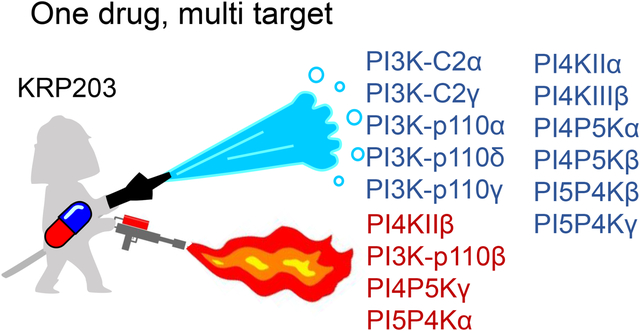
Abstract
Increased phosphoinositide signaling is commonly associated with cancers. While “one-drug one-target” has been a major drug discovery strategy for cancer therapy, a “one-drug multi-targets” approach for phosphoinositide enzymes has the potential to offer a new therapeutic approach. In this study, we sought a new way to target phosphoinositides metabolism. Using a high-throughput phosphatidylinositol 5-phosphate 4-kinase-alpha (PI5P4Kα) assay, we have identified that the immunosuppressor KRP203/Mocravimod induces a significant perturbation in phosphoinositide metabolism in U87MG glioblastoma cells. Despite high sequence similarity of PI5P4K and PI4K isozymes, in vitro kinase assays showed that KRP203 activates some (e.g., PI5P4Kα, PI4KIIβ) while inhibiting other phosphoinositide kinases (e.g., PI5P4Kβ, γ, PI4KIIα, class I PI3K-p110α, δ, γ). Furthermore, KRP203 enhances PI3P5K/PIKFYVE’s substrate selectivity for phosphatidylinositol (PI) while preserving its selectivity for PI(3)P. At cellular levels, three hours of KRP203 treatment induces a prominent increase of PI(3)P and moderate increase of PI(5)P, PI(3,5)P2, and PI(3,4,5)P3 levels in U87MG cells. Collectively, the finding of multimodal activity of KRP203 towards multi-phosphoinositide kinases may open a novel basis to modulate cellular processes, potentially leading to more effective treatments for diseases associated with phosphoinositide signaling pathways.
Keywords: KRP203/Mocravimod, Drug repurposing, Polypharmacology, Multitarget drug, One drug, multi targets, Phosphoinositide metabolism, PI3K, PI5P4K/Type II PIPK/ phosphatidylinositol 5-phosphate 4-kinase, PI3P5K/Type III PIPK/PIKFYVE, drug screening
Graphical Abstract
1. Introduction
Phosphoinositides, phosphorylated forms of phosphatidylinositol (PI), are second messenger lipids important for fundamental cellular processes, and they are critically involved in multiple human diseases, including cancers [1–4]. In pursuing novel therapeutic strategies, most pharmacological interventions have focused on perturbing a specific phosphoinositide kinase, such as class I PI3K. This “one-drug one-target” approach has been the major drug discovery strategy in cancer therapy. On the other hand, another less explored approach is the “one-drug multi-targets” approach, which uses a single drug with the ability to simultaneously modulate multiple targets associated with the pathogenesis of the diseases. For instance, salicylic acid and its prodrug form acetylsalicylic acid, aspirin, exert their effects (e.g., suppression of pain, fever, and inflammation) through modulating divergent targets, in addition to the originally expected targets, cyclooxygenase-1 (COX-1) and COX-2 [5]. A dichotomic advantage of the one-drug multi-target approach is to be able to decrease or technically eliminate, risk of drug−drug interactions compared to the cocktail approach [6,7].
In this study, we explore if the one-drug multi-targets approach could be a paradigm for multilevel modulation of phosphoinositide metabolism resulting in an altered phosphoinositide equilibrium. In general, most identified kinase inhibitors are structural ATP analogs that bind to the catalytic pocket of the kinases. In the present work, we hypothesized that some activators interact with kinase at a site allosteric to the ATP-binding sites and may inhibit or activate other kinases, leading to the imbalance of phosphoinositides metabolism. By taking advantage of our previously developed high-throughput screening format to identify small molecules that inhibit PI5P4K activity [8, 9], we explored PI5P4Kα activators, leading to the discovery of an immunosuppressive compound—KRP203/Mocravimod—that possesses a novel type of multimodal effect on phosphoinositide metabolism by activating some, while inhibiting other, phosphoinositide kinases despite their high sequence similarity.
