Abstract
Purpose
Recent guidelines provide broader support for the use of less invasive imaging modalities for the evaluation of patients with stable chest pain. Coronary CT angiography (CCTA) uses increasingly sophisticated techniques to improve evaluation of coronary lesions. The purpose of this study is to describe one center's experience implementing AI-assisted advanced imaging techniques to diagnose coronary artery disease.
Materials & methods
Retrospective study of patients who had AI-assisted CCTA interpretation, including a subgroup who underwent fractional flow reserve CT (FFR-CT) and invasive coronary angiography. Descriptive statistics summarized baseline characteristics and univariate statistics compared findings between groups of patients with and without anatomically and hemodynamically significant lesions based on FFR-CT. For patients who underwent invasive coronary angiography, concordance between CCTA and angiography was evaluated.
Results
Of 532 included patients, AI-assisted CCTA identified statistically significant difference in calcification scores, plaque types and total plaque volume between lesions <50% and ≥50% stenosis. CCTA results were mostly concordant with invasive coronary angiography. Importantly, we identified a subset of patients with less than 50% anatomical stenosis that demonstrated physiologically significant stenosis on FFR-CT and invasive coronary angiography.
Conclusions
AI-assisted CCTA and other advanced techniques are a tool to support high quality diagnostic assessment of coronary lesions in a clinical environment. Combined CCTA with FFRCT in mild to moderate coronary stenosis identifies patients with hemodynamically significant stenosis even when quantitative stenosis is <50%. Implementation of AI-assisted coronary CT angiography is feasible in a community hospital setting, but these technologies do not replace the need for expert review and clinical correlation.
Keywords: Coronary artery disease, Computed tomography, Artificial intelligence, Fractional flow reserve
Highlights
-
•
Advanced CT techniques, including artificial intelligence-assisted coronary CT angiography are improving non-invasive approaches to the identification and quantification of coronary artery plaques.
-
•
Implementation of AI-assisted coronary CT angiography is feasible in a clinical setting, but these technologies do not replace the need for expert review and clinical correlation.
Abbreviations
- AI
Artificial intelligence
- CAD
Coronary artery disease
- CCTA
Coronary Computed Tomography Angiography
- FFR-CT
Fractional flow reserve – computed tomography
- HU
Hounsfield units
- ICA
Invasive coronary angiography
- ML
Machine learning
1. Introduction
Coronary artery disease (CAD) is a significant cause of morbidity and mortality worldwide. Recent American College of Cardiology/American Heart Association guidelines have shifted to provide broader support for the use of less invasive, lower cost evaluations, particularly for patients with stable chest pain [1]. Among these evaluations is coronary CT angiography (CCTA), which provides a direct and more complete assessment of coronary stenosis and identifies obstructive and non-obstructive CAD. CCTA has the added benefit of evaluating both the intraluminal aspect as well as the wall of the vessel.
For more than a decade, studies and consensus statements have supported the wider use of CCTA as an alternative to invasive coronary angiography (ICA) [[1], [2], [3], [4]]. Technological advances such as CT Fractional Flow Reserve (FFR-CT) – in which computational fluid dynamic modeling of the coronary artery flow can estimate the hemodynamic significance of coronary stenosis – have been shown to accurately predict the likelihood of finding significant stenosis on ICA [5,6], and provide sufficient information to change treatment recommendations in as many as two-thirds of patients [7]. As well, dual-energy computed tomography (DECT) has recently shown promise in providing clinicians with additional functionality information for patients with CAD [8,9].
This improvement in CCTA and advancement in clinical applications of plaque characterization/coronary phenotyping, however, creates additional burdens for the diagnostic team. Analysis of CCTA requires accurate segmentation of coronary arteries which is time-consuming, and significant training is required to accurately quantify the severity of coronary stenosis. Machine learning (ML) algorithms and artificial intelligence (AI)-aided interpretation of CCTA improves diagnostic accuracy and efficiency [10]. Of particular note, Choi and colleagues found that AI-aided CCTA compared very favorably with expert readers, and demonstrated excellent accuracy, sensitivity, and specificity, and the algorithm performed better than any individual reader [11]. Recent reviews acknowledge that many AI applications remain limited to research purposes, but there is a clear potential to improve workflows and optimize many aspects of the cardiac radiologist's work [12].
