10.1002/mnfr.201800865. [DOI] [PubMed] [Google Scholar]
- 133.Colosia A.D., Palencia R., Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes, Metabolic Syndrome and Obesity. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abbasnezhad A., et al. Effect of different dietary approaches compared with a regular diet on systolic and diastolic blood pressure in patients with type 2 diabetes. A systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2020;163 doi: 10.1016/j.diabres.2020.108108. [DOI] [PubMed] [Google Scholar]
- 135.Evans C.E., et al. Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals. J. Hypertens. 2015;33(5):897–911. doi: 10.1097/HJH.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 136.Noureddin S., Mohsen J., Payman A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: a randomized trial in patients with type 2 diabetes and chronic constipation. Compl. Ther. Med. 2018;40:1–7. doi: 10.1016/j.ctim.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 137.López Vega, R.L S., Quintanar Vidal, Fernandez M.L. Sex and hormonal status influence plasma lipid responses to psyllium. Am. J. Clin. Nutr. 2001;74(4):435–441. doi: 10.1093/ajcn/74.4.435. [DOI] [PubMed] [Google Scholar]
- 138.Zhou X., et al. Hypolipidaemic effects of oat flakes and β‐glucans derived from four Chinese naked oat (Avena nuda) cultivars in Wistar–Lewis rats. J. Sci. Food Agric. 2016;96(2):644–649. doi: 10.1002/jsfa.7135. [DOI] [PubMed] [Google Scholar]
- 139.Julius M., et al. Glutathione and morbidity in a community-based sample of elderly. J. Clin. Epidemiol. 1994;47(9):1021–1026. doi: 10.1016/0895-4356(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 140.Koay Y.C., et al. Ingestion of resistant starch by mice markedly increases microbiome-derived metabolites. Faseb. J. 2019;33(7):8033–8042. doi: 10.1096/fj.201900177R. [DOI] [PubMed] [Google Scholar]
- 141.Zhang N., et al. Functional oligosaccharide fermentation in the gut: improving intestinal health and its determinant factors-A review. Carbohydr. Polym. 2022;284 doi: 10.1016/j.carbpol.2021.119043. [DOI] [PubMed] [Google Scholar]
- 142.Soltanian N., Janghorbani M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: a randomized trial in constipated patients with type 2 diabetes. Clinical Nutrition ESPEN. 2019;29:41–48. doi: 10.1016/j.clnesp.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 143.Chengxiao Y., et al. Challenges of pectic polysaccharides as a prebiotic from the perspective of fermentation characteristics and anti-colitis activity. Carbohydr. Polym. 2021;270 doi: 10.1016/j.carbpol.2021.118377. [DOI] [PubMed] [Google Scholar]
- 144.Lomer M.C.E. Dietary and nutritional considerations for inflammatory bowel disease. Proc. Nutr. Soc. 2011;70(3):329–335. doi: 10.1017/S0029665111000097. [DOI] [PubMed] [Google Scholar]
- 145.Dehghan P., et al. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014;65(1):117–123. doi: 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- 146.Chu H., et al. Galactooligosaccharides protects against DSS-induced murine colitis through regulating intestinal flora and inhibiting NF-κB pathway. Life Sci. 2020;242 doi: 10.1016/j.lfs.2019.117220. [DOI] [PubMed] [Google Scholar]
- 147.Wilson B., et al. Prebiotic galactooligosaccharide supplementation in adults with ulcerative colitis: exploring the impact on peripheral blood gene expression, gut microbiota, and clinical symptoms. Nutrients. 2021;13(10):3598. doi: 10.3390/nu13103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Preter V., et al. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn's disease patients: a double-blinded randomized controlled trial. Clin. Transl. Gastroenterol. 2013;4(1):e30. doi: 10.1038/ctg.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang M., et al. In vitro colonic fermentation of dietary fibers: fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019;88:1–9. [Google Scholar]
- 150.Sun P., et al. Eucommiae cortex polysaccharides mitigate obesogenic diet-induced cognitive and social dysfunction via modulation of gut microbiota and tryptophan metabolism. Theranostics. 2022;12(8):3637–3655. doi: 10.7150/thno.72756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yarandi S.S., et al. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22(2) doi: 10.5056/jnm15146. 201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Banks W.A., et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation. 2015;12:223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Farhangi M.A., et al. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: targeting the hypothalamic–pituitary–adrenal axis and immune system. Clin. Nutr. 2018;37(4):1216–1223. doi: 10.1016/j.clnu.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 155.Holzer P. In: International Review of Neurobiology. Cryan J.F., Clarke G., editors. Academic Press; 2016. Chapter four - neuropeptides, microbiota, and behavior; pp. 67–89. [DOI] [PubMed] [Google Scholar]
- 156.An L., et al. Inulin‐type oligosaccharides extracted from yacon produce antidepressant‐like effects in behavioral models of depression. Phytother Res. 2016;30(12):1937–1942. doi: 10.1002/ptr.5698. [DOI] [PubMed] [Google Scholar]
- 157.Weir T.L., Trikha S.R.J., Thompson H.J. Diet and cancer risk reduction: the role of diet-microbiota interactions and microbial metabolites. Semin. Cancer Biol. 2021;70 doi: 10.1016/j.semcancer.2020.06.007. 53-60. [DOI] [PubMed] [Google Scholar]
- 158.Ben Q., et al. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology. 2014;146(3):689–699.e6. doi: 10.1053/j.gastro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 159.Sze M.A., et al. Fecal short-chain fatty acids are not predictive of colonic tumor status and cannot Be predicted based on bacterial community structure. mBio. 2019;10(4) doi: 10.1128/mBio.01454-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.O'Keefe S.J.D., et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015;6(1):6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Earle Kristen A., et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe. 2015;18(4):478–488. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johansson M.E.V., et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou J., et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23(1):41–53.e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Makarem N., et al. Consumption of whole grains and cereal fiber in relation to cancer risk: a systematic review of longitudinal studies. Nutr. Rev. 2016;74(6):353–373. doi: 10.1093/nutrit/nuw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deschasaux M., et al. Dietary total and insoluble fiber intakes are inversely associated with prostate cancer risk. J. Nutr. 2014;144(4) doi: 10.3945/jn.113.189670. 504-510. [DOI] [PubMed] [Google Scholar]
- 166.Huxley R.R., Woodward M., Clifton P. The epidemiologic evidence and potential biological mechanisms for a protective effect of dietary fiber on the risk of colorectal cancer. Current Nutrition Reports. 2013;2(1):63–70. [Google Scholar]
- 167.Pensinger D.A., et al. Butyrate differentiates permissiveness to Clostridioides difficile infection and influences growth of diverse C. difficile isolates. Infect. Immun. 2023;91(2) doi: 10.1128/iai.00570-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pruss K.M., et al. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. ISME J. 2021;15(2):577–591. doi: 10.1038/s41396-020-00798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.