2. Results
2.1. Chemical library screening identifies KRP203 as a multi targets drug for phosphoinositide kinases.
To identify phosphoinositide kinases modulators, we sought compounds that could activate PI5P4K signaling, as opposed to the general approach to screen the inhibitors. Among the three PI5P4K isotypes, PI5P4Kα uses ATP and GTP equally as phospho-donor [10–13]. We took advantage of our high-throughput screening system for PI5P4Kα [8, 9], which utilizes a DMSO-based 1,536-well format to assay PI5P4Kα activity with D-myo-di16-PI(5)P substrate by bioluminescence readout. The product, ADP was coupled through a two-step reaction to luminescence produced by firefly luciferase (ADP-Glo™). We screened the 339,992 compounds in the NIH Molecular Libraries Small Molecule Repository (MLSMR) library, annotated collections in 4-point dose-response, and ranked the hits by curve class, AC50 values, and maximum activation obtained from the primary screen. Following the removal of synthetically intractable and promiscuous hits (defined as compounds active in >50% efficacy of NCATS-run assays), the counter-screening assays were performed to eliminate luciferase activators. The remaining seven compounds (Fig. 1B, Suppl. Table 1) were then tested by in vitro kinase assay with radio-labeled γ−32P -ATP or -GTP using recombinant PI5P4Kα and PI(5)P. Most of the compounds failed to activate PI5P4Kα; however, KRP203 caused an increase of the PI5P4Kα activity at 10 μM concentration (Fig. 1C).
Fig. 1. Chemical library screening identifies KRP203 as a potential modifier for phosphoinositide metabolism.
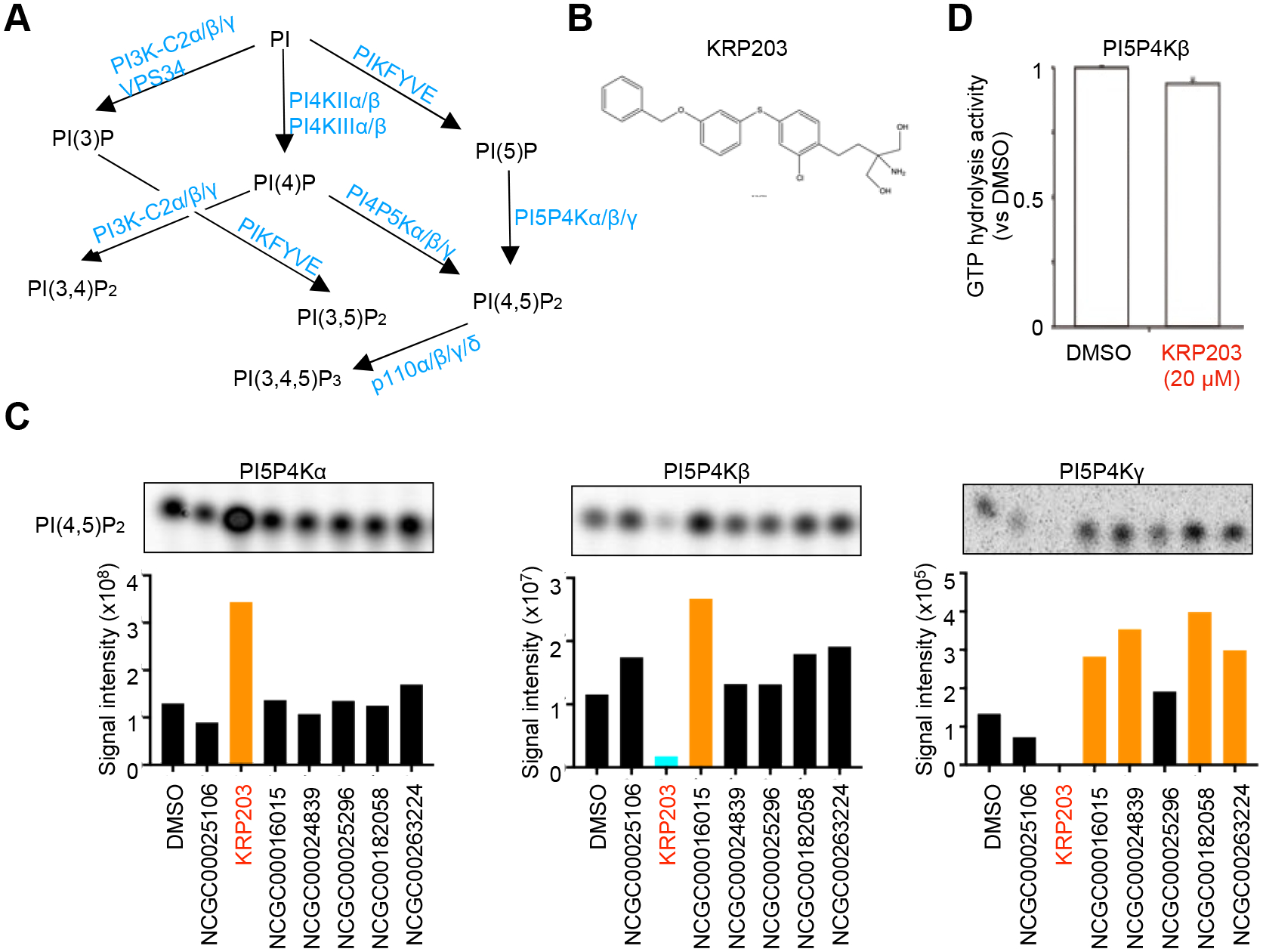
A. Phosphoinositide metabolism pathways.
B. Chemical screening to modulate the activity of type II PIP4Ks by kinase assay. A chemical structure of KRP203 (NCGC00250388), 2-amino-2-{2-[4-(3-benzyloxyphenylthio)-2-chlorophenyl]ethyl}−1,3-propanediol hydrochloride, is shown below.
