Abstract
Objective
Elucidating the concurrence and interdependence of three precipitating factors as contributors of a subset of secondary burning mouth syndrome (BMS), which is defined having detectable precipitating factors.
Design
47 secondary BMS and 15 non-BMS cases were sourced from medical records of an Oral Pathology Specialty Clinic in Canada (2017–2021). Each case had Cytology, Hematology, and Sialometry tests to detail the state of three precipitating factors (the presence of fungal hyphae, hypovitaminosis D, and objective oral dryness). Three factors were compared between secondary BMS and non-BMS groups independently, in pairs, and as a triple-factor by Fisher's exact tests, Contingency Coefficients, and Logistic Regressions.
Results
Rates of objective oral dryness (89.36%) and hypovitaminosis D (74.47%) in the secondary BMS group significantly differ from the non-BMS group (p = 0.0013, p = 0.0016). No difference was found in the incidence of fungal hyphae between BMS (91.49%) and non-BMS groups (p = 0.0881). Rates of three precipitating factors in pairs and as a triple-factor within the secondary BMS group significantly differ from the non-BMS group (p-values from 0.0011 to <0.0001). Their significant correlations with secondary BMS are found independently (excluding fungal hyphae), in pairs, and as a triple-factor (C-values from 0.371 to 0.461, p-values from 0.002 to <0.001). The highest C-value belongs to the triple-factor. Objective oral dryness (p = 0.009) and hypovitaminosis D (p = 0.008) are confirmed as significant predictors for secondary BMS.
Conclusions
The presence of fungal hyphae contribute to a subset of secondary BMS only when coinciding with objective oral dryness, hypovitaminosis D, or both. This interdependent relationship leads to a hypothesis that hypovitaminosis D, which is commonly called “a low value of vitamin D”, and objective oral dryness make an oral environment conducive to insidious Candida invasion, which is an intermediate status of the host-fungal interaction staying between healthy oral mucosa (non-infection) and oral candidiasis (infection).
Keywords: Secondary burnin g mouth syndrome, The presence of fungal hyphae, Hypovitaminosis D, A low value of vitamin D, Objective oral dryness, Insidious candida invasion, Host-fungal interaction
Highlights
-
•
What is already known? Burning mouth syndrome (BMS) secondary to detectable multiple precipitating factors is defined as secondary BMS.
-
•
What does this study add? A subset of secondary BMS is associated with the concurrence of three precipitating factors including the presence of fungal hyphae, objective oral dryness, and hypovitaminosis.
1. Introduction
Burning mouth syndrome (BMS) is a unique oral condition that has inconsistent diagnostic criteria and definitions [1] although the prevalence had reached to 3.7% [2]. In 2016 the International Association for the Study of Pain (IASP) defined BMS as a chronic intraoral burning sensation without identifiable cause associated with either local or systemic disease [3]. However not having a disease does not mean the patient does not have precipitating factors that can contribute to the development of BMS. Scholars introduced idiopathic BMS (primary BMS) and BMS with precipitating factors (secondary BMS) to the field in 2003 [4]. BMS-related precipitating factors were grouped as psychological, oral, and systemic factors, which can be identified by psychological profiles, and microbiological and hematology tests [4]. Three major precipitating factors for BMS patients were found to be: subjective oral dryness (66%) [2], low Vitamin D levels (14.9%) [5], and the growth of Candida species (21.4%) [6]. Table 1 posts the pertinent details of the three studies.
Table 1.
General comparison of previous studies in three precipitating factors of burning mouth syndrome (BMS). The incidences came from different data sources with dissimilar inclusion criteria.
| Authors | The title of the original research article | Case number, Female: Male |
Incidence of three factors | Inclusion criteria of BMS cases | Investigating method |
|---|---|---|---|---|---|
| Bergdahl et al., 1999, Sweden [2] |
BMS: prevalence and associated factors | 53, 1: 0.26 |
Subjective oral dryness: 66% | Study cases with Diabetes Mellitus, Hypertension, anxiety, perceived stress, and depression. | Sialometry |
| Verenzuela et al., 2017, USA [5] | BMS: results of screening tests for vitamin and mineral deficiencies, thyroid hormone, and glucose levels - experience at Mayo Clinic over a decade | 87, 1: 0.24 (generated from the data: 80.7% female) |
A low value of Vitamin D: 14.9% | Study cases with Diabetes Mellitus and Hypothyroidism. | Hematology tests |
| Jankovskis et al., 2022, Latvia [6] | Candidiasis and other bacterial infections among patients diagnosed with BMS | 173, 1: 0.15 |
The growth of Candida: 21.4% | Study cases without any type of local and/or systemic factors. | Culture test |
Note BMS: burning mouth syndrome.
Theoretically, these three precipitating factors are interdependent, leading to the development of secondary BMS. Candida Albicans is a dimorphic yeast that transitions from a commensal state (harmless status) to pathogenicity (harmful status) in response to various environmental stimuli [7]. Common environmental predisposing factors in the oral cavity include reduced saliva flow (objective oral dryness) and impaired immune response [8]. Vitamin D is a well-known regulator of innate immunity that up-regulates Cathelicidin antimicrobial peptide within macrophages and keratinocytes as first-barrier defenses for fighting pathogens [9]. Hypovitaminosis D may cause an impaired immune response in the oral cavity. As such, the presence of objective oral dryness and hypovitaminosis D, which is commonly called “a low value of Vitamin D”, would contribute to promoting an environment for the transition from commensal (budding form) towards pathogenicity (hyphae form). In our view, the host-fungal interaction may end up with one of three outcomes: healthy oral mucosa (non-infection), oral candidiasis (infection), or an intermediate status that is present of fungal transition but absent of fungal infection. The intermediate status does not qualify to be identified as oral candidiasis due to the lack of visible oral lesions, while not qualifying for healthy oral mucosa due to the presence of consistent burning mouth sensation. This status could be a subset of secondary BMS.
