Abstract
Introduction
Emerging evidence suggests that metastasis is better described as a spectrum of disease rather than a binary state. A greater understanding of the genomic features that determine extent and location of metastatic spread may inform risk stratification and monitoring. Here, we identify genomic alterations from primary prostate carcinomas that are predictive of wide-spread metastatic potential.
Methods
Genomic and clinical data from 1,312 patients with primary prostate carcinoma were extracted from the MSK-MET cohort through cBioPortal. Metastatic site counts and overall survival (OS) data were publicly available and used as outcome. Primary tumor samples were profiled using the MSK-IMPACT targeted sequencing platform. We focused on 58 genes frequently altered in prostate cancer. Cox proportional hazard analyses defined hazard ratios (HRs) and 95% confidence intervals (CIs) for overall mortality in patients with different metastatic outcomes.
Results
Out of the 1,312 patients in our cohort, 939 (71%) developed metastases, of whom 113 (8.6%) had metastases to 5 or more distinct anatomical sites (defining wide-spread metastases, WSM). Bone was the most common site of metastasis (36%), and 80% of patients with liver metastases had 4 or more additional sites of metastasis. Among patients with metastasis, increasing number of metastatic sites was associated with increased risk of death (HR:1.8,95%CI:1.63–1.99, p<0.001).
Alterations in the following genes were enriched in tumors from patients with WSM vs others: TP53 (40% vs 20%, p<0.0001), FOXA1-amplification (8% vs 3%, p=0.02), AR-amplification (4.4% vs 1%, p=0.01), RB1-deletion (5.3% vs 0.7%,p=0.001), and BRCA2-deletion (4.4% vs 0.7%, p=0.01). Univariable survival analysis showed all these alterations were predictive of OS (p<0.05). On multivariable analysis, only TP53 mutations, and FOXA1 and AR amplifications were independent prognostic factors. FOXA1 (n=37) and AR (n=13) amplifications were mutually exclusive and patients with these experienced very poor OS (HR:3.57, 95%CI:2.26–5.6, p<0.001].
Conclusions
We identified genomic alterations (TP53 mutations, FOXA1/AR amplification, RB1/BRCA2 deletion) from primary prostate carcinomas that are predictive of wide-spread metastases and poor outcome.
Keywords: wide-spread metastasis, prostate cancer, cancer genomics, FOXA1-amplification, TP53-mutation
Patient summary
Patients with multiple metastatic sites are at higher risk of cancer-specific death; metastases to bone and to liver were independently associated with worse outcomes. TP53 mutation, FOXA1 amplification, AR amplification, RB1 deletion, and BRCA2 deletion were associated with WSM. Therefore, the extent, location, and mutations associated with metastatic prostate cancer should be considered in risk stratification and treatment planning.
Introduction
Primary prostate carcinomas demonstrate molecular heterogeneity and are associated with diverse clinical outcomes and metastatic potential. While metastatic disease has long been considered a binary state (i.e., metastatic vs. non-metastatic), emerging evidence suggests that patients with metastases are better described along a spectrum of disease states associated with varying degrees of metastatic proclivity, numbers of metastatic lesions, and locations of metastatic spread.1 Such work suggests an underlying biological distinction between early so-called oligometastatic disease and more advanced widespread disease, with unique risk factors and response to treatment.2 Early work has demonstrated that the number and location of metastases are potential predictors of outcomes, but less is known about the genomic underpinnings of oligometastatic biology.
Importantly, much of our understanding of genomic predictors of metastatic potential come from studies that consider metastasis as a binary outcome and do not distinguish between different types of metastases. For example, work that led to the development of the Decipher genomic classifier used the binary presence/absence of metastasis as the primary outcome.3,4 Thus, less is known about the association between genomic alterations present in the primary tumor and the extent and location of subsequent metastatic spread.
