Abstract
A sensor can be called ideal or perfect if it is enriched with certain characteristics viz., superior detections range, high sensitivity, selectivity, resolution, reproducibility, repeatability, and response time with good flow. Recently, biosensors made of nanoparticles (NPs) have gained very high popularity due to their excellent applications in nearly all the fields of science and technology. The use of NPs in the biosensor is usually done to fill the gap between the converter and the bioreceptor, which is at the nanoscale. Simultaneously the uses of NPs and electrochemical techniques have led to the emergence of biosensors with high sensitivity and decomposition power. This review summarizes the development of biosensors made of NPssuch as noble metal NPs and metal oxide NPs, nanowires (NWs), nanorods (NRs), carbon nanotubes (CNTs), quantum dots (QDs), and dendrimers and their recent advancement in biosensing technology with the expansion of nanotechnology.
Keywords: Nanowires, Nanorods, Carbon nanotubes, Quantum dots, Dendrimers
1. Introduction
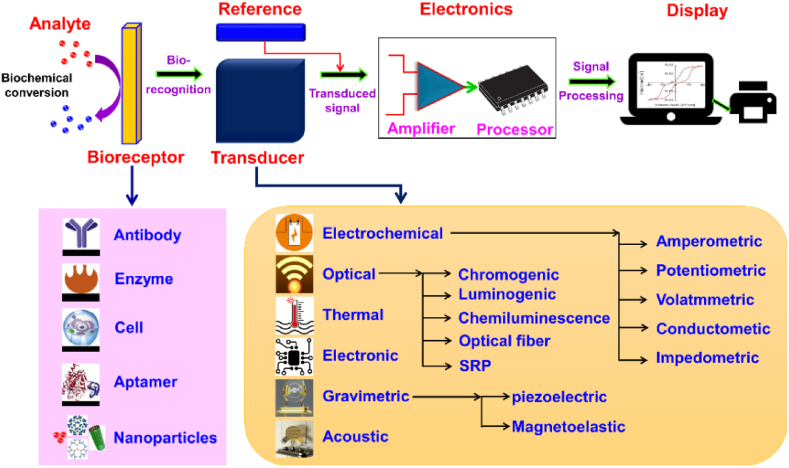
Diseases if detected in early stages can increase the chance of successful treatments and survival, hence it is the need of hour to develop a device which can detect or sense the trouble causing organic/inorganic biomolecule in the living organism [1]. The American biochemist L.L Clark was the first person to invent the biosensor in year 1956. He used this biosensor to detect percentage of oxygen in the blood and the electrode he used in this sensor was named as the Clark electrode or oxygen electrode [2]. The term biosensor was first used by Cammann in 1977 [1] and it can be defined as analytical device which is designed by the combination of bioreceptor (cell, enzyme, antibody, DNA etc.), transducer (translate the observed signal into a useful output) and an amplifier (amplifies and processes the final signal) as depicted in Fig. 1. Further, a substance of curiosity, which needs to be detected, is called an analyte.
Fig. 1.
Schematic diagram of biosensor consisting of bioreceptor, transducer, and amplifier. Reproduced with permission from Ref. [3], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2021, MDPI.
The development in the area of biosensors is divided into mainly five generations:–In the first generation, the biosensors measure two different things, first the composition of the analyte and second the bioreceptor reactions product, which finally produces the signal as a response. Leland Charles Clark Jr. was the first one to work on this type of biosensor [2]. In 1962, Clark further employed an amperometric enzyme electrode for glucose detection [4]. Later, in 1967, Updike and Hicks, modified the Clark's work and prepared the first enzyme electrode [5]. Further in the year 1969, Guilbault and Montalvo, designed a potentiometric electrode sensor for sensing urea [6]. Similarly in 1973, Guilbault and Lubrano detected hydrogen peroxide using lactate/glucose enzyme-based sensor [7]. The design and development of thermistors was carried out by Klaus Mosbach group in 1974 for detecting temperature variations [8]. In 1975, Lubbers and Opitz prepared an optical biosensor for the sensing of alcohol [9]. Fig. 1 shows the schematic diagram for the fundamental components of a biosensor, highlighting the critical elements of the bioreceptor, transducer, and amplifier. This visual representation provides a clear understanding of the essential building blocks that collaborate to enable accurate and sensitive biosensing capabilities.
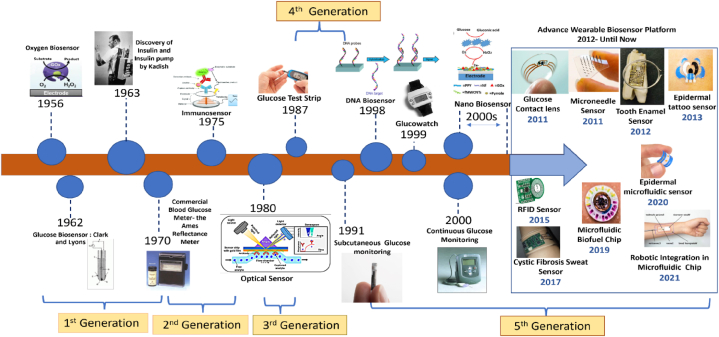
In the 2nd generation biosensors, the analytical efficiency of the biosensor was enhanced by the addition of auxiliary enzymes and co-reactants [10]. These sensors were named as mediator amperometric biosensors. In the third to fifth generation, the bio-receptors become an essential part of the sensing element. The direct interface was created between the bioreceptor (enzymes) and electrode via the transfer of electrons, without involving any intermediate. The main advantages of these generation sensors were their low cost and repeatability with high sensitivity [11]. The different milestones achieved in the field of biosensors are shown in Fig. 2. In Fig. 2, a visual representation illustrates the significant milestones that have been accomplished in the dynamic field of biosensors. This comprehensive overview highlights the key advancements and breakthroughs that have shaped the evolution of biosensing technologies, highlighting the remarkable progress achieved over time.
Fig. 2.
The different milestones achieved in the field of biosensors. Reproduced with permission from Ref. [12], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2022, MDPI.
1.1. Characteristics and classification of biosensors
The designed and prepared biosensor prototype must possess the following characteristics to the better end so that the desired results can be achieved for the upliftment and betterment of the health of the society.
-
(i)
Selectivity: Before designing any biosensor, the main thought in the mind of the designer is to think about the selectivity of the biosensor so that it can detect the desired analyte from sample containing different or nearly similar analytes/contaminants [3]. Hence selectivity is the most important feature of the biosensor.
-
(ii)
Reproducibility: The ability of the biosensor to reproduce the same results again and again for duplicated experiments is an immense matter of concern [13]. The biosensors with high reproducibility quality are really in high demand at present. Along with reproducibility, the results obtained should be of high accuracy and precision and altogether these properties of the biosensor make it the more dependable one.
-
(iii)
Stability: The stability of the biosensor is quite crucial factor which counts for the commercial successes of the biosensor. The biosensor does lose their strength of signals with age, hence this factor needs more consideration and attention [3]. Further the ageing or instability get accelerated with rise in temperature or in other words ageing in directly temperature dependent.
-
(iv)
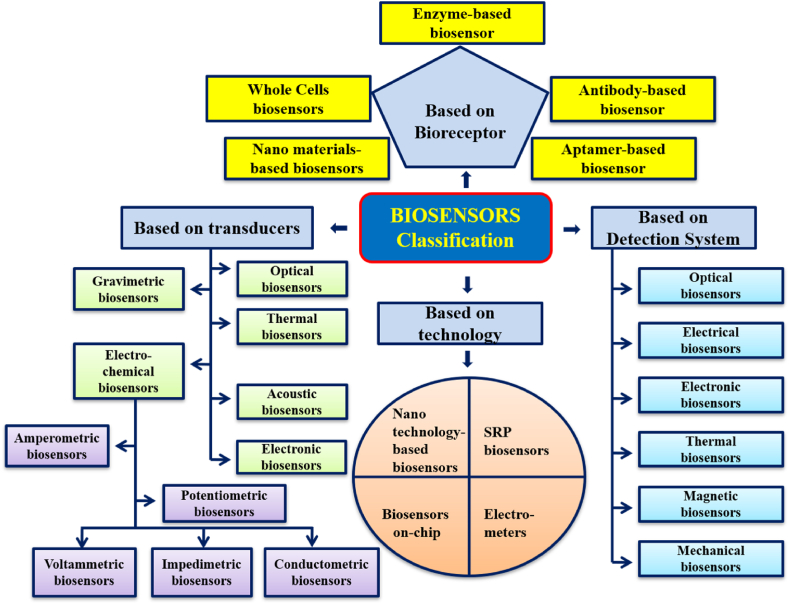
Sensitivity and Linearity: The biosensors are rated high only if they possess high sensitivity. In today's world especially in air, water and soil pollutant detection, the requirement is a ppm level whereas in medical field it goes from nanograms per milliliter to femtograms per milliliter [13]. Further linearity of the device highlights the accuracy of the given response for a given set of measurements versus different concentration of an analyte. Fig. 3 illustrates a comprehensive classification of biosensors, categorized based on their detection system, transducer technology, and choice of bio-receptors. This classification offers insights into the diverse range of biosensing approaches, aiding researchers in selecting the most suitable biosensor design for their specific applications. Regarding the classification of biosensors, the different criterion and factors can be employed. Here in this review article the classification has been chalked out based on four main criterions as shown in Fig. 3 and are detailed as below:
-
(i)
Biosensor based on the type of bio-receptors used for preparing the device
-
(ii)
Biosensor based on the type of transducer used for making the device
-
(iii)
Biosensor based on the type of technology used for designing the device
-
(iv)
Biosensor based on the type of detection system used.
Fig. 3.
Classification of biosensors based on detection system, transducer, technology and bio-receptors.
Further, this article will be focused on only the nano material-based biosensors as they are finding more importance and have wider applications.
2. Advancement of nanotechnology and NPs-based biosensors
To meet up the heavy demand of biosensors nearly in all fields of science and technology, scientist community have been forced to explore new materials at nanoscale level which could be employed in the sensor technology to achieve good results. Opioids, widely employed as potent analgesics for pain management, bear the dual nature of therapeutic benefits and potential risks. Instances of overdose and the risk of developing addictive behaviors underscore the need for vigilant monitoring. The surge in illicit drug consumption and misuse on a global scale necessitates precise and efficient detection methodologies across confiscated samples, biological matrices, and environmental effluents. In this context, the integration of advanced nanostructures into biosensing platforms offers a promising avenue for opioid detection, enabling rapid and accurate identification. A recent scholarly contribution by Saman Sargazi and colleagues delves into the realm of nanobiosensors tailored for opioids, offering a comprehensive analysis of the burgeoning field [14]. This review meticulously navigates the landscape of nanomaterials, exemplifying their application as biosensing tools targeting opioids. The focus extends to the molecular entities under scrutiny and the associated limits of detection, collectively shaping the precision and scope of these nanobiosensor systems.
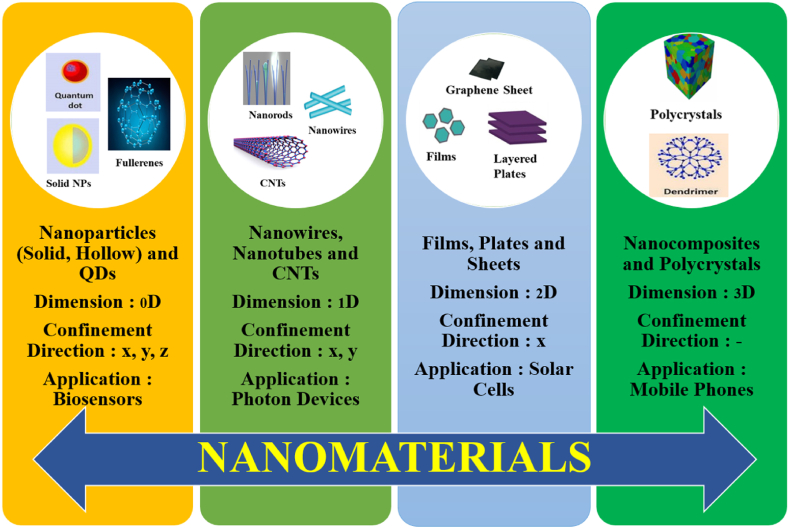
In last two to three decades, nanotechnology has shown great advancement in its development and applications [15]. Numerous NPs, nanomaterials have been synthesized, designed and are utilized in enhancing the overall performances of the biosensors [16]. In Fig. 4, a depiction showcases the diverse array of nanomaterials (NMs) employed in the design of biosensors, highlighting their distinct dimensions and characteristics. This visual representation offers insights into the various types of nanomaterials utilized, underlining their significant role in enhancing the performance and sensitivity of biosensing platforms.
Fig. 4.
Different types of NMs with different dimensions utilized in designing biosensors.
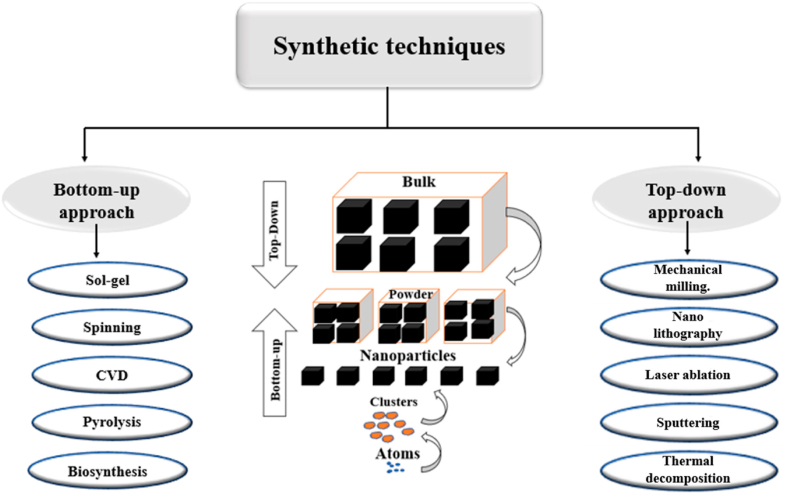
Several methods and technologies [17] have been designed and opted for preparing nanomaterials, including a “top-down” (bulk materials are reconstituted to form nanoscale materials) and a “bottom-up” methodologies (nano-scale materials are assembled at the molecular level) as shown in Fig. 5 [18]. Fig. 5 exhibits the schematic for the top-down and bottom-up methodologies employed for the preparation of nanomaterials. This illustration provides a clear overview of these distinct approaches, showcasing how the nanomaterials are synthesized from macroscopic to nanoscale dimensions (top down) or prepare from individual components to form larger structures (bottom up). The bottom-up approach encompasses a multitude of techniques, including sol-gel, spinning, chemical vapor deposition (CVD), pyrolysis, biosynthesis, hydrothermal synthesis, and more. Similarly, the top-down approach comprises a diverse array of methods such as mechanical milling, lithography, laser ablation, sputtering, thermal decomposition, and others. This comprehensive range of techniques underscores the versatility and complexity of both bottom-up and top-down methodologies in nanoparticle synthesis. The intricate investigation and systematic development of metal oxide-based nanomaterials, such as nanowires (NWs), nanorods (NRs), carbon nanotubes (CNTs), quantum dots (QDs), and nanocomposite dendrimers, have the potential to revolutionize the landscape of biosensor technology. By delving into the design and synthesis of these nanomaterials, it becomes possible to unlock new dimensions in enhancing the detection capabilities of biosensors. These nanomaterials offer a remarkable platform for manipulation and customization, allowing researchers to finely tune their properties to precisely match the requirements of diverse biosensing applications. This level of precision engineering not only empowers biosensors to achieve higher sensitivities but also opens avenues to controlling their selectivity and overall performance.
Fig. 5.
Top-down and bottom-up methodologies for preparing nanomaterials. Reproduced with permission from Ref. [18], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2022, MDPI.
2.1. Metal oxide-based biosensors
Metal oxide-based nanomaterials, known for their unique physicochemical properties at the nanoscale, hold the promise of elevating the sensitivity and responsiveness of biosensors. Nanowires and nanorods provide a one-dimensional architecture that can facilitate efficient charge transfer and signal transduction. In addition, such nanomaterials offer exceptional mechanical, electrical, and thermal properties, and all of which can be harnessed to enhance biosensor functionality. Quantum dots, with their tunable optical properties, enable precise signal amplification and multiplexing, adding a new layer of sophistication to biosensor designs. Moreover, the incorporation of nanocomposite dendrimers offers a versatile platform for functionalizing biosensor surfaces, enhancing binding interactions, and improving stability, thereby contributing to the overall robustness of biosensor performance.
As the realms of nanotechnology and biosensor development converge, the potential for groundbreaking advancements in detection capabilities becomes evident. The careful manipulation and incorporation of these nanomaterials into biosensor designs pave the way for unparalleled sensitivity, specificity, and efficiency in detecting target analytes. By harnessing the inherent advantages of these nanoscale structures, researchers can push the boundaries of biosensor technology and redefine its role in various fields, ranging from healthcare diagnostics to environmental monitoring and beyond.
In the past two decades, oxides of copper (CuO), nickel (NiO), iron (Fe2O3), cobalt (Co3O4), manganese (MnO2), zinc (ZnO), tin (SnO2), titanium (TiO2) and cadmium (CdO) etc. have been extensively engaged in a variety of fields virtue of their extensive range of electrical, chemical and physical properties. Among the above-mentioned metal oxides, oxides of zinc, copper, iron and manganese are adopted as the best magnetic nanomaterials showing high electron movement rate hence utilized in designing electrochemical biosensors [19].
