Abstract
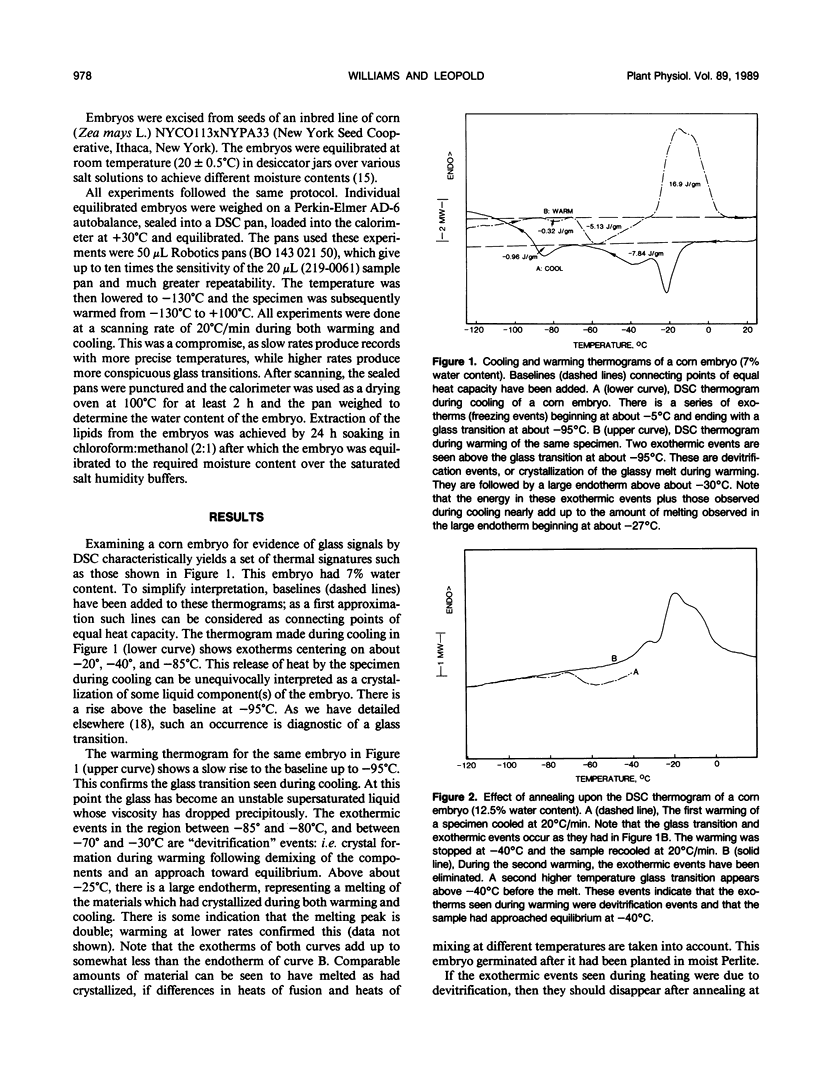
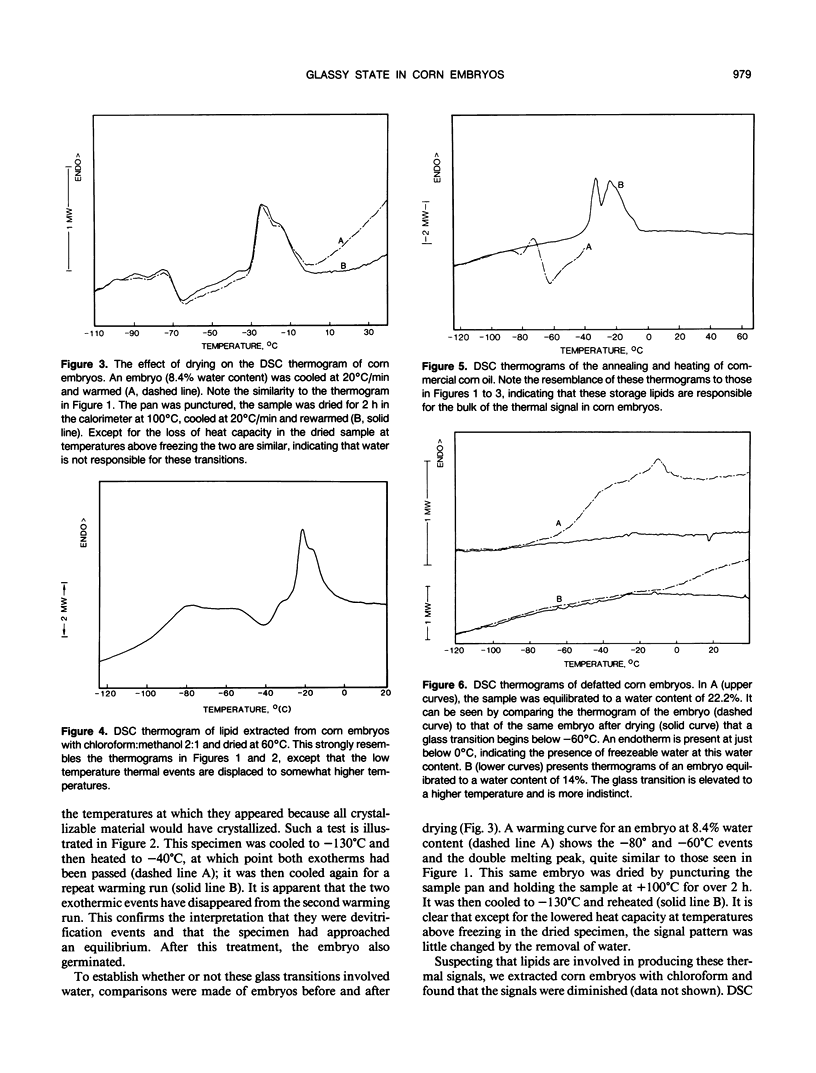
The possibility is examined whether seeds may survive the desiccated state in part by vitrification, or the formation of a glassy state. Embryos excised from viable corn (Zea mays L.) seeds at low moisture contents show a series of low temperature first- and second-order phase transitions in the differential scanning calorimeter. These embryos produce normal seedlings if moistened. The thermal events can be duplicated almost entirely in both extracted lipids and purified commercial corn oil. They are therefore associated primarily with these bulk lipids, since membrane phospholipids are present in too small an amount to produce a detectable signal. When the bulk lipids have been extracted, a glass transition appears in the remaining material. At low water contents, it occurs above +40°C and systematically falls to below −60°C as the water content of the embryo rises to 20%. These data are consistent with our hypothesis that the desiccated state in seeds is a glassy state, and that imbibition of water reduces the glass transition temperature below ambient, allowing biochemical activity to resume.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clegg J. S., Seitz P., Seitz W., Hazlewood C. F. Cellular responses to extreme water loss: the water-replacement hypothesis. Cryobiology. 1982 Jun;19(3):306–316. doi: 10.1016/0011-2240(82)90159-6. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Fahy G. M., MacFarlane D. R., Angell C. A., Meryman H. T. Vitrification as an approach to cryopreservation. Cryobiology. 