Despite several research studies, there is relatively little real-world experience in the use of combined AI-assisted diagnostics incorporating coronary phenotyping and FFR-CT in evaluating CAD. The objective of this study is to describe one center's real-world experience implementing AI-assisted CCTA interpretation in conjunction with selective FFR-CT to provide advanced CAD evaluation and correlate the findings with clinically indicated ICA.
2. Methods
2.1. Study design
This was a retrospective, descriptive study of consecutive patients undergoing coronary CT between July 26, 2021–December 10, 2021 at a community hospital. Patients who underwent AI-assisted CCTA interpretation were included in the study. Patients who underwent coronary artery bypass grafting (CABG) and those with poor technical image quality were excluded.
Ethical approval for the conduct of this study was sought and received from Western Institutional Review Board (WCG IRB, Puyallup, WA) under protocol number 1-1-1608312-1. The study was determined to be exempt from the need for informed consent due to its nature as a retrospective secondary use of data, and study procedures were run in compliance with relevant regulations for research involving human subjects. The STROBE checklist was used to guide the writing of this manuscript.
2.2. CCTA image acquisition and analysis
Image acquisition was performed on a 256–MDCT scanner (GE Revolution; GE Healthcare Inc., Milwaukee, WI) following our center's typical protocols for CCTA. Typical protocol at our institution includes the use of low-osmolar non-ionic iodinated contrast agent (Iohexol) injected via high flow rate compatible catheter, with a contrast dose of 70–85 ml depending on the patient's body mass index. Bolus tracking of the ascending aorta is performed to automatically trigger the diagnostic scan, with ECG-gated acquisition performed using autogating and a smart arrhythmia management system. Sublingual nitroglycerine, oral and/or intravenous metoprolol or diltiazem are used to achieve appropriate target heart rate and coronary hyperemia. We utilized the CT platform's deep learning image reconstruction (TrueFidelity; GE Healthcare Inc., Milwaukee, WI) to optimize image quality.
CCTA image analysis was performed using AI methodology (Cleerly Inc., New York, NY) that has been previously described [9]. Briefly, two convolutional neural networks were used to produce a centerline along the coronary vessel and then to produce vessel segmentation. The top two optimal series for each vessel/segment are further analyzed for plaque and stenosis quantification. Percent diameter stenosis is calculated based on normal proximal reference vessel cross-sectional slide at the start and end of the lesion. Atherosclerotic plaques were quantified similarly (including the calculation of plaque volume, which were summated to compute the plaque volume at the patient level), and further characterized using Hounsfield Units (HU) as low-attenuation non-calcified plaque (<30 HU), non-calcified plaque (−189 to +350 HU) and calcified plaque (>350 HU). The results were then reviewed by one of three Board-certified cardiologists or cardiac radiologists (HG, EL, GK) with a combined more than 40 years' experience interpreting CCTA. Non-contrast coronary calcium scans were reconstructed at 2.5 mm slice thickness using standard algorithms as per the recommended guidelines. Calcium scores were calculated using Smart Score (version 4.0, GE Healthcare Inc., Milwaukee, WI). Additional coronary artery segmentation was performed in select cases based on the reviewers’ clinical judgement using GE AWS (version 3.2, GE Healthcare Inc., Milwaukee, WI) and Philips PACS DICOM viewer. During the early phase of implementing AI-assisted CCTA interpretation, the reviewers performed additional segmentation on the majority of cases; as comfort with the technology grew, additional segmentation was performed selectively to confirm outputs (e.g. branch points, ostial locations, calcified lesions and uncertain severity of stenoses). The final assessment of coronary stenosis was based on AI-assisted analysis in conjunction with qualitative and quantitative assessment of native CCTA images. Stenoses were graded as being absent (no stenosis), minimal (<24% stenosis), mild (25–49% stenosis), moderate (50–69% stenosis), severe (70–99% stenosis) and occluded (100% stenosis).
2.3. FFR-CT
In a subset of patients, FFR-CT was calculated using HeartFlow analysis (HeartFlow Inc., Redwood City, CA) using techniques that have been previously described [6,13]. The decision to obtain FFR-CT was based on the clinical judgement of the reporting physician, taking into account the lesion location and type, severity, clinical presentation, and selective referring physicians’ input. In our analysis, FFR values were measured distal to the anatomical stenosis and physiological significance was based on FFR-CT values of ≤0.8 with anatomical correlation [13].