2.1.1. Zinc oxide-based biosensor
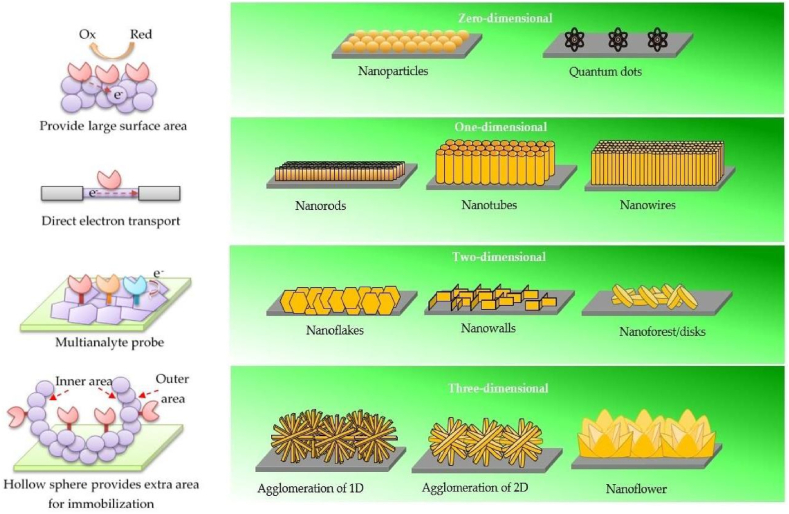
ZnO has been identified an excellent candidate for designing a biosensor virtue of its high isoelectric point (IEP), cost effectiveness, eco-friendly nature, and chemical stability. A high value of IEP allows enhanced absorption process of the analytes such as enzymes, DNA, and proteins by electrostatic interactions. Further its properties viz.; an n-type semiconductor with broad band gap (3.37eV), high exciton binding energy (6.0 meV) and good electron mobility makes it more promising material in fabrications of biosensors [20]. The broad band gap helps ZnO to sustain large electric fields, which allow a high breakdown voltage and stable semiconductor in the visible region [21]. Along with this, to enhance its application to wider range ZnO NPs have four different dimensions starting from zero dimension (0-D) to 3-D. The illustration in Fig. 6 highlights the diverse dimensions of ZnO nanostructures, each offering distinct advantages for biosensor applications. These advantages play a pivotal role in enhancing the overall performance and functionality of biosensors, catering to specific detection needs.
Fig. 6.
The four different shapes of ZnO nanostructures with their characteristics. Reproduced with permission from Ref. [22], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2019, MDPI.
0-D Nanostructures (Zero-Dimensional): Zero-dimensional nanostructures present a vast surface area. This expansive surface offers ample room for immobilizing biomolecules, enabling efficient interactions between target analytes and sensing elements. The large surface-to-volume ratio enhances sensitivity, making these structures well-suited for ultra-sensitive biosensing applications.
1-D Nanostructures (One-Dimensional): One-dimensional nanostructures provide stable and direct electron transport pathways. The elongated structure facilitates efficient charge transfer, leading to enhanced signal transduction. This stability in electron transport results in improved sensor response and accuracy, making 1-D nanostructures an ideal choice for robust biosensor designs.
2-D Nanostructures (Two-Dimensional): Two-dimensional nanostructures offer specific planes for immobilization processes. These immobilization planes enable the simultaneous detection of multiple analytes, making them highly valuable for multi-analyte biosensing. The versatility of 2-D nanostructures in accommodating various sensing elements enhances the sensor's capability to target and distinguish different analytes in complex samples.
3-D Nanostructures (Three-Dimensional): Three-dimensional nanostructures encompass both outer and inner surfaces, providing additional sites for immobilization. This extra surface area facilitates the attachment of a higher number of biomolecules, thereby improving the sensitivity and binding efficiency of the biosensor. The increased surface area of 3-D nanostructures enhances the sensor's ability to capture and detect trace amounts of target analytes.
Collectively, the distinct advantages associated with each dimension of ZnO nanostructures contribute to the advancement of biosensor performance. By harnessing these advantages, biosensor designers can tailor their platforms to achieve specific detection goals, enabling applications ranging from medical diagnostics to environmental monitoring and beyond.
ZnO has been used extensively used to sense various compounds such as glucose, ascorbic acid, cholesterol, uric acid, and cancer cells etc. In 2014, Tashkhourian et al. fabricated an effective naproxen electrochemical sensor by using carbon paste electrode modified with ZnO NPs and multiwalled carbon nanotubes. It was detected by square wave voltammetry with a linear concentration range of 1.0 × 10−6 M to 2.0 × 10−4 M and the detection limit was 2.3 × 10−7 M [23]. In year 2015, Roy et al. prepared a novel Ag–ZnO bimetallic, graphene oxide coated polymer-based sensor for the detection, of E. coli bacteria. It detected concentrated in the range of 105 CFU mL−1 and detected as low as 10 CFUmL−1 [24]. In the same year, Bashami et al. fabricated the ZnO coated carbon electrode for the sensitive detection of para-nitrophenol. It was detected over a linear concentration range from 2.1 μM to 6.3 μM and the lower detection limit was 0.02 μM [25]. Further in the year 2016, Fang et al. developed 3-D ZnO sensors using trisodium citrate-assisted solution phase method for sensing glucose. It detected glucose in linear range from 1 to 20 mM and the lower detection limit was 0.02 mM [26].
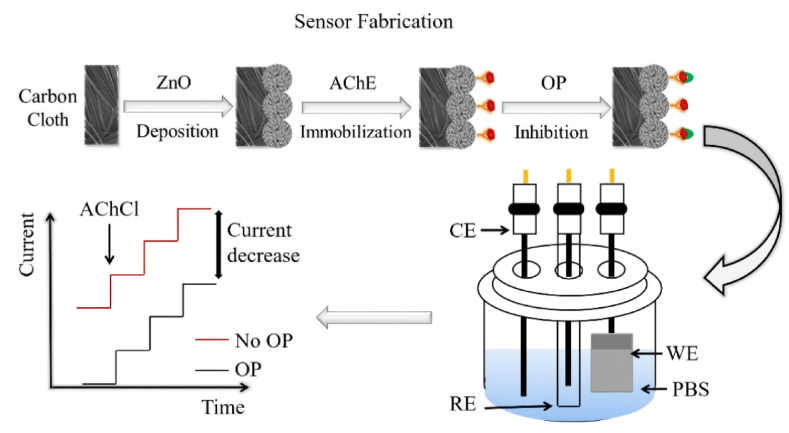
The development of an affordable, sensitive, and portable biosensor for detecting pesticides holds significance in various applications, including food packaging, agriculture, and environmental monitoring. In the year 2022, Fallatah et al. prepared a zinc oxide (ZnO) nanostructure-based biosensor that can be formed on a flexible porous surface for the detection of pesticides, as shown in Fig. 7. The biosensors were constructed by immobilizing the acetylcholinesterase (AChE) enzyme on ZnO, which was directly grown on the flexible substrates. Notably, the ZnO biosensors developed on carbon cloth exhibited superior performance characteristics, including a detection limit for OP ranging from 0.5 nM to 5 μM, heightened sensitivity, and enhanced stability [27].
Fig. 7.
ZnO based biosensor for pesticide detection. Reproduced with permission from Ref. [27], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2022, MDPI.
2.1.2. Copper oxide-based biosensor
The oxides of copper viz.; CuO and Cu2O are non-poisonous nanomaterials and that could be easily fabricated in the abundant amount at quite effective low cost. Further their synthesis can be tailored to obtain the NPs of high crystalline nature with required size and shape that can be employed in fabricating biosensors of severe demand. Along with fabricating sensor devices, the oxides of copper being p-type semiconductor are also in high demand for making batteries, supercapacitors, photovoltaic cells and field emission devices etc. [28,29].
Hydrogen peroxide (H2O2) serves as a potent oxidant and bleaching agent with widespread applications across biomedicine, households, and industries. Additionally, H2O2 functions as a significant reactive oxygen species (ROS) implicated in various physiological and pathological processes. Its connection to a range of human diseases, including cardiovascular disorders, diabetes, neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, Huntington's disease, as well as metabolic disorders and cancers, underscores its critical role. Consequently, the accurate detection of H2O2 holds paramount importance for both academic research and industrial applications. Addressing this need, the development of H2O2 sensors that are cost-effective, rapid, sensitive, and selective is imperative. In current times, a plethora of sensor platforms has emerged to detect hydrogen peroxide.
Ping et al. fabricated carbon ionic liquid electrode with copper oxide NPs for sensing H2O2. It was detected over a continuous range from 1.0 μM to 2.5 mM and the lower detection limit was 0.5 μM [30]. Dhara et al. prepared a biosensor by decorating reduced reduce graphene oxide with palladium-copper oxide NPs for the detection of glucose, with the linear concentration range from 6 μM to 22 mM and the lower limit of detection was 30 nM [31]. Z. Monsef Khoshhesab developed electrochemical sensor based on CuO-graphene nanocomposite for the simultaneous detection of acetaminophen, ascorbic acid and caffeine. It was detected over a linear range from 0.025 to 5.3 μmol L−1 and the limit of detection were 0.008, 0.011 and 0.010 μmol L−1 respectively [32]. Similarly, Zhang et al. synthesized CuO NPs decorated with carbon spheres (CuONPs-CSs) for the electrochemical determination of glucose with a linear concentration range from 5.0 × 10−7 to 2.3 × 10−3 M, the detection limit was 0.1 μM and the high sensitivity of 2981 μA mM−1 cm−2 [33]. Table 1 highlights the different cuprous/cupric oxide NPs based electrochemical biosensors used in recent years.
Table 1.
The different cuprous/cupric oxide NPs based electrochemical biosensors.
| S.No. | MOs Nanomaterials | Analyte | Sensing Method | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| 1. | CuO/g-C3N4 nanocomposites | Dopamine | Electrochemical | 2 × 10−9–7.11 × 10−5 molL−1 | 1 × 10−10 molL−1 | [34] |
| 2. | CuO/GO | Glucose | Electrochemical | 0.0028––2.03 mM | 0.69 μM | [35] |
| 3. | CuxO/ERGO | Dopamine | Electrochemical | 0.1––400 μM | 12 nM | [36] |
| 4. | CuO NPs on Carbon ceramic electrode | Tyrosine | Amperometry | 2––1350 μM | 160 nM | [37] |
| 5. | CuO-rGO | Glucose | Amperometry | 0.0004––12 mM | 0.1 μM | [38] |
| 6. | Cu2O–TiNTs | Eugenol | Cyclic Voltammetry | 4.6––130 μM | 1.3 μM | [39] |
| 7. | Cu2O–rGO/GCE | H2O2 | Amperometry | 0.03––12.8 mM | 21.7 μM | [40] |
| 8. | CuO-Gr/CPE | Acetaminophen | DPV | 0.025––5.3 μM | 0.008 μM | [41] |
| 9. | Cu2O-BSA NPs | Glucose | Cyclic Voltammetry | Up to 10 mM | 0.4 μM | [42] |
| 10. | CuO-Gr/CPE | Caffeine | DPV | 0.025––0.3 μM | 0.010 μM | [41] |
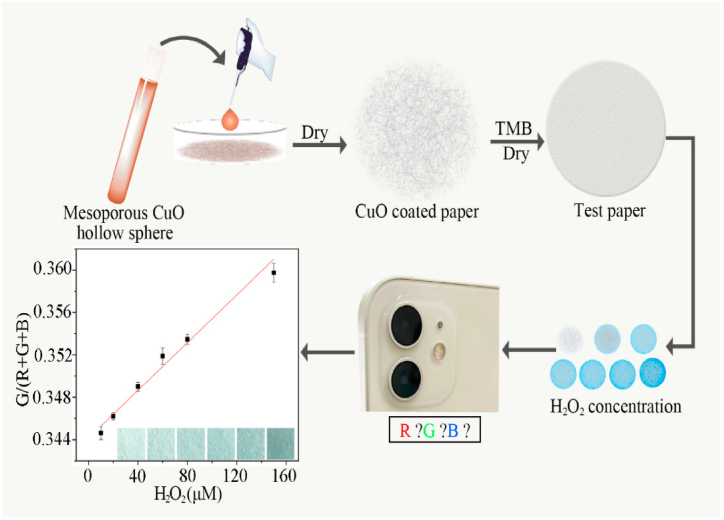
Another illustrative example by Cheng et al. introduces a paper-based colorimetric sensor utilizing mesoporous copper oxide (CuO) hollow spheres for H2O2 detection. These mesoporous CuO hollow spheres exhibit noteworthy characteristics, including a substantial specific surface area (58.77 m2/g), appreciable pore volume (0.56 cm3/g), accessible mesopores (5.8 nm), a hollow morphology, and uniform diameter (∼100 nm). Importantly, they demonstrate excellent peroxidase-like activities, with Km and Vmax values of 120 mM and 1.396 × 10−5 M s−1, respectively (Fig. 8). Leveraging these properties, the mesoporous CuO hollow spheres are employed on low-cost, disposable filter paper test strips. The resultant paper-based sensor exhibits efficacy in detecting H2O2 across a range of 2.4–150 μM. This innovative approach presents a promising avenue for efficient and reliable H2O2 detection, offering significant benefits for various applications [43].
Fig. 8.
Schematic for the paper based colorimetric sensor using mesoporous copper oxide CuO hollow sphere for the detection of hydrogen peroxide. Reproduced with permission from Ref. [43], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2021, MDPI.
2.1.3. Iron oxide-based biosensor
The oxides of iron Fe2O3 and Fe3O4 have been utilized extensively in fabricating different types of electrodes for designing numerous types of biosensors for detecting heavy metal ions, organic molecules etc. In year 2009, Kaushik et al. fabricated a urea sensor by coating glass plate with indium-tin oxide and then depositing thin film of Fe3O4NPs/chitosan. Urea was detected with a concentration range of 5–100 mg/dL and with limit of detection was 0.5 mg/dL [44]. In year 2015, Li et al. developed a nitrite sensor by using the Ag–Fe3O4–graphene oxide magnetic nanocomposite, with a linear range of 0.5 μM–0.72 mM and the lower limit of detection was 0.17 μM [45]. In 2016, Lee et al. used Fe2O3/graphene NPs for fabricating electrochemical sensor for detecting Zn2+, Cd2+ and Pb2+ metal ions. These were detected over a linear range of 1–100 μg L−1 for Zn2+, Cd2+, and Pb2+ and the lower limit of detection were 0.11 μg L−1, 0.08 μg L−1, and 0.07 μg L−1 [46]. Further Table 2 depicts the Iron oxide NPs based electrochemical biosensors employed recently in different fields.
Table 2.
The various iron oxide NPs based electrochemical biosensors.
| S.No. | MOs Nanomaterials | Analyte | Sensing Method | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| 1. | Fe2O3 NPs | Uric Acid | Electrochemical | 10––100 μM | 2.5 nM | [47] |
| 2. | Fe3O4/rGO nanocomposite | Dopamine | Amperometry | 0.010––0.270 μM | 5 nM | [48] |
| 3. | Fe3O4/rGO nanocomposite | Ascorbic Acid | DPV | 1––9 mM | 0.42 μM | [49] |
| 4. | Fe3O4 modified Carbon paste electrode |
Tyrosine | DPV | 0.4––270.0 μM | 50 nM | [50] |
| 5. | rGO/Fe3O4/Gelatin | Glucose | Cyclic Voltammetry | 0.1––10 mM | 0.024 μM | [51] |
| 6. | Polypyrrole-chitosan-Iron oxide | Glucose | Electrochemical | 1––16 mM | 234 μM | [52] |
| 7. | Ag@Fe2O3/SPCE | Nitrate | Amperometry | 0––1000 μM | 30 μM | [53] |
| 8. | Fe3O4NPs-CB/GCE | Bisphenol A | DPV | 0.1 nM–50 μM | 0.031 nM | [54] |
| 9. | Fe2O3/GCE | Pyrocatechol | Chronoamperometry | 7.9––130 μM | [55] | |
| 10. | PEG- Fe3O4/GE | l- Dopa | DPV | 0.05––10 μM | 9.5 nM | [56] |
Iron oxide (Fe2O3) has emerged as a versatile transition metal oxide renowned for its affordability, abundance, favorable biocompatibility, and impressive electrochemical attributes, rendering it a material of significant interest across various domains. Among its myriad applications, α-Fe2O3 nanoparticles (α-Fe2O3 NPs) have garnered substantial attention as an exceptional modifying agent. This is attributed to the inherent capability of iron oxides to undergo in situ electrochemical reduction or oxidation, owing to their variable valence state, thereby inducing heterogeneous redox reactions pertinent to the target analyte. Notably, investigations have showcased the influential role of nanostructured α- Fe2O3 morphologies on optical, magnetic, photocatalytic, and electrochemical properties. Intriguingly, the impact of morphology on electrochemical sensing, particularly concerning small biomolecules, remains an area warranting exploration.