Here, we define a set of genomic alterations in primary prostate carcinomas that are associated with the development of widespread metastases (WSM, defined as 5 or more distinct sites) using a retrospective cohort of 1,312 patients from the Memorial Sloan-Kettering - Metastatic Events and Tropisms (MSK-MET) cohort, which is an integrated pan-cancer cohort of tumor genomic and clinical outcome data from more than 25,000 cancer patients.5
Methods
Samples
Genomic data from 1,312 primary prostate carcinoma samples and clinical data from patients were extracted from the MSK-MET cohort through cBioPortal.org (Table S1). Only one sample per patient was used in this study. More details on characterizing metastatic sites and selecting samples per patient are available in the MSK-MET study.5 Counts of distinct anatomical sites of metastases (based on imaging, pathology, and electronic health record data) and overall survival (OS) data were available for all patients with primary cancer. A separate cohort of 860 metastatic prostate carcinoma samples was also analyzed from MSK-MET cohort. All tumors were profiled using the Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT) clinical sequencing assay, a hybridization capture-based, next-generation sequencing platform.6 Genomic data for 72 genes frequently altered in prostate cancer were obtained.7 Our study focused on 58 genes (out of 72) that were present in all three MSK-IMPACT platforms (MSK-341, MSK-410, MSK-468). All alterations in these genes were included in our analyses.
Statistical analyses
First, Cox proportional hazard analyses defined hazard ratios (HRs) and 95% confidence intervals (CIs) for overall mortality based on number of metastases. Survival data were calculated from the time of presentation with metastatic disease. Multivariable Cox analysis was also performed adjusting for metastatic site count, age, and location of metastasis.
Next, to assess molecular patterns of primary tumors based on the number of metastatic sites, we compared genomic alterations based on the number of metastatic sites (1–2, 3–4, and 5-or-more). Subsequently, we analyzed mutational patterns based on the location of metastasis, comparing primary tumors associated with liver vs. non-liver metastases, and bone vs. non-bone sites. We also assessed gene alteration frequencies in 860 metastatic prostate tissue samples from the MSK-MET cohort compared with gene alteration frequencies in primary tumors that went on to metastasize to 5 or more sites. X2 test was used to assess for associations between genomic alterations and number of metastasis sites. The BenjaminiHochberg method controlled for multiple testing correction and false discovery rate (FDR).
Lastly, we characterized the prognostic implications of TP53-mutation, FOXA1-amplification, AR-amplification, RB1-deletion, and BRCA2-deletion in the 1,312 primary patients using both univariable and multivariable Cox regression analyses.
Results
A total of 1,312 patients with prostate carcinoma underwent IMPACT sequencing. 939 (71%) developed metastasis to at least one distant site, and 190 (14.5%) patients died (Table S1). Among the 1,312 patients, the most frequent sites of metastases were bone (36.3%), distant lymph nodes (19%), bladder (12%), and liver (5%). 60/67 (87%) patients with liver metastases and 33/35 (95%) with brain metastases also had metastases to bone (Table S2). Notably, 113 patients (8.6%) had widespread metastases (WSM), defined here as metastatic disease located in 5 or more distinct anatomic sites. In our cohort, patients with high metastatic site count (MSC) were most likely to have disease spread to the liver, lung, and brain.
We first characterized the impact of MSC on overall survival (Figure 1.A). Among patients who developed metastases, a higher MSC was associated with increased risk of death (HR: 1.8, 95% CI: 1.63–1.99, p<e−16). Within the metastatic spectrum, WSM (i.e., 5 or more sites of metastasis) was also associated with higher risk of death compared to patients with 3–4 sites of metastasis (23% vs. 57% 5yr OS, HR: 2.46, 95% CI: 1.75–3.46, p=1.8e−7). Patients with 3–4 sites of metastases were also at higher risk of death compared to patients with 1–2 sites of metastasis (57% vs. 86% 5yr OS, HR: 3.56, 95% CI: 2.44–5.3, p=4.4e−11).
Figure 1.