Invasive Angiogram: Patients were referred to ICA at the discretion of their treating cardiologist. For the subset of patients who underwent clinically indicated ICA within 30 days of CCTA, we compared the results of these two imaging modalities.
2.4. Statistical analysis
Statistical analysis was conducted using SPSS (version 22. IBM Corp. Armonk, NY). Numeric continuous variables measured in this study were descriptively summarized using means and standard deviations. Categorical variables were descriptively summarized using frequencies and percentages.
Means of the continuous variables were compared using independent samples t-tests in cases of two-group comparisons and one-way analysis of variance (ANOVA) for comparisons among more than two groups. Chi-squared tests were used to analyze associations between categorical variables. P-values ≤0.05 were considered to be statistically significant.
3. Results
A total of 583 patients underwent CCTA during our study period (see Fig. 1). Patients with CABG (n = 8), those not analyzed using AI-assisted CCTA analysis (n = 38), and those with poor technical image quality (n = 5) were excluded. In the final cohort of patients (N = 532), 48% were female, 56% had hypertension, 20% had heart disease and 27% had a history of smoking (Table 1).
Fig. 1.
Flowchart of patients included in retrospective analysis.
Table 1.
Baseline patient characteristics.
| Characteristic | N (%) or Mean ± SD |
|---|---|
| Demographics: | |
| Female | 254 (48%) |
| Age (years) | 62.5 ± 13.3 |
| BMI (kg/m2) | 29.3 ± 6.1 |
| Medical History: | |
| Smoking | 146 (27%) |
| Heart disease | 106 (20%) |
| Hypertension | 298 (56%) |
| Diabetes | 77 (15%) |
| Congestive heart failure | 21 (4%) |
AI-assisted CCTA analysis characterized lesions as having no stenosis (n = 17, 3.2%), minimal stenosis (n = 261, 49.1%), mild stenosis (n = 157, 29.5%), moderate stenosis (n = 67, 12.6%), severe stenosis (n = 16, 3.0%) and occlusion (n = 14, 2.6%). Those patients with a stenosis ≥50% (moderate, severe, or occluded) had significantly higher mean calcification scores (1295.8 vs 213.3, p < 0.001) and a higher proportion of calcified, non-calcified, and low-density non-calcified plaques compared to patients with stenoses <50% (Table 2).
Table 2.
Comparison of coronary lesion characteristics in the overall cohort stratified by lesion stenosis, and in the FFR-CT sub-group stratified by hemodynamic significance.
| Overall CT cohort (N = 532), stratified by stenosis group | |||
|---|---|---|---|
| Less than 50% Stenosis (n = 435) | Greater than or equal to 50% Stenosis (n = 97) | p-value | |
| Agatston calcification score | 213.3 ± 420.9 | 1295.8 ± 1310.9 | <0.001 |
| Percentile rank | 36.5 ± 33.6 | 76.9 ± 17.8 | <0.001 |
| Total atherosclerotic plaque volume (mm3) | 219.6 ± 270.4 | 764.4 ± 468.9 | <0.001 |
| Percent atheroma volume | 6.68 ± 6.6 | 21.23 ± 10.97 | <0.001 |
| Total calcified plaque (mm3) | 88.4 ± 164.6 | 400.2 ± 351.9 | <0.001 |
| Total non-calcified plaque (mm3) | 130.7 ± 146.1 | 366.4 ± 212.1 | <0.001 |
| Low density non-calcified plaque (mm3) | 1.6 ± 2.7 | 5.6 ± 7.8 | <0.001 |
| Non-calcified plaque (mm3) |
129.1 ± 144.9 |
360.8 ± 207.3 |
<0.001 |
| FFR-CT sub-group (n = 121) by hemodynamic significance | |||
| Not hemodynamically significant (n = 66) |
Hemodynamically significant (n = 55) |
p-value |
|
| Agatston calcification score | 540 ± 657 | 1353.3 ± 1324.1 | <0.001 |
| Percentile rank | 64.8 ± 25.4 | 79.6 ± 13.4 | <0.001 |
| Total atherosclerotic plaque volume (mm3) | 417.1 ± 396.2 | 770.8 ± 468.6 | <0.001 |
| Percent atheroma volume | 11.99 ± 7.25 | 21.3 ± 10 | <0.001 |
| Total calcified plaque (mm3) | 193 ± 209.9 | 425.6 ± 356.6 | <0.001 |
| Total non-calcified plaque (mm3) | 219.1 ± 234.9 | 349.3 ± 198.8 | 0.001 |
| Low density non-calcified plaque (mm3) | 2.4 ± 3.9 | 4.5 ± 5.7 | 0.02 |
| Non-calcified Plaque (mm3) | 216.7 ± 233.5 | 344.7 ± 194.8 | 0.002 |
A subgroup of 121 patients underwent FFR-CT, of whom just under half (n = 55, 45.5%) had hemodynamically significant lesions. Patients with hemodynamically significant stenoses were significantly more likely to have a higher calcification score (1353.3 HU vs 540.0 HU, p < 0.001) and higher total plaque volume (Table 2).