C. Quantification of the effect of screened chemicals (10 μM) on the activity of type II PIP4Ks by in vitro kinase assay with γ−32P -ATP or -GTP
D. The GTP hydrolysis activity of PI5P4Kβ. KRP203 (20 μM) did not affect the GTP hydrolysis activity of PI5P4Kβ. The color of orange signifies values that are significantly higher than the control, whereas blue indicates values that are lower than the control. N =3, error bars indicate SD.
KRP203 is a prodrug that exerts its immunosuppressive effect through a unique inside-out activation mechanism. Specifically, upon in vivo administration, KRP203 becomes phosphorylated intracellularly, is secreted into plasma, and binds to sphingosine 1-phosphate (S1P) receptors, leading to the S1P receptor internalization [12–14]. Various in vivo animal experiments show that KRP203 possesses a potent immunosuppressive effect in organ transplantation models and autoimmune diseases [13, 15–17].. While the in vivo effect of KRP203 on immunity through S1PR binding is compelling, its effect requires phosphorylation. Whether non-phosphorylated KRP203 has another bioactivity remained unknown.
2.2. KRP203 could differentially modulate each isotype of phosphoinositide kinases
To further characterize, we focused on the highly homologous PI5P4Kα isozymes, PI5P4Kβ, and PI5P4Kγ. Surprisingly, regardless of their high sequence identity, KRP203 inhibits PI5P4Kβ and γ activities (Fig. 1C). These results suggest that KRP203 possesses a novel activity to modulate PI5P4K activity in vitro. It should be noted that KRP203 did not inhibit the GTPase activity of PI5P4Kβ (Fig. 1D). This indicates that KRP203 does not directly compete the nucleotide-binding site with GTP in aqueous condition to exert the inhibition of kinase activity—i.e., conversion of PI(5)P to PI(4,5)P2.
Given that KRP203 increases the activity of PI5P4Kα while suppressing the PI5P4Kβ and γ isotypes, we were interested in the possible effect of KRP203 on the other families of phosphoinositide kinases. For this purpose, we took advantage of a kinase panel assay and selected representative kinases in the phosphoinositide metabolism (Suppl. Table 2). Well-studied isotypes were selected for the pathways containing multiple isotypes. As shown in Figure 2, KRP203 inhibited class I PI3K isotypes α, δ, and γ, but not isotype β. KRP203 inhibited PI4K isotypes, type-IIα and type-IIIβ, whereas KRP203 increased the activity of isotype type-IIβ. KRP203 inhibited PI4P5K isotypes α and β, while KRP203 increased the activity of isotype γ. Both class II PI3K C2α and C2γ are slightly suppressed. These results suggest that KRP203 has multiple targets within phosphoinositide kinases. Furthermore, KRP203 can activate one isotype while suppressing another isotype within the same family of enzymes. To the best of our knowledge, this is the first report of a chemical drug that possesses multimodal activity against phosphoinositide kinases, activating one isotype while inhibiting the other isotype.