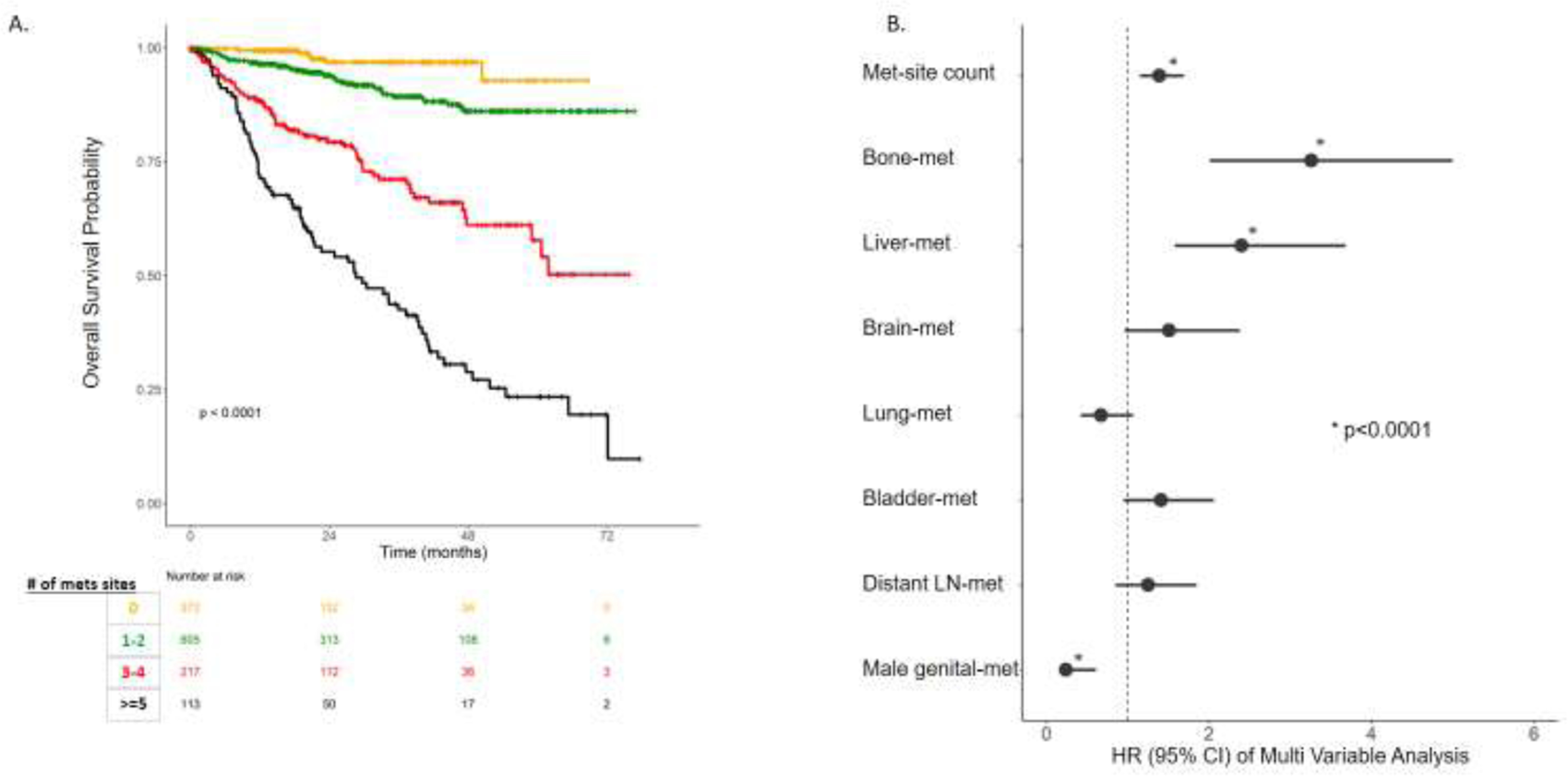
Survival analysis of metastasis sites. A. Kaplan Meier Curve of overall survival of number of Metastasis Site Count (MSC).B. Hazard Ratio (HR) of Multi-variable analysis (MVA) of numbers of MSC adjusted for other sites and age at sequencing.