Patients were divided into groups based on whether they had <50% and ≥50% stenosis by CCTA, and lesions that were hemodynamically significant vs non-significant by FFR-CT. For patients with ≥50% stenosis (n = 58), calcification scores were significantly higher (337.2 HU vs 1642.2 HU, p < 0.001), as were plaque volumes (401.1 mm3 vs 910.0 mm3, p < 0.001) in patients with hemodynamically significant lesions (n = 39) compared to patients with hemodynamically non-significant lesions (n = 19). In contrast, for patients with <50% stenosis by CCTA (n = 63), patients with hemodynamically significant lesions (n = 16) did not demonstrate significantly different atherosclerotic characteristics (Fig. 2).
Fig. 2.
Relationship of CCTA quantified lesion severity and hemodynamic significance based on FFR-CT.
Among patients referred for ICA, we compared the concordance of ICA results against AI-assisted CCTA (n = 45) and FFR-CT where available (n = 25). Overall CCTA impressions varied from concordant (n = 18, 40%) to having one (n = 24, 53.3%) to two category discordances (n = 3, 6.7%) (Fig. 3a and b). Among patients in our subgroup who underwent both FFR-CT and ICA (n = 25), 12% (3/25) were found to have physiologically significant stenosis on FFR-CT and ICA that was <50% on CCTA. For patients that demonstrated moderate or greater stenosis on CCTA and underwent FFR-CT and ICA (n = 19), there was complete concordance with ICA and FFR-CT (Fig. 3b).
Fig. 3.
Concordance between CCTA impression and either ICA impression in the sub-group of patients who underwent ICA (A., n = 45 patients); or in the sub-group of patients who underwent ICA and FFR-CT (B., n = 25 patients). Concordance is shown in green, while one and two category discordance are shown in yellow and orange respectively. † FFR-CT suggested hemodynamically significant stenosis ^ FFR-CT suggested that coronary stenosis was not hemodynamically significant.
4. Discussion
We report on a retrospective study of our single center's experience implementing advanced CCTA techniques, including the use of FFR-CT and correlation with ICA in a subset of patients. We identified significant differences in calcification scores, plaque types and total plaque volume between lesions <50% and ≥50% by AI-assisted CCTA, as well as lesions that were and were not hemodynamically significant by FFR-CT. For patients that demonstrated moderate or greater stenosis on CCTA and underwent ICA, we noted that all of these patients demonstrated hemodynamically significant stenosis on FFRCT. An important observation in our study is that we identified a subset of patients with less than 50% anatomical stenosis that demonstrated physiologically significant stenosis on FFR-CT and ICA (n = 3 of 25, 12%).
Our results are consistent with a new and growing area of the literature investigating the use of advanced CCTA techniques in both research and clinical settings. Results overall show promise for the new technologies, while also raising awareness about their limitations. The recent ISCHEMIA trial, for example, demonstrated high concordance for excluding significant (≥50%) left main coronary artery stenosis and identifying patients with at least 1 vessel CAD in a cohort with very high a primary likelihood of obstructive CAD [14]. However, concordance based on the number of diseased vessels was modest at 54.5%. CCTA overestimated disease burden in 25% of the patients and underestimated disease burden in 20%. Moreover, in approximately 3% of patients in the ISCHEMIA cohort CCTA suggested a stenosis of <50% in the left main coronary artery when follow-up ICA identified a >50% stenosis.
