Abstract
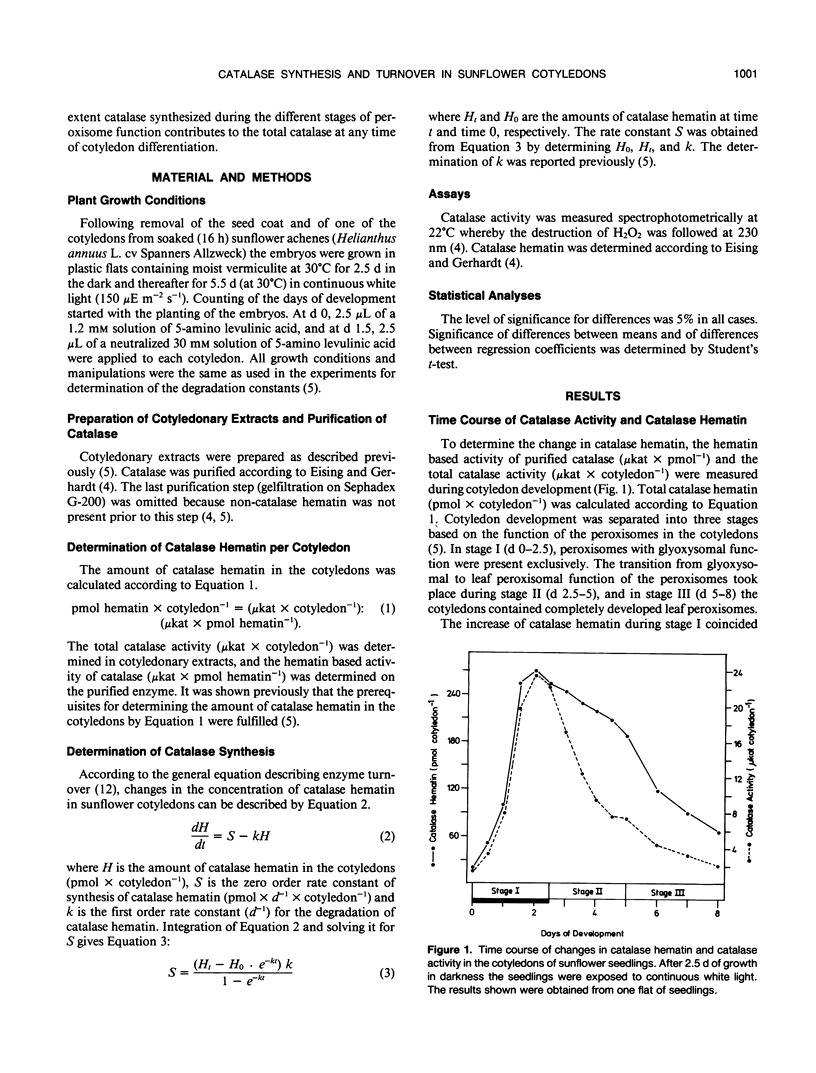
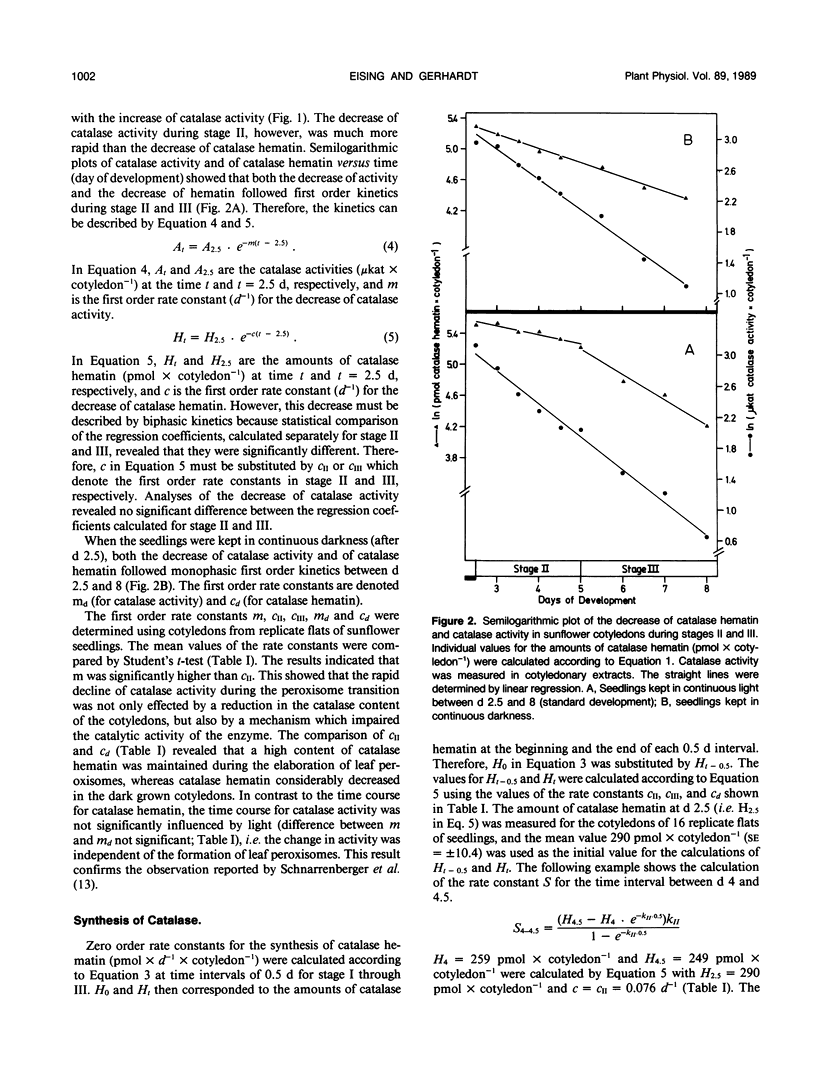
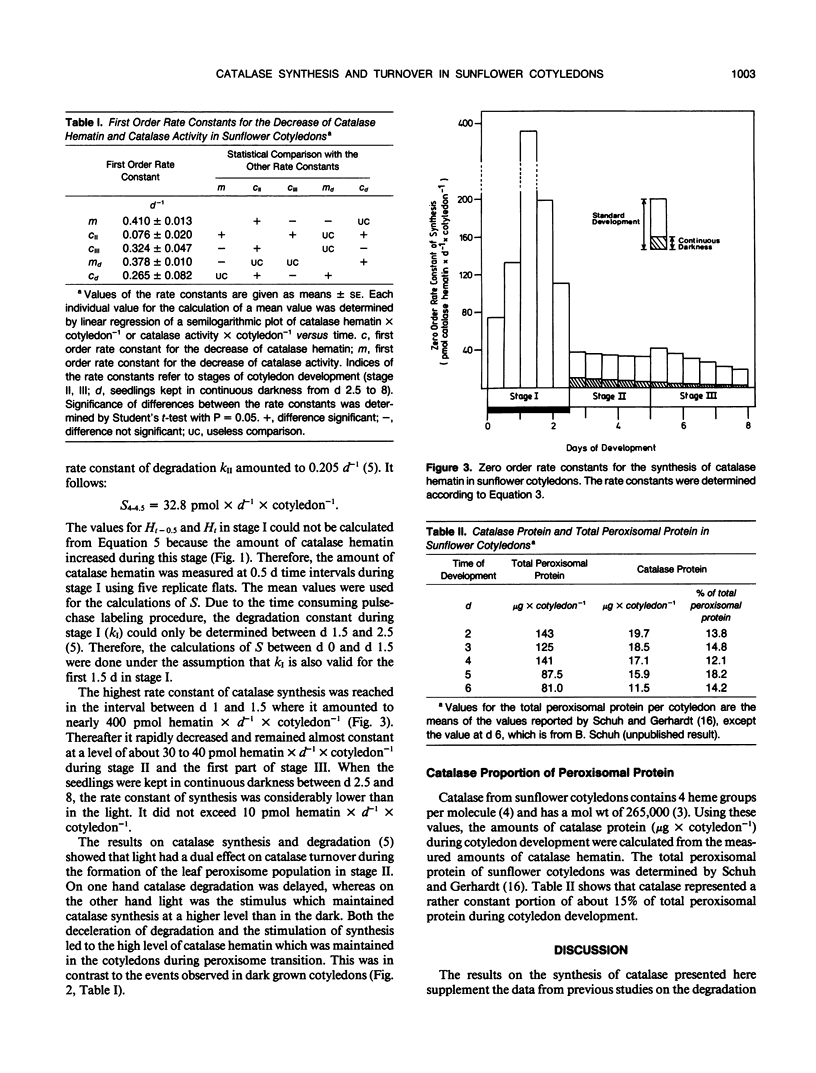
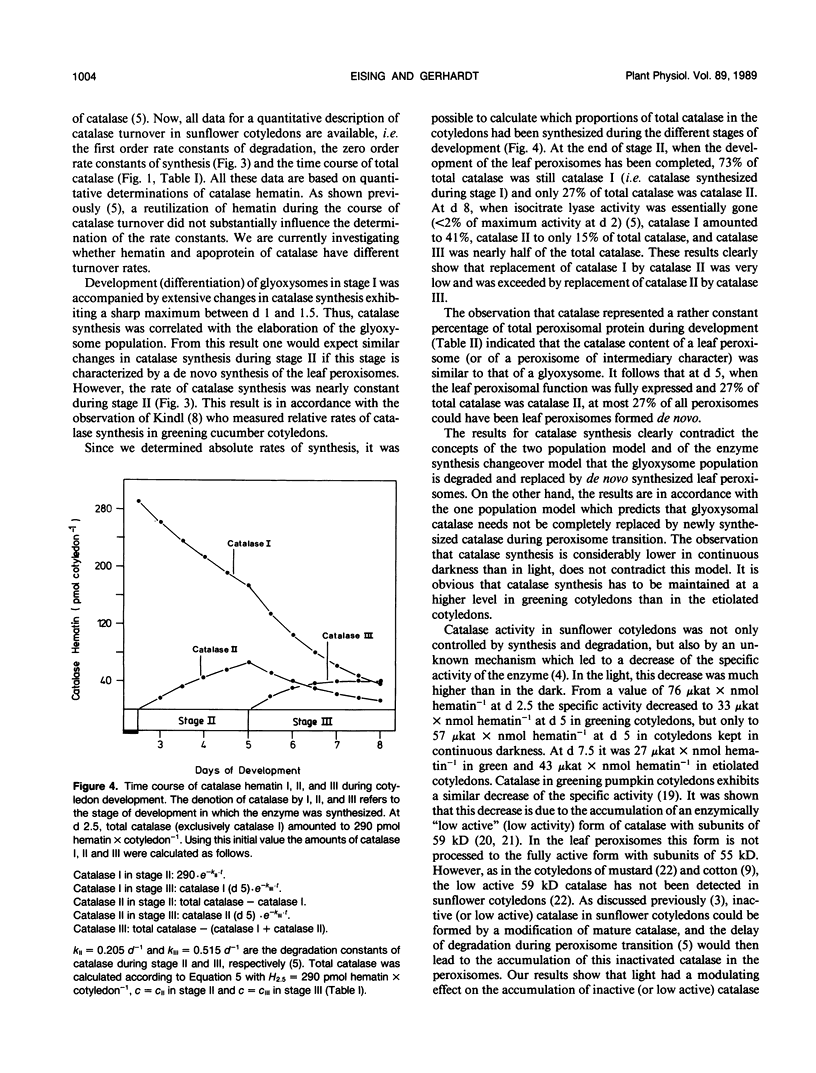
Based on measurements of total catalase hematin and the degradation constants of catalase hematin, zero order rate constants for the synthesis of catalase were determined during the development of sunflower cotyledons (Helianthus annuus L.). Catalase synthesis reached a sharp maximum of about 400 picomoles hematin per day per cotyledon at day 1.5 during the elaboration of glyoxysomes in the dark. During the transition of glyoxysomes to leaf peroxisomes (greening cotyledons, day 2.5 to 5) catalase synthesis was constant at a level of about 30 to 40 picomoles hematin per day per cotyledon. In the cotyledons of seedlings kept in the dark (day 2.5 to 5) catalase synthesis did not exceed 10 picomoles hematin per day per cotyledon. During the peroxisome transition in the light, total catalase hematin was maintained at a high level, whereas total catalase activity rapidly decreased. In continuous darkness, total catalase hematin decreased considerably from a peak at day 2. The results show that both catalase synthesis and catalase degradation are regulated by light. The turnover characteristics of catalase are in accordance with the