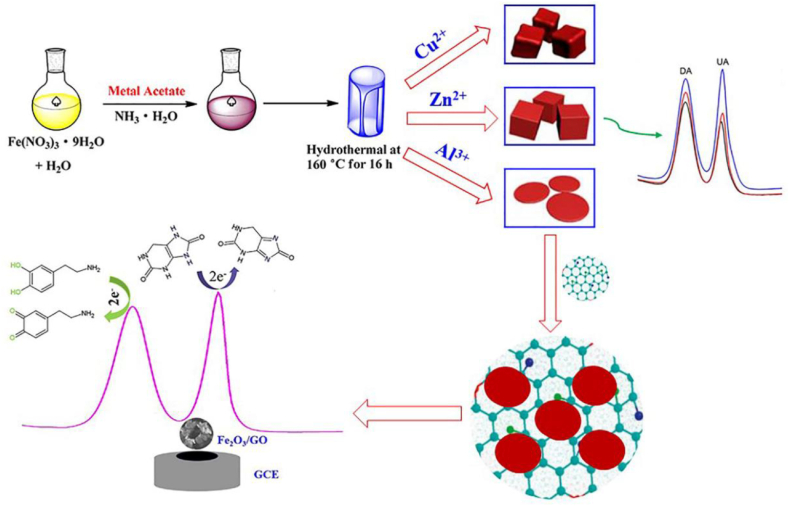
Ran et al. fabricated electrochemical sensor using bromocresol green and Fe3O4 embedded in chitosan matrix for sensing serotonin, with a linear concentration range of 0.5–100 mM with lower limit of detection 80 nM [57]. In light of this imperative, Cai et al. have unveiled the morphology–dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid (Fig. 9) [58]. Leveraging a facile meta-ion mediated hydrothermal method, the research yielded distinct morphologies of iron oxide nanoparticles (Fe2O3 NPs) encompassing cubic, rhombic, and discal configurations. In a quest to elevate electrochemical sensing prowess, the research team harnessed the exceptional electrocatalytic activity of discal Fe2O3 NPs (d- Fe2O3), coupling them with graphene oxide (GO) nanosheets. The synergistic interplay between discal Fe2O3 NPs and GO engendered remarkable electrocatalytic efficiency in the oxidation of dopamine (DA) and uric acid (UA). Significantly, this collaboration facilitated linear electrochemical responses for both DA and UA within concentration ranges of 0.02–10 μM and 10–100 μM, respectively. Impressively low limits of detection (LOD), specifically 3.2 nM for DA and 2.5 nM for UA, further underscored the sensitivity of the approach. Notably, the d- Fe2O3/GO nanohybrids showcased commendable selectivity and reproducibility, offering promising avenues for advanced electrochemical sensing applications.
Fig. 9.
Fe2O3/GO/GCE based electrochemical sensor for the detection of dopamine and uric acid. Reproduced with permission from Ref. [58], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2019, MDPI.
2.1.4. Manganese oxide-based biosensor
The utilization of manganese oxide in biosensing applications has gained substantial attention due to its unique physicochemical properties and potential for interfacing with biological systems. Manganese oxide, a transition metal oxide, possesses a diverse range of oxidation states, allowing it to facilitate redox reactions and electron transfer processes relevant to biosensing mechanisms. This distinctive property has led to the exploration of manganese oxide -based biosensors across various fields. Manganese oxide exhibits remarkable catalytic activity, making it an excellent candidate for electrochemical biosensors. Its inherent ability to mediate electron transfer between biomolecules and electrode surfaces has paved the way for the development of sensitive and efficient biosensing platforms. The tunable electrocatalytic behavior of manganese oxide, coupled with its compatibility with various biomolecules, holds promise for the detection of a wide array of analytes. Furthermore, manganese oxide nanostructures, including nanoparticles, nanowires, and nanosheets, offer high surface area-to-volume ratios, enhancing the immobilization of biomolecules and enabling signal amplification. These nanostructured forms of manganese oxide have been integrated into biosensing devices to achieve enhanced sensitivity and improved detection limits.
In the context of enzyme-based biosensors, manganese oxide has demonstrated its ability to facilitate the direct electron transfer between enzymes and electrodes. This feature eliminates the need for additional redox mediators, simplifying the sensor design and enhancing its stability. Enzymes immobilized on manganese oxide surfaces retain their bioactivity, allowing for reliable and reproducible biosensing. The diverse applications of manganese oxide -based biosensors encompass the detection of various analytes, including glucose, hydrogen peroxide, heavy metals, and environmental pollutants.
The MnO, MnO2, and Mn3O4 are three different oxides of manganese which are extensively studied and opted successfully as an electrode substance used in different biosensors. These oxides are non-hazardous, ecofriendly, easily available with low synthesis cost. Virtue of their quite high energy density and activity in alkaline medium, they have emerged an optimum material for designing biosensor for different analytes [[59], [60], [61], [62], [63], [64]]. In addition to this, oxides of manganese have four different dimensions starting from zero dimensions (0-D) to 3-D as oxides of zinc. Table 3 highlights all the four dimensions along with their applications in biosensing field. In comparison to 0-D, 1-D nanostructures 3-D NPs have more surface area (outer as well as inner) providing more reaction sites.
Table 3.
Different dimensions of MnO2 nanostructures used in electrochemical biosensors.
| Dimensions | Improved Electrode | Sensing Techniques | Sample | Biochemicals | Detection Limit | References |
|---|---|---|---|---|---|---|
| 0-D | MnO2 NSPs@ GNR composites | Electrochemical | Honey | Glucose | 0.1––1.4 mM | [65] |
| MnO2 NPs/Polythiophene composite on GCE | Electrochemical | Human Serum | Dopamine | 0.04––9.0 μM | [66] | |
| MnO2 NPs/Ta Electrode | Cyclic Voltammetry and Amperometry | Milk | H2O2 | 1––2 μM | [67] | |
| MnO2 NSPs GNR/SPCE | Cyclic Voltammetry and Amperometry | Honey | Glucose | 0.1––1.4 mM | [65] | |
| 1-D | MnO2 NTs/Ag@C shell nanocomposites | Electrochemical | Toothpaste | H2O2 | 0.5 μM–5.7 mM | [68] |
| M13-E4 @MnO2 NWs | Electrochemical | Human Serum, Local Peach Juice | Glucose | 5 μM–2 mM | [69] | |
| Au/MnO2 NNDs/SPCE | Amperometry | Blood Plasma | Histamine | 0.3––5.1 μM | [70] | |
| MnO2 NRs HBCs Nanocomposites/SPE | Cyclic Voltammetry and Chrono amperometry | Blood Sample | Glucose | 28––93 μg/mL | [71] | |
| 2-D | MnO2 NSs/GCE | Electrochemical | SP2/0 Cells | H2O2 | 2––10 μM | [72] |
| Lucigenin/MnO2 NSs/GCE | Electrochemiluminescence | Human Serum | Glutathione | 10––2000 nM | [73] | |
| MWCNT-MnO2/rGO/Au electrode | Cyclic Voltammetry | Serum | Acetylcholine | 0.1––100 μM | [74] | |
| 3-D | MnO2 NFs/3D-RGO @Ni foam | Electrochemical | Pork Sample | Ractopamine (RAC) | 17––962 nM | [75] |
| MNO2 NFs/N -rGO | Electrochemical | Human Serum | Dopamine | 6––100 μM | [76] | |
| 3D-MnO2 nanofibrous-mesh @GCE | Electrochemical | Blood and Urine Samples | Ascorbic Acid | 0.20––10 mM | [77] |
2.2. Quantum dot-based biosensors
Quantum dots (QDs) are semiconducting nanocrystalline materials with the diameter usually ranging from 2.0 nm to 10.0 nm [78]. Depending upon the size, these nanomaterials exhibit different colors viz.; QDs of diameter 5.0–6.0 nm give orange or red color while smaller QDs of diameter 2.0–3.0 nm are blue and green in appearance. The properties of QDs mainly depends on their size, shape, and structures. One of the main strategies for the synthesis of QDs is a top-down method in which large-sized carbon materials, such as graphite, graphene oxide, carbon nanotubes, carbon fibers extracted from different sources are broken down into small nano-sized quantum dots. They have been extensively employed as a substitute as the mimic fluorophores, for fabricating optical biosensors to detect organic compounds along with macromolecules [79]. Table 4 highlights the different types of QDs based biosensor used for detecting various analytes.
Table 4.
The different types of QDs based biosensor.
| S. No. | Type of QDs | Sensor type | Real sample | Analyte | Linear Range | References |
|---|---|---|---|---|---|---|
| 1. | Ni-doped CdTe | Fluorescence | Plasma samples | Pyrazinamide | 2–100 μM | [80] |
| 2. | CdTe | Fluorescence | Biological fluids | Dopamine | 0.5–10 μM | [81] |
| 3. | MoS2/CdTe | Fluorescence | Milk samples | Tetracycline | 0.1–1 μM | [82] |
| 4. | CdS@MOF | Electrochemiluminescence | Human serum | Carcinoembryonic antigen | – | [83] |
| 5. | CdTeS @SiO2 | ImageJ software | Serum samples | Folic acid | 5–80 μM | [84] |
| 6. | α-FeOOH@ CdS/Ag | Electrochemiluminescence | – | 17β-estradiol | 0.01–10 pg mL−1 | [85] |
| 7. | ZnCdS QDs@MIP | Fluorescence | Vitamin C tablets | Ascorbic acid | 1–500 μM | [86] |
| 8. | MoS2/GQD | Electrochemical | Red wine samples | Caffeic acid | 0.38–100 μM | [87] |
| 9. | CdTe | Photoinduced electron transfer | Synthetic samples | Double-Stranded DNA |
0.0874 μg mL−1 20 μg mL−1 |
[88] |
| 10. | Polymer CdTe/CdS | Fluorescence | Human body fluids | Glucose | 0.2–5 mM | [89] |
In 2013, Zhang et al. used nitrogen-doped carbon quantum dots (N-CQDs) for an effectual detection of mercury (II) ions with a lower detection limit of 0.23 μM [90]. In 2017, Saini et al. demonstrated a thiol functionalized fluorescent CQDs chemo sensor for arsenite detection, with a wide detection range of 5–100 ppb [91]. In 2017, Amjadi et al. demonstrated chemiluminescence sensor for the determination of indomethacin based on sulfur and nitrogen co doped CQDs, with a limit of detection of 65 μg L−1, and concentration range of 0.1–1.5 mg L−1 [92]. In the year 2017, Wang et al. used GQDs for designing a photoelectrochemical apta-biosensor for zeatin detection, with broad range [93].
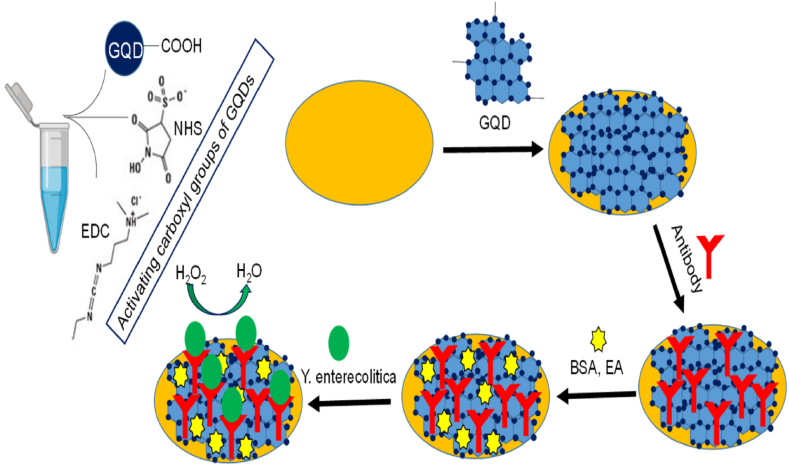
Yersinia enterocolitica, a gram-negative bacillus with its distinct rod-shaped morphology, is the causative agent of yersiniosis, a significant zoonotic ailment. This infection manifests through clinical symptoms such as mesenteric adenitis, acute diarrhea, terminal ileitis, and pseudoappendicitis, necessitating a robust detection strategy. To address this, an innovative approach was introduced by Sumeyra Savas and Zeynep Altontas in 2019, wherein an electrochemical sensor employing graphene quantum dots (GQDs) as nanozymes was meticulously developed and reported in their seminal work (Fig. 10) [94].
Fig. 10.
Graphene quantum dots (GQDs)-based immunosensor for Y. enterocolitica detection. Reproduced with permission from Ref. [94], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2019, MDPI.
The study meticulously optimized the conditions for the assay, ensuring precision and accuracy in the detection process. This pioneering immunosensor, enriched with GQDs, was meticulously designed to specifically target Yersinia enterocolitica. The sensor showcased a remarkable ability to quantify the bacterium across a broad concentration range with extraordinary sensitivity. Notably, the limit of detection (LOD) was found to be impressively low, with LOD values of 5 cfu mL−1 for milk samples and 30 cfu mL−1 for serum samples. The system also exhibited remarkable specificity, further underscoring its reliability for targeted pathogen detection.
This novel electrochemical approach transcends its application to the detection of Yersinia enterocolitica, displaying the potential to revolutionize clinical and food sample analysis. With the elimination of pre-sample treatment, this methodology presents an efficient, swift, and cost-effective means to detect various pathogenic bacteria in diverse sample matrices. Thus, the innovative GQD-immunosensor holds the promise to transform the landscape of diagnostic and detection strategies for infectious agents, benefitting both clinical and food safety applications.
2.3. Nanowire-based biosensors
NWs are the solid wire like structures with nanometer diameters synthesized from semiconducting metal oxides, carbon and metal nanotubes. Virtue of their size, nanowire shows excellent mechanical, thermal, chemical, optical and electronic properties which are not seen in bulk materials. They are been highly exploited for the synthesis of biosensors with enhanced sensing/detecting limits [95,96]. Table 5 highlights some different metal NWs-based biosensors used for detecting different analytes.
Table 5.
Different Metal NWs based biosensors.
| Type of Sensor | Methods of Synthesis | Mechanism Used | Targeted Molecule | Range Limit | References |
|---|---|---|---|---|---|
| Pd@Ag Hollow NWs | LPNE/GRR | Chemiresistive | H2 | 900–100 ppm (100 ppm) | [97] |
| PAN@Pd yarn | Electrospinning | Chemiresistive | H2 | 4––0.0001% (1 ppm) | [98] |
| Pt NW PtOx NW | Electron beam lithography | Chemiresistive | H2 | 1000––0.5 ppm (100 ppm) | [99] |
| CoS2 NWs with Au NPs | Hydrothermal | Optical (Chemiluminescence) | H2O2 | 100–1 μM (0.03 μM) | [100] |
| Au NWs with DNA zyme | CVT | Optical (SERS) | UO22+ | 10−7–10−12 M (1 pM) | [101] |
| PtNi jagged NWs | Solvothermal | Electrochemical | Caffeic Acid | 0.75––600 μM (0.05 μM) | [102] |
| Cu3P NWs | Hydrothermal | Electrochemical | Glucose | 1––0.005 mM (0.32 μM) | [103] |
| Ni/Au Multilayer NWs | Electrodeposition | Electrochemical | Glucose | 2––0.0025 mM (0.1 μM) | [104] |
| G/Au NWs | Hydrothermal | Electrochemical (Cyclic Voltammetry) | Tulobuterol | 7.6––0.076 μmolL−1 (0.0136 μmolL−1) | [105] |
| Ag NWs | Commercial | Piezoresistive | Strain | 80–0% Strain (0.2%) | [106] |
| Au NWs | Oriented attachment | Chemiresistive | DNA | 1––0.001 nM (1 pM) | [107] |
| Au NWs | Nanoimprint lithography | Electrochemical (SWV) | C-reactive protein | 220–5 fg mL−1 (2.25 fg mL−1) | [108] |
| Ni NWs | Electrodeposition | Electrochemical (Cyclic Voltammetry) | HCHO | 20––0.01 mM (0.8 μM) | [109] |
| AuPt NWs network with PDa Coating | Hydrothermal | Electrochemical | Pesticide | 1000––0.5 ng/L (0.185 ng/L) | [110] |
Further, in the year 2012, Hakim et al. fabricated a poly-silicon NWs biosensor for sensing the joining capacity of two inflammatory biomarkers with wide range of concentration and good detection sensitivity [111]. In 2017, Irrera et al. demonstrated label-free optical silicon Nws-based biosensors to detect the C-reactive protein in human serum, with the detection range of 10−2 μg/mL to 100 μg/ml [112]. In year 2018, Priolo et al. prepared and used silicon nanowires optical biosensors for ultrasensitive genome detection extracted from human blood [113].
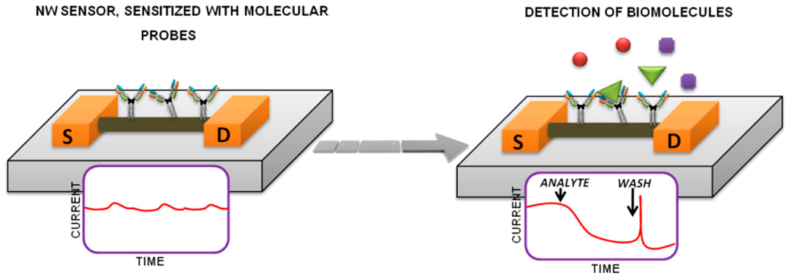
In the year 2021, Ivanov and colleagues embarked on a pivotal scientific endeavor that concentrated on the exploration of cancer-associated genetic markers through the utilization of silicon nanowire field-effect transistors (Si-NW FETs) [114]. This investigation was strategically designed to capitalize on the inherent advantages of Si-NW FETs, particularly their compatibility with established and widely utilized mass production technologies. This pursuit of optimizing and integrating state-of-the-art technologies has significant implications for the advancement of diagnostics and detection in cancer research, as exemplified in Fig. 11.