The purpose of this study is to investigate the existence of this intermediate status by observing 47 secondary BMS and 15 non-BMS participants in the concurrence of three precipitating factors: the presence of fungal hyphae, objective oral dryness, and a low value of Vitamin D. Also, we analyze the correlation and regression between study groups and three precipitating factors independently, in pairs, and as a triple factor.
2. Materials & methods
The real challenge of this study is formulating the inclusion and exclusion criteria since there are no consistent diagnostic criteria for BMS and no widely accepted consensus on the classification of primary and secondary BMS. We applied the most rigorous criteria that integrated almost all recommendations in six articles [1,4,[10], [11], [12], [13]]. Another tough section of this study is the method of excluding uncontrolled systemic diseases and correlative conditions reportedly associated with BMS. The list of those diseases and conditions was generated from the abovementioned references as well. Those were confirmed not only by medical histories but also by laboratory examination results.
2.1. Data source and BMS diagnostic criteria
The Dalhousie University Research Ethics Board approved the case-control study (No. 2021–5902). We searched the consecutive database of an Oral Pathology Specialty Clinic (Nova Scotia, Canada) for records of patients with “consistent burning mouth sensation without any signs of oral candidiasis” from January 1, 2017, to December 31, 2021. Totally 138 cases were collected, which were further sorted by the diagnostic criteria of BMS. The cases that didn't qualify for the BMS diagnostic criteria are non-BMS participants. Fig. 1 outlines the workflow of the case-control study. The diagnostic criteria of BMS applied in this study are referred from the BMS definition posted by WHO in 2018 [10], descriptions in an original research article [11], and a systemic review [1]. The diagnostic criteria of BMS are as follows: the patient has a consistent burning sensation of the tongue, lips, or entire oral mucosa over three months in the absence of any identifiable orofacial abnormality on physical examination, which includes oral mucosal diseases, salivary gland diseases, dental/denture irritation, fissured tongue, geographic tongue, cheilitis, and orofacial pain.
Fig. 1.
The workflow of the case-control study. BMS: burning mouth syndrome.
2.2. Investigation methods
The collected BMS and non-BMS participants fulfilled the basic requirements of having patients' medical histories, demographic information, results of cytology tests, hematology tests, and evaluations of objective dry mouth in the diagnostic period. Besides Vitamin (B9, B12, and D) levels, other results of hematology tests are required for the exclusion procedure. Nova Scotia Health Authority's Pathology and Medicine Laboratories issued hematology reports. Cytology tests reported the positive or negative results of fungal hyphae. Oral Pathology Biopsy Service of the Faculty of Dentistry of Dalhousie University issued cytology reports.
The criteria for confirming objective oral dryness were developed from the diagnostic evaluation methods for dry mouth induced by cancer therapy [14]. A positive result of objective oral dryness must satisfy at least three of the following five items and the Sialometry is the must-have: 1) Chief complaint: consistent dry mouth, sticky lips, and or tongue roughness. 2) Clinical examination: no salivation when milking major salivary glands and or bubbly saliva. 3) Sialometry in the clinic: unstimulated saliva volume <1.5 ml/15 min 4) Oral habits: open mouth-breathing, smoking, alcohol, cannabis, and or drinking too much coffee (> two cups of coffee per day) and too little water (< 1 L of water per day). 5) Offensive drugs: antidepressants, antihistamines, diuretics, and or anticholinergics that contribute to reduced saliva flow.
2.3. Inclusion and exclusion criteria
Fig. 2 depicts the process for applying inclusion and exclusion criteria. First, we excluded BMS participants that did not have all reports of medical history, hematology test, cytology test, and evaluation of objective oral dryness. And then, we threw out cases that had uncontrolled systemic diseases and correlative conditions reportedly associated with BMS. The list of excluded diseases and conditions was generated from review literature [1,4,10,12] and findings in an original research article [13]. Participants could have some common but well-controlled systemic diseases that include gastroesophageal reflux disease (GERD), hypertension (HTN), Diabetes Mellitus (DM), and hypothyroidism. Those are very common among the public and it is impossible to exclude all of them from this study.
Fig. 2.
The flow chart for collecting the eligible cases of burning mouth syndrome (BMS). The 1st step involved searching for cases with “consistent burning sensation without any signs of oral candidiasis” in the medical record system of the Oral Pathology Specialty Clinic (2017–2021), but only the cases that fit for the diagnostic criteria of BMS were collected. The 2nd step eliminated any cases with incomplete data, uncontrolled systemic diseases, and correlative conditions. The 3rd step insured that all the remaining cases included all the necessary data for the study.