Within the same cohort of patients with metastatic disease, we performed a multivariable Cox analysis to assess the prognostic impact of MSC, adjusting for age at sequencing and the different metastatic sites (Figure 1.B, Table S3). High MSC and the presence of liver or bone metastases were independently associated with poor OS (p<0.001).
To define a molecular signature from primary tumors that went on to metastasize to 5 or more sites (WSM), we assessed sequencing data from 58 prostate cancer-related genes and identified genomic alterations that were associated with future number of metastatic sites (1–2, 3–4, or 5-or-more) (Figure 2.A, Table S4). After multiple test correction, our analyses demonstrated that TP53 mutations were enriched in primary tumors from patients with WSM (40% vs. 24% for 3–4 MSC vs. 19% for 1–2 MSC, FDR=0.0001). We also assessed copy number alterations, and found that FOXA1 (8.8% vs. 4.1% for 3–4 MSC vs. 2.3% for 1–2 MSC, FDR=0.02) and AR amplifications (4.4% vs. 2.3 for 3–4 MSC vs. 0.3% for 1–2 MSC, FDR=0.01), and RB1(5.3% vs. 0.9% for 3–4 MSC vs. 0.5% for 1–2 MSC, FDR=0.001) and BRCA2 (4.4% vs. 0.9% for 3–4 MSC vs. 0.5% for 1–2 MSC, FDR=0.01) deletions were enriched in primary tumors from patients with WSM (Figure 2.A). Additionally, we found that primary tumors from patients that went on to develop WSM tended to have a larger fraction of their genomes altered, and increased tumor mutational burden (p<0.001 for both). Notably, when we assessed gene alteration frequencies in 860 metastatic prostate carcinoma samples from the MSK-MET cohort and compared them with gene alteration frequencies in primary tumors that went on to metastasize to 5 or more sites, we found that the frequency of FOXA1-amplification was higher in the latter (Figure 2.A).
Figure 2.

Genomic alterations associated with MSC. A. Genomic alterations significantly associated with primary tumors with different MCS (1–2,3–4,>=5) after FDR correction and compared to actual metastatic samples. B. Venn diagram of alterations common across different analyses of identifying signatures associated with metastasis patterns. C. Hazard ratios of the five genomic alterations in Univariable (UVA) and multivariable analysis (MVA) in a cohort of 1,312 patients with primary prostate tumors. D. Kaplan Meier of patients with either FOXA1 or AR amplifications compared to other patients.
Given our observation that liver metastases and bone metastases were associated with poorer OS, we next asked whether similar genomic predictions could be made for primary tumors that went on to metastasize to liver or bone. We thus conducted similar analyses on the primary tumors from patients that went on to develop liver metastases and patients that went on to develop bone metastases vs. those that developed metastases to non-liver or non-bone sites, respectively (Table S5–6). We found that TP53 mutations, RB1 deletions, and BRCA2 deletions were enriched in primary tumors that led to liver metastases, while TP53 mutations, AR amplifications, and PTEN deletions were enriched in primary tumors that led to bone metastases (Figure 2.B).
Finally, we characterized the prognostic impact of TP53-mutation, FOXA1-amplification, AR-amplification, RB1-deletion, and BRCA2-deletion in our 1,312 primary tumor cohort. In a univariable survival analysis, all these alterations were predictive of OS (p<0.05). However, on multivariable analysis, only TP53 mutation, and FOXA1 and AR amplifications were independent prognostic factors (Figure 2.C). Patients with FOXA1 (n=37) and AR amplifications (n=13) were mutually exclusive (0 overlap). Given their mutual exclusivity and because FOXA1 functions downstream of AR to facilitate AR-dependent transcription8, we combined patients with either FOXA1 or AR amplification into a single group and found that these patients experienced very poor OS (HR: 3.57, 95% CI: 2.26–5.6, p<0.0001] (Figure 2.D).