Fig. 11.
The schematic illustration for Si-NW-sensors to detect biomolecules. Reproduced with permission from Ref. [114], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2021, MDPI.
Central to their study was the intricate scheme of Si-NW sensors, which serves as a foundation for the nanowire-based detection of biomolecules, specifically cancer-related genetic markers. The Si-NW FET configuration is envisaged to offer exceptional sensitivity and precision in the identification of these markers, underpinned by the unique electrical properties of nanowires. The miniaturized dimensions and enhanced surface-to-volume ratio of silicon nanowires inherently enable the detection of molecular interactions at a remarkably intricate level. By capitalizing on these inherent attributes, the researchers aimed to establish a robust and reliable methodology for detecting cancer-specific biomolecular signals.
2.4. Nanorods-based biosensors
NRs as the name suggest are the rods having dimensions range from 1 to 100 nm is synthesized chemically from different materials such as graphene, graphene oxide, oxides of various metals and other semiconducting materials [115,116]. These NRs have shown excellent potential in the field of biosensing for the detection of nucleic acids, different carbohydrates, metal ions etc.
In the year 2013, Sun et al. used graphene NRs and graphene oxide to prepare a biosensor to detect bovine IgG [117]. Later in the year 2017, Hahn et al. designed afield effect transistor (FET) biosensor using zinc oxide NRs for the detection of phosphate [118]. Further Zhu et al. in 2018used the same FET biosensor for glucose monitoring with high sensitivity and concentration detection limit of 1 μM [119]. Liu et al., in 2019 created a fluorescence resonance energy transfer biosensor for sensing lead ions using gold NRs and carbon dots [120]. Bagyalakshmi et al. in year 2020 fabricated a ZnO NRs-based enzymatic glucose biosensor on a chitosan film, with linear range of glucose concentrations from 10 μM to 40 μM [121].
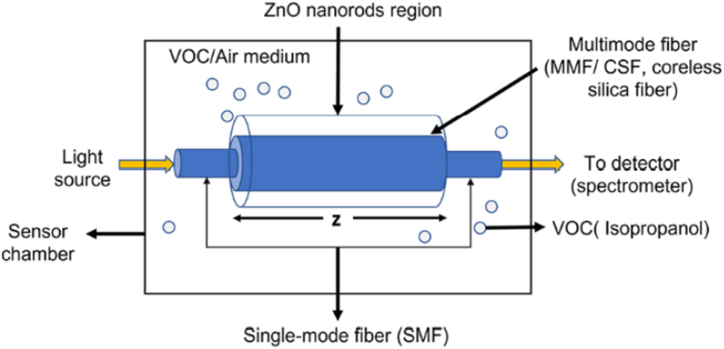
Volatile organic compounds (VOCs) are ubiquitous in the environment, often existing as gaseous species under specific temperature and pressure conditions. These compounds emanate from a diverse array of sources, including household products, paints, fuels, personal care items, waxes, and industrial processes, and become integral components of the atmosphere. The quantification of VOC concentrations in exhaled breath has garnered considerable attention due to their potential as indicative biomarkers for various chronic diseases. Notably, acetone and isopropanol have emerged as significant biomarkers for type 1 diabetes and lung cancer, respectively, emphasizing the clinical relevance of VOC analysis. In a recent groundbreaking study by Kankan Swargiary et al. an innovative optical fiber sensor employing zinc oxide (ZnO) coating was introduced for the selective detection of a volatile organic compound (VOC) biomarker associated with diabetes, specifically targeting isopropanol (IPA) markers [122]. The sensor configuration incorporated a coreless silica fiber (CSF) bridging two single-mode fibers (SMFs), forming a structured SMF–CSF–SMF architecture (depicted in Fig. 12). The CSF region functioned as the sensing zone, harnessing multimode interference (MMI) to intensify light interaction at the interface between the fiber and the sensing medium, thereby enhancing sensitivity levels. Numerical simulations were meticulously employed to optimize the CSF length, ensuring maximal coupling efficiency at the output.
Fig. 12.
ZnO nanorods coated optical fiber sensor for volatile organic compounds (VOC) biomarker detection. Reproduced with permission from Ref. [122], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2022, MDPI.
The surface of the CSF was ingeniously functionalized via a hydrothermal ZnO nanorod growth process, facilitated at low temperatures. This innovative step facilitated the establishment of a robust sensing platform without compromising the structural integrity of the fiber. The constructed optical fiber-based sensor was subjected to rigorous testing using various concentrations (20%, 40%, 60%, 80%, and 100%) of isopropanol (IPA). The sensor exhibited exceptional potential in accurately detecting isopropanol vapor, showcasing an impressive sensitivity of 0.053 nm/% IPA vapor. These findings collectively underscore the sensor's capacity to discriminate and quantify the presence of isopropanol, thereby showcasing its potential utility in non-invasive monitoring for diabetes-related applications and potentially extending its applications to broader medical contexts.
2.5. Carbon nanotubes-based biosensors
Carbon nanotubes (CNTs), also known as buckytubes were first reported by SsumioIjima in year 1991. They are hollow carbon structures having diameters in nanoscale. They display proper arrangement of carbon atoms linked via sp2 bonds [123], making them quite strong and stiff materials. They are most extensively explored class of nanomaterials for making biosensors applied for different diagnostics purposes in medical and other research areas and serve as a scaffold of immobilization of biomolecules at their surface. In the year 2006, Tang et al. prepared a single-walled carbon nanotube (SWNT) based DNA sensors with great sensibility and response [124]. In year 2013, Li et al. fabricated a biosensor using semiconducting single-wall carbon nanotubes (s-SWCNTs) to detect the dopamine, with a very low detection limit of 10−18 mol/L at room temperature [125]. There is a list of CNTs-based biosensors with different analytes as shown in Table 6.
Table 6.
List of CNTs-based biosensors with different analytes.
| Sensor Types | Type of Mechanism | Methods of Synthesis | Analyte | LOD and Range | References |
|---|---|---|---|---|---|
| CNTs | Field–effect transistor | OTS masking | Aquaporin-4 | 1 ng/L, 1–106 ng/L | [126] |
| Amperometric | Dielectrophoresis | Streptavidin | 100 aM, 100––106 aM | [127] | |
| Amperometric | Dielectrophoresis | HER2 Antibody | 10 fM, 10–105 fM | [127] | |
| Chemiresistive | Direct Contact Printing | H5N1 DNA Sequence | SWCNT- 2pM, 2––200 pM; MWCNT- 20 pM, 20––2000 pM | [128] | |
| Chemiresistive | Drop–Coat | Prostate-Specific antigen | 1.18 ng/mL, 0––1000 ng/mL | [129] | |
| Field–effect transistor | Dielectrophoresis | Cortisol | 50 nM, 50––1000 nM | [130] | |
| CNTs | Field–effect transistor | Dielectrophoresis | NPY | 500 pM, 500–106 pM, | [130] |
| Field–effect transistor | Dielectrophoresis | DHEAS | 10 nM, 10––1000 nM | [130] | |
| Field–effect transistor | OTS masking | Aspergillus Niger | N/A | [131] | |
| Field–effect transistor | Immersed in CNTs solution | DNA | 60 aM, 100––1000 aM | [132] | |
| Field–effect transistor | Immersed in CNTs solution | Micro vesicle | 6 particles per mL, 6––6 × 106 particles per mL | [132] | |
| Field–effect transistor | Catalytic Chemical vapor deposition | N2+ ion | Single ion | [133] | |
| CNTs | Amperometric | Drop–Coat (Paper Filter) | Formaldehyde | 0.016 ppm, 0.05––6.7 ppm | [134] |
| Chemiresistive | Dielectrophoresis | H2 | 10 ppm, 10 ppm–4% | [135] | |
| Chemiresistive | Dielectrophoresis | NO2 | 0.5––20 ppm | [136] | |
| Chemiresistive | Immersed in CNTs solution | H2 | 0.89 ppm | [137] | |
| Chemiresistive | Drop–Coat | NH3 | 100 ppb, 1.5––20 ppm | [138] | |
| Chemiresistive | Drop–Coat | N-nitroso dialkylamine | 1 ppb, 0––1000 ppb | [139] | |
| Chemiresistive | Spray deposition | NH3 | 10 ppm, 10––100 ppm | [140] | |
| Chemiresistive | Spray deposition | CO2 | 600 ppm, 600––7000 ppm | [140] | |
| CNTs | Chemiresistive | Spray deposition | CO | 3 ppm, 3––27 ppm | [140] |
| Chemiresistive | Spray deposition | Ethanol | 17 ppm, 17––70 ppm | [140] | |
| Chemiresistive | Dielectrophoresis | Tetrahydrocannabinol | 0.163 ng, 0.0018–0.8262 μg | [141] | |
| Chemiresistive | Drop–Coat | NH3 | 2 ppm, 2––40 ppm | [142] | |
| Chemiresistive | Drop–Coat | NO2 | 2 ppm, 2––40 ppm | [142] | |
| Chemiresistive | Catalytic Chemical vapor deposition | Toluene | 50 ppm, 50––500 ppm | [143] | |
| Spin-coat | Field–effect transistor | DNA (cDNA from Cancer Cell) | 880 ng/L, 50––5 × 106 pM | [144] |
Abbreviations: LOD = Limit of detection, CNT = Carbon Nanotubes, HER2 = Human epidermal growth factor receptor 2, DHEAS = Dehydroepiandrosterone sulfate.
Neurotransmitters play a fundamental role in orchestrating crucial physiological functions within the human body, particularly in mediating intricate chemical communications within neuronal networks of the brain. However, a comprehensive understanding of their intricate mechanisms remains largely uncharted territory, primarily due to the scarcity of effective tools capable of capturing their concentration dynamics with spatiotemporal precision. Over the last few decades, significant strides have been made in devising analytical methodologies aimed at quantifying neurotransmitter levels.
Janssen et al. prepared CNTs-based biosensor for detecting bovine serum albumin (BSA), with excellent detection limit [145,146].
In an another notable contribution to this field, Florian et al. presented an innovative approach encompassing the development and thorough characterization of fluorescent carbon nanotube-based sensors dedicated to neurotransmitter detection [147]. In their study, Florian and colleagues employed a systematic manipulation of the organic phase surrounding single-walled carbon nanotubes (SWCNTs) to engineer a spectrum of sensors, each endowed with distinct selectivity and sensitivity profiles tailored for catecholamine neurotransmitters (Fig. 13). The investigation yielded a comprehensive understanding of the sensors' performance, establishing a nuanced interplay between the DNA sequences and the SWCNT platform. Of particular significance is the sensors' capacity to distinguish between diverse catecholamine neurotransmitters or detect them amidst the presence of structurally similar interfering compounds. This remarkable capability addresses a critical limitation in existing methodologies, allowing for more accurate and nuanced measurements.
Fig. 13.
Fluorescent carbon nanotube-based neurotransmitter sensors. Reproduced with permission from Ref. [147], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2017, MDPI.
The implications of this research are noteworthy, as DNA-functionalized SWCNT-based sensors exhibit the potential to revolutionize our capacity to delve into neurotransmitter signaling within complex biological milieus. By virtue of their heightened selectivity and sensitivity, these sensors hold promise as invaluable tools in deciphering the intricate interplay of neurotransmitters in both health and disease contexts, thereby offering a stepping stone toward unraveling the complexities of neurological processes at unprecedented levels of detail.
2.6. Dendrimer-based biosensors
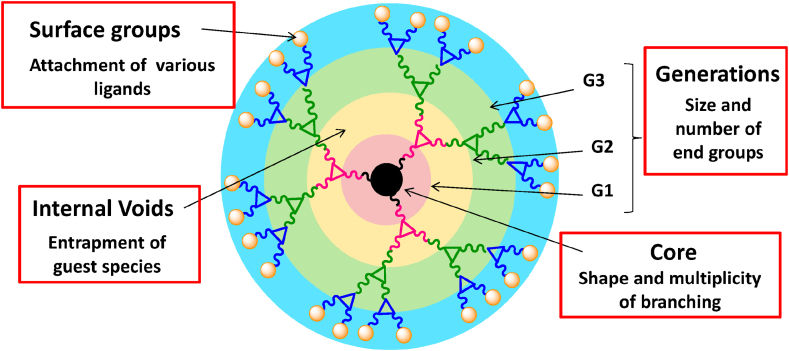
In recent years, dendrimers have garnered significant attention as versatile nanoscale architectures with promising applications in the field of biosensors. Dendrimers, three-dimensional hyperbranched macromolecules, offer a unique combination of properties, including well-defined structures, tunable surface functionalities, and high branching densities (Fig. 14). These attributes make them well-suited for engineering biosensing platforms with enhanced sensitivity, selectivity, and stability.
Fig. 14.
Different elements of Dendrimers. Reproduced with permission from Ref. [148], with the permission of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2015, MDPI.
The incorporation of dendrimers into biosensor design leverages their multifunctional nature. Dendrimers can serve as molecular scaffolds for immobilizing biomolecules such as enzymes, antibodies, and nucleic acids. This controlled immobilization not only maintains the bioactivity of these recognition elements but also facilitates their precise arrangement, leading to improved interactions with target analytes.
Moreover, dendrimers possess intrinsic signal amplification capabilities owing to their high surface area and numerous functional groups. This unique feature enables the immobilization of multiple reporter molecules or signal tags, resulting in an amplified response upon target binding. This enhanced signal output enhances the biosensor's detection limit and dynamic range, rendering it more suitable for accurately quantifying low concentrations of analytes.
Dendrimer-based biosensors find applications across diverse domains, including medical diagnostics, environmental monitoring, and food safety. For instance, in medical diagnostics, dendrimer-enhanced biosensors have shown promise in detecting specific biomarkers associated with diseases such as cancer, diabetes, and infectious disorders. Their tunable surface chemistry allows for tailoring sensor surfaces to interact with distinct target molecules, enabling the development of highly specific assays. Furthermore, dendrimer-modified electrodes have demonstrated improved electron transfer kinetics, enhancing the overall sensor performance in terms of response time and stability. This attribute is especially advantageous in electrochemical biosensors, where rapid and accurate measurements are paramount.
The remarkable potential of dendrimer-based biosensors lies in their ability to bridge the gap between nanotechnology and biorecognition elements. By synergistically combining the unique attributes of dendrimers with the specificity of biomolecular recognition, these biosensors hold the promise of advancing the boundaries of biosensing technology. As research in this field continues to evolve, it is anticipated that dendrimer-enhanced biosensors will play an increasingly pivotal role in addressing pressing challenges in healthcare, environmental monitoring, and beyond.
The extensive branching in the dendrimers provides immense outside area for binding of different biologically active molecules. They are basically made up of three subunits viz.; the central core unit, the branching dendrones, and the outer surface ligands [149]. Tumor markers, esteemed for their diagnostic potential, offer invaluable insights into the intricacies of diverse tumors, aiding in prognosis and unraveling the molecular underpinnings of tumorigenesis. Within this context, the emergence of nanotechnology-driven approaches has ushered in novel avenues for detecting these markers, promising heightened accuracy and clinical utility.
In a recent contribution, Ushna Laraib orchestrates a comprehensive literature review that accentuates the pivotal role of nanomaterials in the realm of tumor biomarker detection [150]. Notably, the review encapsulates an array of prominent tumor markers, including prostate-specific antigen (PSA), human carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), human epidermal growth factor receptor-2 (HER2), cancer antigen 125 (CA125), cancer antigen 15-3 (CA15-3, MUC1), and cancer antigen 19-9 (CA19-9).
The landscape of theranostics is undergoing a paradigm shift with the advent of RNA aptamers, emerging as a potent arsenal against a myriad of disorders. Leveraging their remarkable structural flexibility, RNA aptamers demonstrate a unique capability to intricately fold and engage with a diverse array of nanostructures, macromolecules, cells, and viruses. This multifaceted potential has propelled them to the forefront of cutting-edge theranostic research.
In a recent scholarly endeavor, Mahtab and colleagues have orchestrated a comprehensive literature review, unveiling a treasure trove of insights into the evolutionary landscape of RNA aptamers [151]. With a meticulous exploration, the review delves into the intricate facets of their development, classification, nanomerization, and strategic modification. Notably, it proffers an incisive account of their applications within the realm of cancer theranostics, signifying a substantial leap forward in precision medicine.
Dendrimers have been extensively used for designing numerous biosensors based on electrochemical, fluorescence and impedance methods applied for different diagnostic purposes. This sensor possesses high analytical sensitivity, stability, and reproducibility [152,153]. In 2016, Ou et al. fabricated electrochemiluminescence (ECL) biosensor using Ag nanocubes–polyamidoamine dendrimer–luminol–glucose oxidase (AgNCs–PAMAM–luminol–GOx) to detect the concanavalin A (Con A), with two wide linear response ranges from 0.005 to 0.1 ng/mL and 0.1–20 ng/mL [154]. In the year 2017, Dervisevic et al. fabricated a novel electrochemical urea biosensor based on ferrocene poly(amidoamine) (Fc-PAMAM) dendrimers combined with multi walled carbon nanotubes (MWCNTs), with detection limit of 0.05 mM, and sensitivity of 1.085 μA/cm2/mM [155]. Further in the year 2019, Baker et al. used polyamidoamine (PAMAM) dendrimer sensor for the detection of dengue fever [156]. To conclude the application of various nanomaterials used for biosensor development, Table 7 was prepared so that the reader can get proper idea of the work carried out in last two decades.