The excluded uncontrolled systemic diseases were referred to GERD, HTN, DM, and hypothyroidism. The excluded correlative conditions were as follows: fibromyalgia, multiple sclerosis, Anemia, autoimmune conditions, renal and liver dysfunctions, and the administration of angiotensin-converting enzyme inhibitors or dopamine agonists that may cause burning mouth sensation. They were picked out not only by medical histories but also by the results of hematology tests, for example, uncontrolled DM (higher fasting glucose or HgA1c), uncontrolled thyroid disorders (abnormal thyrotropin (TSH) or thyroxin (T4)), Autoimmune conditions (higher antinuclear antibody (ANA), rheumatoid factor (RF), C-reactive protein (CRP), or abnormal numbers of white blood cells), Anemia (lower numbers of erythrocytes or hemoglobin or Iron or Ferritin), renal dysfunction (abnormal renal panel), and liver dysfunction (abnormal liver panel).
2.4. BMS group and non-BMS group
In total, 49 eligible BMS cases were selected according to our strict inclusion and exclusion criteria. Of them, 47 were secondary BMS cases based on the presence of one precipitating factor at least, while two were primary BMS cases being the absence of any precipitating factors and any well-controlled systemic diseases (Fig. 1, Fig. 2). Fig. 3(a–f) discloses three cases in the secondary BMS group that exhibit the lack of visible oral lesions and the presence of two or three precipitating factors.
Fig. 3.
Photos and pertinent information of three cases of secondary burning mouth syndrome (BMS). Case 1 (a and b): A 73-year-old female with well-controlled gastroesophageal reflux disease presented with a one-year history of burning mouth sensation along with Vitamin D deficiency (18.8 nmol/L), objective oral dryness, and the presence of fungal hyphae (arrow, PAS stain). Case 2 (c and d): A 57-year-old male with well-controlled hypertension and hypothyroidism presented with a six-month history of burning mouth sensation along with Vitamin D insufficiency (43.9 nmol/), objective oral dryness, and the presence of fungal hyphae (arrow, PAS stain). Case 3 (e and f): A 44-year-old healthy female presented with a three-month history of burning mouth sensation along with Vitamin D insufficiency (42.1 nmol/L), the presence of fungal hyphae (arrow, PAS stain), but without objective oral dryness.
The non-BMS group covered the following oral conditions: myalgia of the tongue that were caused by the shortness of lingual frenulum) (4 cases), oral lichenoid mucositis (4 cases), geographic tongue (2 cases), fissured tongue (2 cases), dysgeusia (1 case), plasmacytic gingivitis (1 case), and a traumatic ulcer (1 case). The exclusion and inclusion criteria of the non-BMS group are the same as the secondary BMS group (Fig. 1, Fig. 2). Only 15 eligible non-BMS cases had the complete results in hematology tests, cytology tests, and the evaluation of objective oral dryness. These tests were conducted as per their medical requests during the diagnostic period.
Apart from the different diagnoses, both groups applied the same exclusion and inclusion criteria (Fig. 1). The demographic baseline of the two groups is consistent. No significant difference was found in gender (female rate: 72.34% vs 80%), well-controlled systematic diseases (presence: 40.43% vs 40%), mean age (years old: 53.39 vs 62.93), and age range (years old: 25–95 vs 34–75) between secondary BMS and non-BMS groups.
2.5. Statistical analysis
Fisher's exact test was used to compare nominal variables between the two groups. A p-value ≤0.05 indicates statistical significance. The correlation between precipitating factors and their relations to secondary BMS was determined using Contingency Coefficients. A regression model in R version 4.20 software evaluated the prediction ability of three precipitating factors independently.
The statistical analysis was not performed on Vitamin B12 and B9 levels as no participant had an abnormal level of B9 and only two eligible secondary BMS cases had low values of B12 which were matched by low values of Vitamin D. Of the 49 eligible BMS cases, only two were primary. Hence, only 47 secondary BMS cases are analyzed statistically in the study (Fig. 1).
3. Results
3.1. The general distribution
Based on the consecutive medical records (2017–2011) in the Oral Pathology Specialty Clinic, the diagnostic BMS only shared 68.11% (94/134) of cases that had consistent burning sensations without any signs of oral candidiasis (Fig. 1, Fig. 2). Within the diagnostic BMS, only 2.13% (2/94) cases were primary BMS. Inside the eligible secondary BMS, only 4.25% (2/47) cases had a lower level of Vitamin B12. The abnormal level of Vitamin B9 was not identified in the secondary BMS group.
3.2. The comparison and the correlation
The incidence of fungal hyphae in the secondary BMS group (91.49%) and the non-BMS group (73.33%) had no significant difference (p = 0.0881), but the incidence of objective oral dryness (89.36% vs 46.67%, p = 0.0013) and a low value of Vitamin D (74.47% vs 26.67%, p = 0.0016) were significantly higher in the secondary BMS group compared with the non-BMS group (Table 2, Table 3). Similarly, all contingency coefficients correlating secondary BMS with precipitating factors are significant except for the presence of fungal hyphae (C = 0.166, p = 0.178). When looking at the correlation between individual precipitating factors and secondary BMS, a low value of vitamin D and objective oral dryness are equally correlated to secondary BMS (C = 0.362, 0.364, p = 0.002) (Table 3). Similarly, the pairs of precipitating factors containing a low value of Vitamin D show an equivalent correlation to secondary BMS (C = 0.432, 0.432, p < 0.001), while the pairs consisting of objective oral dryness and fungal hyphae have a relatively lower correlation to secondary BMS (C = 0.371, p = 0.001). The combined three precipitating factors have the highest correlation to secondary BMS of all groupings (C = 0.461, p < 0.001) (Table 3).