Fig. 2. Chemical library screening identifies KRP203 as a potential modifier for phosphoinositide metabolism.
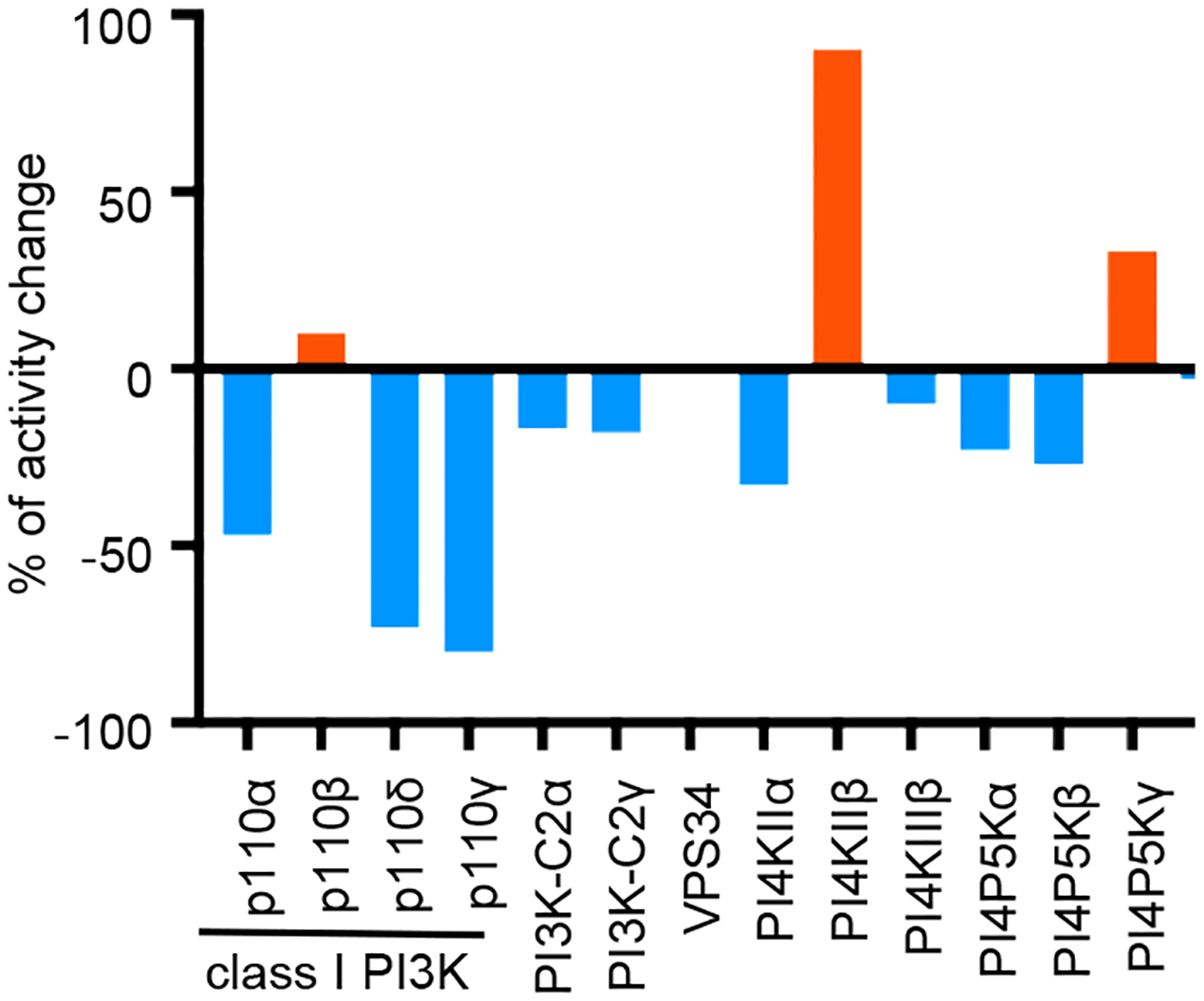
Effects of 10 μM KRP203 on the activity of each phosphoinositide kinase were tested using of a kinase panel assay (see method).
2.3. KRP203 increases PI-dependent, but not PI(3)P-dependent, kinase activity of PIKFYVE.
PI3P5K/PIKFYVE is considered to produce two phosphoinositides, PI(5)P and PI(3,5)P2, from PI and PI(3)P, respectively [18–20]. However, the relative contribution to these pathways remains unclear [21, 22]. Consistent with a previous report [23], the purified PIKFYVE phosphorylates PI and PI(3)P to generate PI(5)P and PI(3,5)P2, respectively (Fig.3). Surprisingly, KRP203 significantly increased PIKFYVE’s kinase activity against PI, forming more PI(5)P (Fig.3). On the other hand, KRP203 showed negligible effects on the PIKFYVE-dependent formation of PI(3,5)P2 from PI(3)P. The results suggest that KRP203 could alter the substrate selectivity of phosphoinositide kinase. Given its hydrophobic feature as well as its dissimilarity to phosphodonor purine nucleotide (Fig. 1B), it is plausible that KRP203 likely affects the substrate recognition site of phosphoinositide kinase.
Fig. 3. KRP203 changes PIKFYVE substrate selectivity.

A. Kinase assay of PIKFYVE using phosphatidylinositol (PI) and PI(3,5)P2 as a substrate.
B. Quantification of the effect of KRP203 on the activity of PIKFYVE. Data were shown as mean+SD of three independent experiments. The orange color indicates the samples treated with KRP203.
2.4. KRP203 induces an imbalance of phosphoinositide metabolism in glioblastoma cells.
Next, we examined the in vivo effect of KRP203 on phosphoinositide metabolism. Cellular phosphoinositides were radio-labeled by 3H-myoinositol and treated with or without KRP203 for 3 h. The phosphoinositides were extracted and deacylated for high-performance liquid chromatography (HPLC) analysis [24]. Among the seven measured phosphoinositides, the most dramatic changes were observed in increased PI(3)P levels in KRP203. While such robust PI(3)P accumulation was known to occur upon PI3P5K inhibition, it was also accompanied by depletion of PI(3,5)P2 and PI(5)P [18, 19, 22, 25]. Thus, it is surprising that KRP203-treated cells exhibited increased PI(3,5)P2 and PI(5)P levels more than untreated cells (Fig. 4). The KRP203-dependent elevation of PI(5)P synthesis from PI by PIKFYVE may also be a part of this phenomenon. It is worth noting that PI(4)P and PI(4,5)P2 were the most abundant phosphoinositides. The ratio of PI(4)P to PI(4,5)P2 is slightly but significantly increased by the KRP203 treatment. This could be a consequence of the increased PI 4-kinase activity detected in the kinase assay (Fig. 2). Collectively, these results show that KRP203 has a novel activity that modulates isozymes of phosphoinositide kinases differentially in vitro and induces the unique changes in phosphoinositide profiles.
Fig. 4. KRP203 modulates the entire phosphoinositide metabolism.