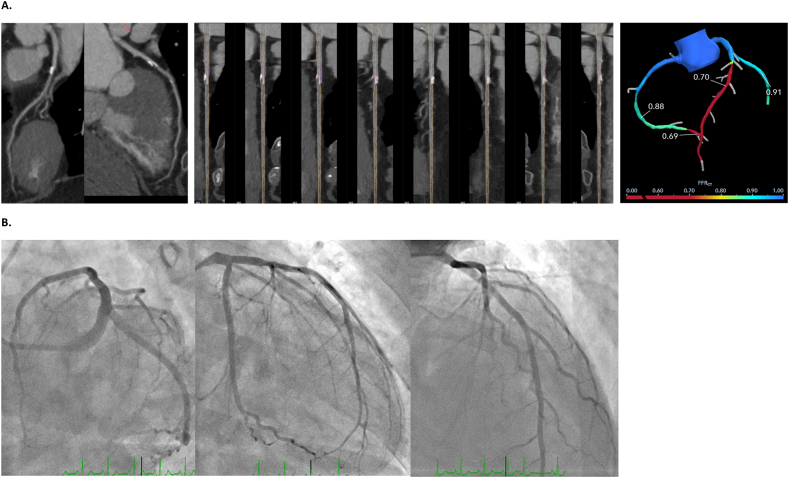
In our experience, awareness of the limitations of AI-assisted CCTA, even with FFR-CT, is important; Two cases from our cohort help to illustrate how we have carefully integrated AI-assisted CCTA and FFRCT into our clinical practice. The first patient was a 71-year-old male with past medical history significant for COVID-19, symptomatic high degree AV block requiring dual chamber permanent pacemaker, hypertension and hyperlipidemia demonstrated abnormal exercise stress test. Although the initial CCTA quantitative analysis identified a 32% stenoses in the left main (LM) and 34% stenosis in the proximal left anterior descending (LAD), qualitatively it appeared severe which was validated by FFR-CT that revealed hemodynamically significant lesions in both the LM and LAD (0.60 to <0.50) (Fig. 4A). This was confirmed as a 90% lesion in the LM and 95% lesion in the ramus intermedius, and a 70% lesion in the LAD (Fig. 4B). For this patient, CCTA alone was inadequate, but the use of FFR-CT correctly identified the severity of the lesions. The second patient was a 61-year-old female with past medical history of hypertension, hyperlipidemia, COVID-19 and abnormal exercise stress test. The initial CCTA suggested a 30% stenosis in the proximal LAD, but FFR-CT suggested a hemodynamically significant lesions of 0.70 (Fig. 5A). Interestingly, the patient underwent ICA, which identified no significant lesions (Fig. 5B). Despite much optimism, including our own, this technology should be used judiciously with in depth review of all pertinent images and output by cardiac imagers. Creating a quality assessment program that includes periodic correlations with invasive procedures and clinical outcomes is critical to monitor the use of these technologies and flag any deviation from the norm.
Fig. 4.
CCTA images from a 71-year-old male patient referred after abnormal exercise stress test. Left main stenosis was quantitatively evaluated less than 50%, but qualitatively appeared more severe, which was validated by FFR-CT (A). A subsequent invasive coronary angiogram demonstrated a severe 90% stenosis in the left main (B). A.
Fig. 5.
CCTA images from a 61-year-old female patient referred after abnormal exercise stress test. Left anterior descending lesion stenosis was quantitatively evaluated at 32%, but FFR CT suggested hemodynamically significant stenosis (A). A subsequent invasive coronary angiogram demonstrated no significant stenosis (B).
In the ongoing exploration of the value of these new AI-assisted technologies, researchers and clinicians are identifying a variety of potential improvements and areas for additional study [15,16]. As previously mentioned, one study reported that AI interpretation of CCTA compares quite favorably against expert readings, indicating a strong supportive role for the technology [6]. Van Rosendael and colleagues describe a machine-learning-based algorithm that has improved risk stratification over current CCTA integrated risk scores [17]. In another study, Doris and colleagues broadened the use of FFR-CT beyond predicting lesion-specific ischemia and reported on its potential to quantify more diffuse CAD and plaque burden, independent of stenosis severity [18]. Further research into the use of CCTA to identify patients are at increased risk of acute coronary syndromes is ongoing [19], and the integration of these insights into AI algorithm is sure to follow.