Discussion
Mounting evidence suggests that metastasis is a spectrum of disease rather than a binary entity. The growing recognition of a distinct oligometastatic biology has implications for the role of local therapy, such as surgery and/or radiation in the oligometastatic setting.2,9 In this large single-institution cohort of patients with metastatic prostate carcinoma, we found primary tumors that went on to develop multiple metastatic sites (particularly, five or more sites) had a distinct profile of genomic alterations compared to patients with 1–2 metastatic sites and was associated with worse overall survival. We also found that metastases to bone and to liver were independently associated with worse outcomes, suggesting that not only the number of sites, but also location of metastases plays a role in patients’ outcome. The MSK-MET study previously defined pan-cancer genomic features associated with metastasis to specific target organs; here we focused on primary prostate carcinomas that went on to metastasize to 5 or more distinct sites and compared them to both primary tumors with fewer metastatic sites and to samples from metastatic tissue.5 We demonstrate that TP53 mutations are associated with both wide-spread metastases and specifically with metastases to liver or bone, and that FOXA1-amplification, AR-amplification, RB1-deletion, and BRCA2-deletion were also associated with wide-spread metastases. Our analyses showed that FOXA1-amplification was specific to wide-spread metastasis and was not seen in tumors that went on to develop more limited metastatic disease. When characterizing the impact of genomic alterations on clinical outcome, we found TP53, FOXA1, and AR amplifications to be independent prognostic biomarkers. Interestingly, FOXA1 and AR amplifications were mutually exclusive with no patients harboring both alterations. Patients with either of these two alterations in their primary tumors were at significantly higher risk of death.
Our work adds to a growing body of epidemiologic, clinical, and genomic work that supports a distinction between oligometastatic and widely metastatic disease.2 First proposed as a clinically distinct entity by Hellman and Weichselbaum in 1995, more recent prospective work in diverse clinical settings has shown that primary tumor-directed and oligometastasis-directed local therapy may be associated with improved outcomes, including OS.2,10–11 It is important to note that most of these studies define the oligometastatic state based on number of metastases, with some consideration given to the location of metastatic spread.11 This work adds to the biologic understanding of oligometastatic prostate cancer, implicating specific genomic alterations present in the primary tumor that may determine the extent of future metastatic spread.
Our study concurs with prior literature identifying unique metastatic patterns impacting patient survival. Cui et al. showed that both number of metastatic sites and the location of metastases were independent prognostic factors.12 In the current study, our previous work, and the Cui et al. study, liver metastases were shown to be associated with worse overall survival; Cui et al. further observed that lung and brain metastases were independent predictors of survival, while our study highlighted bone metastases as a prognostic factor for lower overall survival.13 Our findings are also consistent with the limited number of prior studies exploring potential mutations responsible for widespread metastases in prostate cancer, and expand on these particularly because of our study’s large sample size. Like our study, Deek et al. observed that among populations with metastatic prostate cancer, patients with TP53 mutations were more likely to develop widespread metastasis relative to patients without TP53 mutations.1 Sutera et al. also observed that rates of TP53 mutations were higher in patients with “high volume” disease when compared to patients with “low volume” disease.9