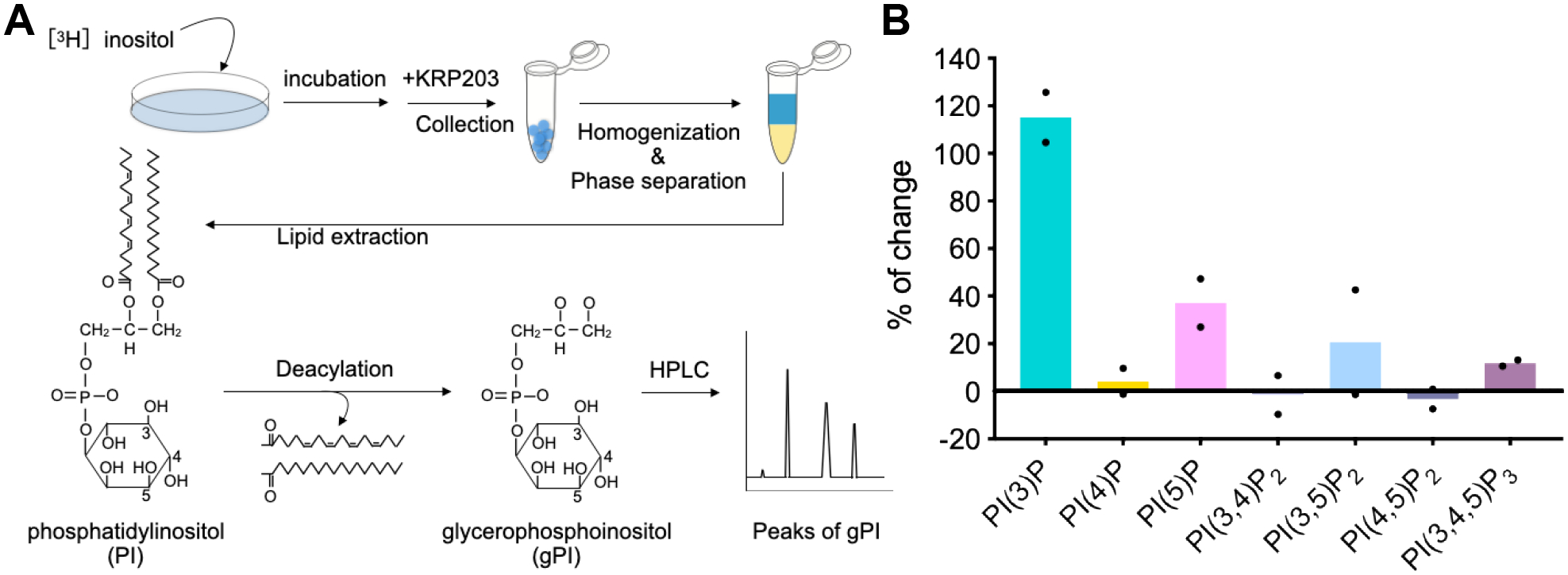
A. Scheme of the quantification of phosphoinositides.
B. Effect of KRP203 on the level of each phosphoinositide. Data were shown as mean of two independent experiments.
3. Discussion
Discovery of a multimodal activity of KPR203 to phosphoinositide kinases.
Most pharmacological or genetic interventions have focused on perturbing a specific phosphoinositide kinase. For example, to inhibit the enzyme activity of PI3K, researchers have sought inhibitors for the kinase activity of class I PI3K. In this study, we describe a previously identified compound KRP203 that, in contrast, modulates more than one phosphoinositide kinase simultaneously. This has the effect of altering the balance of phosphoinositides in a way that a single inhibitor cannot accomplish. KRP203 was developed as an immunosuppressive S1P functional antagonist. Mechanistically, KRP203 requires phosphorylation in a cell and secretion to outside cells to react with S1P receptors [14]. Thus, our finding is the first instance that non-phosphorylated KRP203 has another activity to modulate phosphoinositide kinases in vitro.
KRP203 changes the intracellular balance of phosphoinositide metabolism.
Our results show that KRP203 induces unique changes in phosphoinositide profiles, namely, the elevation of PI(3)P levels without decreases of PI(3,5)P2 and PI(5)P (Fig. 4). This suggests that the elevation of PI(3)P is not due to the inhibition of PIKFYVE, but rather due to the enhanced production of PI(3)P, or PI5P4K inhibition. Alternatively, given that the activities of tested class II and class III PI3K were unaffected by KRP203 (Fig. 2), another possible model would be the enhanced PI(3,4,5)P3 synthesis followed by dephosphorylation to PI(3)P, or specific class II PI3K isotype (e.g., PI3KC2β), might be increased. It is also possible that KRP203’s effect on phosphoinositide kinases may be influenced by their subcellular localization and upstream regulators.
Discovery of a pharmacologically tunable feature of PIKFYVE for its PI dependent activity.
Our results also show an unexpected effect of KRP203 on substrate reactivity of PIKFYVE; KRP203 increased the rate of PI phosphorylation by PIKFYVE, whereas the rate of PI(3)P phosphorylation was marginally affected (Fig. 3). To our knowledge, this is the first evidence that substrate specificity of phosphoinositide kinases, or the kinase activity to specific substrate among the multiple, could be tuned pharmacologically. These results are reminiscent of the history of class I PI3K, which were originally designated to PI as a substrate and phosphorylate 3-position of the hydroxy group of the inositol ring, leading to the name of PI 3-kinase or PI3K [26–28]. Further mechanistic study of KRP203-dependent increase of PI 5-kinase activity of PIKFYVE may clarify the mystery of why in vivo and in vitro substrate selectivity is different not only in class I PI3K but also perhaps in the other phosphoinositide kinases.
