Abstract
Amoebiasis is a parasitic infection that represents a public health problem in developing countries including Asia and Latin America where it is endemic (1000–5000 cases/100,000 habitants/year). The majority of patients have an asymptomatic course; however, 10% of patients develop complications with high morbidity and mortality, such as colonic perforation or fulminant amoebic colitis. We report a case in which a 73-year-old female presented with an acute abdomen that was initially attributed to a bowel obstruction that rapidly progressed to fulminant colitis with bowel perforation requiring total colectomy. Pre-surgical endoscopic histopathological examination revealed findings suggestive of Entamoeba histolytica trophozoites that were later confirmed in the colon post-surgical specimen leading to a diagnosis of fulminant amoebic colitis. This atypical presentation of amoebiasis, further expands the already broad differential diagnosis of acute abdominal pathology in the elderly population. A high index of suspicion is required for its prompt treatment and to prevent life-threatening complications.
Keywords: Amoebiasis, fulminant colitis, colonic perforation, case report
Introduction
Amoebiasis is caused by Entamoeba histolytica and it is the second cause of death by parasites worldwide. 1 This parasite infects around 480 million people per year, it is endemic in developing countries and tropical climates like India, Mexico, Central American, and South America countries such as Ecuador, where it causes the death of 40,000–100,000 people per year. Ten percent of the world population is infected, 90% of whom are asymptomatic while the remaining 10% can present with symptoms like fever, bloody/watery diarrhea, and right upper quadrant pain. Only a small subset of symptomatic patients develop severe complications such as liver abscess and acute fulminant colitis (<0.5%–3%), 2 the latter carrying a mortality rate up to 75% 3 and presenting with signs and symptoms of acute abdomen. 4
The E. histolytica is acquired through fecal–oral route. Infection occurs following ingestion of the amoebic cysts, usually via contaminated food or water; these cysts hatch in the stomach and trophozoites emerge, continuing their replication by binary fission in the small intestine, and invading the epithelium followed by migration to other organs (i.e., liver, lung, and brain).3,5
Case report
A 73-year-old woman, of Ecuadorian indigenous ancestry, was evaluated in the emergency department with 2-day history of intense and diffuse colicky abdominal pain, with associated abdominal distension and inability to eliminate flatus. Initial vital signs were within normal limits and on physical examination was remarkable for signs of peritonism on the left lower quadrant of the abdomen. Paraclinical tests showed an increased polymerase chain reaction (307 mg/L), no leukocytosis (5600/mm3), no neutrophilia, no acidosis, and mild hypokalemia (3.3 mEq/L). Her past medical history included hypothyroidism, conventional cholecystectomy 20 years prior to presentation and no prior history of colitis or any gastrointestinal disease. In the context of surgical history and clinical picture, we presumed an obstructive abdomen. Simple and contrast-enhanced tomography of the abdomen and pelvis did not reveal a transition zone or pneumoperitoneum, and demonstrated a small amount of fluid in Douglas sac. A stool studies did not show parasites or blood.
At this time, there was no established diagnosis; non-surgical medical treatment was initiated (i.e., analgesia, bowel rest, and hydration), after which the patient showed clinical improvement; however, when the oral route was restarted (fourth day of hospitalization), the gastrointestinal disturbances recurred (i.e., pain, distension, and an inability to eliminate flatus).
A colonoscopy was performed on the seventh day of hospitalization and reported numerous mucosal patches, erosions, and edema localized at the sigmoid, descendant colon, and transverse colon. The lesions were biopsied and sent to pathology and microbiology. Sections under the microscope showed fragments of colonic mucosa with ulceration of the epithelium and a lymphoplasmacytic infiltration with eosinophiles, accompanied with edema, congestion, hemorrhage, and parasitic bodies with phagocyted red blood cells suggestive of E. histolytica trophozoites. The serum antibodies against E. histolytica on the seventh day of hospitalization were negative. With these findings, we started metronidazole 500 mg every 8 h orally.
Unfortunately, the patient’s evolution was unfavorable, with gradual worsening abdominal distension and pain as well as feeding intolerance. The laboratory evaluation remained overall stable with persistent mild hypokalemia (3.4 mEq/L), normal renal and liver function, and down trending leukocytes (4000/mm3). Repeat abdominal tomography on the 14th day of hospitalization demonstrated pneumoperitoneum, consisting with colonic perforation (Figure 1).
Figure 1.
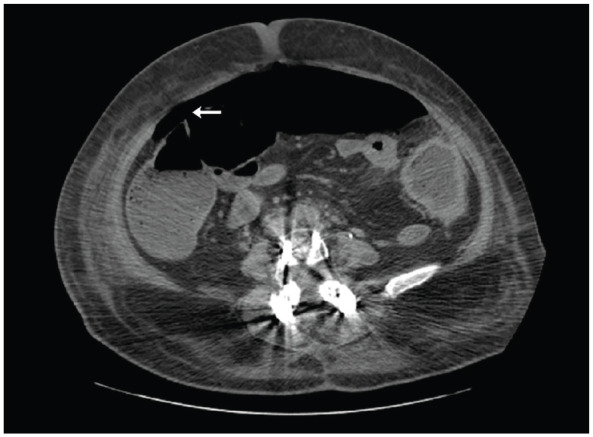
Simple and contrast-enhanced computed tomography in the late phase, axial slice at L3–L4 level. Pneumoperitoneum (←) is evidenced. Consisting with colon perforation.