Table 2.
The distribution of three precipitating factors in secondary BMS and non-BMS groups.
|
1. Secondary BMS group (n = 47) |
Fungal hyphae (Cytology test) | Objective oral dryness | A low value of Vitamin D | Total number | Ratio (%) |
|---|---|---|---|---|---|
| Existence of three precipitating factors | Positive | Yes | Yes | 30 | 30/47 (63.83) |
| Only low Vitamin D value and fungal hyphae | Positive | No | Yes | 4 | 4/47 (8.51) |
| Only fungal hyphae and objective oral dryness | Positive | Yes | No | 8 | 8/47 (17.02) |
| Only low Vitamin D and objective oral dryness | Negative | Yes | Yes | 1 | 1/47 (2.13) |
| Only fungal hyphae | Positive | No | No | 1 | 1/47 (2.13) |
| Only objective oral dryness | Negative | Yes | No | 3 | 3/47 (6.38) |
| Only low Vitamin D value | Negative | No | Yes | 0 | 0/47 (0.00) |
| Total case number | 43 | 42 | 35 | – | |
| Ratio (%) - over secondary BMS cases | 43/47 (91.49) | 42/47 (89.36) | 35/47 (74.47) | ||
| 2. Non-BMS group (n = 15) | |||||
| Existence of three precipitating factors | Positive | Yes | Yes | 0 | 0/15 (0.00) |
| Only low Vitamin D value and fungal hyphae | Positive | No | Yes | 2 | 2/15 (13.3) |
| Only fungal hyphae and objective oral dryness | Positive | Yes | No | 5 | 5/15 (33.3) |
| Only low Vitamin D and objective oral dryness | Negative | Yes | Yes | 1 | 1/15 (6.67) |
| Only fungal hyphae | Positive | No | No | 5 | 5/15 (33.3) |
| Only objective oral dryness | Negative | Yes | No | 1 | 1/15 (6.67) |
| Only low Vitamin D value | Negative | No | Yes | 1 | 1/15 (6.67) |
| Total case number | 11 | 7 | 4 | – | |
| Ratio (%) - over non-BMS cases | 11/15 (73.33) | 7/15 (46.67) | 4/15 (26.67) | ||
Note BMS: burning mouth syndrome.
Table 3.
The comparison and correlation of three precipitating factors between secondary BMS and non-BMS groups independently, in pairs, and as a triple-factor. All have significant differences and correlations except the independent factor of the presence of fungal hyphae.
| Precipitating factors | Secondary BMS group Ratio (%) |
Non-BMS group Ratio (%) |
Fisher's exact test (two-tailed P value) | Contingency coefficients and significance |
|---|---|---|---|---|
| Independent factor | ||||
| Fungal hyphae positive | 43/47 (91.49) | 11/15 (73.33) | p = 0.0881 | C = 0.166, p = 0.178 |
| Objective oral dryness | 42/47 (89.36) | 7/15 (46.67) | p = 0.0013 | C = 0.364, p = 0.002 |
| A low value of Vitamin D | 35/47 (74.47) | 4/15 (26.67) | p = 0.0016 | C = 0.362, p = 0.002 |
| Double factors | ||||
| A low value of Vitamin D + Objective oral dryness | 31/47 (65.96) | 1/15 (6.67) | p < 0.0001 | C = 0.432, p < 0.001 |
| A low value of Vitamin D + fungal hyphae positive | 34/47 (72.34) | 2/15 (13.3) | p = 0.0001 | C = 0.432, p < 0.001 |
| Objective oral dryness + fungal hyphae positive | 38/47 (80.85) | 5/15 (33.3) | p = 0.0011 | C = 0.371, p = 0.001 |
| Triple factors | ||||
| Fungal hyphae positive + a low value of Vitamin D + objective oral dryness | 30/47 (63.83) | 0/15 (0.00) | p < 0.0001 | C = 0.461, p < 0.001 |
| Total case number | 47 | 15 | – | – |
Note BMS: burning mouth syndrome.
3.3. The regression and prediction
Using a two-step backward regression process, a low value of Vitamin D and objective oral dryness were found to be significant predictors of the diagnosis of secondary BMS. Each step of the logistic regression accounted for the same Nagelkerke R square (0.347) and predictability of the model (80%). The exponent with a P-value in the final step was 6.698 (p = 0.008) for a low value of Vitamin D and 6.655 (p = 0.009) for objective oral dryness.
3.4. The comparison of a low value of Vitamin D
A Canadian cohort study found that 59.73% of Canadians over 35 years old have a low value of Vitamin D (<75 nmol/L) and 2.3% were Vitamin D deficient (<27.5 nmol/L) [15]. Referring to these results it was found that the incidences of low (74.47%) and deficient (12.77%) Vitamin D levels were higher in the secondary BMS group than the national average (p = 0.0493, p = 0.001). Conversely, the rate of the low value of Vitamin D (26.67%) in the non-BMS group is even lower than the finding in Canadian adults (p = 0.0147). Table 4 exhibits the different ranges of Vitamin D in Canadian adults and the mean value of Vitamin D within each range of secondary BMS and non-BMS groups, which were analyzed for significance using Fisher's exact test.