Limitation of the work and future directions
While this study identified KRP203 as a novel phosphoinositide kinase modulator with dual action in vitro, we did not test all 19 inositol phospholipid kinases. The specific molecular mechanism of KRP203 remains unknown and needs clarification. It is also important to analyze how the multimodal regulation of inositol phospholipid metabolism by KRP203 affects cellular functions and whether it can be used as a therapeutic strategy for cancer cells. Future research should clarify the effect of KRP203 on cell functions and the growth of normal and transformed cells.
3. Materials and Methods
3.1. Materials
Bacterially expressed PI5P4K isozymes were prepared as in [10, 11]. For PIKFYVE, the plasmid encoded FLAG-tagged PIKFYVE was transfected into HEK293T cells and and subjected to immunoprecipitation with anti-FLAG-M2-agarose beads followed by 3x FLAG peptide elution as in [31]. 3X FLAG peptide was prepared in 20 mM Tris-HCl, pH7.5, 100 mM NaCl, 0.5 mM EGTA, and 0.1% fattyfree BSA was added to the eluates.
3.4. In vitro kinase assay
The in vitro kinase reaction for PI5P4Ks was performed as previously [10, 11]. Using similar format, PIKFYVE assay was carried out. Briefly, a total of 50 μl of reaction buffer (6.5 mM HEPES, pH7.4, 10 mM MgCl2, 2.5 mM MnCl2, 1 mM β-glycerophosphate, 50 μM ATP and 50 μCi/ml γ−32P radiolabeled ATP) was prepared with 1 μg of PI (D-myo-phosphatidylinositol, diC16, Echelon Bioscience #P-0016) or 1 μg of PI(3)P (D-myo-phosphatidylinositol 3-phosphate, diC16, Echelon Bioscience #P-3016) and 1 μg of phosphatidylserine (Avanti #840037) that were suspended by sonication before addition to form liposomes. 1 μg of recombinant PIKFYVE was added to start the reaction and incubated for 15 min at room temperature, followed by lipid extraction and thin-layer chromatography (TLC) assay as in previously [10], and signals were quantified with a phosphoimager (Typhoon FLA7000, GE Healthcare, 100 μm/pixel image). The data were analyzed and drawn by Prism7 software.
3.4. Lipid kinase assay
We used a commercially available compound profiling assay for lipid kinases (SignalChem). Lipid protein kinases targeted were prepared at SignalChem using proprietary methods. The various lipid substrates were purchased from Echelon Biosciences. The activity of each kinase was examined in duplicate using ADP-Glo™ assay (Promega) in the presence of 20 μM KRP203.
3.5. 3H-Labeling of Phosphoinositides and HPLC Analysis
Subconfluent cells in 10-cm dishes were labeled in 8 mL of inositol-free DMEM and 10% dialyzed FBS supplemented with 160 μCi 3H-myo-inositol (PerkinElmer, NET1168001MC, specific activity = 20.1 Ci/mmol) for 48 h. Cells were washed with ice-cold PBS and then incubated with 1.5 mL of ice-cold aqueous solution (1M HCl, 5 mM tetrabutylammonium bisulfate, 25 mM EDTA). Then 2 mL of ice-cold methanol and 4 mL of CHCl3 were added to each sample. Samples were vortexed and then centrifuged at 1,000 rpm for 5 min. The organic (lower) phase was cleaned using theoretical upper, while the aqueous layer was cleaned using theoretical lower (theoretical upper and lower made by combining CHCl3: methanol: aqueous solution at 8:4:3 v/v ratio). Organic phases were collected and dried under nitrogen gas. Lipids were deacylated using monomethylamine solution (47% methanol, 36% of 40% methylamine, 9% butanol, and 8% water, by volume). Samples were incubated at 55° for 1 hour and dried under nitrogen. To the dried vials, 1 mL of theoretical upper and 1.5 mL of theoretical lower were added (theoretical upper and lower made by combining CHCl3:methanol: water in 8:4:3 v/v ratio). Samples were vortexed and spun at 1000 rpm. The aqueous phase was collected and dried under a speed-vac. Samples were resuspended in 150 μL of 1 mM EDTA and filter prior to HPLC analysis.
Deacylated phosphoinositides were resolved by HPLC using an Agilent 1200 Quaternary system, and radioactivity detected in-line using a Packard Flo-one Radiomatic detector. HPLC Buffer A is 1 mM EDTA, and Buffer B is 1 mM EDTA and 1 M NaH2PO4. Two Partisphere SAX columns (5 μm, 4.6 × 250 mm) in tandem were eluted by a gradient program (from 100% A to 2% B at 1 min, 14% B at 30 min, 30% B at 31 min, 66% at 60 min, 100% B at 85 min, 100% A at 86 min, and hold at 100% A until 110 min) at a flow rate of 1 ml/min.