Table 7.
List of various nanomaterials used in biosensor development over the last two decades.
| Nanomaterial | Transducer | Analyte | Detection Limit | Linear Range | Ref. |
|---|---|---|---|---|---|
| Au NBPs | SPR Impedimetric |
Aflatoxin B1(AFB1) Aflatoxin B1 (AFB1) |
0.4 nM 0.1 nM |
0.1––500 nM 0.1––25 nM |
[157] |
| Au NPs | Electrochemical | Uranyl | 0.3 μg L−1 | 2.4––480 μg L−1 | [158] |
| Au NPs | Fluorescent | Pb2+ | 16.7 nm | 50 nm–4 μm | [159] |
| Au/CdS QDs/TNTs | Electrochemical Electrochemical |
Cholesterol H2O2 |
0.012 μM 0.06 μM |
0.024––1.2 mM 18.73––355.87 μm |
[160] |
| Au NPs | Electrochemical | E. coli | 15 CFU mL−1 | 10––106 CFU mL−1 | [161] |
| Au NP-MoS2 -rGO | SAW | Carcinoembryonic antigen (CEA) | 0.084 ngmL−1 | 36.58 ng mL−1 | [162] |
| Au/rGO | Electrochemical | miENA-122 | 1.73 pM | 10 μm–10 p.m. | [163] |
| Au NPs/TiO2 | Electrochemical | H2O2 | 5 μm | 65––1600 μm | [164] |
| Ag NPs | Colorimetric | H2O2 Glucose Fe2+ |
0.032 μm 0.29 μm 0.54 μm |
0.05––7.5 μm 1.5––3.0 μm 1––90 μm |
[165] |
| Ag/Pd NPs | Electrochemical | Ractopamine Clenbuterol Salbutamol |
1.52 pg mL−1 1.44 pg mL−1 1.38 pg mL−1 |
0.01––100 ng mL−1 0.01––100 ng mL−1 0.01––100 ng mL−1 |
[166] |
| Ag@CQDs-rGO | Electrochemical | Dopamine | 0.59 nm | 0.1––300 μm | [167] |
| Ag NP-MWNT | Electrochemical | Glucose | 0.01 mM | 0.025––1.0 mM | [168] |
| Ag NPs | Electrochemilumi-nescence | Mucin 1 | 0.37 fg mL−1 | 1.135 fg mL−1 -0.1135 ng mL−1 | [169] |
| Pt NPs | Voltammetric | Adrenaline | 2.93 × 10−4 mol L−1 | 9.99 × 10−1–2.13 × 10−4 mol L−1 | [170] |
| Pt NPs/RGO–CS–Fc | Electrochemical | H2O2 | 20 nm | 2.0 × 10−8 M − –3.0 × 10−8 M | [171] |
| Pt–Fe3O4@C | Amperometric | Sarcosine | 0.43 μm | 0.5––60 μm | [172] |
| Pt NFs/PANi | Cyclic Voltammetry |
Urea | 10 μm | 20 mM | [173] |
| Pt@CeO2 NM | Electrochemical | Dopamine | 0.71 nM | 2––180 nM | [174] |
| Pd/Co-NCNT | Electrochemical | Hydrazine | 0.007 μm | 0.05––406.045 μm | [175] |
| Pd/CNF/[M3O A]+[NTF2]− |
H2 | 0.33 nM | 1.00––35.0 nM | [176] | |
| Cu NPs/Rutin/MWCNTs/IL/Chit/GCE | Cyclic Voltammetry |
H2O2 | 0.11 μm | 0.35––2500 μM | [177] |
| Cu/rGO-BP | Electrochemical | Glucose | 11 μm | 0.1––2 mM | [178] |
| Cu2O@CeO2–Au | Amperometric | PSA | 0.0001––100.0 ng mL−1 | 0.03 pg mL−1 | [179] |
| Ni/Cu MOF | FET | Glucose | 0.51 μM | 1 μM–20 mM | [180] |
| NiO/PANINS | Amperometric | Glucose | 0.06 μM | 1––3000 μM | [181] |
| NiO@Au | Electrochemical | Lactic acid | 11.6 μM | 100.0 μM–0.5 M | [182] |
| Co3O4 NCs | Electrochemical chip | Glutamate | 10 μM | 10––600 μM | [183] |
| Co3O4–Au | Photoelectricalche-mical | miRNA-141 | 0.2 pM | 1 pM–50 nM | [184] |
| MnO–Mn3O4 @rGO |
Impedimetric | H2O2 | 0.1 μM | 0.004––17 mM | [185] |
| MnO2 NFs | Impedimetric | Salmonella | 19 CFU mL−1 | 3.0 × 101––3.0 × 106 | [186] |
| Fe2O3/NiO/Mn2O3 NPs | Electrochemical | Folic acid | 96.89 ± 4.85 pM | 0.1 nM–0.01 mM | [187] |
| ZnO-rGO | Cyclic Voltammetric | Dopamine | 8.75 ± 0.64pM | 0.1––1500 pM | [188] |
| ZnO NRs | FET | Phosphate | 0.5 mM | 0.1 μM–7.0 mM | [189] |
| ZnO NFs | Optical | Amyloid | 2.76 μg | 2––20 μL | [190] |
| Ca/Al–ZnO NPs | Semiconductor | CO2 | 200 ppm | 0.25––5 RH% | [191] |
| Cr doped SnO2 NPs | Voltammetric | Riboflavin | 107 nM | 0.2 × 10−6––1.0 × 10−4 M | [192] |
| TiO2/APTES | Impedimetric | Glucose | 24 μmol | 50––1000 μmol | [193] |
| TiO2 NTs | Photoelectrochemical | Asulam | 4.1 pg mL−1 | 0.02––2.0 ng mL−1 | [194] |
| MoO3@RGO | Electrochemical | Breast cancer | 0.001 ng mL−1 | 0.001––500 ngmL−1 | [195] |
| Graphene QDs | Electrochemical | Cu2+ | 1.34 nM | 0.015––8.775 μM | [196] |
| Graphene QDs | Fluorescence | Lung cancer+ | 0.09 pg mL−1 | 0.1 pg mL−1–1000 ng mL−1 | [197] |
| CdTe/CdS//ZnS core/shell /shell QDs |
Fluorescence | l-ascorbic acid | 1.8 × 10−9 M | 8.0 × 10−9––1.0 × 10−7 M | [198] |
| NSETamptamer@Fe3O4@GOD and MoS2 | Magnetic fluorescence | Tumor cell (EpCAM) | 1.19 nM | 2––64 nM | [199] |
| Au NPs@PDA @CuInZnSQDs |
Electrochemiluminescence | P53 gene | 0.03 nmol L−1 | 0.1––15 nmol L−1 | [200] |
| CaM/SiNW FETs |
FET | Protein | 7 nM | 10−8–10−6 M | [201] |
| Si NWs | FET | Dengue virus | 2.0 fM | 1 μM–10 fM | [202] |
| ZnO NRs | FET | Phosphate | 0.5 mM | 0.1 μM–7.0 mM | [203] |
| G/Au NR/PT | Electrochemical | HPV DNA | 4.03 × 10−14 m L−1 | 1.0 × 10−13––1.0 × 10−10 m L−1 | [204] |
| Graphene-Au NRs | Amperometric Voltammetric | NADHEthanol | 6 μM 1.5 μM |
20––160 μM 5––377 μM |
[205] |
| LAC-CNTs-SPCE | Electrochemical | Para-cresol | 0.05 ppm | 0.2––25 ppm | [206] |
| Co3O4-CNT/TiO2 | Photoelectrochemical | Glucose | 0.16 μM | 0––4 mM | [207] |
| CNT thin-film transistor (TFT) | Thin film transistor (TFT) | DNA | 0.88 μg L−1 | 1.6 × 10−4–5 μmol L−1 | [208] |
| GQDs-MWCNTs | Electrochemical | Dopamine | 0.87 nM | 0.005––100.0 μM | [209] |
| CNT/Au NPs | Amperometric | Choline | 15 μM | 0.05––0.8 mM | [210] |
| PAMAM dendrimer | Optical fiber | DENV 2E | 19.53 nm nM−1 | 0.1 pM–1 μM | [211] |
| SAM/NH2rGO/PAMAM | SPR | DENV 2E | 0.08 pM | 0.08 pM––0.5 pM | [212] |
3. Challenges and emerging trends in nanobiosensors
The impending global population projection of 8.5 billion by 2030 brings forth significant challenges to the healthcare infrastructure, including the availability of diagnostic resources, testing facilities, and affordable medical care. Such a scenario may lead to increased costs associated with diagnostic procedures, impacting healthcare accessibility, particularly in developing countries like India. The pressing need for immediate and portable diagnostics has spurred the development of Point-of-Care Technologies (POCT) that integrate cutting-edge technologies to provide rapid results [213,214].
The realm of nanotechnology has witnessed remarkable advancements, with nanomaterials like quantum dots, graphene, carbon nanotubes, and nanocomposites prominently harnessed for diagnostic applications. While nanobiosensors initially made their debut in glucose detection [215], several challenges have surfaced in bringing nanoparticle-based biosensors to the commercial market. Critical challenges include addressing public and regulatory concerns surrounding safety, ethical considerations, and the establishment of universal standards for assessing nanobiosensor safety.
The escalating demand for POCT has extended to various biological sample analyses, encompassing blood, urine, saliva, and DNA, and even encompassing environmental pollution monitoring, biochemical testing, and pathogen detection. The integration of artificial intelligence, cyber-physical systems, and cutting-edge technologies has propelled the intelligent nanobiosensors market [216]. However, the multidisciplinary nature of nanobiosensors calls for advances in sciences, electronics, and mechanical design to enhance sensitivity and selectivity for applications spanning in vitro diagnostics, pharmaceuticals, drug delivery, and pathogen detection [217].
Furthermore, nanobiosensors offer substantial promise to healthcare practitioners, researchers, and scientists by enabling precise detection of nucleic acid sequences, proteins, enzymes, and biomarkers associated with various conditions and diseases. Conventional assays, though available, often suffer from extended processing times, a need for multiple analytes, and the risk of erroneous outcomes. Consequently, there is a compelling demand for rapid, reliable, and cost-effective multiplexed screening capable of detecting diverse analytes.
Focusing on the fusion of nanoelectronics, sensors, and materials, the pursuit of eco-friendly nanobiosensors with applications in diverse fields like food analysis, environmental monitoring, and diagnostics has gained momentum. The evolution of diagnostic technologies remains pivotal, allowing healthcare professionals and researchers to glean accurate insights into disease pathways. To address these challenges and propel the nanobiosensor field forward, emphasis must be placed on pioneering nanomaterials and sensor technologies that efficiently bridge the gap between nanoscience and diagnostics, ultimately serving healthcare, environmental monitoring, and other industries requiring precision detection and monitoring [218].
4. Conclusions and future perspectives
Biosensors, a remarkable fusion of bioreceptors, transducers, and amplifiers, stand as versatile analytical tools capable of detecting a wide spectrum of analytes including heavy metal ions, carbohydrates, amino acids, gases, and disease-associated substances. This comprehensive review underscores the diverse types, classifications, and applications of biosensors. It particularly highlights the pervasive utilization and recent advancements in metal oxide nanoparticles (NPs), nanowires (NWs), nanorods (NRs), carbon nanotubes (CNTs), quantum dots (QDs), and dendrimers in designing NPs-based biosensors for a plethora of applications.
The adoption of these nanomaterials in biosensors capitalizes on their inherent attributes of heightened sensitivity, selectivity, reproducibility, and stability. The exceptional charge mobility, expansive surface area, and superior electrochemical characteristics of nanomaterials underpin their enhanced performance. As we cast a glance toward the future, the potential of nanobiosensors is boundless, with a trajectory marked by automation, integration, and miniaturization. The synergy of advanced technologies such as the Internet of Things (IoT), deep learning (DL), cloud computing, data analysis, cyber-physical systems (CPS), and artificial intelligence (AI) promises to drive their commercialization.
Nanobiosensors, borne from the convergence of nanotechnology, biotechnology, and sensor engineering, are primed to revolutionize Point-of-Care Testing (POCT). Looking ahead, these innovative devices hold immense promise in healthcare and beyond, offering real-time, on-site diagnostics and monitoring. In essence, the emergence of nanomaterials-based biosensors stands as a monumental achievement of our time, poised to reshape the landscape of diagnostics and catalyze transformative advancements in a wide array of domains.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of interest's statement: The authors declare no conflict of interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to the Deanship of Scientific Research at Najran University, Najran, Kingdom of Saudi Arabia for funding under the Research Group funding program grant no. NU/RG/SERC/12/49. The authors are thankful to Head of Chemistry Department, Maharishi Markandeshwar (Deemed to be University), Mullana Ambala, India, for providing research facilities.
Contributor Information
Joginder Singh, Email: joginderchem@mmumullana.org.
Ahmad Umar, Email: ahmadumar786@gmail.com.
Sotirios Baskoutas, Email: bask@upatras.gr.