Much the way that recent work on the use of FFR in ICA has focused on identifying thresholds of clinical significance [20,21], but there is a need for additional data to support interpretation and clinical decision-making in a real-world environment. In our series, we observed the greatest discordance between CCTA and ICA in mild and moderate stenoses, with FFR-CT correctly classifying the hemodynamic significance of most but not all lesions. As others have noted, future studies should focus on generating data to support the development of evidence-based, guideline-driven treatment algorithms to support clinical decision-making, improve outcomes, and reduce costs [22]. Results from the forthcoming PRECISE trial would provide that additional information to support clinical decision-making based on CCTA and FFR-CT [23].
At our center, these advanced CCTA modalities – including deep-learning image reconstruction, AI-assisted CCTA interpretation, and computational fluid dynamics to evaluate the hemodynamic significance of coronary lesions – are used as an increasingly-valued tool to support the work of our general and interventional cardiologists, cardiac surgeons, and cardiac radiologists. Initially there was a feeling that these technologies might reduce the role of the diagnostic and interventional cardiologist and radiologist. With time, we became aware of the limitations of the AI-assisted CCTA interpretation, and there was perhaps some pessimism about the technology not living up to expectations. We have reached a stage now that we see AI-assisted CCTA interpretation for what it is – a tool to support, rather than replace, the clinical judgement of our diagnostic and interventional cardiologists and radiologists in assessing patients with CAD. We have found that AI-assisted CCTA has improved the quality of our images and supported faster processing and interpretation of results. It provides our patients with a less costly, non-invasive option to evaluate coronary lesions. Anecdotally, our interventional cardiologists report that the highly detailed results of AI-assisted CCTA have helped them to better tailor their invasive procedure to a particular patient including optimizing invasive angiographic views and use of ancillary technologies such as intravascular ultrasound and invasive FFR reducing the procedure time, radiation exposure, and contrast agent volume used for each case. Our cardiac surgeons report that the AI-assisted CCTA results allow for better preoperative design of the conduit and construct selection in cases of surgical coronary revascularization. Our use of these technologies is ongoing and will be guided by additional evidence and guidelines that we anticipate are forthcoming.
Our study has some important limitations. This is a retrospective analysis of a single center's experience implementing AI-assisted CCTA into our clinical processes. There was no control group, which precludes wider comparisons, and it was smaller subgroups of our cohort that underwent FFR-CT and ICA; both factors may limit the generalizability of our findings. At our center, the decision to obtain FFR-CT is made at the discretion of the reporting physician, and the findings in this sub-group should be interpreted with caution. As with any retrospective clinical study, there is likely to be referral bias among the patients referred for AI-assisted CCTA and FFR-CT. We also acknowledge that our patient population has a relatively low rate of diabetes (15%) compared to a general population undergoing a diagnostic workup for CAD.
In conclusion, we report on our experience using AI-assisted CCTA interpretation and FFR-CT at our single center in a cohort of 532 patients. These advanced technologies are being used to support the work of cardiologists, cardiac surgeons, and cardiac radiologists by improving image quality, assisting in CCTA interpretation, and facilitating non-invasive assessments of hemodynamics using FFR-CT. These technologies represent a promising tool in providing appropriate and less-invasive assessments of coronary lesions to support individualized patient care.
Author contribution statement
Himanshu Gupta: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Basil Spanopoulous; John Rutledge: Analyzed and interpreted the data.
Edward Lubat: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Glenn Krinsky: Contributed reagents, materials, analysis tools or data.
Jacqueline H. Fortier; Juan B. Grau: Analyzed and interpreted the data; Wrote the paper.
Rajiv Tayal: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
The authors do not have permission to share data.
Additional information
No additional information is available for this paper.
Clinical perspectives
Technological advancements are providing additional supports to cardiologists and radiologists in the use of CT angiography to diagnose and manage coronary artery disease. Additional research is needed to develop validated, evidence-informed algorithms to support clinical decision-making based on these technologies.
Financial support
None.