Table 4.
Comparison of low Vitamin D levels between Canadian adults and two groups respectively. The second BMS group has significantly higher rates in the low value and the deficiency of Vitamin D. The non-BMS group has a significantly lower rate in the low value of Vitamin D.
| The ranges of Vitamin D (VD) value in Canadian Adults [15] | A. Case numbers in each range were generated from [15] | B. Secondary BMS group Note comparing corresponding cells between columns B and A. |
C. Non-BMS group Note comparing corresponding cells between columns C and A. |
||||
|---|---|---|---|---|---|---|---|
| VD value (mmol/L) | Ratio (%) | VD value (Mean) | Ratio (%) | Fisher's exact test | VD value (Mean) | Ratio (%) | Fisher's exact test |
| Normal: 76–200 | 770/1912 (40.27) | 85.41 | 12/47 (25.53) | P = 0.0493 | 118.75 | 11/15 (73.33) | P = 0.0147 |
| Abnormal: low < 75 | 1142/1912 (59.73) | 42.17 | 35/47 (74.47) | P = 0.0493 | 51.88 | 4/15 (26.67) | P = 0.0147 |
| Suboptimal 51-75 | 752/1912 (39.33) | 61.68 | 15/47 (31.91) | P = 0.3648 | 67.30 | 2/15 (13.33) | P = 0.0594 |
| Insufficiency 27.5–50 | 346/1912 (18.10) | 42.01 | 14/47 (29.79) | P = 0.0543 | 36.45 | 2/15 (13.33) | P = 1.000 |
| Deficiency <27.5 | 44/1912 (2.30) | 22.84 | 6/47 (12.77) | P = 0.001 | – | 0/15 (0.00) | P = 1.000 |
| Total case number | 1912 | 47 | 15 | ||||
Note BMS: burning mouth syndrome, VD: Vitamin D.
4. Discussion
4.1. Objective oral dryness and fungal hyphae accumulation
Candida Albicans is a commensal constituent of the normal microbiota in the oral cavity. Oral environmental stress triggers the gene-driven signal pathways to start a reversible transition from budding yeasts into a hyphae form [16]. Following the transition to hyphae, Candida must adhere for colonization and invasion into the mucosal epithelium leading to further epithelial damage [17]. This process along with transition is usually blocked by saliva, the epithelial barrier, and the innate and adaptive immune responses [8]. Saliva flow is the primary barrier to the adhesion and colonization of fungal hyphae on the oral mucosa and is assisted by saliva's antimicrobial components, including Lysozyme, Lactoferrin, Cathelicidins, Histatin, Calprotectin, and Defensins [18]. Objective oral dryness represents the deficit of the saliva flow following the cessation of the defense mechanism. The logistic regression found objective oral dryness (p = 0.009) was a significant predictor for the diagnosis of secondary BMS. The frequency (89.36%) of objective oral dryness in secondary BMS cases significantly differs from the non-BMS group (p = 0.0013). The contingency coefficient analysis showed the correlation (C = 0.364, p = 0.002) between objective oral dryness and secondary BMS as well (Table 3). This result is consistent with the finding in a previous study but the frequency of subjective oral dryness in BMS is 66% [2]. The difference could come from the different definitions for oral dryness (objective vs subjective) (Table 1). For instance, the objective oral dryness in our study includes cases who didn't feel mouth dryness (subjective oral dryness), but the Sialometry test found hyposalivation.
4.2. A low value of Vitamin D and fungal hyphae invasion
After the adhesion of fungal hyphae to the mucosal epithelium, the invading process continues via a hyphae-driven penetration and epithelial cell-driven endocytosis [17]. The essential contributor to penetrating the keratin layer of the oral epithelium is the secreted aspartic proteases (Saps) released from fungal hyphae, which degrade intercellular E-cadherin leading to a breakdown of epithelial integrity [18]. Vitamin D has been shown to enhance the intercellular bond by increasing the expression of RhoA and Ezrine proteins of vaginal mucosal epithelium via Vitamin D receptors (VDR) [19]. A histopathological study identified no significant differences between human buccal mucosa and vaginal mucosa in the epithelial pattern and the distribution of intercellular lipid lamellae [20]. Furthermore, Vitamin D upgrades the expression of Cathelicidin within epithelial cells and dendritic cells to enhance innate immunity via the intracellular VDR [21]. As such, a low value of Vitamin D corrupts the adequate host response against the fungal invasion that occurs due to the decreased intercellular connection of mucosal epithelium and the diminished innate immunity response.
Our study found that 74.47% of secondary BMS cases had a low value of Vitamin D, significantly higher than the incidences in the non-BMS group (p = 0.0016) (Table 3) and in the Canadian adults (Table 4). The contingency coefficient analysis showed the correlation (C = 0.362, p = 0.002) between a low value of Vitamin D and secondary BMS (Table 3). A former study found 14.9% of BMS cases had a low value of Vitamin D [5], but the article did not mention the low value of Vitamin D was lower than 75 nmol/L (the normal level) or 27.5 nmol/L (the deficiency level) (Table 1). At the same time, their discussion paragraph concluded that Vitamin D deficiency may be associated with BMS. If this is their point, the Vitamin D deficiency rate of secondary BMS (12.77%) in our study is quite close to their result (14.9%). Only 2.3% of Canadian adults maintained the Vitamin D deficiency status [15], however, it is significantly lower than the finding (12.77%) in secondary BMS (p = 0.001) (Table 4). This result further supports the conclusion that a low value of Vitamin D is correlated to secondary BMS. The logistic regression confirmed the low Vitamin D level (p = 0.008) as a significant predictor for the diagnosis of secondary BMS.