References
- 1.Theavenot D.R., Toth K., Durst R.A., Wilson G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001;16:121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 2.Heineman W.R., Jensen W.B., Clark Leland C., Jr. Biosens. Bioelectron. 2006;8(21):1403–1404. 1918–2005. [Google Scholar]
- 3.Naresh V., Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors. 2021;21(4):1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark L.C., Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 5.Updike S.J., Hicks G.P. The enzyme electrode. Nature. 1967;214:986–988. doi: 10.1038/214986a0. [DOI] [PubMed] [Google Scholar]
- 6.Guilbault G.G., Montalvo J.G., Jr. Urea-specific enzyme electrode. J. Am. Chem. Soc. 1969;91:2164–2165. doi: 10.1021/ja01036a083. [DOI] [PubMed] [Google Scholar]
- 7.Guilbault G.G., Lubrano G.J. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta. 1973;64:439–455. doi: 10.1016/S0003-2670(01)82476-4. [DOI] [PubMed] [Google Scholar]
- 8.Mosbach K., Danielsson B. An enzyme thermistor. Biochim. Biophys. Acta. 1974;364:140–145. doi: 10.1016/0005-2744(74)90141-7. [DOI] [PubMed] [Google Scholar]
- 9.Lübbers D.W., Opitz N. The pCO2-/pO2-optode: a new probe for measurement of pCO2 or pO2 in fluids and gases (authors transl) Z. Naturforsch C Biosci. 1975;30:532–533. [PubMed] [Google Scholar]
- 10.Singh A., Sharma A., AhmedA, Sundramoorthy A.K., Furukawa H., Arya S., Khosla A. Recent advances in electrochemical biosensors: applications, challenges, and future scope. Biosensors. 2021;11(9):336. doi: 10.3390/bios11090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Guan P., Yu F., Li W., Xie X. CeO2 nanorods embedded in Ni(OH)2 matrix for the non-enzymatic detection of glucose. Nanomaterials. 2017;7:205. doi: 10.3390/nano7080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafar H., Channa A., Jeoti V., Stojanović G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors. 2022;22(2):638. doi: 10.3390/s220206389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karunakaran C., Rajkumar R., Bhargava K. Biosensors and Bioelectronics. Elsevier; 2015. Introduction to biosensors; pp. 1–68. [Google Scholar]
- 14.Razlansari M., Ulucan-Karnak F., Kahrizi M., Mirinejad S., Sargazi S., Mishra S., Rahdar A., Díez-Pascual A.M. Nanobiosensors for detection of opioids: a review of latest advancements. Eur. J. Pharm. Biopharm. 2022;179:79–94. doi: 10.1016/j.ejpb.2022.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Yuvaraj G., Ramesh M., Rajeshkumar L. Carbon and cellulose-based nanoparticle-reinforced polymer nanocomposites: a critical review. Nanomaterials. 2023;13(11):1803. doi: 10.3390/nano13111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan F.A. In: Applications of Nanomaterials in Human Health. Khan F., editor. Springer; Singapore: 2020. Nanomaterials: types, classifications, and sources; pp. 1–13. [Google Scholar]
- 17.Karim R.A., Reda Y., Fattah A.A. Review-Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020;167 [Google Scholar]
- 18.Barabadi H., Najafi M., Samadian H., Azarnezhad A., Vahidi H., Mahjoub M.A., Koohiyan M., Ahmadi A. A systematic review of the genotoxicity and antigenotoxicity of biologically synthesized metallic nanomaterials: are green nanoparticles safe. Enough for Clinical Marketing. Medicina. 2019;55(8):439. doi: 10.3390/medicina55080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi X., Gu W., Li B., Chen N., Zhao K., Xian Y. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta. 2014;181:1–22. [Google Scholar]
- 20.Li P.X., Yang A.Y., Xin L., Xue B., Yin C.H. Photocatalytic activity and mechanism of Cu2+ doped ZnO nanomaterials. c. 2022;14(10):1599–1604. [Google Scholar]
- 21.Arshi N., Ahmed F., Kumar S., Umar A., Aljaafari A., Alshoaibi A., Alsulami A., Alshahrie A., Melaibari A. Construction of dye-sensitized solar cells using coffee as a natural dye and ZnO nanorods based photoanode. Sci. Adv. Mater. 2022;14(8):1388–1393. [Google Scholar]
- 22.Napi M.L.M., Sultan S.M., Ismail R., How K.W., Ahmad M.K. Electrochemical-based biosensors on different zinc oxide nanostructures: a review. Materials. 2019;12(18):2985. doi: 10.3390/ma12182985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashkhourian J., Hemmateenejad B., Beigizadeh H., et al. ZnO nanoparticles and multiwalled carbon nanotubes modified carbon paste electrode for determination of naproxen using electrochemical techniques. J. Electroanal. Chem. 2014;103(1):714–715. [Google Scholar]
- 24.Roy E., Patra S., Tiwari A., et al. Single cell imprinting on the surface of Ag–ZnO bimetallic nanoparticle modified graphene oxide sheets for targeted detection, removal and photothermal killing of E. coli. Biosens. Bioelectron. 2017;89:620–662. doi: 10.1016/j.bios.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 25.Bashami R.M., Hameed A., Aslam M. The suitability of ZnO film-coated glassy carbon electrode for the sensitive detection of 4-nitrophenol in aqueous medium. Anal. Methods. 2015;7:1794–1801. [Google Scholar]
- 26.Fang L., Liu B., Liu L. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bio electrochemical glucose sensor. Sens Actuators B Chem. 2016;222:1096–1102. [Google Scholar]
- 27.Fallatah A., Kuperus N., Almomtan M., Padalkar S. Sensitive biosensor based on shape-controlled ZnO nanostructures grown on flexible porous substrate for pesticide detection. Sensors. 2022;22(9):3522. doi: 10.3390/s22093522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji X., Yang Y., Wang A., Zhao Q. One-step hydrothermal synthesis of CuO micro-crystals for non-enzymatic glucose sensors. Sci. Adv. Mater. 2022;14(4):638–643. [Google Scholar]
- 29.Li Y., Wang Q. Design and infrared spectral modulation properties of Cu/CuO one-dimensional photonic crystals. Sci. Adv. Mater. 2022;14(2):372–382. [Google Scholar]
- 30.Ping J., Ru S., Fan K., Wu J., Ying Y. Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim. Acta. 2010;171(1):117–123. [Google Scholar]
- 31.Dhara K., Thiagarajan R., Nair B.G., Thekkedath G.S.B. Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen-printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim. Acta. 2015;182(13):2183–2192. [Google Scholar]
- 32.Khoshhesab Z.M. Simultaneous electrochemical determination of acetaminophen, caffeine and ascorbic acid using a new electrochemical sensor based on CuO–graphene nanocomposite. RSC Adv. 2015;5(115):95140–95148. [Google Scholar]
- 33.Zhang J., Ma J., Zhang S., Wang W., Chen Z. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles decorated carbon spheres. Sensor. Actuator. B Chem. 2015;211:385–391. [Google Scholar]
- 34.Zou J., Wu S., Liu Y., Sun Y., Cao Y., Hsu J.P., Wee A.T.S., Jiang J. An ultrasensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon. 2018;130:652–663. [Google Scholar]
- 35.Song J., Xu L., Zhou C., Xing R., Dai Q., Liu D., Song H. Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces. 2013;5(24):12928–12934. doi: 10.1021/am403508f. [DOI] [PubMed] [Google Scholar]
- 36.Dogana H.O., Urhan B.K., Çepni E., Eryigit M. Simultaneous electrochemical detection of ascorbic acid and dopamine on Cu2O/CuO/electrochemically reduced graphene oxide (CuxO/ERGO)-nanocomposite-modified electrode. Microchem. J. 2019;150 [Google Scholar]
- 37.Razmi H., Nasiri H., Mohammad-Rezaei R. Amperometric determination of L-tyrosine by an enzyme less sensor based on a carbon ceramic electrode modified with copper oxide nanoparticles. Microchim. Acta. 2011;173:59–64. [Google Scholar]
- 38.Wang X., Liu E., Zhang X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta. 2014;130:253–260. [Google Scholar]
- 39.Kang S.Z., Liu H., Li X., Sun M., Mu J. Electrochemical behavior of eugenol on TiO2 nanotubes improved with Cu2O clusters. RSC Adv. 2014;4:538–543. [Google Scholar]
- 40.Xu F., Deng M., Li G., Chen S., Wang L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta. 2013;88:59–65. [Google Scholar]
- 41.Khoshhesab Z.M. Simultaneous electrochemical determination of acetaminophen, caffeine and ascorbic acid using a new electrochemical sensor based on CuO-graphene nanocomposite. RSC Adv. 2015;5:95140–95148. [Google Scholar]
- 42.Dai Z., Yang A., Bao X., Yang R. Facile non-enzymatic electrochemical sensing for glucose based on Cu2O–BSA nanoparticles modified GCE. Sensors. 2019;19(12):2824. doi: 10.3390/s19122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng D., Qin J., Feng Y., Wei J. Synthesis of mesoporous CuO hollow sphere nanozyme for paper-based hydrogen peroxide sensor. Biosensors. 2021;11(8):258. doi: 10.3390/bios11080258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaushik A., Solanki P.R., Ansari A.A., Sumana G., Ahmad S., Malhotra B.D. Iron oxide-chitosan nanobiocomposite for urea sensor. Sensor. Actuator. B Chem. 2009;138(2):572–580. [Google Scholar]
- 45.Li B.Q., Nie F., Sheng Q.L., Zheng J.B. An electrochemical sensor for sensitive determination of nitrites based on Ag-Fe3O4-graphene oxide magnetic nanocomposites. Chem. Pap. 2015;69(7):911–920. [Google Scholar]
- 46.Lee S., Oh J., Kim D., Piao Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta. 2016;160:528–536. doi: 10.1016/j.talanta.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 47.Cai Z., Ye Y., Wan X., Liu J., Yang S., Xia Y., Li G., He Q. Morphology–dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials. 2019;9:835. doi: 10.3390/nano9060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenita Rani G., Justice Babu K., Gnana Kumar G., Jothi Rajan M.A. Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J. Alloys Compd. 2016:500–512. [Google Scholar]
- 49.Peik-See T., Pandi Kumar A., Nay-Ming H., Hong-Ngee L., Sulaiman Y. Simultaneous electrochemical detection of dopamine and ascorbic acid using an iron oxide/reduced graphene oxide modified glassy carbon electrode. Sensors. 2014;14:15227–15243. doi: 10.3390/s140815227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arani N.H., Ghoreishi S.M., Khoobi A. Increasing the electrochemical system performance using a magnetic nanostructured sensor for simultaneous determination of L-tyrosine and epinephrine. Anal. Methods. 2019;11:1192–1198. [Google Scholar]
- 51.Naghib S.M., Rahmanian M., Keivan M., Asiaei S., Vahidi O. Novel magnetic nanocomposites comprising reduced graphene oxide/Fe3O4/gelatin utilized in ultrasensitive non-enzymatic biosensing. Int. J. Electrochem. Sci. 2016;11:10256–10269. [Google Scholar]
- 52.AL-Mokaram A.M.A.A., Yahya R., Abdi M.M., Mahmud H.N.M.E. One-step electrochemical deposition of Polypyrrole–Chitosan–Iron oxide nanocomposite films for non-enzymatic glucose biosensor. Mater. Lett. 2016;183:90–93. [Google Scholar]
- 53.Bonyani M., Mirzaei A., Leonardi S.G. Electrochemical properties of Ag@iron oxide nanocomposite for application as nitrate sensor. Electroanalysis. 2015;27:2654–2662. [Google Scholar]
- 54.Hou C., Tang W., Zhang C. A novel and sensitive electrochemical sensor for bisphenol a determination based on carbon black supporting ferro ferric oxide nanoparticles. Electrochim. Acta. 2014;144:324–331. [Google Scholar]
- 55.Suresh R., Vijayaraj A., Giribabu K. Fabrication of iron oxide nanoparticles: magnetic and electrochemical sensing property. J. Mater. Sci. Mater. Electron. 2013;24:1256–1263. [Google Scholar]
- 56.Ali M., Barman K., Jasimuddin S., Ghosh S.K. Fluid interface-mediated nanoparticle membrane as an electrochemical sensor. RSC Adv. 2014;4:61404–61408. [Google Scholar]
- 57.Ran G., Chen X., Xia Y. Electrochemical detection of serotonin based on a poly (bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017;7(4):1847–1851. [Google Scholar]
- 58.Cai Z., Ye Y., Wan X., Liu J., Yang S., Xia Y., Li G., He Q. Morphology–dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials. 2019;9(6):835. doi: 10.3390/nano9060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou S., Wang Q., Chen J., Shen Y., Liu L., Wang C. Preparation and optimization of MnO2 nanoparticles. Sci. Adv. Mater. 2022;14(5):927–933. [Google Scholar]
- 60.Battilocchio C., Hawkins J.M., Ley S.V. Mild and selective heterogeneous catalytic hydration of nitriles to amides by flowing through manganese dioxide. Org. Lett. 2014;16:1060–1063. doi: 10.1021/ol403591c. [DOI] [PubMed] [Google Scholar]
- 61.Xiao W., Wang D., Lou X.W. Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C. 2010;114:1694–1700. [Google Scholar]
- 62.Zhang J., Chu W., Jiang J., Zhao X.S. Synthesis, characterization and capacitive performance of hydrous manganese dioxide nanostructures. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/12/125703. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z., Chen Z., Yu A. Manganese dioxide nanotube and nitrogen-doped carbon nanotube based composite bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta. 2012;69:295–300. [Google Scholar]
- 64.Devaraj S., Munichandraiah N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C. 2008;112:4406–4417. [Google Scholar]
- 65.Vukojevic V., Djurdjic S., Ognjanovic M., Fabian M., Samphao A., Kalcher K., Stankovic D.M. Enzymatic glucose ´biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018;823:610–616. [Google Scholar]
- 66.Shoja Y., Rafati A.A., Ghodsi J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of D-amino acid oxidase and hemoglobin as efficient bio-nanocomposite in fabrication of dopamine BiEnzyme biosensor. Mater. Sci. Eng., C. 2017;76:637–645. doi: 10.1016/j.msec.2017.03.155. [DOI] [PubMed] [Google Scholar]
- 67.Vijayalakshmi K., Renitta A., Alagusundaram K., Monamary A. Novel two-step process for the fabrication of MnO2 nanostructures on tantalum for enhanced electrochemical H2O2 detection. Mater. Chem. Phys. 2018;214:431–439. [Google Scholar]
- 68.Zhang S., Zheng J. Synthesis of single-crystal α-MnO2 nanotubes-loaded Ag@C core-shell matrix and their application for electrochemical sensing of nonenzymatic hydrogen peroxide. Talanta. 2016;159:231–237. doi: 10.1016/j.talanta.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 69.Han L., Shao C., Liang B., Liu A. Genetically engineered phage-templated MnO2 nanowires: synthesis and their application in electrochemical glucose biosensor operated at neutral PH condition. ACS Appl. Mater. Interfaces. 2016;8(22):13768–13776. doi: 10.1021/acsami.6b03266. [DOI] [PubMed] [Google Scholar]
- 70.Knezevic S., Ognjanovic M., Nedic N., Mariano J.F.M.L., Milanovic Z., Petkovic B., Antic B., Djuric S.V., Stankovic D.A. Single drop histamine sensor based on AuNPs/MnO2 modified screen-printed electrode. Microchem. J. 2020;155 [Google Scholar]
- 71.Abd El-Haleem H.S., Hefnawy A., Hassan R.Y.A., Badawi A.H., El-Sherbiny I.M. Manganese dioxide-core-shell hyperbranched chitosan (MnO2-HBCs) nano-structured screen-printed electrode for enzymatic glucose biosensors. RSC Adv. 2016;6(110):109185–109191. [Google Scholar]
- 72.Shu Y., Xu J., Chen J., Xu Q., Xiao X., Jin D., Pang H., Hu X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators, B. 2017;252:72–78. [Google Scholar]
- 73.Gao W., Liu Z., Qi L., Lai J., Kitte S.A., Xu G. Ultrasensitive glutathione detection based on lucigenin cathodic electrochemiluminescence in the presence of MnO2 nanosheets. Anal. Chem. 2016;88(15):7654–7659. doi: 10.1021/acs.analchem.6b01491. [DOI] [PubMed] [Google Scholar]
- 74.Chauhan N., Balayan S., Jain U. Sensitive biosensing of neurotransmitter: 2D material wrapped nanotubes and MnO2 composites for the detection of acetylcholine. Synth. Met. 2020;263 [Google Scholar]
- 75.Wang M.Y., Zhu W., Ma L., Ma J.J., Zhang D.E., Tong Z.W., Chen J. Enhanced simultaneous detection of ractopamine and salbutamol–via electrochemical-facial deposition of MnO2 nanoflowers onto 3D RGO/Ni foam templates. Biosens. Bioelectron. 2016;78:259–266. doi: 10.1016/j.bios.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 76.Wan X., Yang S., Cai Z., He Q., Ye Y., Xia Y., Li G., Liu J. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials. 2019;9(6):847. doi: 10.3390/nano9060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tehseen B., Rehman A., Rahmat M., Bhatti H.N., Wu A., Butt F.K., Naz G., Khan W.S., Bajwa S.Z. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosens. Bioelectron. 2018;117:852–859. doi: 10.1016/j.bios.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 78.Ramesh M., Janani R., Deepa C., Rajeshkumar L. Nanotechnology-enabled biosensors: a review of fundamentals, design principles, materials, and applications. Biosensors. 2022;13(1):40. doi: 10.3390/bios13010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma F., Li C.C., Zhang C.Y. Development of quantum dot-based biosensors: principles and applications. J. Mater. Chem. B. 2018;6:6173–6190. doi: 10.1039/c8tb01869c. [DOI] [PubMed] [Google Scholar]