Ethics statement
Ethical approval for the conduct of this study was sought and received from Western Institutional Review Board (WCG IRB, Puyallup, WA) under protocol number 1-1-1608312-1. The study was determined to be exempt from the need for informed consent due to its nature as a retrospective secondary use of data, and study procedures were run in compliance with relevant regulations for research involving human subjects.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., Blankstein R., Boyd J., Bullock-Palmer R.P., Conejo T., Diercks D.B., Gentile F., Greenwood J.P., Hess E.P., Hollenberg S.M., Jaber W.A., Jneid H., Joglar J.A., Morrow D.A., O'Connor R.E., Ross M.A., Shaw L.J. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. 2021 Nov 30. [DOI] [PubMed] [Google Scholar]
- 2.Paech D.C., Weston A.R. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc. Disord. 2011 Jun 16;11:32. doi: 10.1186/1471-2261-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdulla J., Abildstrom S.Z., Gotzsche O., Christensen E., Kober L., Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur. Heart J. 2007 Dec;28(24):3042–3050. doi: 10.1093/eurheartj/ehm466. [DOI] [PubMed] [Google Scholar]
- 4.Narula J., Chandrashekhar Y., Ahmadi A., Abbara S., Berman D.S., Blankstein R., Leipsic J., Newby D., Nicol E.D., Nieman K., Shaw L., Villines T.C., Williams M., Hecht H.S. SCCT 2021 expert consensus document on coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2021 May-Jun;15(3):192–217. doi: 10.1016/j.jcct.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M.T., Ferencik M., Roberts R.S., Lee K.L., Ivanov A., Adami E., Mark D.B., Jaffer F.A., Leipsic J.A., Douglas P.S., Hoffmann U. Noninvasive FFR derived from coronary CT angiography: management and outcomes in the PROMISE trial. JACC Cardiovasc Imaging. 2017 Nov;10(11):1350–1358. doi: 10.1016/j.jcmg.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor C.A., Fonte T.A., Min J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J. Am. Coll. Cardiol. 2013 Jun 4;61(22):2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 7.Fairbairn T.A., Nieman K., Akasaka T., Nørgaard B.L., Berman D.S., Raff G., Hurwitz-Koweek L.M., Pontone G., Kawasaki T., Sand N.P., Jensen J.M., Amano T., Poon M., Øvrehus K., Sonck J., Rabbat M., Mullen S., De Bruyne B., Rogers C., Matsuo H., Bax J.J., Leipsic J., Patel M.R. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE Registry. Eur. Heart J. 2018 Nov 1;39(41):3701–3711. doi: 10.1093/eurheartj/ehy530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo T., Martin S., Micari A., Booz C., Steyer A., Blandino A., Lanzafame L.R., Koch V., Ascenti G., Mazziotti S. Coronary angiography using spectral detector dual-energy CT: is it the time to assess myocardial first-pass perfusion? Eur Radiol Exp. 2022 Dec 8;6(1):60. doi: 10.1186/s41747-022-00313-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Angelo T., Bucher A.M., Lenga L., Arendt C.T., Peterke J.L., Caruso D., Mazziotti S., Blandino A., Ascenti G., Othman A.E., Martin S.S., Leithner D., Vogl T.J., Wichmann J.L. Optimisation of window settings for traditional and noise-optimised virtual monoenergetic imaging in dual-energy computed tomography pulmonary angiography. Eur. Radiol. 2018 Apr;28(4):1393–1401. doi: 10.1007/s00330-017-5059-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu C.Y., Tang C.X., Zhang X.L., et al. Deep learning powered coronary CT angiography for detecting obstructive coronary artery disease: the effect of reader experience, calcification and image quality. Eur. J. Radiol. 2021;142 doi: 10.1016/j.ejrad.2021.109835. [DOI] [PubMed] [Google Scholar]