4.3. A low value of Vitamin D and a burning mouth sensation
The frequency of oral carriers of Candida Albicans in healthy dentate men is 44.4%, but only 5.5% of healthy subjects showed the presence of fungal hyphae [22]. Our study found that 91.49% of the secondary BMS cases have positive findings of fungal hyphae. However, there is no significant difference between the secondary BMS group and the non-BMS group and no correlation between the presence of fungal hyphae with the diagnosis of secondary BMS (Table 3). A recent study found that the frequency of Candida growth in the BMS group (21.4%) resembled the control group [6] (Table 1). They confirmed our findings, but they used culture tests, and we applied cytology tests. Thus, fungal hyphae can't cause consistent burning mouth sensations independent of other contributing factors. When the presence of fungal hyphae is combined with a low Vitamin D level, a consistent burning mouth sensation may emerge.
Vitamin D is an endogenous partial agonist of the transient receptor-potential cation channel subfamily V member 1 (TRPV1) and binds with the vanilloid-binding pocket to initiate calcium-induced desensitization of pain signals [23]. TRPV1 is a Vanilloid receptor subtype −1 (VR-1) present at the nerve endings of unmyelinated C-fibers that are responsible for sensing burning sensations. VR-1 has been found within the circumvallate, foliate, and fungiform papillae of the entire tongue of rats by penetrating the apical epithelium and the trench wall epithelium [24]. The partial blocking of the VR-1 sensitivity by Vitamin D limits the amount of pain felt by an induvial. In addition, the Vitamin D receptor is selectively present in rats’ unmyelinated calcitonin-gene-related peptide (CGRP)-positive neurons in dorsal root ganglions [25]. As such, normal levels of Vitamin D may downgrade pain perception by blocking the peripheral pathway (at nerve endings) and the central pathway (at neurons) together. A randomized clinical trial found that a topical oral Vitamin D gel did reduce the pain sensation of oral mucositis during radiotherapy [26]. As such, we propose a potential mechanism in that those fungal hyphae invasion initiates a burning sensation by disturbing VR-1 receptors within the mucosal epithelium. Coincidently the low value of Vitamin D strengthens the VR-1 sensitivity due to the lack of the agonist function in the peripheral region and the loss of control to CGRP-positive neurons in the central nervous system. This impaired inhibition upgrades a burning sensation from transient to consistent.
4.4. Intermediate status and three precipitating factors
Three precipitating factors in pairs or as a triple factor in the secondary BMS group extremely differ from the non-BMS group (p-value from 0.0011 to <0.0001) (Table 3). The correlation between secondary BMS and precipitating factors in pairs or as a triple-factor significantly differs from the non-BMS group as well (p-value from 0.001 to <0.001) (Table 3). The interdependent relationship of three precipitating factors seems obvious.
The host-fungal interaction leads to one of three consequences: healthy oral mucosa (non-infection), oral candidiasis (infection), or an intermediate status. Healthy oral mucosa is the result of a successful blockage of fungal invasion. In contrast, oral candidiasis results from a successful fungal invasion followed by visible epithelial damage. The intermediate status represents the stage where a compromised immunity prevents fungal hyphae from infecting but not invading. Fungal hyphae invade oral mucosa following their natural course, such as adhesins and secreted aspartic proteases [8]. The invasion is enhanced by objective oral dryness first, which ceases the defense against the adhesion and colonization of fungal hyphae and then low Vitamin D levels, which loosens the intercellular connection of mucosal epithelium and weakens the innate immune response. Further, the lower value of Vitamin D could amplify the burning sensation by losing the inhibition to the sensitivity of VR-1 at nerve endings and CGRP-positive neurons. The term, insidious Candida invasion that we proposed, may reflect the interdependent relationship of three precipitating factors and the way of developing a subset of a secondary BMS. Fig. 4 exhibits this hypothesis.
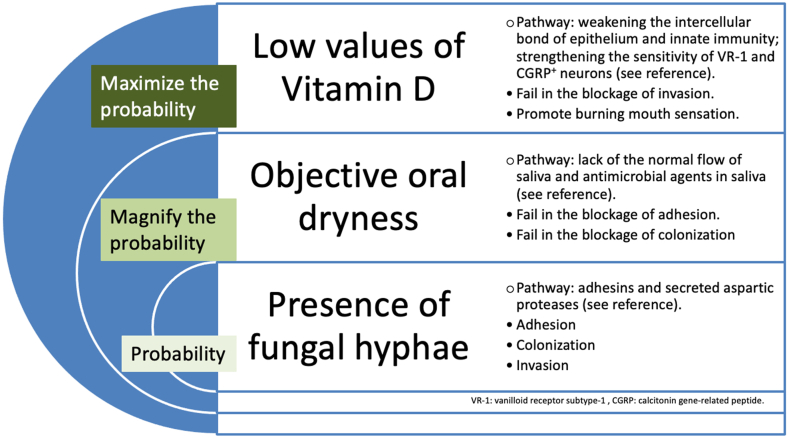
Fig. 4.