- 80.Safari M., Najafi S., Arkan E., Amani S., Shahlaei M. Facile aqueous synthesis of Ni-doped CdTe quantum dots as fluorescent probes for detecting pyrazinamide in plasma. Microchem. J. 2019;146:293–299. [Google Scholar]
- 81.Pourghobadi Z., Mirahmadpour P., Zare H. Fluorescent biosensor for the selective determination of dopamine by TGA-capped CdTe quantum dots in human plasma samples. Opt. Mater. 2018;84:757–762. [Google Scholar]
- 82.Liang N., Hu X., Li W., Wang Y., Guo Z., Huang X., et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022;378 doi: 10.1016/j.foodchem.2022.132076. [DOI] [PubMed] [Google Scholar]
- 83.Wei X.-H., Qiao X., Fan J., Hao Y.-Q., Zhang Y.-T., Zhou Y.-L., et al. A label-free ECL aptasensor for sensitive detection of carcinoembryonic antigen based on CdS QDs@MOF and TEOA@ Au as bi-coreactants of Ru(bpy)32+ Microchem. J. 2022;173 [Google Scholar]
- 84.Yang M., Wang C., Yan Y., Liu E., Hu X., Hao H., et al. Visual detection of folic acid based on silica coated CdTeS quantum dots in serum samples. Mater. Res. Bull. 2021;144 [Google Scholar]
- 85.Liu Y., Li B., Yao Y., Yang B., Tian T., Miao Y., et al. An electrochemilumine scence sensor for 17β-estradiol detection based on resonance energy transfer in α-FeOOH@CdS/Ag NCs. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121479. [DOI] [PubMed] [Google Scholar]
- 86.Yang M., Wang C., Liu E., Hu X., Hao H., Fan J. A novel ascorbic acid ratiometric fluorescent sensor based on ZnCdS quantum dots embedded molecularly imprinted polymer and silica-coated CdTeS quantum dots. J. Mol. Liq. 2021;337 [Google Scholar]
- 87.Vasilescu I., Eremia S.A.V., Kusko M., Radoi A., Vasile E., Radu G.-L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016;75:232–237. doi: 10.1016/j.bios.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 88.Jamei H.R., Rezaei B., Ensafi A.A. Ultra-sensitive and selective electrochemical biosensor with aptamer recognition surface based on polymer quantum dots and C60/MWCNTs- polyethylenimine nanocomposites for analysis of thrombin protein. Bioelectrochemistry. 2021;138 doi: 10.1016/j.bioelechem.2020.107701. [DOI] [PubMed] [Google Scholar]
- 89.Yu M., Zhao K., Zhu X., Tang S., Nie Z., Huang Y., et al. Development of near infrared ratiometric fluorescent probe based on cationic conjugated polymer and CdTe/CdS QDs for label-free determination of glucose in human body fluids. Biosens. Bioelectron. 2017;95:41–47. doi: 10.1016/j.bios.2017.03.065. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R., Chen W. Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014;55:83–90. doi: 10.1016/j.bios.2013.11.074. [DOI] [PubMed] [Google Scholar]
- 91.Pooja D., Saini S., Thakur A., Kumar B., Tyagi S., Nayak M.K. A “Turn-On” thiol functionalized fluorescent carbon quantum dot based chemosensory system for arsenite detection. J. Hazard Mater. 2017;328:117–126. doi: 10.1016/j.jhazmat.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Hallaj T., Amjadi M., Manzoori J.L., Azizi N. A novel chemiluminescence sensor for the determination of indomethacin based on sulfur and nitrogen co‐doped carbon quantum dot–KMnO4 reaction. Luminescence. 2017;32(7):1174–1179. doi: 10.1002/bio.3306. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y., Zhou Y., Xu L., Han Z., Yin H., Ai S. Photoelectrochemical apta-biosensor for zeatin detection based on graphene quantum dots improved photoactivity of graphite-like carbon nitride and streptavidin induced signal inhibition. Sensor. Actuator. B Chem. 2018;257:237–244. [Google Scholar]
- 94.Savas S., Altintas Z. Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocoliticain milk and human serum. Materials. 2019;12:2189. doi: 10.3390/ma12132189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramanathan K., Bangar M.A., Yun M., Chen W., Myung N.V., Mulchandani A. Bioaffinity sensing using biologically functionalized conducting polymer nanowire. J. Am. Chem. Soc. 2005;127:496–497. doi: 10.1021/ja044486l. [DOI] [PubMed] [Google Scholar]
- 96.Patolsky F., Zheng G., Lieber C.M. Nanowire-based biosensors. Anal. Chem. 2006;78:4260–4269. doi: 10.1021/ac069419j. [DOI] [PubMed] [Google Scholar]
- 97.Jang J.S., Qiao S., Choi S.J., Jha G., Ogata A.F., Koo W.T., Kim D.H., Kim I.D., Penner R.M. Hollow Pd–Ag composite nanowires for fast responding and transparent hydrogen sensors. ACS Appl. Mater. Interfaces. 2017;9(45):39464–39474. doi: 10.1021/acsami.7b10908. [DOI] [PubMed] [Google Scholar]
- 98.Kim D.H., Kim S.J., Shin H., Koo W.T., Jang J.S., Kang J.Y., Jeong Y.J., Kim I.D. High-resolution, fast, and shape-conformable hydrogen sensor platform: polymer nanofiber yarn coupled with nanograined Pd@ Pt. ACS Nano. 2019;13(5):6071–6082. doi: 10.1021/acsnano.9b02481. [DOI] [PubMed] [Google Scholar]
- 99.Prajapati C.S., Bhat N. Self-heating oxidized suspended Pt nanowire for high performance hydrogen sensor. Sensor. Actuator. B Chem. 2018;260:236–242. [Google Scholar]
- 100.Zhu Q., Huang J., Yan M., Ye J., Wang D., Lu Q., Yang X. N-(Aminobutyl)-N-(ethylisoluminol)-functionalized gold nanoparticles on cobalt disulfide nanowire hybrids for the non-enzymatic chemiluminescence detection of H2O2. Nanoscale. 2018;10(31):14847–14851. doi: 10.1039/c8nr03990a. [DOI] [PubMed] [Google Scholar]
- 101.Gwak R., Kim H., Yoo S.M., Lee S.Y., Lee G.J., Lee M.K., Rhee C.K., Kang T., Kim B. Precisely determining ultralow level UO22+ in natural water with plasmonic nanowire interstice sensor. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J., Yang B., Gao F., Song P., Li L., Zhang Y., Lu C., Goh M.C., Du Y. Ultra-stable electrochemical sensor for detection of caffeic acid based on platinum and nickel jagged-like nanowires. Nanoscale Res. Lett. 2019;14(1):1–7. doi: 10.1186/s11671-018-2839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie L., Asiri A.M., Sun X. Monolithically integrated copper phosphide nanowire: an efficient electrocatalyst for sensitive and selective nonenzymatic glucose detection. Sensor. Actuator. B Chem. 2017;244:11–16. [Google Scholar]
- 104.Qin L., He L., Zhao J., Zhao B., Yin Y., Yang Y. Synthesis of Ni/Au multilayer nanowire arrays for ultrasensitive non-enzymatic sensing of glucose. Sensor. Actuator. B Chem. 2017;240:779–784. [Google Scholar]
- 105.Huang H., Nie R., Song Y., Ji Y., Guo R., Liu Z. Highly sensitive electrochemical sensor for tulobuterol detection based on facile graphene/Au nanowires modified glassy carbon electrode. Sensor. Actuator. B Chem. 2016;230:422–426. [Google Scholar]
- 106.Zhang S., Liu H., Yang S., Shi X., Zhang D., Shan C., Mi L., Liu C., Shen C., Guo Z. Ultrasensitive and highly compressible piezoresistive sensor based on polyurethane sponge coated with a cracked cellulose nanofibril/silver nanowire layer. ACS Appl. Mater. Interfaces. 2019;11(11):10922–10932. doi: 10.1021/acsami.9b00900. [DOI] [PubMed] [Google Scholar]
- 107.Yu Y., Zhu Q., Xiang F., Hu Y., Zhang L., Xu X., Liu N., Huang S. Applying AuNPs/SWCNT to fabricate electrical nanogap device for DNA hybridization detection. Carbon. 2020;157:40–46. [Google Scholar]
- 108.Vilian A.E., Kim W., Park B., Oh S.Y., Kim T., Huh Y.S., Hwangbo C.K., Han Y.K. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: a predictive strategy for heart failure. Biosens. Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111549. [DOI] [PubMed] [Google Scholar]
- 109.Trafela Š., Zavašnik J., Šturm S., Rožman K.Ž. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta. 2019;309:346–353. [Google Scholar]
- 110.Wu Y., Jiao L., Xu W., Gu W., Zhu C., Du D., Lin Y. Polydopamine‐capped bimetallic AuPt hydrogels enable robust biosensor for organophosphorus pesticide detection. Small. 2019;15(17) doi: 10.1002/smll.201900632. [DOI] [PubMed] [Google Scholar]
- 111.Hakim M.M., Lombardini M., Sun K., Giustiniano F., Roach P.L., Davies D.E., Howarth P.H., de Planque M.R., Morgan H., Ashburn P. Thin film polycrystalline silicon nanowire biosensors. Nano Lett. 2012;12(4):1868–1872. doi: 10.1021/nl2042276. [DOI] [PubMed] [Google Scholar]
- 112.Irrera A., Leonardi A.A., Di Franco C., Lo Faro M.J., Palazzo G., D'Andrea C., Manoli K., Franzo G., Musumeci P., Fazio B., Torsi L. New generation of ultrasensitive label-free optical Si nanowire-based biosensors. ACS Photonics. 2018;5(2):471–479. [Google Scholar]
- 113.Leonardi A.A., Faro M.J.L., Petralia S., Fazio B., Musumeci P., Conoci S., Irreara A., Priolo F. Ultrasensitive label- and PCR-free genome detection based on cooperative hybridization of silicon nanowires optical biosensors. ACS Sens. 2018;3:1690–1697. doi: 10.1021/acssensors.8b00422. [DOI] [PubMed] [Google Scholar]
- 114.Ivanov Y.D., Romanova T.S., Malsagova K.A., Pleshakova T.O., ArchakovA I. Use of silicon nanowire sensors for early cancer diagnosis. Molecules. 2021;26(12):3734. doi: 10.3390/molecules26123734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao J., Sun T., Grattan K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: a review. Sens. Actuators B Chem. 2014;195:332–351. [Google Scholar]
- 116.Ibupoto Z.H., Ali S.M.U., Khun L., Chey C.O., Nur O., Willander M. ZnO nanorods based enzymatic biosensor for selective determination of penicillin. Biosensors. 2011;1:153–163. doi: 10.3390/bios1040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H., Song D., Gao S., Zhang H., Zhang J., Sun Y. Enhanced wavelength modulation SPR biosensor based on gold nanorods for immunoglobulin detection. Talanta. 2013;115:857–862. doi: 10.1016/j.talanta.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 118.Ahmad R., Ahn M.S., Hahn Y.B. ZnO nanorods array-based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017;498:292–297. doi: 10.1016/j.jcis.2017.03.069. [DOI] [PubMed] [Google Scholar]
- 119.Zong X., Zhu R. ZnO nanorod-based FET biosensor for continuous glucose monitoring. Sens. Actuator B Chem. 2018;255:2448–2453. [Google Scholar]
- 120.Liu G., Feng D.Q., Qian Y., Wang W., Zhu J.J. Construction of FRET biosensor for off-on detection of lead ions based on carbon dots and gold nanorods. Talanta. 2019;201:90–95. doi: 10.1016/j.talanta.2019.03.101. [DOI] [PubMed] [Google Scholar]
- 121.Bagyalakshmi S., Sivakami A., Balamurugan K.S. A Zno nanorods based enzymatic glucose biosensor by immobilization of glucose oxidase on a chitosan film. Obesity Medicine. 2020;18 [Google Scholar]
- 122.Swargiary K., Metem P., Kulatumyotin C., Thaneerat S., Ajchareeyasoontorn N., Jitpratak P., Bora T., Mohammed W.S., Dutta J., Viphavakit C. ZnO nanorods coated single-mode–multimode–single-mode optical fiber sensor for VOC biomarker detection. Sensors. 2022;22:6273. doi: 10.3390/s22166273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pan R., Zhang Y., Yu M., Zhang S., Wu S. Value of flexible nano-sensor with carbon nanotube and graphene in ultrasound screening of congenital heart malformations in early pregnancy. Sci. Adv. Mater. 2022;14(1):34–42. [Google Scholar]
- 124.Xiaowu Tang X., Bansaruntip S., Nakayama N., Yenilmez E., Chang Y.L., Wang Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006;6:1632–1636. doi: 10.1021/nl060613v. [DOI] [PubMed] [Google Scholar]
- 125.Li W.S., Hou P.X., Liu C., Sun D.M., Yuan J., Zhao S.Y., Yin L.C., Cong H., Cheng H.M. High-quality, highly concentrated semiconducting single-wall carbon nanotubes for use in field effect transistors and biosensors. ACS Nano. 2013;7(8):6831–6839. doi: 10.1021/nn401998r. [DOI] [PubMed] [Google Scholar]
- 126.Son M., Kim D., Park K.S., Hong S., Park T.H. Detection of aquaporin-4 antibody using aquaporin-4 extracellular loop-based carbon nanotube biosensor for the diagnosis of neuromyelitis optica. Biosens. Bioelectron. 2016;78:87–91. doi: 10.1016/j.bios.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 127.Li D., Wang C., Sun G., Senapati S., Chang H.C. A shear-enhanced CNT-assembly nano sensor platform for ultra-sensitive and selective protein detection. Biosens. Bioelectron. 2017;97:143–149. doi: 10.1016/j.bios.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 128.Fu Y., Romay V., Liu Y., Ibarlucea B., Baraban L., Khavrus V., Oswald S., Bachmatiuk A., Ibrahim I., Rümmeli M., Gemming T. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sensor. Actuator. B Chem. 2017;249:691–699. [Google Scholar]
- 129.Ji S., Lee M., Kim D. Detection of early-stage prostate cancer by using a simple carbon nanotube@ paper biosensor. Biosens. Bioelectron. 2018;102:345–350. doi: 10.1016/j.bios.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 130.Xu X., Clément P., Eklof-Osterberg J., Kelley-Loughnane N., Moth-Poulsen K., Chávez J.L., Palma M. Reconfigurable carbon nanotube multiplexed sensing devices. Nano Lett. 2018;18(7):4130–4135. doi: 10.1021/acs.nanolett.8b00856. [DOI] [PubMed] [Google Scholar]
- 131.Park M., Kim H.S., Kim T., Kim J., Seo S., Lee B.Y. Real-time monitoring of microbial activity using hydrogel-hybridized carbon nanotube transistors. Sensor. Actuator. B Chem. 2018;263:486–492. [Google Scholar]
- 132.Liang Y., Xiao M., Wu D., Lin Y., Liu L., He J., Zhang G., Peng L.M., Zhang Z. Wafer-scale uniform carbon nanotube transistors for ultrasensitive and label-free detection of disease biomarkers. ACS Nano. 2020;14(7):8866–8874. doi: 10.1021/acsnano.0c03523. [DOI] [PubMed] [Google Scholar]
- 133.Bushmaker A.W., Oklejas V., Walker D., Hopkins A.R., Chen J., Cronin S.B. Single-ion adsorption and switching in carbon nanotubes. Nat. Commun. 2016;7(1):1–8. doi: 10.1038/ncomms10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ishihara S., Labuta J., Nakanishi T., Tanaka T., Kataura H. Amperometric detection of sub-ppm formaldehyde using single-walled carbon nanotubes and hydroxylamines: a referenced chemiresistive system. ACS Sens. 2017;2(10):1405–1409. doi: 10.1021/acssensors.7b00591. [DOI] [PubMed] [Google Scholar]
- 135.Li X., Le Thai M., Dutta R.K., Qiao S., Chandran G.T., Penner R.M. Sub-6 nm palladium nanoparticles for faster, more sensitive H2 detection using carbon nanotube ropes. ACS Sens. 2017;2(2):282–289. doi: 10.1021/acssensors.6b00808. [DOI] [PubMed] [Google Scholar]
- 136.Kumar D., Chaturvedi P., Saho P., Jha P., Chouksey A., Lal M., Rawat J.S.B.S., Tandon R.P., Chaudhury P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sensor. Actuator. B Chem. 2017;240:1134–1140. [Google Scholar]
- 137.Xiao M., Liang S., Han J., Zhong D., Liu J., Zhang Z., Peng L. Batch fabrication of ultrasensitive carbon nanotube hydrogen sensors with sub-ppm detection limit. ACS Sens. 2018;3(4):749–756. doi: 10.1021/acssensors.8b00006. [DOI] [PubMed] [Google Scholar]
- 138.Panes-Ruiz L.A., Shaygan M., Fu Y., Liu Y., Khavrus V., Oswald S., Gemming T., Baraban L., Bezugly V., Cuniberti G. Toward highly sensitive and energy efficient ammonia gas detection with modified single-walled carbon nanotubes at room temperature. ACS Sens. 2018;3(1):79–86. doi: 10.1021/acssensors.7b00358. [DOI] [PubMed] [Google Scholar]
- 139.He M., Croy R.G., Essigmann J.M., Swager T.M. Chemiresistive carbon nanotube sensors for N-nitrosodialkylamines. ACS Sens. 2019;4(10):2819–2824. doi: 10.1021/acssensors.9b01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aniello Falco F.C., Loghin M., Becherer P., Lugli José F.S., Almudena R. Low-cost gas sensing: dynamic self-compensation of humidity in CNT-based devices. ACS Sens. 2019;4(12):3141–3146. doi: 10.1021/acssensors.9b01095. [DOI] [PubMed] [Google Scholar]
- 141.Hwang S.I., Franconi N.G., Rothfuss M.A., Bocan K.N., Bian L., White D.L., Burkert S.C., Euler R.W., Sopher B.J., Vinay M.L., Sejdic E. Tetrahydrocannabinol detection using semiconductor-enriched single-walled carbon nanotube chemiresistors. ACS Sens. 2019;4(8):2084–2093. doi: 10.1021/acssensors.9b00762. [DOI] [PubMed] [Google Scholar]
- 142.Chen J., Lotfi A., Hesketh P.J., Kumar S. Carbon nanotube thin-film-transistors for gas identification. Sensor. Actuator. B Chem. 2019;281:1080–1087. [Google Scholar]
- 143.Seekaew Y., Wisitsoraat A., Phokharatkul D., Wongchoosuk C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sensor. Actuator. B Chem. 2019;279:69–78. [Google Scholar]
- 144.Li W., Gao Y., Zhang J., Wang X., Yin F., Li Z., Zhang M. Universal DNA detection realized by peptide-based carbon nanotube biosensors. Nanoscale Adv. 2020;2(2):717–723. doi: 10.1039/c9na00625g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Janssen J., Lambeta M., White P., Byagowi A. Carbon nanotube-based electrochemical biosensor for label-free protein detection. Biosensors. 2019;9:144. doi: 10.3390/bios9040144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee B.Y., Seo S.M., Lee D.J., Lee M., Lee J., Cheon J.H., Cho E., Lee H., Chung I.Y., Park Y.J., et al. Biosensor system-on-a-chip including CMOS-based signal processing circuits and 64 carbon nanotube-based sensors for the detection of a neurotransmitter. Lab Chip. 2010;10:894–898. doi: 10.1039/b916975j. [DOI] [PubMed] [Google Scholar]