1984 Aug;21(4):407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Hirsh A. G., Williams R. J., Meryman H. T. A novel method of natural cryoprotection : intracellular glass formation in deeply frozen populus. Plant Physiol. 1985 Sep;79(1):41–56. doi: 10.1104/pp.79.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster K. L., Leopold A. C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988 Nov;88(3):829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A. P. Non-equilibrium freezing behaviour of aqueous systems. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):167–189. doi: 10.1098/rstb.1977.0036. [DOI] [PubMed] [Google Scholar]
- Singh J., Miller R. W. Spin-Probe Studies during Freezing of Cells Isolated from Cold-Hardened and Nonhardened Winter Rye : MOLECULAR MECHANISM OF MEMBRANE FREEZING INJURY. Plant Physiol. 1982 Jun;69(6):1423–1428. doi: 10.1104/pp.69.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., de La Roche I. A., Siminovitch D. Differential scanning calorimeter analyses of membrane lipids isolated from hardened and unhardened black locust bark and from winter rye seedlings. Cryobiology. 1977 Oct;14(5):620–624. doi: 10.1016/0011-2240(77)90173-0. [DOI] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiol. 1984 May;75(1):114–117. doi: 10.1104/pp.75.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci C. W., Leopold A. C. The relationship between water binding and desiccation tolerance in tissues. Plant Physiol. 1987;85:232–238. doi: 10.1104/pp.85.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]