The hypothesis of insidious Candida invasion as a subset of secondary burning mouth syndrome. The fungal hyphae invade oral mucosa following their nature course, such as adhesins and secreted aspartic proteases [8,17,18]. Objective oral dryness magnifies the probability on account of the deficit of saliva flow and the lack of antimicrobial agents in saliva [8,18]. The low value of Vitamin D results in the weakness of the intercellular bond of mucosal epithelium and the innate immune response [9,19,21], also the strength of the sensitivity of VR-1 receptors and CGRP-positive neurons [23,25]. This maximizes the probability of fungal hyphae invasion and promotes a consistent burning mouth sensation.
The ICHD (3rd edition) indicated secondary BMS contributed to local or systemic disorders should be considered as an entity is a matter for debate [27]. We tried to extract three precipitating factors from multiple disorders to outline a subset of secondary BMS. Our findings may draw a picture that the burning sensation (in a subset, but not for all subsets of secondary BMS) is the sign of insidious Candida invasion, while hyposalivation and hypovitaminosis D aggravate the process. This study may initiate the debate again.
4.5. Remaining questions for three precipitating factors
Four secondary BMS cases had negative results of fungal hyphae in cytology tests, of which three only had objective oral dryness while the fourth had objective oral dryness plus a low value of Vitamin D. This may suggest that the four cases belong to another subset of secondary BMS defined by objective oral dryness and other types of deficiencies. A study from the Mayo Clinic reported frequencies of B2 (15%), B6 (5.3%), Zinc (5.7%), or B1 (5.3%) deficiencies in BMS cases [6]. Exploring these aspects was outside the scope of our study.
4.6. Limitations of the study
Due to restricted access to data, all secondary BMS participants (47 cases) came from an Oral Pathology Specialty Clinic in Canada. Our study results and conclusion were generated from the clinical and laboratory findings. The mechanism of the insidious Candida invasion is hypothesized mainly in the light of experimental studies. A multicenter and large-databased study is expected.
We would like to list the limitations in detail. This could be helpful for researchers to design a future study. 1. We didn't check the values of Zinc, and Vitamins B1, B2, and B6 because those are not common items in the laboratory requisition form here. 2. We didn't apply the Sialochemistry method to evaluate the qualitative salivary characteristics due to the lack of funds. We may lose cases that had a normal value in Sialometry but did have a qualitative abnormality in saliva (e. g. the lack of antimicrobial components). 3. We excluded correlative conditions including fibromyalgia, multiple sclerosis, and autoimmune diseases by the medical history and the hematology test. However, rheumatic diseases that affect salivation, the immune response, and or pain perception may not be identified in this way, especially before a definitive diagnosis. 4. Our study participants lived in a place with a northern latitude of over 45°, where the public can't have enough Vitamin D even through daily sunshine exposure because the best zone of having Vitamin D generation is within the latitude 35° between north and south. Hypovitaminosis D is a precipitating factor of secondary BMS in Canada but maybe not the one for BMS patients who live in the south. 5. The eligible 47 secondary BMS cases were extracted from a five-year consecutive database that held 138 patients who presented with burning mouth sensations without any diagnostic signs of oral candidiasis (please see Fig. 1). However, it is a relatively small size study. Hopefully, a large sample size study from multiple centers can repeat and reproduce the study findings.
5. Conclusion
The presence of fungal hyphae is a common event of oral mucosal diseases. Fungal hyphae only induce a consistent burning mouth sensation when coinciding with objective oral dryness, a low value of vitamin D, or both. The finding is proved by the correlation and regression analyses. We propose that a low value of vitamin D and objective oral dryness make an oral environment conducive to insidious Candida invasion that we define as a subset of secondary BMS. This intermediate status of the host-fungal interaction is characterized by a consistent burning mouth sensation without any visible oral lesions. The highest correlation of the presence of all three precipitating factors with secondary BMS further supports the hypothesis.
Author contribution statement
Yang Gu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Samuel Baldwin: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Carl Canning: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge our appreciation to Mr. Jialiu Wang. He was a clinical assistant and was responsible for collecting raw data of all BMS and non-BMS cases from the medical record system of the Oral Pathology Specialty Clinic.