- 147.Mann F.A., Herrmann N., Meyer D., Kruss S. Tuning selectivity of fluorescent carbon nanotube-based neurotransmitter sensors. Sensors. 2017;17(7):1521. doi: 10.3390/s17071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharma A., Kakkar A. Designingdendrimer and miktoarm polymer based multi-tasking nanocarriers for efficient medical therapy. Molecules. 2015;20(9):16987–17015. doi: 10.3390/molecules200916987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abbasi E., Aval S.F., Akbarzadeh A., Milani M., Nasrabadi H.T., Joo S.W., Hanifehpour Y., Koshki K.N., Asl R.P., Dendrimers Synthesis applications, and properties. Nanoscale Res. Lett. 2014;9:247. doi: 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Laraib U., Sargazi S., Rahdar A., Khatami M., Pandey S. Nanotechnology-based approaches for effective detection of tumor markers: a comprehensive state-of-the-art review. Int. J. Biol. Macromol. 2022;195:356–383. doi: 10.1016/j.ijbiomac.2021.12.052. [DOI] [PubMed] [Google Scholar]
- 151.Razlansari M., Jafarinejad S., Rahdar A., Shirvaliloo M., Arshad R., Fathi-Karkan S., Mirinejad S., Sargazi S., Sheervalilou R., Ajalli N., Pandey S. Development and classification of RNA aptamers for therapeutic purposes: an updated review with emphasis on cancer. Mol. Cell. Biochem. 2023;478(7):1573–1598. doi: 10.1007/s11010-022-04614-x. [DOI] [PubMed] [Google Scholar]
- 152.Satija J., Sai V.V.R., Muherji S. Dendrimers in biosensors: concept and applications. J. Mater. Chem. 2011;21:14367–14386. [Google Scholar]
- 153.Caminade A.M., Turrin C.O. Dendrimers for drug delivery. J. Mater. Chem. B. 2014;2:4055–4066. doi: 10.1039/c4tb00171k. [DOI] [PubMed] [Google Scholar]
- 154.Ou X., Fang C., Fan Y., Chen H., Chen S., Wei S. Sandwich-configuration electrochemiluminescence biosensor based on ag nanocubes–polyamidoamine dendrimer–luminol nanocomposite for con a detection. Sensor. Actuator. B Chem. 2016;228:625–633. [Google Scholar]
- 155.Dervisevic E., Dervisevic M., Nyangwebah J.N., Şenel M. Development of novel amperometric urea biosensor based on Fc-PAMAM and MWCNT bio-nanocomposite film. Sensor. Actuator. B Chem. 2017;246:920–926. [Google Scholar]
- 156.Kamil Y.M., Al-Rekabi S.H., Yaacob M.H., Syahir A., Chee H.Y., Mahid M.A., Bakar M.H.A. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Castro A.C., Alves L.M., Siquieroli A.C., Madurro J.M., Brito-Madurro A.G. Label-free electrochemical immunosensor for detection of on comarker CA125 in serum. Microchem. J. 2020;155 [Google Scholar]
- 158.Shi S., Wu H., Zhang L., Wang S., Xiong P., Qin Z., Chu M., Liao J. Gold nanoparticles based electrochemical sensor for sensitive detection of uranyl in natural water. J. Electroanal. Chem. 2021;880 [Google Scholar]
- 159.Niu X.F., Zhong Y., Chen R., Wang F., Liu Y., Luo D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018;255:1577–1581. [Google Scholar]
- 160.Khaliq N., Rasheed M.A., Khan M., Maqbool M., Ahmad M., Karim S., Nisar A., Schmuki P., Cho S.O., Ali G. Voltage switchable biosensor with gold nanoparticles on TiO2 nanotubes decorated with CdS quantum dots for the detection of cholesterol and H2O2. ACS Appl. Mater. Interfaces. 2021;13:3653–3668. doi: 10.1021/acsami.0c19979. [DOI] [PubMed] [Google Scholar]
- 161.Vu Q.K., Tran Q.H., Vu N.P., Anh Y.L., Dang T.T.L., Tonezzer M., Ngyyen T.H.H. A label-free electrochemical biosensor based on screen-printed electrodes modified with gold nanoparticles for quick detection of bacterial pathogens. Mater. Today Commun. 2020 [Google Scholar]
- 162.Jandas P.J., Luo J., Prabakaran K., Chen F., Fu Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020;246 [Google Scholar]
- 163.Kasturi S., Eom Y., Torati S.R., Kim C.G. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021;93:186–195. [Google Scholar]
- 164.Liu X., Zhang J., Liu S., Zhang Q., Liu X., Wong D.K.Y. Gold nanoparticle encapsulated-tubular TiO2 nanocluster as a scaffold for development of thiolated enzyme biosensors. Anal. Chem. 2013;85:4350–4356. doi: 10.1021/ac303420a. [DOI] [PubMed] [Google Scholar]
- 165.Basiri S., Mehdinia A., Jabbari A. A sensitive triple colorimetric sensor based on plasmonic response quenching of green synthesized silver nanoparticles for determination of Fe2+, hydrogen peroxide, and glucose. Colloids Surf. A Physicochem. Eng. Asp. 2018;545:138–146. [Google Scholar]
- 166.Wang H., Zhang Y., Du B., Ma H., Wu D., Wei Q. A silver–palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens. Bioelectron. 2013;49:14–19. doi: 10.1016/j.bios.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 167.Han G., Cai J., Liu C., Ren J., Wang X., Yang J., Wang X. Highly sensitive electrochemical sensor based on xylan-based Ag@CQDs-rGO nanocomposite for dopamine detection. Appl. Surf. Sci. 2021;541 [Google Scholar]
- 168.Chen L., Xie H., Li J. Electrochemical glucose biosensor based on silver nanoparticles/multiwalled carbon nanotubes modified electrode. J. Solid State Electrochem. 2012;16:3323–3329. [Google Scholar]
- 169.Wei U.P., Zhang Y.W., Mao C.J. A silver nanoparticle-assisted signal amplification electrochemiluminescence biosensor for highly sensitive detection of mucin 1. J. Mater. Chem. B. 2020;8:2471–2475. doi: 10.1039/c9tb02773d. [DOI] [PubMed] [Google Scholar]
- 170.Brondani D., Scheeren C.W., Dupont J., Vieira I.C. Biosensor based on platinum nanoparticles dispersed in ionic liquid and laccase for determination of adrenaline. Sens. Actuators, B. 2009;140:252–259. [Google Scholar]
- 171.Bai Z., Li G., Liang J., Su J., Zhang Y., Chen H., Huang Y., Sui W., Zhao Y. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016;82:185–194. doi: 10.1016/j.bios.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 172.Yang Q., Li N., Li Q., Chen S., Wang H.L., Yang H. Amperometric sarcosine biosensor based on hollow magnetic Pt–Fe3O4@C nanospheres. Anal. Chim. Acta. 2019;1078:161–167. doi: 10.1016/j.aca.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 173.Jia W., Su L., Lei Y. Pt nanoflower/polyaniline composite nanofibers-based urea biosensor. Biosens. Bioelectron. 2011;30:158–164. doi: 10.1016/j.bios.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 174.Fu C., Sun Y., Huang C., Wang F., Li N., Zhang L., Ge S., Yu J. Ultrasensitive sandwich-like electrochemical biosensor based on core-shell Pt@CeO2 as signal tags and double molecular recognition for cerebral dopamine detection. Talanta. 2019;223 doi: 10.1016/j.talanta.2020.121719. [DOI] [PubMed] [Google Scholar]
- 175.Zhang Y., Huang B., Ye J., Ye J. A sensitive and selective amperometric hydrazine sensor based on palladium nanoparticles loaded on cobalt-wrapped nitrogen-doped carbon nanotubes. J. Eelctroanal. Chem. 2017;801:215–223. [Google Scholar]
- 176.Afzali M., Mostafavi A., Nekooie R., Jahromi Z. A novel voltammetric sensor based on palladium nanoparticles/carbon nanofibers/ionic liquid modified carbon paste electrode for sensitive determination of anti-cancer drug pemetrexed. J. Mol. Liq. 2019;282:456–465. [Google Scholar]
- 177.Roushani M., Dizajdizi B.Z. Development of nonenzymatic hydrogen peroxide sensor based on catalytic properties of copper nanoparticles/Rutin/MWCNTs/IL/Chit. Catal. Commun. 2015;69:133–137. [Google Scholar]
- 178.Zhu T., Wang X., Chang W., Zhang Y., Maruyama T., Luo L., Zhao X. Green fabrication of Cu/rGO decorated SWCNT buckypaper as a flexible electrode for glucose detection. Mater. Sci. Eng. C. 2021;120 doi: 10.1016/j.msec.2020.111757. [DOI] [PubMed] [Google Scholar]
- 179.Li F., Li Y., Feng J., Dong Y., Wang P., Chen L., Chen Z., Liu H., Wei Q. Ultrasensitive amperometric immunosensor for PSA detection based on Cu2O@CeO2 -Au nanocomposites as integrated triple signal amplification strategy. Bisensor Bioelectron. 2017;87:630–637. doi: 10.1016/j.bios.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 180.Wang B., Luo Y., Gao L., Liu B., Duan G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112736. [DOI] [PubMed] [Google Scholar]
- 181.Kailasa S., Rani B.G., Reddy M.S.B., Jayarambabu N., Munindra P., Sharma S., Rao K.V. NiO nanoparticles -decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2020;242 [Google Scholar]
- 182.Maduraiveeran G., Chen A. Design of an enzyme-mimicking NiO@Au nanocomposite for the sensitive electrochemical detection of lactic acid in human serum and urine. Electrochim. Acta. 2021;368 [Google Scholar]
- 183.Hu F., Liu T., Pang J., Chu Z., Jin W. Facile preparation of porous Co3O4nanocubes for directly screen-printing an ultrasensitive glutamate biosensor microchip. Sens. Actuators B Chem. 2020;306 [Google Scholar]
- 184.Zhao J., Fu C., Huang C., Zhang S., Wang F., Zhang Y., Zhang L., Ge S., Yu J. Co3O4 -Au polyhedron mimic peroxidase- and cascade enzyme-assisted cycling process-based photoelectrochemical biosensor for monitoring of miRNA-141. Chem. Eng. J. 2021;406 [Google Scholar]
- 185.Li Y., Tang L., Deng D., He H., Yan X., Wang J., Luo L. Hetero-structured MnO-Mn3O4@rGO composites: synthesis and nonenzymatic detection of H2O2. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111443. [DOI] [PubMed] [Google Scholar]
- 186.Xue L., Guo R., Huang F., Qi W., Liu Y., Cai G., Lin J. An impedance biosensor based on magnetic nanobead net and MnO2 nanoflowers for rapid and sensitive detection of foodborne bacteria. Biosens. Bioelectron. 2021;173 doi: 10.1016/j.bios.2020.112800. [DOI] [PubMed] [Google Scholar]
- 187.Alam M.M., Rahman M.M., Uddin M.T., Asiri A.M., Uddin J., Islam M.A. Fabrication of enzyme-less folic acid sensor probe based on facile ternary doped Fe2O3/NiO/Mn2O3nanoparticles. Curr. Res. Biotechnol. 2020;2:176–186. [Google Scholar]
- 188.Verma S., Arya P., Singh A., Kaswan J., Shukla A., Kushwaha H.R., Gupta S., Singh S.P. ZnO-rGO nanocomposite based bioelectrode for sensitive and ultrafast detection of dopamine in human serum. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112347. [DOI] [PubMed] [Google Scholar]
- 189.Ahmad R., Ahn M.S., Hahn Y.B. ZnO nanorods array-based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017;498:292–297. doi: 10.1016/j.jcis.2017.03.069. [DOI] [PubMed] [Google Scholar]
- 190.Akthar N., Metkar S.K., Girigoswami A., Girigoswami K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C. 2017;78:960–968. doi: 10.1016/j.msec.2017.04.118. [DOI] [PubMed] [Google Scholar]
- 191.Dhahri R., Leonardi S.G., Hjiri M., El Mir L., Bonavita E., Donato N., Iannazzo D., Neri G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B Chem. 2017;239:36–44. [Google Scholar]
- 192.Lavanya N., Radhakrishna S., Sekar C., Navaneethan M., Hayakawa Y. Fabrication of Cr doped SnO2nanoparticles-based biosensor for the selective determination of riboflavin in pharmaceuticals. Analyst. 2013;138:2061–2067. doi: 10.1039/c3an36754a. [DOI] [PubMed] [Google Scholar]
- 193.Ognjanovic M., Stankovic V., Knezevic S., Antic B., Djuric S.V., Stankovic D.M. TiO2/APTES cross-linked to carboxylic graphene based impedimetric glucose biosensor. Microchem. J. 2020;158 [Google Scholar]
- 194.Tian J., Li Y., Dong J., Huang M., Lu J. Photoelectrochemical TiO2 nanotube arrays biosensor forasulam determination based on in-situ generation of quantum dots. Biosens. Bioelectron. 2018;110:1–7. doi: 10.1016/j.bios.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 195.Augustinbe S., Kumar P., Malhotra B.D. Amine-functionalized MoO3@RGO nanohybrids-based biosensor for breast cancer detection. ACS Appl. Bio Mater. 2019;2:5366–5378. doi: 10.1021/acsabm.9b00659. [DOI] [PubMed] [Google Scholar]
- 196.Wang Y., Zhao S., Li M., Li W., Zhao Y., Qi J., Cui X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper (II) J. Electroanal. Chem. 2017;797:113–120. [Google Scholar]
- 197.Kalkal A., Pradhan R., Kadian S., Manik G., Packirisamy G. Biofunctionalized graphene quantum dots based fluorescent biosensor toward efficient detection of small cell lung cancer. ACS Appl. Bio Mater. 2020;3:4922–4932. doi: 10.1021/acsabm.0c00427. [DOI] [PubMed] [Google Scholar]
- 198.Huang S., Zhu F., Xiao Q., Su W., Sheng J., Huang C., Hu B. A CdTe/CdS/ZnS core/shell/shell QDs-based “off–on” fluorescent biosensor for sensitive and specific determination of l-ascorbic acid. RSC Adv. 2014;4:46751–46761. [Google Scholar]
- 199.Cui F., Ji J., Sun J., Wang J., Wang H., Zhang Y., Ding H., Lu Y., Xu D., Sun X. A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 2019;411:985–995. doi: 10.1007/s00216-018-1501-0. [DOI] [PubMed] [Google Scholar]
- 200.Liu Y., Chen X., Ma Q. A novel amplified electrochemiluminescence biosensor based on Au NPs@PDA@CuInZnS QDs nanocomposites for ultrasensitive detection of p53 gene. Biosens. Bioelectron. 2018;117:240–245. doi: 10.1016/j.bios.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 201.Lin T.W., Hsieh P.J., Lin C.L., Fang Y.Y., Yang J.X., Tsai C.C., Chiang P.L., Pan C.Y., Chen Y.T. Label-free detection of protein-protein interactions using a calmodulin-modified nanowire transistor. Proc. Natl. Acad. Sci. USA. 2010;107:1047–1052. doi: 10.1073/pnas.0910243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nuzaihan M.N.M., Hashim U., Md Arsad M.K., Kasjoo S.R., Rahaman S.F.A., Rusilnda A.R., Fathil M.F.M., Adzhri R., Shahimin M.M. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens. Bioelectron. 2016;83:106–114. doi: 10.1016/j.bios.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 203.Ahmad R., Ahn M.S., Hahn Y.B. ZnO nanorods array-based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017;498:292–297. doi: 10.1016/j.jcis.2017.03.069. [DOI] [PubMed] [Google Scholar]
- 204.Huang H., Bai W., Dong C., Guo R., Liu Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015;68:442–446. doi: 10.1016/j.bios.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 205.Li L., Lu H., Deng L. A sensitive NADH and ethanol biosensor based on graphene–Au nanorods nanocomposites. Talanta. 2013;113:1–6. doi: 10.1016/j.talanta.2013.03.074. [DOI] [PubMed] [Google Scholar]
- 206.Zhao K., Veksha A., Ge L., Lisak G. Near real-time analysis of para-cresol in wastewater with a laccase-carbon nanotube-based biosensor. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.128699. [DOI] [PubMed] [Google Scholar]
- 207.Cakiroglu B., Ozacar M. A self-powered photoelectrochemical glucose biosensor based on supercapacitor Co3O4 -CNT hybrid on TiO2. Biosens. Bioelectron. 2018;119:34–41. doi: 10.1016/j.bios.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 208.Li W., Gao Y., Zhang J., Wang X., Yin F., Li Z., Zhang M. Universal DNA detection realized by peptide-based carbon nanotube biosensors. Nanoscale Adv. 2020;2:717–723. doi: 10.1039/c9na00625g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huang Q., Lin X., Tong L., Tong Q.X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020;8:1644–1650. [Google Scholar]
- 210.Wu B., Ou Z., Ju X., Hou S. Carbon nanotubes/gold nanoparticles composite film for the construction of a novel amperometric choline biosensor. J. Nanomater. 2011 2011. [Google Scholar]
- 211.Kamil Y.M., Al-Rekabi S.H., Yaacob M.H., Syahir A., Chee H.Y., Mahid M.A., Bakar M.H.A. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Omar N.A.S., Fen Y.W., Abdullah J., Kamil Y.M., Ebtisyam W.M., Daniyal M.M., Sadrohosseini A.R., Mahdi M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020;10:2374. doi: 10.1038/s41598-020-59388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Thakur M., Wang B., Verma M.L. Development and applications of nanobiosensors for sustainable agricultural and food industries: recent developments, challenges, and perspectives. Environ. Technol. Innov. 2022;26 [Google Scholar]
- 214.SiH, Xu G., Jing F., Sun P., Zhao D., Wu D. A multi-volume microfluidic device with no reagent loss for low-cost digital PCR application. Sens. Actuators B Chem. 2020;318 [Google Scholar]
- 215.Banigo A., Azeez T., Ejeta K., Lateef A., Ajuogu E. Nanobiosensors: applications in biomedical technology. IOP Conf. Ser. Mater. Sci. Eng. 2020;805 [Google Scholar]
- 216.Koo S.-B., Jo D.-G., Park C.-Y., Kim Y.-S., Song H.-J., Kim J.-D. Low-cost miniaturization of gel document system using blue LED. Sens. Mater. 2019;31:377–385. [Google Scholar]
- 217.Huang X., Duan Y., Zhao L., Liu S., Qin D., Zhang F., Lin D. Dexamethasone pharmacokinetics characteristics via sub-tenon microfluidic system in uveitis rabbits. J. Drug Deliv. Sci. Technol. 2020;57 [Google Scholar]
- 218.Singh S., Numan A., Cinti S. Electrochemical nano biosensors for the detection of extracellular vesicles exosomes: from the benchtop to everywhere? Biosens. Bioelectron. 2022;216 doi: 10.1016/j.bios.2022.114635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
Declaration of interest's statement: The authors declare no conflict of interest.