3.6. NMR based GTP hydrolysis assay
All experiments were performed on Bruker Avance 700 MHz spectrometer equipped with a triple resonance probe. All spectra were collected in the NMR buffer (10 mM sodium phosphate (pH 6.8), 100 mM NaCl, 10 mM MgCl2, 2 mM DTT, and 99.6% D2O) at 25 °C. Spectra were processed using TOPSPIN (Bruker). For the GTP-hydrolysis assay, 250 μM GTP were mixed with 2 μM PI5P4Kβ, and hydrolysis reactions were carried out at 25 °C for 20 h. Sample volume was 500 μL. The reaction was monitored by the ratio of the intensity of the H8 position signal from the dinucleotide- over trinucleotide-forms in the 1D 1H experiments and the amount of produced dinucleotide-forms was normalized against DMSO condition and plotted as the GTP hydrolysis activity.
Supplementary Material
Highlight.
KRP203 is identified as a multi-target drug for phosphoinositide kinases.
KRP203 differentially modulates phosphoinositide kinases in vitro.
KRP203 enhances PIKFYVE’s substrate selectivity for PI.
KRP203 significantly alters cellular phosphoinositide metabolism.
Acknowledgements
We thank to Drs. Eric P. Smith and Danielle Tapp for their diligent proofreading and editing of our work, and Drs. Ryo Kamata, Yuki Fujii and Doshun Ito for illustrations and feedback. This work was supported in part by Project for Promotion of Cancer Research and Therapeutic Evolution (P-PROMOTE; 22ama221112h0001 to K.T., T. S., and A.T.S.) and Project for Cancer Research and Therapeutic Evolution (P-CREATE; 20cm0106173 to K.T., T. S., and A.T.S.) from Japan Agency for Medical Research and Development (AMED), Creation of fundamental technologies by interdisciplinary research to coexist with infectious diseases including COVID-19 from Japan Science and Technology Agency (JST), CREST program (JP20356709 to K.T., A.T.S., and T.S.), Generating research infrastructure and novel technologies for anti-infective drug and vaccine discovery from AMED (21gm1610003h0201 to K.T.), the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Numbers 22ama121001 (T.S.), KAKENHI (grant numbers 21K11709 and 18KK0455 to Y.H., 22K18374, 21H02436 and 20H03378 to K.T., and 20H03165 to A.T.S.) from Japan Society for the Promotion of Science (JSPS), Human Frontier Science Program (HFSP) (RGP0028/2022) and NIH grants (R01NS089815, R01CA255331 and R01GM144426) (A.T.S.), Swiss Light Source (proposal numbers 20191094 and 20191134), and the public accession beam time of Photon Factory, Institute of Materials Structure Science, High Energy Accelerator Research Organization (Accession numbers: 2017G147, 2019G063, and 2021G054). The research conducted in Keio University was also supported by grants from the Yamagata prefectural government and the city of Tsuruoka. The authors declare there is no competing interests in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of generative AI in scientific writing
During the preparation of this work the authors used the writing-assist tools, including Grammarly, Wordtune, and ChatGPT, in order to improve the clarity, coherence, and overall quality of the manuscript. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Balla T Phosphoinositides: Tiny Lipids With Giant Impact on Cell Regulation. Physiol. Rev 93 (2013)1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT The PI3K Pathway in Human Disease. Cell 170 (2017) 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fruman DA, Rommel C PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 13 (2014) 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, Suzuki A Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res 48 (2009) 307–343. [DOI] [PubMed] [Google Scholar]
- 5.Amann R, Peskar BA Anti-inflammatory effects of aspirin and sodium salicylate. European Journal of Pharmacology 447 (2002) 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Csermely P, Agoston V, Pongor S The efficiency of multi-target drugs: the network approach might help drug design. Trends in Pharmacological Sciences 26 (2005) 178–182. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins AL Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4 (2008) 682–690. [DOI] [PubMed] [Google Scholar]
- 8.Davis MI, Sasaki AT, Shen M, Emerling BM, Thorne N, Michael S, Pragani R, Boxer M, Sumita K, Takeuchi K, Auld DS, Li Z, Cantley LC, Simeonov A A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation. PLoS ONE 8 (2013) e54127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MI, Sasaki AT, Simeonov A Method for Assaying the Lipid Kinase Phosphatidylinositol-5-phosphate 4-kinase α in Quantitative High-Throughput Screening (qHTS) Bioluminescent Format. Methods Mol. Biol 1376 (2016) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumita K, Lo Y-H, Takeuchi K, Senda M, Kofuji S, Ikeda Y, Terakawa J, Sasaki M, Yoshino H, Majd N, Zheng Y, Kahoud ER, Yokota T, Emerling BM, Asara JM, Ishida T, Locasale JW, Daikoku T, Anastasiou D, Senda T, Sasaki AT The Lipid Kinase PI5P4Kβ Is an Intracellular GTP Sensor for Metabolism and Tumorigenesis. Mol Cell 61 (2016) 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi K, Ikeda Y, Senda M, Harada A, Okuwaki K, Fukuzawa K, Nakagawa S, Yu HY, Nagase L, Imai M, Sasaki M, Lo YH, Ito D, Osaka N, Fujii Y, Sasaki AT, Senda T The GTP responsiveness of PI5P4Kβ evolved from a compromised trade-off between activity and specificity. Structure 30 (2022) 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi K, Senda M, Lo YH, Kofuji S, Ikeda Y, Sasaki AT, Senda T Structural reverse genetics study of the PI5P4Kβ-nucleotide complexes reveals the presence of the GTP bioenergetic system in mammalian cells. FEBS J. 283 (2016) 3556–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi K, Senda M, Ikeda Y, Okuwaki K, Fukuzawa K, Nakagawa S, Sasaki M, Sasaki AT, Senda T Functional molecular evolution of a GTP sensing kinase: PI5P4Kβ. FEBS J. (2023) doi: 10.1111/febs.16763. