Abstract
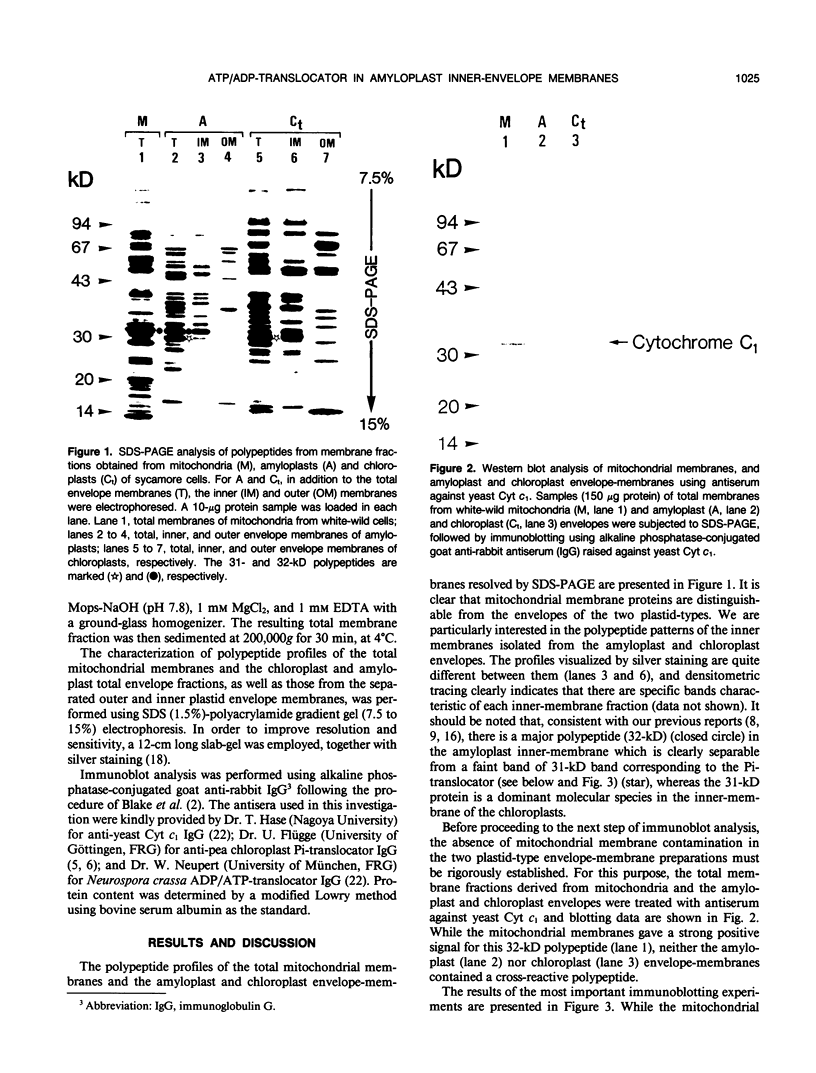
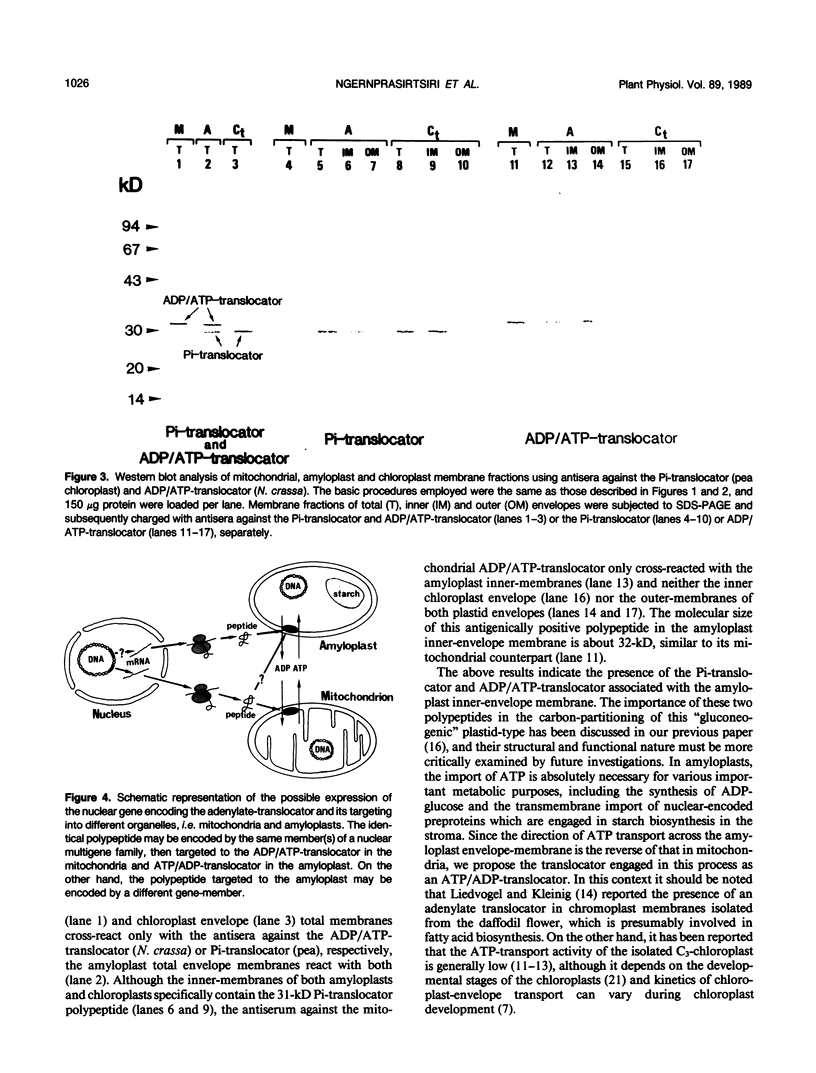
Pure preparations of intact amyloplasts and chloroplasts, free from mitochondrial contamination, were isolated from cultured cells of the white-wild and green-mutant lines of sycamore (Acer pseudoplatanus L.), respectively. A specific rabbit antiserum against yeast mitochondrial cytochrome c1 only cross-reacted with mitochondrial membranes from the white-wild sycamore cells. The outer and inner envelope-membranes of the two plastid-types were isolated and subsequently analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis to characterize polypeptide patterns in each fraction. Analysis by immunoblotting clearly showed that antiserum against the 29-kilodalton inorganic orthophosphate translocator isolated from pea chloroplasts cross-reacted with a 31-kilodalton polypeptide residing in the inner-envelope membranes from both sycamore chloroplasts and amyloplasts. In contrast, antiserum against the ADP/ATP-translocator isolated from mitochondria of Neurospora crassa yielded a positive signal with a 32-kilodalton polypeptide in the inner-membranes isolated from amyloplasts, but not green-mutant chloroplasts. We propose that this 32-kilodalton polypeptide in the amyloplast envelope is a putative ATP/ADP-translocator and its possible functional significance is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Robinson C. Protein import into organelles: hierarchical targeting signals. Cell. 1986 Aug 1;46(3):321–322. doi: 10.1016/0092-8674(86)90650-1. [DOI] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Hampp R. Kinetics of Membrane Transport during Chloroplast Development. Plant Physiol. 1978 Nov;62(5):735–740. doi: 10.1104/pp.62.