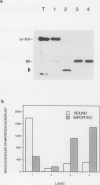
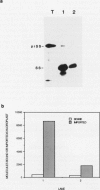
Abstract
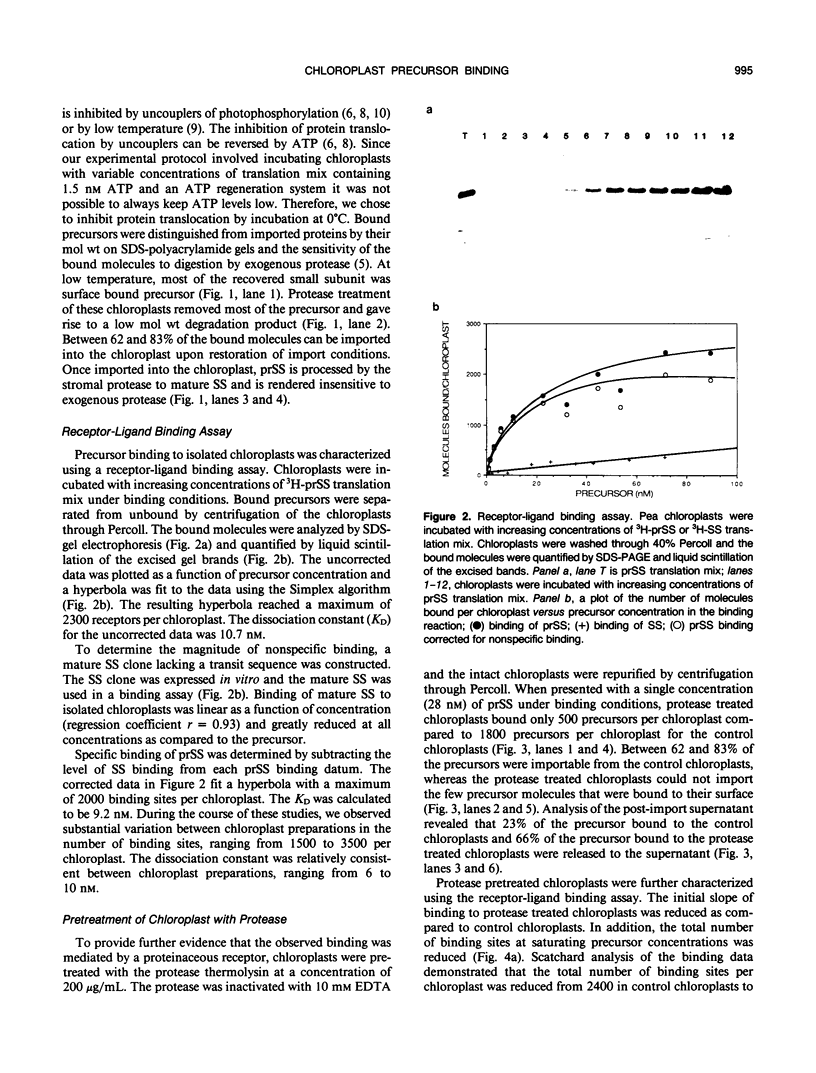
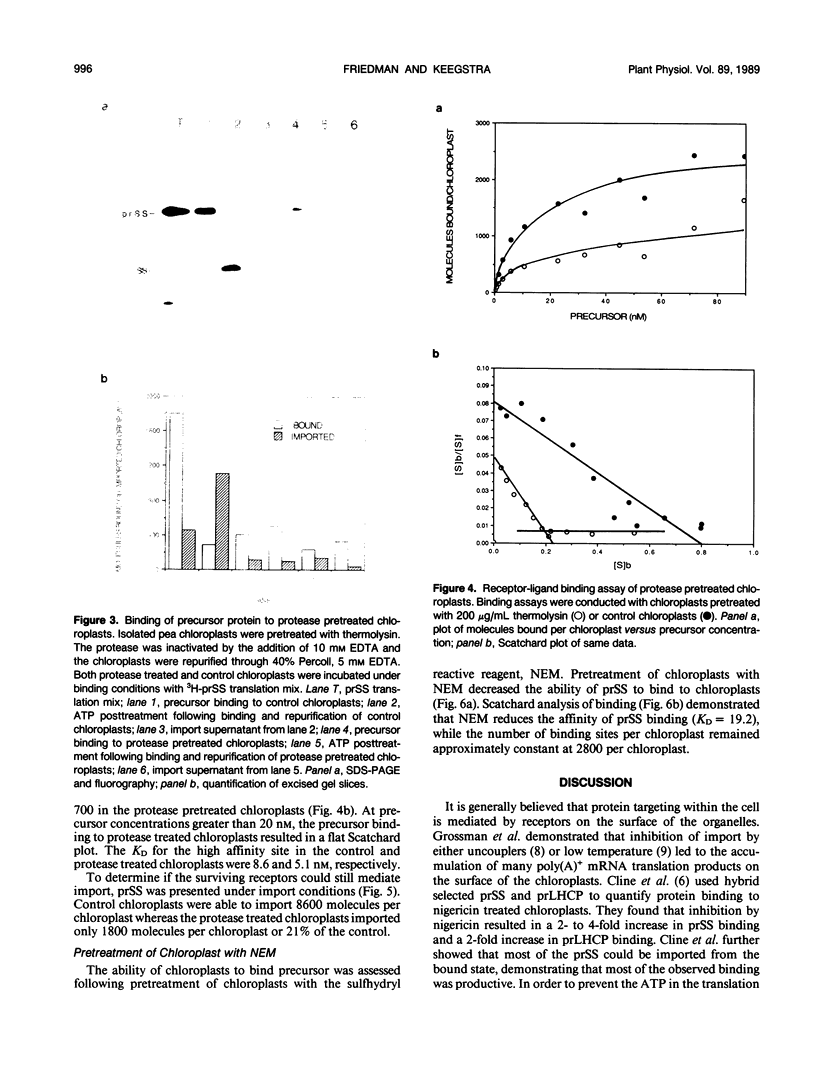
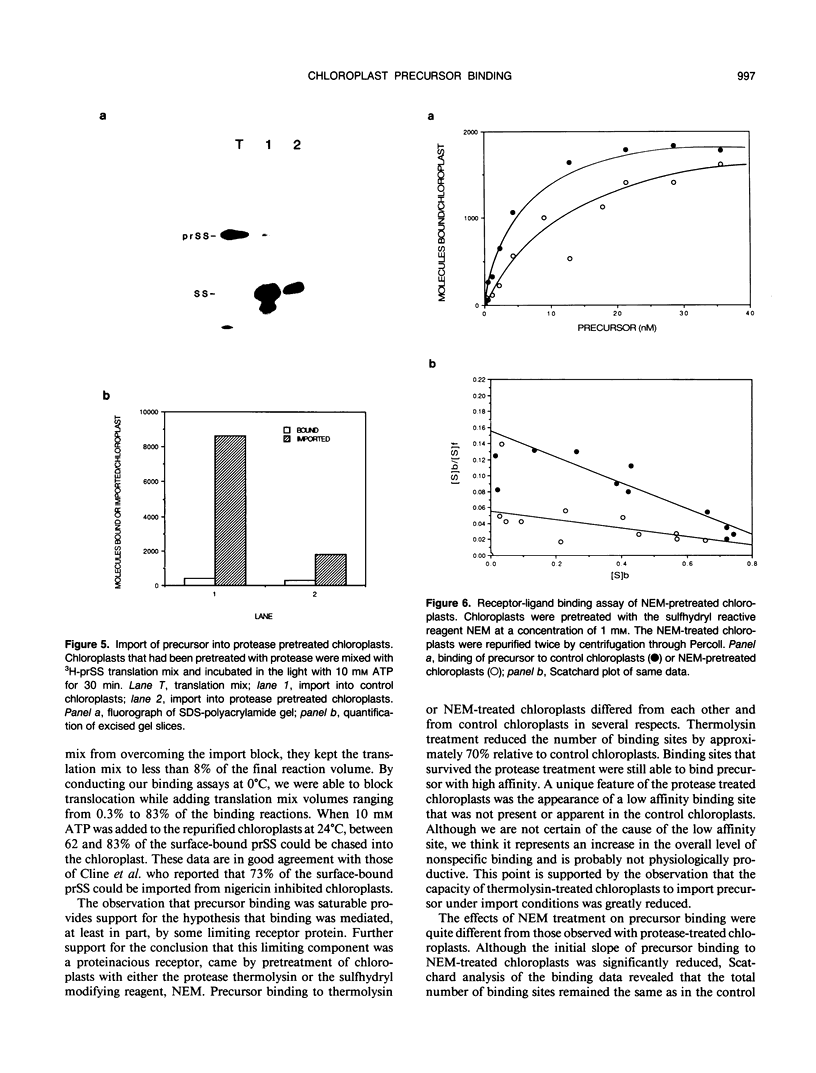
The first step of chloroplast protein import is binding of a precursor protein to the surface of the organelle. Precursor binding for the small subunit of ribulose-1,5-bisphosphate carboxylase to isolated pea chloroplasts was investigated using a receptor-ligand binding assay. Translocation of precursors was blocked by conducting the binding assays at 0°C. Binding of precursor was judged to be receptor mediated by the following criteria: (a) precursor binding was saturable at between 1500 and 3500 molecules per chloroplast; (b) binding is a high affinity interaction with a dissociation constant of 6 to 10 nanomoles; (c) binding is physiologically productive since most of the bound precursors could be imported from the bound state; and (d) precursor binding was sensitive to both protease and the sulfhydryl modifying reagent N-ethylmaleimide. The effects of these two reagents differed in that protease reduced the total number of binding sites from the surface of chloroplasts but had little effect on binding affinity, whereas N-ethylmaleimide reduced the binding affinity but had little or no effect on receptor density.