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billich A Phosphorylation of the Immunomodulatory Drug FTY720 by Sphingosine Kinases. Journal of Biological Chemistry 278 (2003) 47408–47415. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H, Takahashi M, Kaneko T, Murakami T, Hakamata Y, Kudou S, Kishi T, Fukuchi K, Iwanami S, Kuriyama K, Yasue T, Enosawa S, Matsumoto K, Takeyoshi I, Morishita Y, Kobayashi E KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation 111 (2005) 222–229. [DOI] [PubMed] [Google Scholar]
- 16.Takabe K, Paugh SW, Milstien S, Spiegel S “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacological Reviews 60 (2008) 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khattar M, Deng R, Kahan BD, Schroder PM, Phan T, Rutzky LP, Stepkowski SM Novel Sphingosine-1-Phosphate Receptor Modulator KRP203 Combined With Locally Delivered Regulatory T Cells Induces Permanent Acceptance of Pancreatic Islet Allografts. Transplantation 95 (2013) 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki C, Takahashi M, Morimoto H, Izawa A, Ise H, Fujishiro J, Murakami T, Ishiyama J, Nakada A, Nakayama J, Shimada K, Ikeda U, Kobayashi E Efficacy of mycophenolic acid combined with KRP-203, a novel immunomodulator, in a rat heart transplantation model. J. Heart Lung Transplant 25 (2006) 302–309. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama E, Hashimoto D, Hayase E, Ara T, Ogasawara R, Takahashi S, Ohigashi H, Tateno T, Hasegawa Y, Chen X, Teshima T Short-term KRP203 and posttransplant cyclophosphamide for graft-versus-host disease prophylaxis. Bone Marrow Transplant. 55 (2019) 1–9. [DOI] [PubMed] [Google Scholar]
- 20.Sbrissa D Phosphatidylinositol 5-Phosphate Biosynthesis Is Linked to PIKfyve and Is Involved in Osmotic Response Pathway in Mammalian Cells. Journal of Biological Chemistry 277 (2002) 47276–47284. [DOI] [PubMed] [Google Scholar]
- 21.Sbrissa D, Ikonomov OC, Filios C, Delvecchio K, Shisheva A Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636. AJP: Cell Physiology 303 (2012) C436–C446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sbrissa D, Ikonomov OC, Strakova J, Dondapati R, Mlak K, Deeb R, Silver R, Shisheva A A Mammalian Ortholog of Saccharomyces cerevisiae Vac14 That Associates with and Up-Regulates PIKfyve Phosphoinositide 5-Kinase Activity. Mol Cell Biol 24 (2004) 10437–10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zolov SN, Bridges D, Zhang Y, Lee W-W, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, Saltiel AR, Meisler MH, Russell MW, Weisman LS In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proceedings of the National Academy of Sciences 109 (2012) 17472–17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takasuga S, Horie Y, Sasaki J, Sun-Wada GH, Kawamura N, Iizuka R, Mizuno K, Eguchi S, Kofuji S, Kimura H, Yamazaki M, Horie C, Odanaga E, Sato Y, Chida S, Kontani K, Harada A, Katada T, Suzuki A, Wada Y, Ohnishi H, Sasaki T Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proceedings of the National Academy of Sciences 110 (2013) 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sbrissa D, Ikonomov OC, Shisheva A PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J. Biol. Chem 274 (1999) 21589–21597. [DOI] [PubMed] [Google Scholar]
- 26.Sarkes D, & Rameh LE A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5 Pand other phosphoinositides. Biochem J 428 (2010) 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, Rappolee D, Shisheva A The Phosphoinositide Kinase PIKfyve Is Vital in Early Embryonic Development: PREIMPLANTATION LETHALITY OF PIKfyve−/− EMBRYOS BUT NORMALITY OF PIKfyve+/− MICE. Journal of Biological Chemistry 286 (2011) 13404–13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiles ID, Otsu M, Volinia S, Fry MJ, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty NF Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell 70 (1992) 419–429. [DOI] [PubMed] [Google Scholar]
- 29.Whitman M, Downes CP, Keeler M, Keller T, Cantley L Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332 (1988) 644–646. [DOI] [PubMed] [Google Scholar]
- 30.Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 315 (1985) 239–242. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki AT, Carracedo A, Locasale JW, Anastasiou D, Takeuchi K, Kahoud ER, Haviv S, Asara JM, Pandolfi PP, Cantley LC Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci. Signal 4 (2011) ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