An exploratory laparotomy was conducted revealing signs of fecal peritonitis in the left parietocolic groove, pneumoperitoneum, a phlegmonous descending colon, massive colon dilation (diameter of 10 cm), and the presence of multiple transmural perforations in the sigma, descending colon and transverse colon. A total colectomy with ileostomy and rectal stump closure were performed with no surgical complications in the transoperative period (Figure 2).
Figure 2.

Gross specimen: transverse colon showing irregular hemorrhagic adenomatous mucosa with multiple ulcerative lesions along its entire length.
The clinical course was complicated by hypotension in the context of sepsis that was managed in the intensive care unit with fluids and vasoactive drugs for 48 h. The post-operative antimicrobial treatment included meropenem (500 mg IV every 12 h for 7 days), metronidazole (500 mg IV every 12 h for 5 days, followed by 500 mg PO every 12 h) and tinidazole (2000 mg PO daily for 5 days). Another complication that arose was a superficial surgical site infection (7th post-operative day) due to multiresistant organisms: carbapenem-resistant Klebsiella pneumoniae and extended spectrum beta-lactamase producing Escherichia coli (E. coli). The patient was treated with colistin (polymyxin E) (300 mg IV loading dose and 150 mg every 12 h for 7 days), and the wound was opened to heal by secondary intention with serial dressing changes. Patient was discharged 30 days after surgery with no other major complications, and plan for late closure of ileostomy.
The histopathological report of the surgical anatomic piece showed evidence of mucosal ulceration throughout the entire colon that reached the submucosa in several areas. Significant edema and congestion with a rich inflammatory infiltrate with lymphocytes, plasmocytes and numerous eosinophils, and macrophages in a background of abundant necrotic areas and granulation tissue. E. histolytica trophozoites loaded with red blood cells were recognized in the entire bowel wall (Figure 3).
Figure 3.
Microscopic slice of the colonic wall with foci of mucosal ulceration that in areas reaches the submucosa (←), with edema and congestion. Parasitic forms corresponding to Entamoeba histolytica trophozoites (*).
Discussion
Around 90% of the infected people with E. histolytica do not develop symptoms; however, a small percentage of patients can develop fever and dysentery. Acute fulminant amoebic colitis is a rare but deadly complication of invasive intestinal amoebiasis.
Risk factors for a complicated amoebiasis infection are: >60-years-old, pregnancy, lack of access of potable water and sanitation, use of corticosteroids, malignancy, malnutrition, alcoholism, and the presence of chronic disease such as diabetes mellitus and chronic kidney disease. 6 Additionally, in the context of anal sex practices outbreaks of amoebiasis have also been reported. Acute fulminant amoebic colitis typically presents with fever, severe abdominal pain, and bloody/mucous diarrhea, toxic megacolon that ultimately complicates with peritonitis, bowel perforation and sepsis. Although our patient lives in an E. histolytic endemic area, the only risk factor for severe disease was her age, and she was presented with a sub-acute onset disease with no fever or other constitutional symptoms and a bowel obstruction picture.
The diagnosis of intestinal amoebiasis is typically made by serology assays and stool studies. The most sensitive assay is indirect hemagglutination test, although in endemic areas positive results could represent prior infection. 7 Microscopic examination of feces to observe cysts has low sensitivity (25%–60%); however, better results are obtained with stool culture (Robinson’s), stool real-time polymerase chain reaction (sensitivity 79% and specificity 96%), and stool antigen detection (ELISA) (sensitivity 55%–100% and specificity 93%–100%).3,5 In our patient, both the stool and serological studies were strikingly negative, which highlight the need of a high suspicion index of E. histolytica as the presumptive diagnosis in patients with acute abdomen in endemic areas. In this case the confirmation of the diagnosis was done after microscopic examination of the colon surgical specimen (Figure 3).
The treatment of amoebic colitis includes eliminating the cysts and trophozoites. For non-invasive disease treatment with intraluminal agents such as paromomycin, but for invasive and extra intestinal disease, the therapy includes systemic agents (i.e., metronidazole, secnidazole, nitazoxanide). 3 In our patient, oral metronidazole was initiated upon invasive amoebiasis was suspected; however, no improvement was noted given the rapid progression into fulminant colitis and toxic megacolon with perforation requiring emergent surgical intervention with total colectomy and intravenous antimicrobial therapy for E. histolytic and gram-negative enteric organisms.