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Cornwell K. L., Keegstra K. Evidence that a Chloroplast Surface Protein Is Associated with a Specific Binding Site for the Precursor to the Small Subunit of Ribulose-1,5-Bisphosphate Carboxylase. Plant Physiol. 1987 Nov;85(3):780–785. doi: 10.1104/pp.85.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Chua N. H. Post-translational uptake of cytoplasmically synthesized proteins by intact chloroplasts in vitro. Ann N Y Acad Sci. 1980;343:266–274. doi: 10.1111/j.1749-6632.1980.tb47257.x. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubben T. H., Keegstra K. Efficient in vitro import of a cytosolic heat shock protein into pea chloroplasts. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5502–5506. doi: 10.1073/pnas.83.15.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Protein import into yeast mitochondria is inhibited by antibodies raised against 45-kd proteins of the outer membrane. EMBO J. 1987 Jul;6(7):2109–2115. doi: 10.1002/j.1460-2075.1987.tb02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain D., Kanwar Y. S., Blobel G. Identification of a receptor for protein import into chloroplasts and its localization to envelope contact zones. Nature. 1988 Jan 21;331(6153):232–237. doi: 10.1038/331232a0. [DOI] [PubMed] [Google Scholar]
- Pfisterer J., Lachmann P., Kloppstech K. Transport of proteins into chloroplasts. Binding of nuclear-coded chloroplast proteins to the chloroplast envelope. Eur J Biochem. 1982 Aug;126(1):143–148. doi: 10.1111/j.1432-1033.1982.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Riezman H., Hay R., Witte C., Nelson N., Schatz G. Yeast mitochondrial outer membrane specifically binds cytoplasmically-synthesized precursors of mitochondrial proteins. EMBO J. 1983;2(7):1113–1118. doi: 10.1002/j.1460-2075.1983.tb01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Devillers-Thiery A., Desruisseaux H., Blobel G., Chua N. H. NH2-terminal amino acid sequences of precursor and mature forms of the ribulose-1,5-bisphosphate carboxylase small subunit from Chlamydomonas reinhardtii. J Cell Biol. 1979 Dec;83(3):615–622. doi: 10.1083/jcb.83.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Seftor E. A., Schell J., Bohnert H. J. The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J. 1985 Jan;4(1):25–32. doi: 10.1002/j.1460-2075.1985.tb02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Proteinaceous receptors for the import of mitochondrial precursor proteins. J Biol Chem. 1984 Jun 25;259(12):7850–7856. [PubMed] [Google Scholar]
- Zwizinski C., Schleyer M., Neupert W. Transfer of proteins into mitochondria. Precursor to the ADP/ATP carrier binds to receptor sites on isolated mitochondria. J Biol Chem. 1983 Apr 10;258(7):4071–4074. [PubMed] [Google Scholar]