Further work is needed to characterize how best to risk-strategy and treat patients with metastatic prostate cancer, factoring in an improved understanding of the biological underpinnings of oligometastatic biology. Future research should also explore the relationship between genomic alterations in tumor cells or the surrounding microenvironment with response to targeted therapy like AR and PARP inhibitors. For example, recently presented work has shown that metastasis-directed therapy can have a differential degree of benefit based on the primary histology (as has been shown for oligoprogressive lung vs. breast cancer).14 It is possible that within prostate cancer – perhaps as a function of tumor mutational patterns – certain molecular subsets may be found to benefit more from metastasis-directed therapy. Work is also needed to unravel the mechanistic implications of mutations that predict poor outcomes, particularly those that have had relatively less study in the context of prostate cancer, such as FOXA1 and RB1. It is possible that a more nuanced molecular understanding of how these mutations impact metastatic outcome could lead to novel therapeutic strategies. It is particularly interesting that FOXA1 amplification was found more frequently in samples from primary tumors that went on to develop WSM than it was in samples from actual metastatic disease. This suggests one of two things: because we do not have the metastatic count site information for the MSK-MET cohort, it is possible that samples derived from patients with widely metastatic disease do possess FOXA1 amplification but that this signal is diluted by the presence of oligometastatic samples in the cohort.15,16Alternatively, because FOXA1 is known to paradoxically promote AR-dependent cell growth while also inhibiting cell motility and epithelial-to-mesenchymal transition (EMT), it remains possible that FOXA1 amplification promotes the development of aggressive pre-metastatic tumors but is then selected against upon metastatic progression.17
Our work is limited by its retrospective nature and the finite set of mutations assessed in MSK IMPACT. Similarly, selection bias due to the choice of patients for sequencing may impact the generalizability of our results, as patients with non-metastatic prostate cancer do not often receive MSK-IMPACT sequencing at our institution. Furthermore, our study focused on the number of distinct anatomical sites, and not the total number of metastatic sites. Patients with multiple metastases to the same organ might have different genomic signatures. In addition, although the primary clinical outcome of OS was available and relevant for patients, other prostate cancer-specific outcomes were not available; data on treatment received by each patient were similarly lacking. Future work should explore whether local therapies such as surgery and radiation demonstrate differential benefits based not just on the number of metastases (as has been shown in the SABR-COMET, ORIOLE, and STOMP trials, among others), but also based on genomic signatures present in the primary tumor. Furthermore, given the single-institution nature of our study, future work should explore whether genomic predictors of oligometastatic spread (and subsequently, treatment response) are similar in various geographic and sociodemographic settings, especially given the recognition of clear disparities in access to care.
Supplementary Material
Table S1: Characteristics of patients across different metastatic sites count
Table S2: Number of patients with different combination of metastasis sites
Table S3: Multivariable analysis (MVA) of number if metastasis sites and site f metastasis after adjusting for age at sequencing and excluding patients with no-metastasis
Table S4: Genomic alterations associated with number of metastasis sites
Table S5: Genomic alterations associated with liver metastasis vs non-liver metastasis
Table S6: Genomic alterations associated with bone metastasis vs non-bone metastasis
Highlights.
Out of the 1,312 patients, 939(71%) developed metastases, and 113(8.6%) had metastases to 5 or more distinct anatomical sites (defining wide-spread metastases, WSM).
Among patients with metastasis, increasing number of metastatic sites was associated with increased risk of death (HR:1.8, 95%CI:1.63–1.99, p<0.001).
In a univariable survival analysis, the following alterations were predictive of overall survival (OS) (p<0.05): TP53 (40% vs 20%, p<0.0001), FOXA1-amplification (8% vs 3%, p=0.02), AR-amplification (4.4% vs 1%, p=0.01), RB1-deletion (5.3% vs 0.7%, p=0.001), and BRCA2-deletion (4.4% vs 0.7%, p=0.01).
On multivariable analysis, only TP53 mutations, FOXA1, and AR amplifications were independent prognostic factors.
Patients who have either AR or FOXA1 amplifications experienced very poor OS (HR:3.57, 95%CI:2.26–5.6, p p<0.001].
FUNDING:
Dr. Mahal is funded by the Prostate Cancer Foundation and (PCF), the American Society for Radiation Oncology (ASTRO), the Department of Defense, and the Sylvester Comprehensive Cancer Center. Dr. Goglia, Dr. Nguyen, and Dr. Dee are funded in part through the Cancer Center Support Grant from the National Cancer Institute (P30 CA008748).