The amoebic disease can present in the form of an acute abdomen, mimicking appendicitis, intestinal ischemic disease, tuberculosis, or malignancy. 8 An important differential diagnosis is inflammatory bowel disease, although this is more common in people between the ages of 15 and 30, with history of colitis and gastrointestinal disturbances. 9 The endoscopic and histological distinction between inflammatory bowel disease and amoebic colitis is difficult; however, the presence of trophozoites loaded with red blood cells is pathognomonic of invasive disease. 10
Amoebic colitis can evolve into a megatoxic colon and perforation of the intestines, with the development of septic shock as in this case.11,12 Tomography is the imaging method of choice for the diagnosis and assessment of its complications. Findings include thickening of the colonic wall, intestinal pneumatosis, and pneumoperitoneum if there is perforation. 13
Fulminant amoebic colitis usually requires prompt surgical intervention, the extent of the procedure depends on the characteristics of the patient and the severity of the disease. 2 When there is colonic perforation, as in our case, the reported mortality rate is up to 75%. 3
Conclusion
Amoebiasis is prevalent around the globe, it is endemic in several developing countries, especially in areas with poor sanitation. Thus, we should consider this pathogen when evaluating recent immigrants and travelers from those geographic locations and populations at risk. Amoebic colitis should be considered among the differential diagnoses of acute abdomen and a high index of suspicion is necessary to face this pathology, as not all patients present with the typical signs and/or symptoms like fever and dysentery. Education and promotion of essential sanitization practices that break the fecal–oral transmission route are necessary to control this disease and should be coupled with surveillance programs in endemic areas. Additionally, a thorough history and physical examination along with a risk factor analysis for each patient are of utmost importance for an appropriate and timely diagnosis and treatment, especially in the context of rare complications of endemic diseases.
Acknowledgments
Not applicable.
Footnotes
Author contributions: J.C.Y. and C.A.R.C. wrote the manuscript; J.C.Y., C.A.R.C., F.C-Y., and J.K.C.M. participated in the conception of the study; and all authors contributed to the analysis, interpretation and discussion of the data; all authors reviewed and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series. Within the policies of publication of scientific works of the Santa Barbara Hospital, the approval by the Ethics Committee is not required for the publication of descriptive works such as this Case Reports.
Informed consent: Written informed consent was obtained from the patient for the anonymized information to be published in this article. Hospital Santa Barbara, being a teaching hospital, as part of its policies requires a written informed consent from patients about the publication of anonymous information from medical records for purely academic purposes; as was in this case report.
ORCID iDs: Carlos Alberto Romero Cedeño  https://orcid.org/0000-0003-3197-6359
https://orcid.org/0000-0003-3197-6359
Javier Contreras-Yametti  https://orcid.org/0009-0007-4436-1499
https://orcid.org/0009-0007-4436-1499
References
- 1. Guzmán LJ, Molina GA, Cevallos JM, et al. Colonic perforation due to amebiasis, a rare and lethal complication. J Surg Case Rep 2018; 2018: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahadiani N, Habiburrahman M, Putranto AS, et al. Fulminant necrotizing amoebic colitis presenting as acute appendicitis: a case report and comprehensive literature review. J Infect Dev Ctries 2022; 16: 717–725. [DOI] [PubMed] [Google Scholar]
- 3. Kantor M, Abrantes A, Estevez A, et al. Entamoeba histolytica: updates in clinical manifestation, pathogenesis, and vaccine development. Can J Gastroenterol Hepatol 2018; 2018: 4601420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guillén N. Pathogenicity and virulence of Entamoeba histolytica, the agent of amoebiasis. Virulence 2023; 14: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali IKM. Intestinal amebae. Clin Lab Med 2015; 35: 393–422. [DOI] [PubMed] [Google Scholar]
- 6. Kaushik R, Singh S, Punia RS. Case report: Acute Fulminant Necrotising Amoebic Colitis. Trop Gastroenterol 2020; 41: 86–87. [Google Scholar]
- 7. Beg MY, Bains L, Mahajan R, et al. Fulminant necrotising amoebic colitis of whole of large bowel: a rare complication of a common infectious disease. Case Rep Infect Dis 2020; 2020: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinharay AR, Atkin GK, Mohamid W, et al. Caecal amoebic colitis mimicking a colorectal cancer. J Surg Case Rep 2011; 2011: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ekbom A, Helmick C, Zack M, et al. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology 1991; 100: 350–358. [DOI] [PubMed] [Google Scholar]
- 10. Young CA, Gracie DJ, Subramanian V, et al. Amoebic colitis. Diagnostic Histopathol 2017; 23: 563–565. [Google Scholar]
- 11. Chaturvedi R, Gupte PA, Joshi AS. Fulminant amoebic colitis: a clinicopathological study of 30 cases. Postgrad Med J 2015; 91: 200–205. [DOI] [PubMed] [Google Scholar]
- 12. Morris PD, Lee D, Chung KKY, et al. Fulminant amoebic colitis and septic shock in a returning traveller. ANZ J Surg 2019; 89: E50. [DOI] [PubMed] [Google Scholar]
- 13. Goel A, Bansal R, Kaur N, et al. Isolated sigmoid colon perforation in an unsuspected case of amoebic colitis. Indian J Med Spec 2015; 6: 168–169. [Google Scholar]