References
- 1.Ariyawardana A., Chmieliauskaite M., Farag A.M., Albuquerque R., Forssell H., Nasri-Heir C., Miller C.S. World Workshop on Oral Medicine VII: burning mouth syndrome: a systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis. 2019;25(Suppl. 1):141–156. doi: 10.1111/odi.13067. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Bergdahi M., Bergdahi J. Burning mouth syndrome: prevalence and associated factors. J. Oral Pathol. Med. 1999;28:350–354. doi: 10.1111/j.1600-0714.1999.tb02052.x. 1999. [DOI] [PubMed] [Google Scholar]
- 3.IASP . 2016. IASP Orofacial Pain Fact Sheet. Burning Mouth Syndrome. [Google Scholar]
- 4.Scala A., Checchi L., Montevecchi M., Marini L. Update on Burning mouth syndrome: overview and patient management. Crit. Rev. Oral Biol. Med. 2003;14(4):273–291. doi: 10.1177/154411130301400405. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Verenzuela C.S.M., Davis M.D.P., Bruce A.J., Torgerson R.R. Burning mouth syndrome: results of screening tests for vitamin and mineral deficiencies, thyroid hormone, and glucose levels—experience at Mayo Clinic over a decade. Int. J. Dermatol. 2017;56:952–956. doi: 10.1111/ijd.13634. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Jankovskis Viktors, Selga Guntars. Candidiasis and other bacterial infections among patients diagnosed with burning mouth syndrome. Medicina. 2022;58(1029):e1–e16. doi: 10.3390/medicina58081029. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vila T., Sultan A.S., Montelongo-Jauregui D., Jabra-Rizk M.A. Oral candidiasis: a disease of opportunity. Journal of Fungi. 2020;6(15):e1–e28. doi: 10.3390/jof6010015. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebecker B., Naglik J.R., Hube B., Jacobsen I.D. Pathogenicity mechanisms and host response during oral Candida albicans. Expert Rev. Anti-infective Ther. 2014;12(7):867–879. doi: 10.1586/14787210.2014.916210. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Sassi F., Tamone C., D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(1656):1–14. doi: 10.3390/nu10111656. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsabbagh R., Ouanounou A. Burning mouth syndrome: etiology, clinical presentations, and treatment alternatives. Dentistry Review. 2022;2 doi: 10.1016/j.dentre.2022.100036. 2022. e1 - e6. [DOI] [Google Scholar]
- 11.McMillan R., Forssell H., Buchanan J.A.G., Glenny A.M., Weldon J.C., Zakrzewska J.M. Interventions for treating burning mouth syndrome. Cochrane Database Syst. Rev. 2016;(11):1–109. doi: 10.1002/14651858.CD002779.pub3. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurvits G.E., Tan A. Burning mouth syndrome. World J. Gastroenterol. 2013;19(50):665–672. doi: 10.3748/wjg.v19.i5.665. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netto F.O.G., Diniz I.M.A., Grossmann S.M.C., Guimaraes de Abreu M.H.N., Vieira do Carmo M.A., Aguiar M.C.F. Risk factors in burning mouth syndrome: a case-control study based on patient records. Clin. Oral Invest. 2011;15:571–575. doi: 10.1007/s00784-010-0419-5. 2011. [DOI] [PubMed] [Google Scholar]
- 14.AAOM clinical practice statement. Subjectivity: clinical management of cancer therapy-induced salivary gland hypofunction and xerostomia. 2016 doi: 10.1016/j.oooo.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Greene-Finestone L.S., Berger C., De Groh M., Hanley D.A., Hidiroglou N., Sarafin K., Goltzman D. 25-Hydroxyvitamin D in Canadian Adults: biological, environmental, and behavioral correlation. Ostoporos Int. 2011;22(5):1389–1399. doi: 10.1007/s00198-010-1362-7. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadosh D. Pathogenesis in Candida albicans. Curr. Opin. Microbiol. 2019;52:27–34. doi: 10.1016/j.mib.2019.04.005. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudbery P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hofs S., Mogavero H., Hube B. Interaction of Candida Albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016;54(3):149–169. doi: 10.1007/s12275-016-5514-0. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Lee A., Lee M.R., Lee H.-H., Kim Y.-S., Kim J.-M., Enkhbold T., Kim T.-H. Vitamin D proliferates vaginal epithelium through RhoA expression in postmenopausal atrophic vagina tissue. Mol. Cell. 2017;40(9):677–684. doi: 10.14348/molcells.2017.0026. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson I.O.C., Van der Bijl P., Van Wyk C.W., Van Eyk A.D. A comparative light-microscopic, electron-microscopic, and chemical study of human vaginal and buccal epithelium. Arch. Oral Biol. 2001;46:1091–1098. doi: 10.1016/s0003-9969(01)00082-6. 2001. [DOI] [PubMed] [Google Scholar]
- 21.Chung C., Silwal P., Kim I., Modlin R.L., Jo E.-K. Vitamin D-cathelicidin Axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Network. 2020;20(2):e1–e26. doi: 10.4110/in.2020.20.e12. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arendorf T.M., Walker D.M. The prevalence and intra-oral distribution of Candida Albicans in Man. Arch. Oral Biol. 1980;25:1–10. doi: 10.1016/0003-9969(80)90147-8. 1980. [DOI] [PubMed] [Google Scholar]
- 23.Long W.T., Johnson J., Kalyaanamoorthy S., Light P. TRPV1 channels as a newly identified target for vitamin D. Channels. 2021;15(1):360–374. doi: 10.1080/19336950.2021.1905248. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida Y., Ugawa S., Ueda T., Murakami S., Shimada S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Mol. Brain Res. 2002;107:17–22. doi: 10.1016/s0169-328x(02)00441-2. 2002. [DOI] [PubMed] [Google Scholar]
- 25.Tague S.E., Smith P.G. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J. Chem. Neuroanat. 2011;41:1. doi: 10.1016/j.jchemneu.2010.10.001. 2011. e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakr I.S., Zaki A.M., Ei-Moslemany R.M., Elsaka R.O. Vitamin D oral gel for prevention of radiation-induced oral mucositis: a randomized clinical trial. Oral Dis. 2021;27:1197–1204. doi: 10.1111/odi.13650. 2021. [DOI] [PubMed] [Google Scholar]
- 27.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders; Cephalalgia: 2018. pp. 1–178. 2018, 38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.