- 11.Choi A.D., Marques H., Kumar V., Griffin W.F., Rahban H., Karlsberg R.P., Zeman R.K., Katz R.J., Earls J.P. CT evaluation by artificial intelligence for atherosclerosis, stenosis and vascular morphology (clarify): a multi-center, international study. J Cardiovasc Comput Tomogr. 2021 Nov-Dec;15(6):470–476. doi: 10.1016/j.jcct.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Lanzafame L.R.M., Bucolo G.M., Muscogiuri G., Sironi S., Gaeta M., Ascenti G., Booz C., Vogl T.J., Blandino A., Mazziotti S., D'Angelo T. Artificial intelligence in cardiovascular CT and MR imaging. Life. 2023 Feb 11;13(2):507. doi: 10.3390/life13020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabbat M.G., Berman D.S., Kern M., Raff G., Chinnaiyan K., Koweek L., Shaw L.J., Blanke P., Scherer M., Jensen J.M., Lesser J., Nørgaard B.L., Pontone G., De Bruyne B., Bax J.J., Leipsic J. Interpreting results of coronary computed tomography angiography-derived fractional flow reserve in clinical practice. J Cardiovasc Comput Tomogr. 2017 Sep-Oct;11(5):383–388. doi: 10.1016/j.jcct.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Mancini G.B.J., Leipsic J., Budoff M.J., Hague C.J., Min J.K., Stevens S.R., Reynolds H.R., O'Brien S.M., Shaw L.J., Manjunath C.N., Mavromatis K., Demkow M., Lopez-Sendon J.L., Chernavskiy A.M., Gosselin G., Schuchlenz H., Devlin G.P., Chauhan A., Bangalore S., Hochman J.S., Maron D.J. CT angiography followed by invasive angiography in patients with moderate or severe ischemia-insights from the ISCHEMIA trial. JACC Cardiovasc Imaging. 2021 Jul;14(7):1384–1393. doi: 10.1016/j.jcmg.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covas P., De Guzman E., Barrows I., et al. Artificial intelligence advancements in the cardiovascular imaging of coronary atherosclerosis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.839400. Published 2022 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H., Yao Y., Wang L., et al. Research progress of machine learning and deep learning in intelligent diagnosis of the coronary atherosclerotic heart disease. Comput. Math. Methods Med. 2022;2022 doi: 10.1155/2022/3016532. Published 2022 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rosendael A.R., Maliakal G., Kolli K.K., Beecy A., Al'Aref S.J., Dwivedi A., Singh G., Panday M., Kumar A., Ma X., Achenbach S., Al-Mallah M.H., Andreini D., Bax J.J., Berman D.S., Budoff M.J., Cademartiri F., Callister T.Q., Chang H.J., Chinnaiyan K., Chow B.J.W., Cury R.C., DeLago A., Feuchtner G., Hadamitzky M., Hausleiter J., Kaufmann P.A., Kim Y.J., Leipsic J.A., Maffei E., Marques H., Pontone G., Raff G.L., Rubinshtein R., Shaw L.J., Villines T.C., Gransar H., Lu Y., Jones E.C., Peña J.M., Lin F.Y., Min J.K. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr. 2018 May-Jun;12(3):204–209. doi: 10.1016/j.jcct.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Doris M.K., Otaki Y., Arnson Y., Tamarappoo B., Goeller M., Gransar H., Wang F., Hayes S., Friedman J., Thomson L., Slomka P., Dey D., Berman D. Non-invasive fractional flow reserve in vessels without severe obstructive stenosis is associated with coronary plaque burden. J Cardiovasc Comput Tomogr. 2018;12(5):379–384. doi: 10.1016/j.jcct.2018.05.003. Sep-Oct. [DOI] [PubMed] [Google Scholar]
- 19.Puchner S.B., Liu T., Mayrhofer T., Truong Q.A., Lee H., Fleg J.L., Nagurney J.T., Udelson J.E., Hoffmann U., Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014 Aug 19;64(7):684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonino P.A., De Bruyne B., Pijls N.H., Siebert U., Ikeno F., van' t Veer M., Klauss V., Manoharan G., Engstrøm T., Oldroyd K.G., Ver Lee P.N., MacCarthy P.A., Fearon W.F., FAME Study Investigators Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009 Jan 15;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 21.Glineur D., Grau J.B., Etienne P.Y., Benedetto U., Fortier J.H., Papadatos S., Laruelle C., Pieters D., El Khoury E., Blouard P., Timmermans P., Ruel M., Chong A.Y., So D., Chan V., Rubens F., Gaudino M.F. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur. Heart J. 2019 Aug 1;40(29):2421–2428. doi: 10.1093/eurheartj/ehz329. [DOI] [PubMed] [Google Scholar]
- 22.Leipsic J.A., Hurwitz Koweek L. CT fractional flow reserve for stable coronary artery disease: the ongoing journey. Radiology. 2018;287(1):85–86. doi: 10.1148/radiol.2018172838. [DOI] [PubMed] [Google Scholar]
- 23.Douglas P.S., Nanna M., Kelsey M., Yow E.S., Mark D.B., et al. 2022 American Heart Association Scientific Sessions: 06 Nov. 2022. Comparison of a precision care strategy with usual testing to guide management of stable patients with suspected coronary artery disease: the precise randomized trial [abstract] Chicago, IL. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.