Footnotes
Conflict of Interest: PLN reports grants and personal fees from Bayer, Janssen, and Astellas and personal fees from Boston Scientific, Dendreon, Ferring, COTA, Myovant Sciences, Blue Earth Diagnostics, and Augmenix outside the submitted work. All other authors have no relevant COI to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Deek MP, Van der Eecken K, Phillips R, et al. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. European Urology. 2021;80(5):632–640. doi: 10.1016/j.eururo.2020.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: Definitions, clinical outcomes, and treatment considerations. Nature Reviews Urology. 2017;14(1):15–25. doi: 10.1038/nrurol.2016.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Erho N, Crisan A, Vergara IA, et al. Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS ONE. 2013;8(6). doi: 10.1371/journal.pone.0066855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arriaga JM, Panja S, Alshalalfa M, et al. A MYC and RAS co-activation signature in localized prostate cancer drives bone metastasis and castration resistance. Nature Cancer. 2020;1(11):1082–1096. doi: 10.1038/s43018-020-00125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen B, Fong C, Luthra A, et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell. 2022;185(3):563–575.e11. doi: 10.1016/j.cell.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine. 2017;23(6):703–713. doi: 10.1038/nm.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discovery. 2018;8(4):444–457. doi: 10.1158/2159-8290.CD-17-0937 [DOI] [PubMed] [Google Scholar]
- [8].Gao N, Zhang J, Rao MA, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17(8):1484–1507. doi: 10.1210/me.2003-0020 [DOI] [PubMed] [Google Scholar]
- [9].Sutera P, Van Der Eecken K, Kishan AU, et al. Definitions of disease burden across the spectrum of metastatic castration-sensitive prostate cancer: comparison by disease outcomes and genomics. Prostate Cancer and Prostatic Diseases. Published online 2022. doi: 10.1038/s41391-021-00484-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hellman S, Weichselbaum RR. Oligometastases. Journal of Clinical Oncology. 1995;13(1):8–10. doi: 10.1200/jco.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- [11].Ali A, Hoyle A, Haran ÁM, et al. Association of Bone Metastatic Burden with Survival Benefit from Prostate Radiotherapy in Patients with Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncology. 2021;7(4):555–563. doi: 10.1001/jamaoncol.2020.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cui PF, Cong XF, Gao F, et al. Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases: A study of 1358 patients. World Journal of Clinical Cases. 2020;8(1):54–67. doi: 10.12998/wjcc.v8.i1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alshalalfa M, Seldon C, Franco I, et al. Clinicogenomic characterization of prostate cancer liver metastases. Prostate Cancer and Prostatic Diseases. 2022;25(2):366–369. doi: 10.1038/s41391-021-00486-2 [DOI] [PubMed] [Google Scholar]
- [14].Tsai CJ, Yang JT, Guttmann DM, et al. Consolidative Use of Radiotherapy to Block (CURB) Oligoprogression ― Interim Analysis of the First Randomized Study of Stereotactic Body Radiotherapy in Patients With Oligoprogressive Metastatic Cancers of the Lung and Breast. International Journal of Radiation Oncology*Biology*Physics. 2021;111(5):1325–1326. doi: 10.1016/j.ijrobp.2021.09.014 [DOI] [Google Scholar]
- [15].Gerhardt J, Montani M, Wild P, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol. 2012;180(2):848–861. doi: 10.1016/j.ajpath.2011.10.021 [DOI] [PubMed] [Google Scholar]
- [16].Jain RK, Mehta RJ, Nakshatri H, Idrees MT, Badve SS. High-level expression of forkhead-box protein A1 in metastatic prostate cancer. Histopathology. 2011;58(5):766–772. doi: 10.1111/j.1365-2559.2011.03796.x [DOI] [PubMed] [Google Scholar]
- [17].Jin HJ, Zhao JC, Ogden I, Bergan RC, Yu J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res. 2013;73(12):3725–3736. doi: 10.1158/0008-5472.CAN-12-3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Characteristics of patients across different metastatic sites count
Table S2: Number of patients with different combination of metastasis sites
Table S3: Multivariable analysis (MVA) of number if metastasis sites and site f metastasis after adjusting for age at sequencing and excluding patients with no-metastasis
Table S4: Genomic alterations associated with number of metastasis sites
Table S5: Genomic alterations associated with liver metastasis vs non-liver metastasis
Table S6: Genomic alterations associated with bone metastasis vs non-bone metastasis