concept that glyoxysomes are transformed to leaf peroxisomes as described by the one population model and contradict the two population model and the enzyme synthesis changeover model which both postulate de novo formation of the leaf peroxisome population and degradation of the glyoxysome population.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsche T., Gerhardt B. Apparent Catalase Synthesis in Sunflower Cotyledons during the Change in Microbody Function: A Mathematical Approach for the Quantitative Evaluation of Density-labeling Data. Plant Physiol. 1978 Oct;62(4):590–597. doi: 10.1104/pp.62.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising R., Gerhardt B. Catalase Degradation in Sunflower Cotyledons during Peroxisome Transition from Glyoxysomal to Leaf Peroxisomal Function. Plant Physiol. 1987 Jun;84(2):225–232. doi: 10.1104/pp.84.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. Lecithin synthesis during microbody biogenesis in watermelon cotyledons. Arch Biochem Biophys. 1975 Mar;167(1):45–53. doi: 10.1016/0003-9861(75)90439-7. [DOI] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N., Turley R. B. Purification and biosynthesis of cottonseed (Gossypium hirsutum L.) catalase. Biochem J. 1988 Apr 1;251(1):147–155. doi: 10.1042/bj2510147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Yamaguchi J., Mori H., Akazawa T., Yokota S. Immunocytochemical Analysis Shows that Glyoxysomes Are Directly Transformed to Leaf Peroxisomes during Greening of Pumpkin Cotyledons. Plant Physiol. 1986 May;81(1):313–316. doi: 10.1104/pp.81.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus D. E., Becker W. M. Investigation of the glyoxysome-peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J Cell Biol. 1985 Oct;101(4):1288–1299. doi: 10.1083/jcb.101.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Maturation of catalase precursor proceeds to a different extent in glyoxysomes and leaf peroxisomes of pumpkin cotyledons. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4809–4813. doi: 10.1073/pnas.81.15.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Purification and characterization of heme-containing low-activity form of catalase from greening pumpkin cotyledons. Eur J Biochem. 1986 Sep 1;159(2):315–322. doi: 10.1111/j.1432-1033.1986.tb09870.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M. Purification of glyoxysomal catalase and immunochemical comparison of glyoxysomal and leaf peroxisomal catalase in germinating pumpkin cotyledons. Plant Physiol. 1984 Feb;74(2):261–267. doi: 10.1104/pp.74.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]


