Abstract
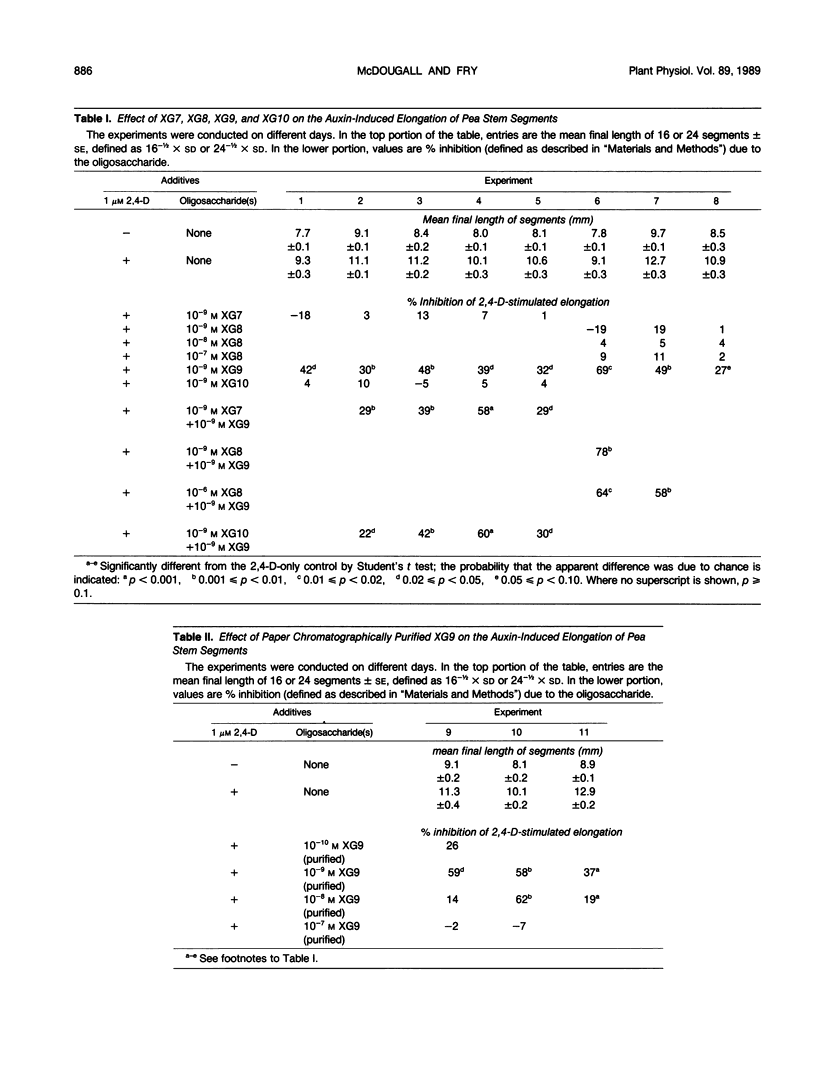
This work was designed to investigate the structural features required for a branched xyloglucan nonasaccharide (XG9; composition: glucose4xylose3galactose1fucose1) to exhibit anti-auxin activity in the pea (Pisum sativum L.) stem segment straight-growth bioassay. Oligosaccharides were prepared by cellulase-catalyzed hydrolysis of Rosa xyloglucan, and tested for auxin antagonism. The quantitatively major hepta-, octa-, and decasaccharides (XG7, XG8, and XG10) showed no antiauxin activity at the concentrations tested and did not interfere with the antiauxin effect of 10−9 molar XG9 when coincubated at equimolar concentrations. The results indicate that the XG9-recognition system in pea stem segments is highly discriminating. A terminal α-l-fucose residue is essential for the antiauxin activity of XG9 and a neighboring terminal β-d-galactose residue can abolish the activity; possible reasons for the effect of the galactose residue are discussed. A sample of XG9 extensively purified by gel-permeation chromatography followed by paper chromatography in two solvent systems still exhibited antiauxin activity with a concentration optimum around 10−9 molar. This diminishes the likelihood that the antiauxin activity reported for previous nonsaccharide preparations was due to a compound other than XG9.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hayashi T., Marsden M. P., Delmer D. P. Pea Xyloglucan and Cellulose: VI. Xyloglucan-Cellulose Interactions in Vitro and in Vivo. Plant Physiol. 1987 Feb;83(2):384–389. doi: 10.1104/pp.83.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Wong Y. S., Maclachlan G. Pea Xyloglucan and Cellulose : II. Hydrolysis by Pea Endo-1,4-beta-Glucanases. Plant Physiol. 1984 Jul;75(3):605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Matsushita J., Kubodera T., Matsuda K. A novel enzyme producing isoprimeverose from oligoxyloglucans of Aspergillus oryzae. J Biochem. 1985 Mar;97(3):801–810. doi: 10.1093/oxfordjournals.jbchem.a135120. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dizet P. Quelques précisions sur la structure de l'amyloïde de capucine. Carbohydr Res. 1972 Oct;24(2):505–509. doi: 10.1016/s0008-6215(00)85085-5. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Soluble Cell Wall Polysaccharides Released from Pea Stems by Centrifugation : I. EFFECT OF AUXIN. Plant Physiol. 1981 Sep;68(3):531–537. doi: 10.1104/pp.68.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent B. S., Darvill A. G., McNeil M., Robertsen B. K., Albersheim P. A general and sensitive chemical method for sequencing the glycosyl residues of complex carbohydrates. Carbohydr Res. 1980 Mar;79(2):165–192. doi: 10.1016/s0008-6215(00)83830-6. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- York W. S., Darvill A. G., Albersheim P. Inhibition of 2,4-dichlorophenoxyacetic Acid-stimulated elongation of pea stem segments by a xyloglucan oligosaccharide. Plant Physiol. 1984 Jun;75(2):295–297. doi: 10.1104/pp.75.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]