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harinasut P., Takabe T., Akazawa T., Tagaya M., Fukui T. Characterization of an ATPase Associated with the Inner Envelope Membrane of Amyloplasts from Suspension-Cultured Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Sep;88(1):119–124. doi: 10.1104/pp.88.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Harinasut P., Macherel D., Strzalka K., Takabe T., Akazawa T., Kojima K. Isolation and Characterization of the Amyloplast Envelope-Membrane from Cultured White-Wild Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Jun;87(2):371–378. doi: 10.1104/pp.87.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Macherel D., Kobayashi H., Akazawa T. Expression of Amyloplast and Chloroplast DNA in Suspension-Cultured Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Jan;86(1):137–142. doi: 10.1104/pp.86.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O., Cannon L. E. Presecretory and cytoplasmic invertase polypeptides encoded by distinct mRNAs derived from the same structural gene differ by a signal sequence. Proc Natl Acad Sci U S A. 1982 Feb;79(3):781–785. doi: 10.1073/pnas.79.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Pfaller R., Neupert W. How finicky is mitochondrial protein import? Trends Biochem Sci. 1988 May;13(5):165–167. doi: 10.1016/0968-0004(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teintze M., Slaughter M., Weiss H., Neupert W. Biogenesis of mitochondrial ubiquinol:cytochrome c reductase (cytochrome bc1 complex). Precursor proteins and their transfer into mitochondria. J Biol Chem. 1982 Sep 10;257(17):10364–10371. [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Transport of proteins into mitochondria. Posttranslational transfer of ADP/ATP carrier into mitochondria in vitro. Eur J Biochem. 1980 Aug;109(1):217–229. doi: 10.1111/j.1432-1033.1980.tb04787.x. [DOI] [PubMed] [Google Scholar]